- 1Department of Radiology, Functional and Molecular Imaging Key Lab of Shaanxi Province, Tangdu Hospital, Fourth Military Medical University (Air Force Medical University), Xi'an, China
- 2Department of Radiology, Gansu Hospital of Chinese Armed Police Force, Lanzhou, China
- 3Department of Radiology, The First Affiliated Hospital, Xi'an Jiatong University, Xi'an, China
- 4Department of Medical Technology, Shaanxi University of Chinese Medicine, Xianyang City, China
Objectives: To quantitatively summarize the specific changes in brain structure and function in migraine patients.
Methods: A literature screening of migraine was conducted from inception to Sept 1, 2022, in PubMed, Web of Science, Cochrane Library, and Medline databases using the keyword combination of “migraine and MRI.” Activation likelihood estimation (ALE) was performed to assess the differentiation of functional connectivity (FC), regional homogeneity (ReHo), and gray matter volume (GMV) of migraine patients.
Results: Eleven voxel-based morphometry (VBM) studies and 25 resting-state fMRI (rs-fMRI) studies (16 FC and 9 ReHo studies) were included in this study. ALE analysis revealed the ReHo increase in the brainstem and left thalamus, with no decreased area. Neither increased nor decreased regions were detected in FC and GMV of migraine patients.
Conclusions: The left thalamus and brainstem were the significantly activated regions of migraine. It is a meaningful insights into the pathophysiology of migraine. The consistent alterated brain areas of morphometrical and functional in migraine patients were far from reached based on current studies.
Introduction
Migraine is a complex neurological dysfunction characterized by recurrent attacks and pulsating headaches susceptible to physical or environmental factors. Broad clinical symptoms, such as nausea, vomiting, photophobia, and phonophobia etc., have been complained by suffers, with a headache duration ranging from 4 to 72 h (1). The estimated 1-year prevalence of migraine is about 15%, with a female-to-male ratio of 3:1 (2).
However, the underlying neuroimaging alterations in migraine patients have previously been studied using functional and structural MRI techniques, with inconsistent conclusions (3–5). Some studies reported the increased functional connectivity (FC) in prefrontal cortex, anterior cingulate cortex (ACC) (6), superior frontal gyrus, and temporal pole (7), while decreased FC in periaqueductal gray (PAG) (6), hypothalamus (8), ACC (9), temporal lobe (10), insular cortex (11) and amygdala (12). Among migraine patients, regional homogeneity (ReHo) was significantly increased in bilateral thalami, middle frontal gyrus and left insula (13), and decreased in putamen (14), cerebellum (15), and posterior cingulate cortex (PCC) (16). Meanwhile, voxel-based morphometry (VBM) studies suggested that brain gray matter volume (GMV) increased in PAG, bilateral fusiform gyri, and cingulate gyri (17), and decreased in cerebellar culmen (18), ACC, hippocampus (17), and orbitofrontal cortex (19).
If there are regions that both function and structure altered in migraine patients. Based on ReHo, amplitude low-frequency fluctuation (ALFF) and positron emission tomography (PET), meta-analysis demonstrated decreased activity in the angular gyrus, visual cortex, and cerebellum, while increased in the caudate, thalamus, pons, and prefrontal cortex (20). On the other hand, GMV decrease in posterior insular-opercular regions, the bilateral prefrontal cortex, and the anterior cingulate cortex were revealed with AES-SDM (3, 5). It is frustrating that a consistent conclusion was not drawn. Meanwhile, there still a lacks meta-analysis on the brain FC alterations in migraine patients. As more studies on the brain structure and function alterations in migraine patients have been published, it is urgent to perform a meta-analysis to draw a comprehensive conclusion including functional and structural studies.
Therefore, we conduct the current neuroimaging meta-analysis on brain structure and function changes in migraine patients, with the hope of drawing a solid conclusion.
Materials and methods
This study was registered on the PROSPERO (https://www.crd.york.ac.uk/PROSPERO/), with the registration number CRD42021257300.
Search strategy
A systematic literature search was conducted in the database of PubMed, Web of Science, Cochrane Library, and MEDLINE from inception to Sept 2022, according to Preferred Reporting Items for Systematic reviews and Meta-Analysis (PRISMA) (21). The subject terms and keywords, (“migraine” OR “primary headache”) AND (“magnetic resonance imaging” OR “neuroimaging” OR “fMRI”) AND (“structure” OR “voxel-based morphometry” OR “morphometrical” OR “functional connectivity” OR “regional homogeneity” OR “function”), were used to identify candidate VBM and rs-fMRI studies. Then, manual screening was conducted in the references of the retrieved studies and reviews.
Inclusion and exclusion criteria
Studies that meet the following criteria were eligible for inclusion in this meta-analysis: (1) migraine patients diagnosed according to the International Classification of Headache Disorders (ICHD) (1); (2) MRI studies employed morphometric approaches of VBM, or functional metrics of FC and ReHo; (3) seed-based FC to whole-brain compared patients with migraine with health controls (HC) group; (4) coordinates were reported in Montreal Neurological Institute space (MNI), or Talairach space, and (5) peer-reviewed. Multiple papers published by the same author were included following the criteria: including the largest number of participants, latest published ones, and reported coordinates underwent stringent correction.
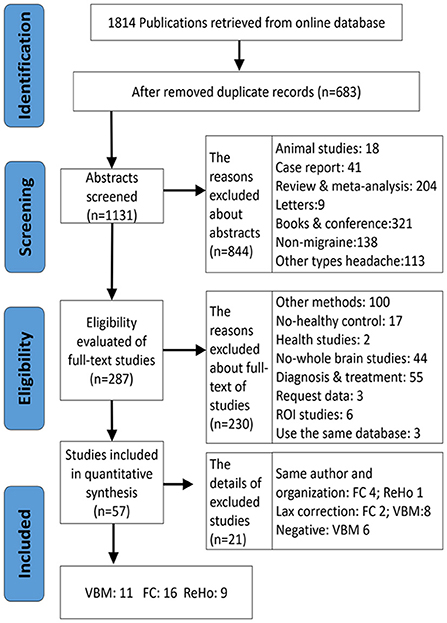
Exclusion criteria were as follows: (1) no HC group, (2) study was neither VBM nor FC and ReHo, (3) studies on the region of interest (ROI)-ROI, seed-ROI or independent component analysis (ICA), (4) intervention studies (pre/post-treatment contrasts such as transcranial magnetic stimulation or acupuncture), (5) seed-points or peak effect coordinates could not be retrieved, or (6) other types of migraine (e.g., vestibular migraine) and studies for comorbidities (Figure 1). We also excluded those studies that adopted lax statistical methods, like small volume correction (SVC) and uncorrected multiple comparisons.
Data extraction
Articles retrieval, assessment, and data extraction were independently implemented by two authors (CZH and SJT) according to the data extraction protocol. Any vagueness or disagreements were discussed with a third author (CYL), and a consensus was reached. The data information was sequentially collected, such as author, published year, sample size, characteristics of participants (e.g., age, gender, disease duration, and attacks), classification of migraine, and technical details (MRI scanner, seed regions, and correction methods, etc.). The peak coordinates of included studies were edited as available files according to the guidelines of AES-SDM 5.15 (http://www.sdmproject.com/) (22) or ALE 3.02 (http://www.brainmap.org/) (23). Talairach coordinates were translated into MNI via a toolbox provided by Ginger ALE.
Literature quality assessment
There is no consensus on the quality evaluation of neuroimaging studies up to now. We performed a customized checklist to assess the quality of included studies based on the assessment items of the Newcastle Ottawa Quality Assessment Scale (http://www.ohri.ca/programs/clinical_epidemiology/oxford.htm). The detailed items and scores of included studies are listed in Supplementary Tables S1, S2.
Activation likelihood estimation (ALE) analysis
ALE evaluated the significant convergence between peak effect foci from different trials (e.g., migraine > controls, migraine < controls) for a given study in comparison with a random distribution of foci. It treated reported significant foci as spatial probability distributions centered on given coordinates rather than as single points. ALE assesses the cumulative probabilities of each voxel based on reported foci. ALE map was acquired after calculating the union probabilities of each voxel. The true convergence of foci and random clustering was tested by permutation tests. Based on the sample size and random effect model, the likelihood of consensus among different experiments is attained. Each focus is modeled as the center of a Gaussian probability distribution. Then, the modeled activation (MA) map for each study is generated. We employed the recommendation setting of cluster-level family-wise error (FWE) (p < 0.05) to carry out multiple comparisons, using an initial cluster threshold of uncorrected p < 0.001, and permutation tests were 5,000 (23).
Sensitivity analysis
To assess the reliability and replicability of main resutls, we conducted a jackknife sensitivity analysis. The method was to repeat the process of removing one study and performing the others with the same meta-analysis at same threshold. If the main results remains significant in all or most of the combinations of the analysis, then it was regarded as rigorous.
Subgroup analysis
Subgroup analyses were performed to evaluate the consistency of findings and to eliminate latent factors affecting main results. We conducted subgroup analysis of patients with migraine without aura to exclud clinical and methodological heterogeniety.
Result
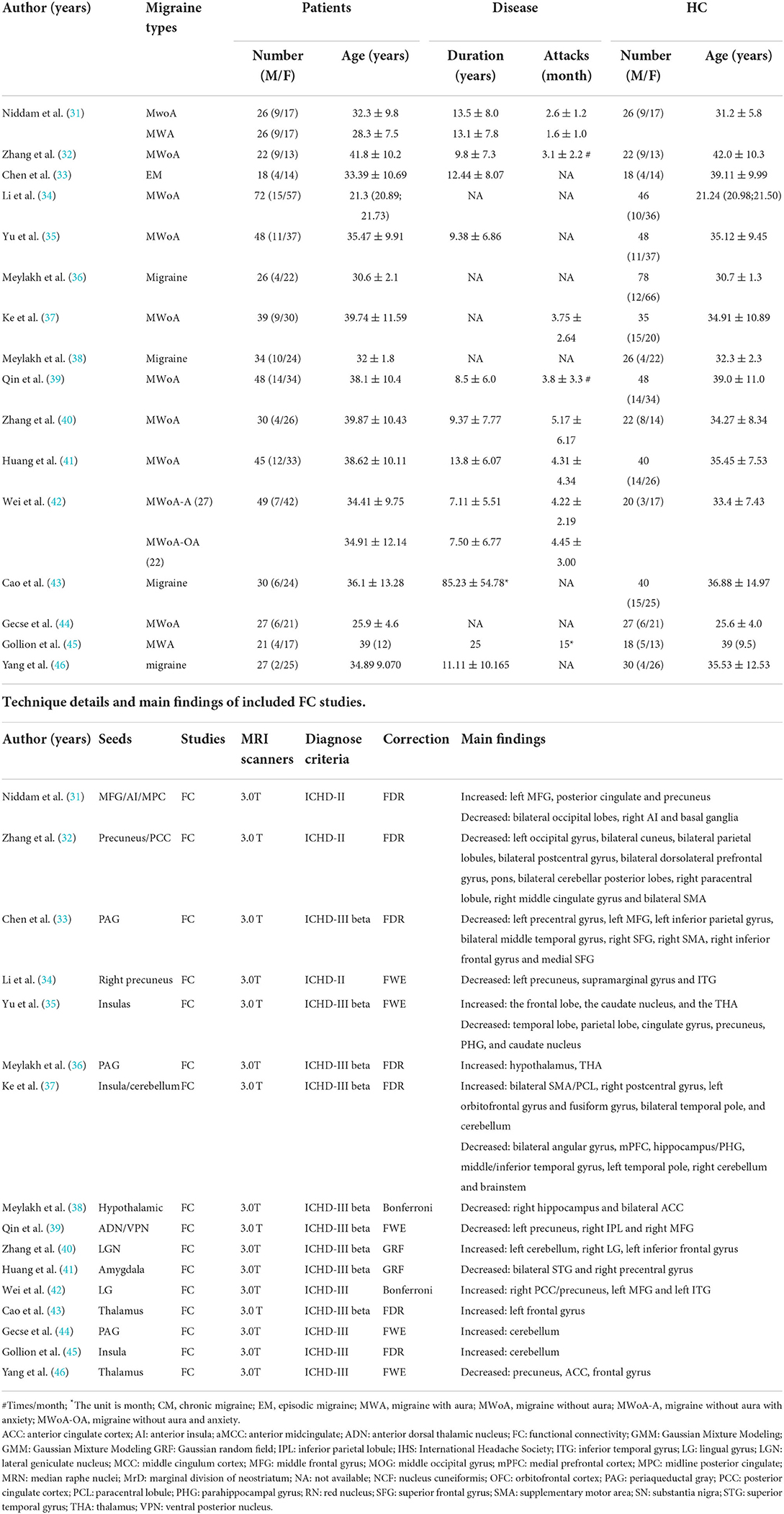
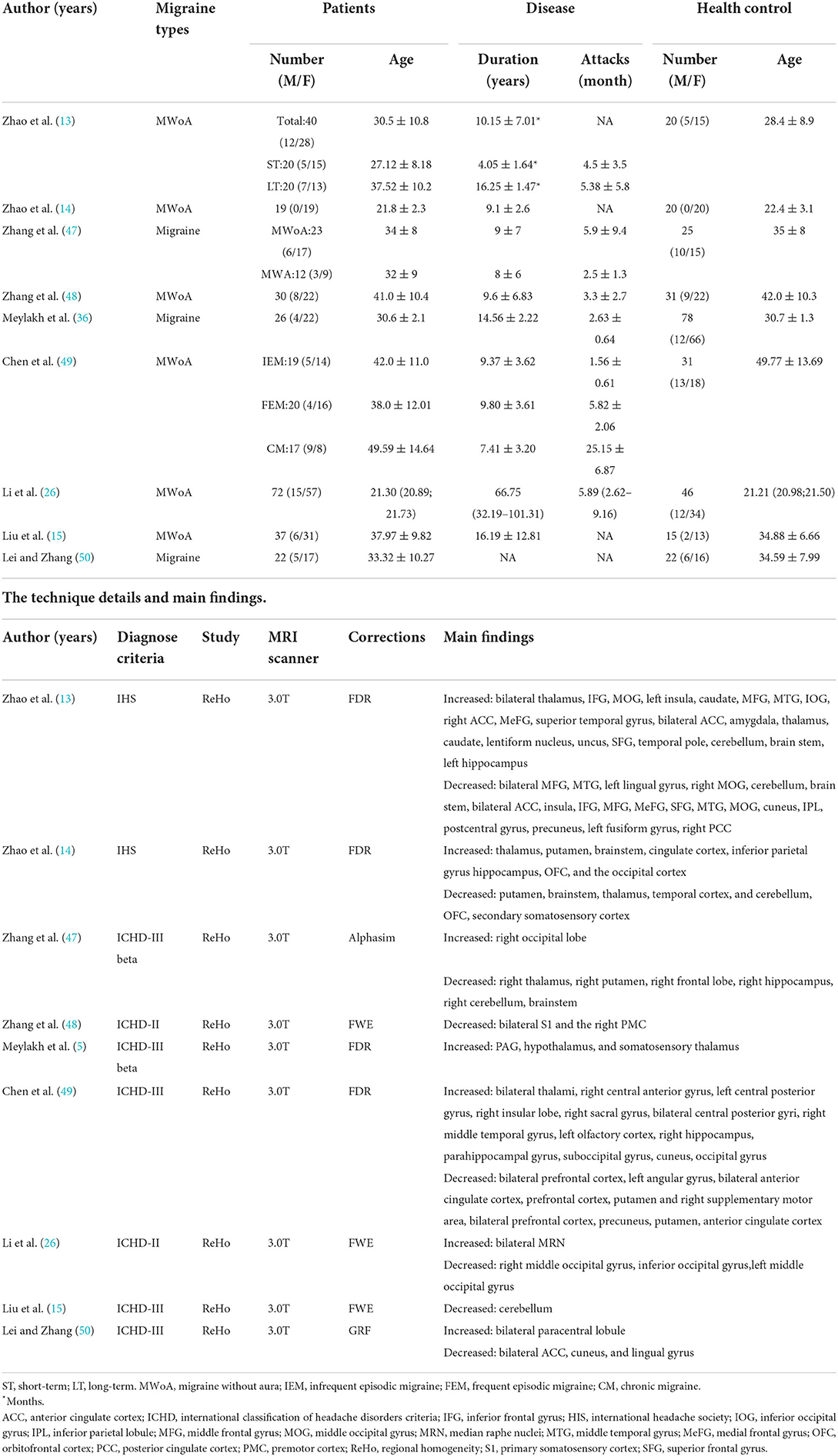
Thirty-nine MRI studies were included in this analysis, covering 11 VBM (Table 1), 16 FC (Table 2), and 9 ReHo studies (Table 3). It was comprised of 1,355 migraine patients (314 males and 1,041 females) and 1,149 (305 males and 844 females) HCs. Among them, VBM studies recruited 430 migraine patients (120 males, 328 females), and 317 HCs (93 males, 224 females); ReHo studies enrolled 337 migraine patients (77 males, 260 females), and 288 HCs (69 males, 219 females); FC studies included 588 migraine patients (135 males, 453 females), and 544 HCs (143 males, 401 females). All included structural and functional studies were performed statistical analyses for age and sex of included patients and controls (t-test or ANOVA, p < 0.05), individually. There were no significant differences in age and ratio of gender between migraine and HC, when the data were independently assessed. The preprocessing of fMRI images in all studies was performed by several steps, such as slice timing, realigning, normalizing, regressing nuisance covariates, filtering, and smoothing.
Brain function alterations
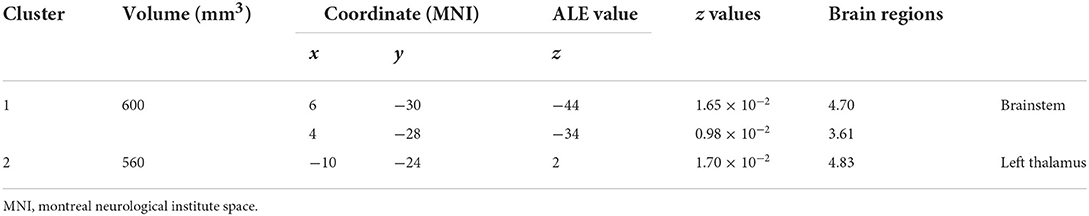
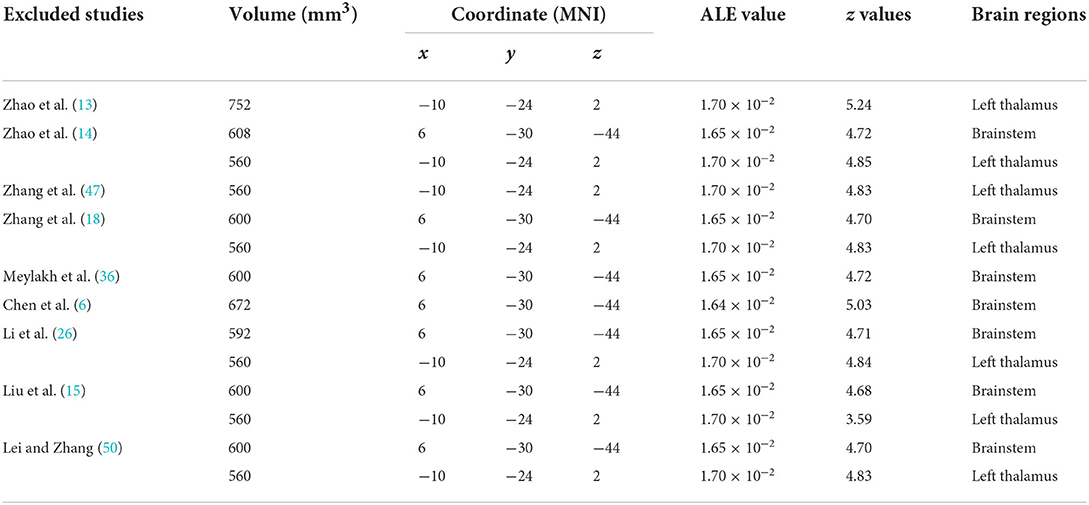
Using the coordinates of functional MRI studies to conduct ALE analysis, the ReHo values of left thalamus (MNI: −10, −24, 2; cluster volume 560 mm3) and brainstem (MNI: 6, −30, −44; 4, −28, −34; cluster volume 600 mm3) were increased, no decreased found. The changes of FC were not found (Figure 2, Table 4).

Figure 2. The spontaneous activity of ReHo in migraine patients. Using whole-brain overview and axial, sagittal, and coronal view, (A) illustrates the brainstem activating cluster (MNI: 6, −30, −44, 600 mm3); (B) shows the left thalamus activating cluster (MNI: −10, −24, 2, 560 mm3).
Brain structure alterations
No VBM alterations were found in this analysis.
Sensitivity analysis
ALE sensitivity analysis repeated the process of removing one study and performing the rest. We found that increased ReHo in brainstem and left thalamus was preserved throughout all studies, in spite of the most coordinates (80 foci) that reported by the Zhao's study (13) were not led to the instability of results (Table 5).
Subgroup analysis
According to the diagnosis classification of migraine, we performed subgroup meta-analysis of migraine patients and migraine without aura to establish the consistency of findings. No clusters were above the threshold.
Discussion
To our knowledge, this is the first study adopting functional and structural fMRI metrics to verify brain alterations (VBM, FC, and ReHo) in migraine patients. The solid conclusion is that the ReHo values of left thalamus and brainstem were consistently increased. While GMV and FC were not illustrated alterations in migraine in terms of current evidence.
Migraine is associated with various central nervous system disorders (2). Profoundly prolonged duration and recurrently attacked headache are the main complaints of migraine suffers (51). Thalamus is thought to have an essential role in the pathophysiology of migraine and has been investigated extensively (52–55). Meanwhile, the migraine genesis is more likely within brainstem, involving dysfunction and plasticity changes (56).
In migraine patients, pain information is transmitted from the meninges to the brain via the trigeminovascular pathway starting from trigeminal ganglion neurons (57). Specifically, the spinal trigeminal nucleus (SpV) neurons convey nociceptive signals to the brainstem (such as periaqueductal gray, reticular formation), hypothalamic, and basal ganglia. Then, the relay thalamic neurons project to the somatosensory, insular, motor, parietal association, auditory, visual, and olfactory cortices to construct the specific properties of migraine pain (58), for instance, nausea, vomiting, lacrimation, anxiety, and hypothalamic-regulated functions like appetite loosing and fatigue (56).
ReHo is specialized in explore local connectivity in a specific region by characterizing its relationship with nearby voxels in a specific region (59). Meta-regression analysis has indicated that migraine patients' visual analog scale score was associated with increased brain activity in the left thalamus (20). Using different meta-analysis method and sensitivity analysis, we also concluded that the left thalamus and brainstem of migraine patients were more spontaneous activated than HCs. Based on these evidences, we speculate that left thalamus and brainstem maybe the biological markers of nociceptive information transmission in frequent migraine attacks.
We postulated that if there is a certain region affected by long-term migraine, the functional connectivity changes of cerebral regions could be convergent at ones regardless of the chosen of the seed-points. Totally, 16 FC studies were included in this study. Among them, the seed-points were distributed the middle frontal gyrus (31), precuneus (32), PAG (33, 36, 44), insulas (35, 37, 45), thalamus (38, 43, 46), pons (26, 39), lateral geniculate nucleus (40), amygdala (41), and lingual gyrus (42). Although, the regions exhibited FC alteration among those studies, involving cortex about pain processing, visual, auditory, affective, and cognitive evaluation, there is no solid conclusion of pain information projecting of migraine temporarily according to our analysis.
Meanwhiles, the GMV changes of migraine assessed by VBM were heterogeneous between previous studies and meta-analysis (3–5). Now, a tendentious consensus of no structural brain alterations is more acceptable by researchers (60, 61). Furthermore, after rigorous literature screening, no morphometrical changes were detected with meta-analysis using different software.
Conclusion
The first quantitative coordinates meta-analysis of whole-brain neuroimaging studies for migraine that synthesized functional and structural MRI metrics, with the aim of providing the most comprehensive insights into brain impairments of migraine patients. Our meta-analysis suggested spontaneous cerebral activity in the left thalamus and brainstem, with no FC and GMV alterations. The findings may be served as the brain dysfunction clue of the underlying pathophysiology of migraine. In addition, neuroimaging meta-analysis, for reliable and robustness results, rigorous literature screening is prerequisite.
Limitation
Firstly, the heterogeneity analysis, and correlation analysis were not carried out due to the ALE software restriction. The number of included studies was insufficient to perform subgroup analysis. Secondly, unpublished studies (“gray studies”) were not included in our meta-analysis, which inevitably leads to publication bias. And the coordinates-based meta-analysis also has inherently biased, as it employs pooled stereotactic coordinates that are statistically significantly different, rather than raw data. Thirdly, this meta-analysis was limited to seed-based to whole-brain fMRI studies of FC, the studies using independent component analysis (ICA) and positron emission tomography (PET) approach not included.
Strengths and limitations of this study
• Functional and structural changes were evaluated simultaneously.
• Robust results were attributed to the rigorous processing analyses.
• More studies are needed to verify the changes in GMV.
Author contributions
Z-HC, Y-LC, J-TS, Y-TL, and CZ devoted to this study equally as the co-first authors. Z-HC wrote the original draft. Y-MZ, Z-YL, Y-XS, and M-HN were constrictive for data abstraction and software analysis for this study. BH and L-FY monitored the analysis procedure. WW supervised the overall procedure. All authors revised and approved the final manuscript.
Funding
This work was supported by the Hovering Program of Fourth Military Medical University (axjhww to WW), and the Talent Foundation of Tangdu Hospital (2018BJ003 to WW).
Acknowledgments
We would like to thank the authors of all included studies for their excellent work. Our gratitude also goes to Profs. Jin-Lian Li, Liang-Wei Chen, and Jun-Ling Zhu from the Department of Radiology of Tangdu Hospital for their constructive comments on this study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2022.1022793/full#supplementary-material
Abbreviations
ACC, anterior cingulate cortex; ALE, activation likelihood estimation; BA, brodmann area; CM, chronic migraine; DMN, default mode network; FC, functional connectivity; FEW, family wise error correction; GMV, gray matter volume; HC, healthy controls; ICA, independent components analysis; ICHD, international classification of headache disorders; HIS, international headache society; MA, modeled activation; MNI, montreal neurological institute space; MWA, migraine with aura; MWoA, migraine without aura; PAG, periaqueductal gray; PCC, posterior cingulate cortex; PHG, parahippocampal gyrus; PRISMA, preferred reporting items for systematic reviews and meta-analysis; ReHo, regional homogeneity; ROI, region of interest; rs-fMRI, resting-state fMRI; SpV, spinal trigeminal nucleus; SVC, small volume correction; VBM, voxel-based morphometry.
References
1. Arnold M. Headache Classification Committee of the International Headache Society (IHS) The International Classification of Headache Disorders, 3rd Edn. New York, NY: Cephalalgia (2018), pp. 1–211.
3. Jia Z, Yu S. Grey matter alterations in migraine: a systematic review and meta-analysis. Neuroimage Clin. (2017) 14:130–40. doi: 10.1016/j.nicl.2017.01.019
4. Masson R, Demarquay G, Meunier D, Leveque Y, Hannoun S, Bidet-Caulet A, et al. Is migraine associated to brain anatomical alterations? New data and coordinate-based meta-analysis. Brain Topogr. (2021) 34:384–401. doi: 10.1007/s10548-021-00824-6
5. Dai Z, Zhong J, Xiao P, Zhu Y, Chen F, Pan P, et al. Gray matter correlates of migraine and gender effect: a meta-analysis of voxel-based morphometry studies. Neuroscience. (2015) 299:88–96. doi: 10.1016/j.neuroscience.2015.04.066
6. Mainero C, Boshyan J, Hadjikhani N. Altered functional magnetic resonance imaging resting-state connectivity in periaqueductal gray networks in migraine. Ann Neurol. (2011) 70:838–45. doi: 10.1002/ana.22537
7. Tessitore A, Russo A, Giordano A, Conte F, Corbo D, De Stefano M, et al. Disrupted default mode network connectivity in migraine without aura. J Headache Pain. (2013) 14:89. doi: 10.1186/1129-2377-14-89
8. Qiu E, Tian L, Wang Y, Ma L, Yu S. Abnormal coactivation of the hypothalamus and salience network in patients with cluster headache. Headache. (2015) 55:1163. doi: 10.1212/WNL.0000000000001442
9. Jia Z, Chen X, Tang W, Zhao D, Yu S. Atypical functional connectivity between the anterior cingulate cortex and other brain regions in a rat model of recurrent headache. Mol Pain. (2019) 15:1744806919842483. doi: 10.1177/1744806919842483
10. Schwedt TJ, Berisha V, Chong CD. Temporal lobe cortical thickness correlations differentiate the migraine brain from the healthy brain. PLoS ONE. (2015) 10:e0116687. doi: 10.1371/journal.pone.0116687
11. Ellingsen DM, Garcia RG, Lee J, Lin RL, Kim J, Thurler AH, et al. Cyclic vomiting syndrome is characterized by altered functional brain connectivity of the insular cortex: a cross-comparison with migraine and healthy adults. Neurogastroenterol Motil. (2017) 29:e13004. doi: 10.1111/nmo.13004
12. Hadjikhani N, Ward N, Boshyan J, Napadow V, Maeda Y, Truini A, et al. The missing link: enhanced functional connectivity between amygdala and visceroceptive cortex in migraine. Cephalalgia. (2013) 33:1264–8. doi: 10.1177/0333102413490344
13. Zhao L, Liu JX, Dong XL, Peng YL, Yuan K, Wu FM, et al. Alterations in regional homogeneity assessed by fMRI in patients with migraine without aura stratified by disease duration. J Headache Pain. (2013) 14:1–9. doi: 10.1186/1129-2377-14-85
14. Zhao L, Liu J, Yan X, Dun W, Yang J, Huang L, et al. Abnormal brain activity changes in patients with migraine: a short-term longitudinal study. J Clin Neurol. (2014) 10:229–35. doi: 10.3988/jcn.2014.10.3.229
15. Liu S, Luo S, Yan T, Ma W, Wei X, Chen Y, et al. Differential modulating effect of acupuncture in patients with migraine without aura: a resting functional magnetic resonance study. Front Neurol. (2021) 12:680896. doi: 10.3389/fneur.2021.680896
16. Zhang YX, Chen H, Zeng M, He JW Qi GQ, Zhang SJ, Liu RB. Abnormal whole brain functional connectivity pattern homogeneity and couplings in migraine without aura. Front Hum Neurosci. (2020) 14:619839. doi: 10.3389/fnhum.2020.619839
17. Yu Y, Zhao HR, Dai LL, Su YY, Wang XM, Chen C, Shang YL, et al. Headache frequency associates with brain microstructure changes in patients with migraine without aura. Brain Imag Behav. (2021) 15:60–7. doi: 10.1007/s11682-019-00232-2
18. Zhang JL, Wu L, Su JJ, Yao Q, Wang MX, Li F, et al. Assessment of gray and white matter structural alterations in migraineurs without aura. J Headache Pain. (2017) 18:1–7. doi: 10.1186/s10194-017-0783-5
19. Chen WT, Chou KH, Lee PL, Hsiao FJ, Niddam DM, Lai KL, et al. Comparison of gray matter volume between migraine and “strict-criteria” tension-type headache. J Headache Pain. (2018) 19:4. doi: 10.1186/s10194-018-0834-6
20. Cai M, Liu J, Wang X, Ma J, Ma L, Liu M, et al. Spontaneous brain activity abnormalities in migraine: a meta-analysis of functional neuroimaging. Hum Brain Mapp. (2022). doi: 10.1002/hbm.26085
21. Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. (2009) 339:b2535. doi: 10.1136/bmj.b2535
22. Albajes-Eizagirre A, Solanes A, Fullana MA, Ioannidis JPA, Fusar-Poli P, Torrent C, et al. Meta-analysis of voxel-based neuroimaging studies using seed-based d mapping with permutation of subject images (SDM-PSI). J Vis Exp. (2019) 153:e59841. doi: 10.3791/59841
23. Eickhoff SB, Bzdok D, Laird AR, Kurth F, Fox PT. Activation likelihood estimation meta-analysis revisited. Neuroimage. (2012) 59:2349–61. doi: 10.1016/j.neuroimage.2011.09.017
24. Neeb L, Bastian K, Villringer K, Israel H, Reuter U, Fiebach JB. Structural gray matter alterations in chronic migraine: implications for a progressive disease? Headache. (2017) 57:400–16. doi: 10.1111/head.13012
25. Bonanno L, Lo Buono V, De Salvo S, Ruvolo C, Torre V, Bramanti P, et al. Brain morphologic abnormalities in migraine patients: an observational study. J Headache Pain. (2020) 21:25. doi: 10.1186/s10194-020-01109-2
26. Li Z, Zhou J, Lan L, Cheng S, Sun R, Gong Q, et al. Concurrent brain structural and functional alterations in patients with migraine without aura: an fMRI study. J Headache Pain. (2020) 21:141. doi: 10.1186/s10194-020-01203-5
27. Chou KH, Lee PL, Liang CS, Lee JT, Kao HW, Tsai CL, et al. Identifying neuroanatomical signatures in insomnia and migraine comorbidity. Sleep. (2021) 44:zsaa202. doi: 10.1093/sleep/zsaa202
28. Hubbard CS, Khan SA, Keaser ML, Mathur VA, Goyal M, Seminowicz DA. Altered brain structure and function correlate with disease severity and pain catastrophizing in migraine patients. eNeuro. (2014) 1:e20.14. doi: 10.1523/ENEURO.0006-14.2014
29. Cao Z, Yu W, Zhang Z, Xu M, Lin J, Zhang L, et al. Decreased gray matter volume in the frontal cortex of migraine patients with associated functional connectivity alterations: a VBM and rs-FC study. Pain Res Manag. (2022) 2022:2115956. doi: 10.1155/2022/2115956
30. Schading S, Pohl H, Gantenbein A, Luechinger R, Sandor P, Riederer F, et al. Tracking tDCS induced grey matter changes in episodic migraine: a randomized controlled trial. J Headache Pain. (2021) 22:139. doi: 10.1186/s10194-021-01347-y
31. Niddam DM, Lai KL, Fuh JL, Chuang CY, Chen WT, Wang SJ. Reduced functional connectivity between salience and visual networks in migraine with aura. Cephalalgia. (2016) 36:53–66. doi: 10.1177/0333102415583144
32. Zhang JL, Su JJ, Wang MX, Zhao Y, Yao Q, Zhang QT, et al. Increased default mode network connectivity and increased regional homogeneity in migraineurs without aura. J Headache Pain. (2016) 17:1–9. doi: 10.1186/s10194-016-0692-z
33. Chen ZY, Chen XY, Liu MQ, Liu SF, Ma L, Yu SY. Disrupted functional connectivity of periaqueductal gray subregions in episodic migraine. J Headache Pain. (2017) 18:1–9. doi: 10.1186/s10194-017-0747-9
34. Li ZJ, Lan L, Zeng F, Makris N, Hwang J, Guo T, et al. The altered right frontoparietal network functional connectivity in migraine and the modulation effect of treatment. Cephalalgia. (2017) 37:161–76. doi: 10.1177/0333102416641665
35. Yu ZB, Lv YB, Song LH, Liu DH, Huang XL, Hu XY, et al. Functional connectivity differences in the insular sub-regions in migraine without aura: a resting-state functional magnetic resonance imaging study. Front Behav Neurosci. (2017) 11:124. doi: 10.3389/fnbeh.2017.00124
36. Meylakh N, Marciszewski KK, Di Pietro F, Macefield VG, Macey PM, Henderson LA. Deep in the brain: changes in subcortical function immediately preceding a migraine attack. Hum Brain Mapp. (2018) 39:2651–63. doi: 10.1002/hbm.24030
37. Ke J, Yu Y, Zhang XD, Su YY, Wang XM, Hu S, et al. Functional alterations in the posterior insula and cerebellum in migraine without aura: a resting-state MRI study. Front Behav Neurosci. (2020) 14:567588. doi: 10.3389/fnbeh.2020.567588
38. Meylakh N, Marciszewski KK, Di Pietro F, Macefield VG, Macey PM, Henderson LA. Altered regional cerebral blood flow and hypothalamic connectivity immediately prior to a migraine headache. Cephalalgia. (2020) 40:448–60. doi: 10.1177/0333102420911623
39. Qin ZX, Su JJ, He XW, Zhu Q, Cui YY, Zhang JL, et al. Altered resting-state functional connectivity between subregions in the thalamus and cortex in migraine without aura. Eur J Neurol. (2020) 27:2233–41. doi: 10.1111/ene.14411
40. Zhang D, Huang XB, Su W, Chen YC, Wang P, Mao CN, et al. Altered lateral geniculate nucleus functional connectivity in migraine without aura: a resting-state functional MRI study. J Headache Pain. (2020) 21:1–9. doi: 10.1186/s10194-020-01086-6
41. Huang X, Zhang D, Wang P, Mao C, Miao Z, Liu C, et al. Altered amygdala effective connectivity in migraine without aura: evidence from resting-state fMRI with Granger causality analysis. J Headache Pain. (2021) 22:25. doi: 10.1186/s10194-021-01240-8
42. Wei HL, Li J, Guo X, Zhou GP, Wang JJ, Chen YC, et al. Functional connectivity of the visual cortex differentiates anxiety comorbidity from episodic migraineurs without aura. J Headache Pain. (2021) 22:40. doi: 10.1186/s10194-021-01259-x
43. Cao ZM, Chen YC, Liu GY, Wang X, Shi AQ, Xu LF, et al. Abnormalities of thalamic functional connectivity in patients with migraine: a resting-state fMRI study. Pain Ther. (2022) 11:561–74. doi: 10.1007/s40122-022-00365-1
44. Gecse K, Dobos D, Aranyi CS, Galambos A, Baksa D, Kocsel N, et al. Association of plasma tryptophan concentration with periaqueductal gray matter functional connectivity in migraine patients. Sci Rep. (2022) 12:739. doi: 10.1038/s41598-021-04647-0
45. Gollion C, Lerebours F, Nemmi F, Arribarat G, Bonneville F, Larrue V, et al. Insular functional connectivity in migraine with aura. J Headache Pain. (2022) 23:106. doi: 10.1186/s10194-022-01473-1
46. Yang Y, Xu H, Deng Z, Cheng W, Zhao X, Wu Y, et al. Functional connectivity and structural changes of thalamic subregions in episodic migraine. J Headache Pain. (2022) 23:119. doi: 10.1186/s10194-022-01491-z
47. Zhang X, Wang Z, Zhang Y, Zhang L, Geng Z, Ren L, et al. [Altered cortical and subcortical local coherence in migraine with and without aura:evidence from resting-state fMRI]. Zhonghua Yi Xue Za Zhi. (2015) 95:3196–200.
48. Zhang J, Su J, Wang M, Zhao Y, Zhang QT, Yao Q, et al. The sensorimotor network dysfunction in migraineurs without aura: a resting-state fMRI study. J Neurol. (2017) 264:654–63. doi: 10.1007/s00415-017-8404-4
49. Chen C, Yan M, Yu Y, Ke J, Xu C, Guo X, et al. Alterations in regional homogeneity assessed by fMRI in patients with migraine without aura. J Med Syst. (2019) 43:298. doi: 10.1007/s10916-019-1425-z
50. Lei M, Zhang J. Brain function state in different phases and its relationship with clinical symptoms of migraine: an fMRI study based on regional homogeneity (ReHo). Ann Transl Med. (2021) 9:928. doi: 10.21037/atm-21-2097
51. Goadsby PJ, Holland PR, Martins-Oliveira M, Hoffmann J, Schankin C, Akerman S. Pathophysiology of migraine: a disorder of sensory processing. Physiol Rev. (2017) 97:553–622. doi: 10.1152/physrev.00034.2015
52. Zhang L, Yu W, Xu M, Cui F, Song W, Yan M, et al. The hypothalamus may mediate migraine and ictal photophobia: evidence from Granger causality analysis. Neurol Sci. (2022) 43:6021–30. doi: 10.1007/s10072-022-06245-y
53. Tu Y, Fu Z, Zeng F, Maleki N, Lan L, Li Z, et al. Abnormal thalamocortical network dynamics in migraine. Neurology. (2019) 92:e2706–16. doi: 10.1212/WNL.0000000000007607
54. Coppola G, Di Renzo A, Petolicchio B, Tinelli E, Di Lorenzo C, Serrao M, et al. Increased neural connectivity between the hypothalamus and cortical resting-state functional networks in chronic migraine. J Neurol. (2020) 267:185–91. doi: 10.1007/s00415-019-09571-y
55. Wei HL, Zhou X, Chen YC Yu YS, Guo X, Zhou GP, et al. Impaired intrinsic functional connectivity between the thalamus and visual cortex in migraine without aura. J Headache Pain. (2019) 20:1–9. doi: 10.1186/s10194-019-1065-1
56. Ashina M, Hansen JM, Do TP, Melo-Carrillo A, Burstein R, Moskowitz MA. Migraine and the trigeminovascular system-−40 years and counting. Lancet Neurol. (2019) 18:795–804. doi: 10.1016/S1474-4422(19)30185-1
57. Charles A. The pathophysiology of migraine: implications for clinical management. Lancet Neurol. (2018) 17:174–82. doi: 10.1016/S1474-4422(17)30435-0
58. Burstein R, Noseda R, Borsook D. Migraine: multiple processes, complex pathophysiology. J Neurosci. (2015) 35:6619–29. doi: 10.1523/JNEUROSCI.0373-15.2015
59. Canario E, Chen D, Biswal B, A. review of resting-state fMRI and its use to examine psychiatric disorders. Psychoradiology. (2021) 1:42–53. doi: 10.1093/psyrad/kkab003
60. Mehnert J, Schulte L, May A. No grey matter alterations in longitudinal data of migraine patients. Brain. (2020) 143:e93. doi: 10.1093/brain/awaa300
Keywords: migraine, magnetic resonance imaging, meta-analysis, systematic review, function, structure
Citation: Chen Z-H, Cui Y-L, Sun J-T, Li Y-T, Zhang C, Zhang Y-M, Li Z-Y, Shang Y-X, Ni M-H, Hu B, Yan L-F and Wang W (2022) The brain structure and function abnormalities of migraineurs: A systematic review and neuroimaging meta-analysis. Front. Neurol. 13:1022793. doi: 10.3389/fneur.2022.1022793
Received: 19 August 2022; Accepted: 18 October 2022;
Published: 07 November 2022.
Edited by:
Lars Neeb, Charité Universitätsmedizin Berlin, GermanyReviewed by:
Alessandro Viganò, Fondazione Don Carlo Gnocchi Onlus (IRCCS), ItalySteffen Naegel, Klinik für Neurologie, Alfried Krupp Krankenhaus, Germany
Copyright © 2022 Chen, Cui, Sun, Li, Zhang, Zhang, Li, Shang, Ni, Hu, Yan and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wen Wang, d2FuZ3dlbkBmbW11LmVkdS5jbg==; NDAyMDQwMjRAcXEuY29t; Lin-Feng Yan, eWxmODM0MkAxNjMuY29t; Bo Hu, cmF5aGJAZm94bWFpbC5jb20=
†These authors have contributed equally to this work
 Zhu-Hong Chen
Zhu-Hong Chen Yu-Ling Cui
Yu-Ling Cui Jing-Ting Sun
Jing-Ting Sun Yu-Ting Li1†
Yu-Ting Li1† Yu-Xuan Shang
Yu-Xuan Shang Min-Hua Ni
Min-Hua Ni Bo Hu
Bo Hu Lin-Feng Yan
Lin-Feng Yan Wen Wang
Wen Wang