- 1Allied Health Research Unit, St Vincent's Health Network Sydney (SVHNS), Sydney, NSW, Australia
- 2Faculty of Health Sciences, Australian Catholic University, Sydney, NSW, Australia
- 3Sydney School of Health Sciences, Faculty of Medicine and Health, The University of Sydney, Camperdown, NSW, Australia
- 4The StrokeEd Collaboration, Ashfield, NSW, Australia
- 5Alfred Health, Melbourne, VIC, Australia
- 6Department of Neuroscience, Faculty of Medicine, Nursing & Health Sciences, Central Clinical School, Monash University, Melbourne, VIC, Australia
- 7School of Clinical Medicine, University of New South Wales, Sydney, NSW, Australia
- 8Department of Rehabilitation, St Vincent's Health Network Sydney (SVHNS), Sydney, NSW, Australia
- 9Physiotherapy Department, Waikato Hospital, Hamilton, New Zealand
- 10Arm's Reach Occupational Therapy, Bristol, United Kingdom
- 11Harrison's Training, Wiltshire, United Kingdom
- 12Centre for Behaviour Change, University College London, London, United Kingdom
- 13Melbourne Medical School, The University of Melbourne, Parkville, VIC, Australia
- 14Department of Rehabilitation, University of La Frontera, Temuco, Chile
- 15Nursing Research Institute, St Vincent's Health Network Sydney, St Vincent's Hospital Melbourne & Australian Catholic University, Sydney, NSW, Australia
Background: Difficulty using the upper extremity in everyday activities is common after stroke. Constraint-induced movement therapy (CIMT) has been shown to be effective in both sub-acute and chronic phases of stroke recovery and is recommended in clinical practice guidelines for stroke internationally. Despite reports of equivalence of outcome when stroke rehabilitation interventions are delivered using telehealth, there has been limited evaluation of CIMT when using this mode of delivery. ReCITE will (a) evaluate the feasibility and acceptability of CIMT when delivered via telehealth to stroke survivors (TeleCIMT) and (b) explore therapists' experiences and use of an online support package inclusive of training, mentoring and resources to support TeleCIMT delivery in clinical practice.
Methods: A prospective single-group, single blinded, study design with embedded process evaluation will be conducted. The study will be conducted at three outpatient services in Sydney, Australia. A multi-faceted therapist support package, informed by the Capabilities, Opportunity, Motivation- Behaviour model (COM-B), will be used to support occupational therapists to implement TeleCIMT as part of routine care to stroke survivors. Each service will recruit 10 stroke survivor participants (n = 30) with mild to moderate upper extremity impairment. Upper extremity and quality of life outcomes of stroke survivor participants will be collected at baseline, post-intervention and at a 4 week follow-up appointment. Feasibility of TeleCIMT will be evaluated by assessing the number of stroke participants who complete 80% of intensive arm practice prescribed during their 3 week program (i.e., at least 24 h of intensive arm practice). Acceptability will be investigated through qualitative interviews and surveys with stroke survivors, supporter surveys and therapist focus groups. Qualitative interviews with therapists will provide additional data to explore their experiences and use of the online support package.
Discussion: The COVID-19 pandemic resulted in a rapid transition to delivering telehealth. The proposed study will investigate the feasibility and acceptability of delivering a complex intervention via telehealth to stroke survivors at home, and the support that therapists and patients require for delivery. The findings of the study will be used to inform whether a larger, randomized controlled trial is feasible.
Introduction
Constraint-induced movement therapy (CIMT) is an intensive motor training approach used following stroke to overcome learned non-use of the upper limb (1, 2). CIMT involves three key components (1) high intensity, task orientated practice using the more affected arm for several hours a day for 2 to 3 weeks, (2) restraint of the unaffected arm using a mitt for up to 90% of waking hours to encourage use of the affected arm, and (3) use of a transfer package of monitoring mechanisms including behavioral contracts, a daily home diary, and daily homework to help participants generalize skills learned into daily life by using their affected arm more (3–5). High level evidence for CIMT exists, with statistically significant and clinically meaningful outcomes for people following stroke in both acute and chronic recovery stages (3, 6). CIMT is recommended as an effective intervention in clinical guidelines internationally for people with upper extremity impairment following stroke (1, 7, 8). The original model of CIMT required stroke survivors to participate in 6 h of daily task practice and to wear a mitt for 90% of waking hours (9). Since this trial, several different models of CIMT delivery have been used within trials which vary in intensity (2, 4, 5, 10–12), duration, group vs. one-on-one (13) and time post stroke (14), however the studies cited in guidelines have all been delivered face-to-face.
Adoption of CIMT has not been universal, and numerous barriers to delivery are cited in the literature (15–17) as well as patient-identified barriers to participation (18). Specifically, the barriers to patient participation include the time commitment and intensity of CIMT, while enablers to completing a CIMT program include support from therapists, carers/supporters and seeing improvements in arm function (19). Recent research has shown that patients (n = 40) receiving either in-person or virtual CIMT were “highly satisfied” with CIMT overall and found it only “moderately difficult” to participate in, based on survey data (20).
More broadly, stroke rehabilitation delivered via telehealth has been shown to attain similar outcomes to face-to-face delivery (21–23). While patients or participants are generally satisfied with this mode of delivery, the presence of family support may be a key variable influencing the success of telehealth interventions (24). The delivery of CIMT using telehealth may address patient barriers to attending the clinic to receive this intensive intervention, but may equally introduce further challenges or be found to be less effective. A previous study of patients completing face-to-face CIMT at home with family supporters and minimal therapist support (5 h total) showed no additional benefit compared to usual upper extremity therapy, suggesting that therapist support may play a key role in CIMT's effectiveness (25). Additionally, the complete transfer package was not used in that study, which may have been another factor contributing to the lack of treatment superiority (25). Other randomized controlled trials of home based CIMT have relied on specifically designed workstations and software (26–28) or gaming devices (29, 30) to deliver CIMT in the home. The use of such devices has shown promise, however may not be feasible to use in practice in health services servicing geographically isolated clients, such as those in regional or remote areas of Australia. What remains unknown is whether CIMT delivered via telehealth (TeleCIMT) can be implemented within usual care, using existing resources and remote therapist support and without the use of specialized equipment, other than a device with internet and video functionality.
The experience of telehealth from the perspective of clinicians has been less favorable (31). Delivering an intervention using telehealth changes workflows and processes, and interventions need to be adapted (32). This raises many barriers, principally time, skills and resources, with clinicians generally preferring to deliver interventions face-to-face (24). The use of behavior change theory can support the implementation of complex health interventions including CIMT (33). A previous study by members of this research group, the ACTIveARM project (34) (published thesis), used a multimodal behavior change intervention targeted at clinicians to successfully increase face-to-face CIMT delivery in public health settings. A behavior change intervention including education of clinicians, provision of resources and support to help them adapt their practice, has the potential to reduce identified barriers to telerehabilitation.
The COVID-19 pandemic impacted the delivery of intensive face-to-face rehabilitation interventions, including CIMT. During this period, the TeleCIMT International Development (TIDE) group of occupational therapists, physiotherapists and researchers from Australia, United Kingdom and New Zealand developed resources to support CIMT delivery via telehealth (TeleCIMT) (35). The free resources for therapists and stroke survivors include educational webinars and videos, step-by-step CIMT practice booklets and exercise libraries (35). While these resources are informed by evidence, their use in clinical practice, the safety of patient participants to complete TeleCIMT, and experiences of TeleCIMT for therapists, stroke survivors and their supporters, have not yet been evaluated.
Therefore the Remote Constraint Induced Therapy of the upper Extremity (ReCITE) pilot will investigate the feasibility and acceptability of CIMT via telehealth, within routine practice. In relation to feasibility, the study will explore;
- Whether it is feasible and safe for community stroke survivors to complete at least 80% of a 3 week TeleCIMT program at home (i.e., 24 h of intensive upper extremity therapy) with remote therapist support
- The clinical outcomes of delivery of CIMT via telehealth
In relation to acceptability, the study will explore:
- The acceptability of TeleCIMT delivery to stroke survivors and their supporters
- Whether therapy teams adopt and deliver CIMT programs via telehealth after receiving a TeleCIMT online therapist support package and the barriers and enablers to TeleCIMT delivery in practice.
Method
Design
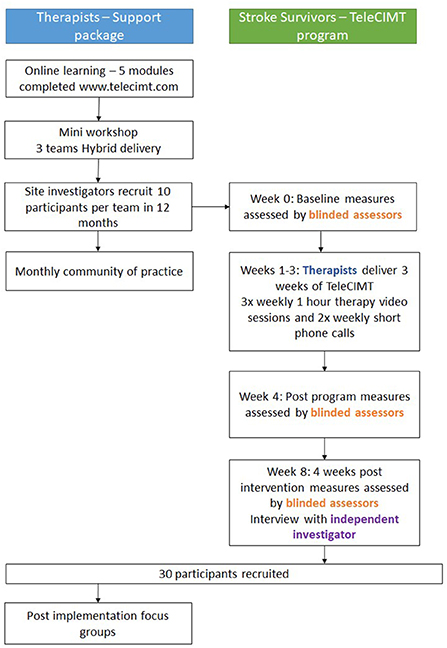
A prospective, single blinded, pre-test post-test study design will be used. To support rigor in this non-randomized design, we will use blinded outcome assessment and embedded qualitative evaluation. The study design is presented in Figure 1, guided by the Consolidated Standards of Reporting Trials (CONSORT) statement (36). The study is reported according to Standard Protocol Items: Recommendations for Interventional Trials (SPIRIT) (37), Standards for Reporting Implementation Studies (StaRI) Statement (38) and the TIDieR framework for describing evidence-based interventions (39).
The study is registered with the Australian New Zealand Clinical Trials Registry (ACTRN12622000082707). Any changes to the trial protocol will be reported. This study was approved by the St Vincent's Hospital and South Western Sydney Local Health District Human Research Ethics Committees (2021/ETH01131).
Setting and participants
This study will involve a sample of three selected public hospital outpatient rehabilitation services within metropolitan Sydney, Australia. Services typically offer publicly funded outpatient occupational therapy and physiotherapy sessions to sub-acute and chronic stroke survivors for 6 to 8 weeks post discharge from hospital.
There will be two groups of participants: therapists and stroke survivors.
Therapist participants
Therapist participants (n = 12) will be occupational therapists currently employed at one of the three outpatient rehabilitation services and involved in delivery of the TeleCIMT program.
Stroke survivor participants
All stroke survivor participants who present for outpatient upper extremity rehabilitation at participating sites will be screened by therapist site investigators during routine therapy. Eligibility criteria are outlined in Box 1. All participants will have been discharged home from hospital and therefore will be either in the subacute or chronic phase post stroke. There are no restrictions placed on maximum time post stroke for study inclusion. Once screened and deemed eligible for study inclusion, potential participants will be provided with an information sheet and consent form. Once consented, baseline assessments will be scheduled with trained, blinded assessors and completed prior to intervention commencement.
Box 1. Inclusion criteria for stroke survivor participants.
1) 18 years or older.
2) Confirmed diagnosis of stroke resulting in mild to moderate upper extremity impairments as measured by the Motor Assessment Scale item 8 (40).
3) Meet CIMT upper limb criteria including recovery of some active finger extension in at least two digits, some active thumb extension and some active wrist extension in the affected upper limb (1, 2, 41). Participants must have at least 10° of active wrist extension, at least 10° of thumb abduction/extension, and at least 10° of extension in at least 2 additional fingers. These movements must be repeated 3 times in 1 min.
4) Medically stable.
5) Able to participate in intensive rehabilitation at least 2 h per day for 3 weeks.
6) Able to read, speak and understand English.
7) Aphasia severity rating (ASR) score of at least three (42).
8) Have adequate cognition to participate in a CIMT program with the assistance of a supporter as determined by a Montreal Cognitive Assessment (MoCA) score of 18 to 25 or with or without a supporter as determined by a MoCA score of 26 and above (range 0–30) (43).
9) Sufficient reading acuity as measured by the Occupational Therapy Adult perceptual screening test—visual acuity item) (44).
Intervention
Therapist support package
The ReCITE therapist support package will be tailored to each therapy team, and support them to deliver TeleCIMT. The therapist support package development was informed by the Theoretical Domains Framework (45) and Behavior Change Wheel (46), and will be described elsewhere. The package was refined with input from occupational therapy managers and occupational therapists during a focus group. Table 1 provides details of support package components in accordance with the TIDieR checklist (39). In summary, the package includes four components:
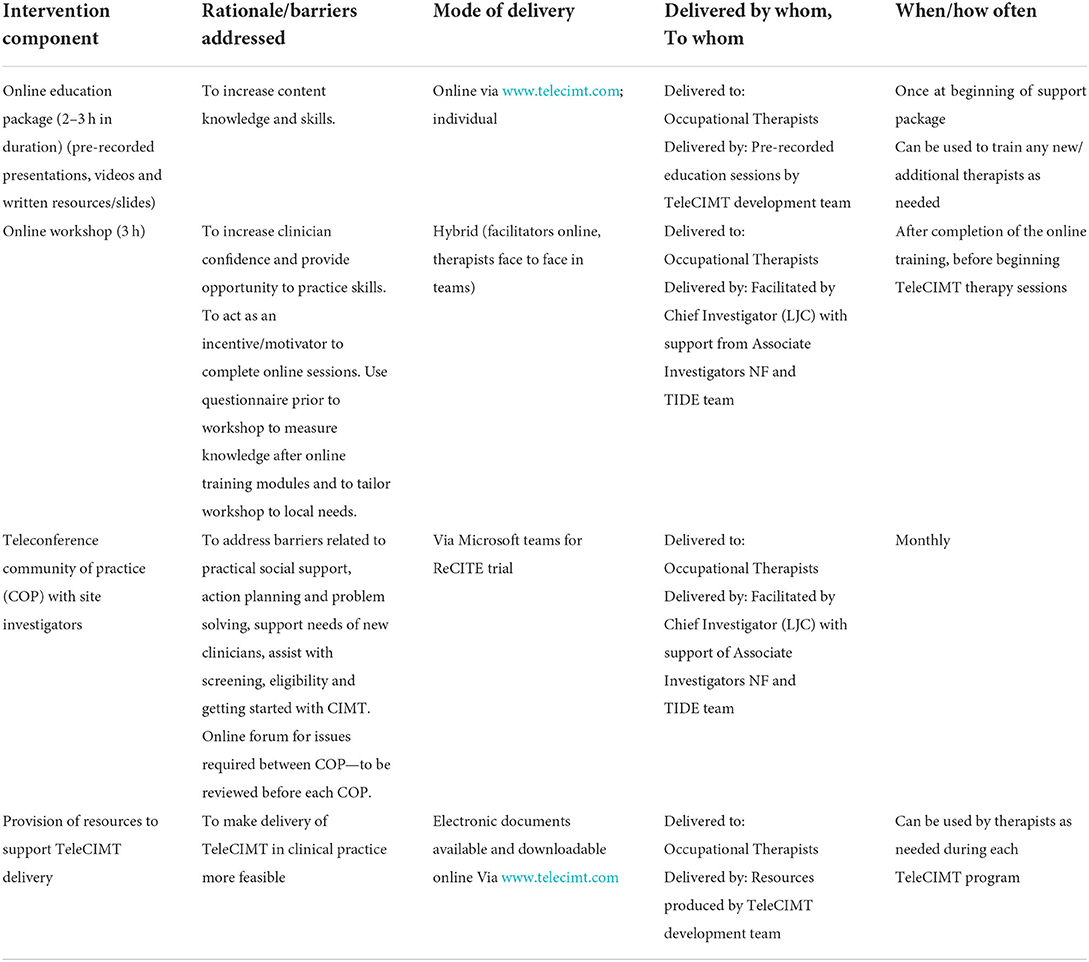
Table 1. Description of multicomponent therapist support package delivered to teams of occupational therapists.
Education: Therapists complete five online, self-directed learning modules about TeleCIMT theory via the www.telecimt.com website (therapist learning resources).
Skills and knowledge consolidation: After completing the online training, a 3 h virtual workshop will be delivered to therapists in their work environment. Therapists will practice delivering CIMT via telehealth.
Patient and therapist resources: Resources for delivering and recording TeleCIMT are available from the www.telecimt.com website. Therapists will select and download the resources required for their team to deliver TeleCIMT.
Community of practice: A monthly virtual community of practice will be facilitated by the research team, supporting therapists' involved in the study with troubleshooting, and discussion of barriers and enablers. Microsoft teams chat will allow therapists to raise (de-identified) problems and ask questions, and provide peer support between monthly meetings.
Evidence-based intervention—TeleCIMT
The evidence-based intervention to be implemented into practice is a 3 week CIMT program delivered via telehealth (TeleCIMT) to stroke survivors. This intervention will be delivered after therapists' receive the therapist support package. Stroke survivor participants will participate in 3 weeks of TeleCIMT delivered by an occupational therapist. The TeleCIMT model was developed by JB, AM, AK, and LJC and is based on current Australian stroke clinical guideline recommendations for CIMT delivery (1): a minimum of 2 h of intensive practice per day [including shaping and functional tasks (2)], 6 h of mitt wearing per day and a transfer package to support program adherence and behavior change by stroke participants for at least 2 weeks (1). The TeleCIMT program uses shaping and functional task practice libraries (www.telecimt.com) and increased involvement of a carer or supporter to deliver a program remotely with reduced, and solely virtual, therapist input.
Shaping is a training method based on principles of behavior training in which tasks are completed in a series of 10 timed trials (30 s to 2 min per timed trial), with feedback provided about performance immediately after each trial (47). Tasks are selected that target a participant's specific motor impairments and movements that have the greatest potential to improve and are set at a level that is challenging but achievable for the participant (48). Tasks are made incrementally more challenging (“shaped”) throughout the participant's program and coaching and encouragement are provided by the therapist throughout all shaping trials (48).
Task practice involves the completion of functional activities (e.g., folding laundry) using only the affected upper limb for 15 to 20 min per activity. The complexity of the prescribed functional tasks are progressed throughout the program (48).
Shaping and functional tasks selected for each program will be individualized to the participant's goals and interests and motor abilities; and will use items already available within the participant's home to support training activities wherever possible.
Participants will receive three, 1 h intensive therapy sessions (shaping and functional tasks) per week via video call, and two brief, 30 min telephone or video calls 2 days per week (total therapist contact 5 days per week for 3 weeks). The shorter telephone or video calls will be used on alternate weekdays to complete components of the transfer package including providing encouragement and positive feedback, to monitor practice completed and to progress practice activities. Outside of these structured times, participants will be required to follow the CIMT program outlined by their treating therapist within their TeleCIMT workbook and complete a minimum of 2 h of active practice per day with or without the support of a carer. Table 2 outlines the structure and components of the TeleCIMT program using the Template for Intervention Description and Replication (TIDIeR) checklist (39).

Table 2. TeleCIMT intervention description using the Template for Intervention Description and Replication (TIDIeR) checklist (39).
Data collection and outcome measures
Feasibility and acceptability of delivering TeleCIMT in routine practice will be investigated in depth using several outcomes.
Therapist outcomes
Feasibility of recruitment will be evaluated by measuring the number of stroke survivors recruited for TeleCIMT at each site over the period of 12 months, as a proportion of those deemed eligible for CIMT. Pace of recruitment is anticipated to be a minimum of 10 participants per site per year to indicate feasibility for a larger randomized controlled trial to be conducted.
Feasibility of delivering the therapist support package will be evaluated by measuring the number/proportion of therapist participants completing each learning module, and download rates of TeleCIMT resources using Google analytic web usage data. Participation rates in the monthly community of practice will also be evaluated for each of the therapy teams.
Perceived barriers to, and enablers of behavior change and TeleCIMT delivery will be captured using the self-evaluation Capabilities, Opportunities, Motivation- Behaviour (COM-B) questionnaire (50) at three time points; prior to delivery of the support package, after completion of online learning and after the mini-workshop. TeleCIMT knowledge will be assessed using an online knowledge quiz following completion of online learning modules.
Post-implementation, focus group interviews will be conducted with site therapists involved in the delivery of two or more TeleCIMT programs, using an interview schedule informed by the reach, effectiveness, adoption, implementation and maintenance (RE-AIM) framework (51). Focus groups will explore TeleCIMT feasibility and acceptability, and barriers and enablers to delivery perceived by clinicians, with respect to their clinical setting. The focus groups will be facilitated by an experienced qualitative researcher within the research team who is independent of all participating health services.
Stroke survivor outcomes
Feasibility outcomes
Program fidelity will be determined by evaluating process outcomes, including (a) number of sessions completed, supervised and unsupervised, as a proportion of planned sessions, (b) repetitions and amount of practice time recorded during supervised and unsupervised sessions. Delivery of TeleCIMT in practice will be deemed feasible if stroke survivor participants complete at least 80% of intensive arm practice during their 3 week TeleCIMT program (i.e., at least 24 h of intensive arm practice). Amount of practice will be measured as the number of minutes of practice completed per day, as reported by each stroke survivor and/or their supporter during their daily telephone/video call.
Acceptability will be determined by qualitative semi-structured interviews, conducted individually with up to 20 TeleCIMT participants by telephone, video call or in person, dependent on participant preference, within 1 month of completing TeleCIMT. Interviews will be recorded and transcribed verbatim to explore stroke survivors' experiences of completing TeleCIMT. Acceptability will also be determined quantitatively using the participant opinion survey (POS) (20).
Clinical outcomes
Upper limb and quality of life outcomes will be measured prior to commencement of TeleCIMT (week 0), immediately post TeleCIMT program (week 4) and 1 month follow-up (week 8) by trained therapists, blinded to the intervention. Change in upper extremity function will be assessed using five standardized, reliable and valid assessments: the Action Research Arm Test (ARAT) (52), Box and Block Test (BBT) (53), Nine Hole Peg Test (NHPT) (54), Grip Strength (55), and the Motor Activity Log (MAL-30) (56, 57). Quality of life outcomes will also be collected using the EuroQOL 5 dimensions, 5 level (EQ 5D 5L) (58), and Stroke Impact Scale-16 (SIS-16) (59).
Supporter outcomes
Supporters who provide assistance to participants during their TeleCIMT program will be invited to participate in an online survey following program completion to assess TeleCIMT acceptability to supporters. The survey consists of demographic questions and the generic theoretical framework of acceptability (TFA) questionnaire (60), tailored to the role of the supporter in a TeleCIMT program. The TFA questionnaire consists of seven component constructs of acceptability and an overall acceptability measure.
Safety outcomes for TeleCIMT
The number and type of adverse events experienced by participants during TeleCIMT, such as illness, extreme fatigue, muscle soreness, shoulder pain or injuries, trips (a near miss but did not fall to the ground and no injury sustained) and falls (fall to the ground, with or without sustaining an injury) will be recorded after each contact with a blinded assessor or therapist. Therapists delivering TeleCIMT and blinded assessors completing outcome measures will report any adverse events reported or experienced by stroke survivors to the site principal investigator and chief investigator. They will record whether or not the event appears to be caused by / associated with TeleCIMT or other concurrent activities. A register of any adverse events will be maintained and any serious adverse events reported to the Human Research Ethics Committee. Dependent on the nature of the event, escalation may also include referral to a general practitioner, hospital and/or discontinuation or suspension of the intervention (TeleCIMT).
Data management
Study data will be collected and managed using Research Electronic Data Capture (REDCap) tools hosted at St Vincent's Health Network Sydney. Site investigators will enter screening data, demographic information and CIMT program data for all included participants. De-identified screening information will also be collected by site investigators to explore reasons why potential participants were not eligible for, or refused study participation.
Blinded assessors will enter outcome measurement data following each assessment timepoint. The chief investigator and senior research officer will regularly review completeness of data entered by therapists and blinded assessors.
Data analysis
Sample size for TeleCIMT participants
We will recruit 30 TeleCIMT participants (up to 10 stroke survivors per site). A sample size of 29 stroke survivors is required to assess the feasibility of 90% of participants completing at least 24 h (80% of planned hours) of intensive practice during their TeleCIMT program with precision (half-width 95% confidence interval) of 11% (61). Outcomes will be reported with 95% confidence intervals and analyses will assume a two-sided level of significance of p < 0.05.
Feasibility outcomes
Descriptive statistics (mean [SD] or number [%]) will be used to analyse the proportion of participants recruited from those eligible for TeleCIMT, therapist adherence to the therapist support package, including completion of online education modules, download rates, and participation in the community of practice and changes in perceived barriers and enablers on the COM-B questionnaire. The proportion of participants completing 80% of a TeleCIMT program (at least 24 h of intensive upper extremity practice) will be analyzed and reported using descriptive statistics to explore TeleCIMT participant program feasibility, adherence and fidelity. The TeleCIMT recruitment will be considered feasible if 75% of eligible stroke participants are enrolled in the study and each team can recruit 10 stroke participants over a period of 12 consecutive months.
Upper extremity outcomes
Outcomes for learned non-use (MAL-30) and upper extremity outcomes (NHPT, ARAT, BBT, and grip strength) will be compared using paired t-tests (pre-post TeleCIMT program) and 95% confidence intervals, and linear mixed effect models (multiple time points) or non-parametric methods if data are not normally distributed. The proportion of participants achieving the minimal clinically important difference (MCID) post TeleCIMT will be analyzed using mixed effects logistic regression for the MAL-30 (1.0) (62), ARAT [12 points for acute stroke (63), and 5.7 points for chronic stroke (64)], and grip strength (62).
Quality of life outcomes
EQ5D5L raw scores will be converted to a utility-based index based on an Australian population (65). Health-related quality of life scores from the EQ5D5L and SIS-16 will be analyzed using paired t-tests (pre-post) and 95% confidence intervals, and linear mixed effect models (multiple time points). The proportion of participants achieving the MCID on the SIS-16 (9.4–14.1 points) (66) will be analyzed using mixed effects logistic regression. The relationship between the results from the EQ5D5L and the SIS-16 will be evaluated using Spearman bivariate correlation analysis.
Acceptability outcomes
Responses by stroke survivors to the POS and supporters to the TFA questionnaire will be analyzed descriptively.
Transcripts from individual interviews with stroke survivors and focus groups with therapists will undergo thematic analysis, using NVIVO software for data management. Focus group interviews will be deductively mapped to the RE-AIM framework. Tables will be generated to summarize team experiences of barriers and enablers to TeleCIMT delivery within clinical practice. Stroke survivor interviews will be coded using an inductive thematic analysis approach. Triangulation of data will be used to ensure consistency of coding and categories. Peer examination will be used to ensure the trustworthiness of the data collection and analysis (67). Figure 2 presents the logic model showing how therapist support package components are hypothesized to influence the outcomes of the study.
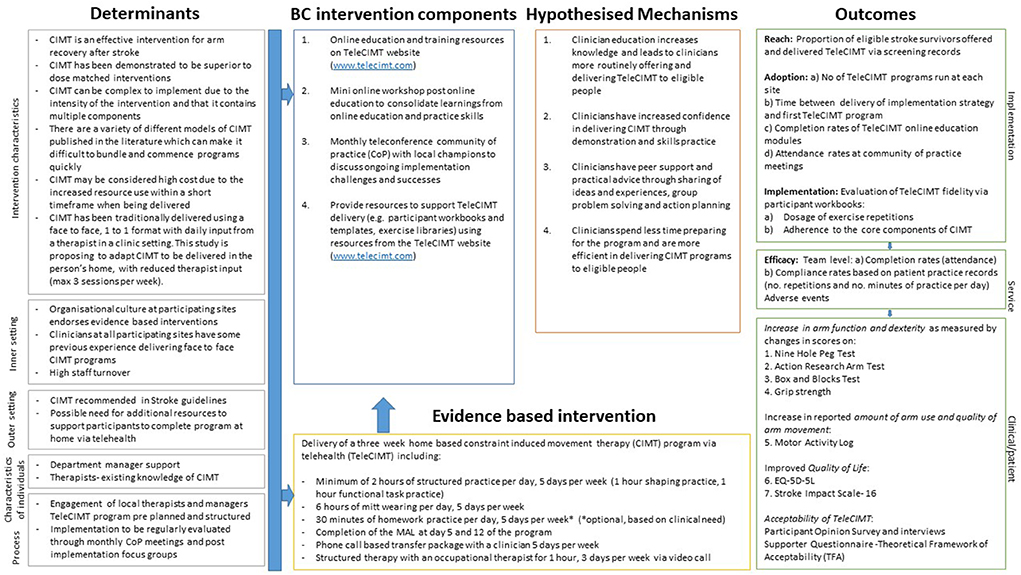
Figure 2. Implementation research logic model for ReCITE study (49).
Summaries of the results will be presented to the services involved, and shared on the TIDE website. Consumers involved in the development of resources will also be provided with a summary of results from the study.
Discussion
This study will present findings of the feasibility of TeleCIMT in an Australian context without the need for specialist devices other than a device with internet access and video capabilities. Distance from specialist rehabilitation services in Australia is an issue for many stroke survivors (68). In addition, the COVID-19 pandemic resulted in a rapid transition to delivering telehealth, however, research on the safety, feasibility and acceptability of delivering complex interventions via telehealth, and the support that therapists and patients require, has not been previously explored (69). Upper extremity outcomes will be evaluated and qualitative feedback from therapists, stroke survivors and supporters on TeleCIMT will explore these factors.
The intensive and complex nature of CIMT may not lend itself to remote delivery. Reduced therapist input and increased reliance on a support person, for the majority of stroke survivors (especially those with a cognitive impairment), to complete a program may impact on the feasibility and acceptance of TeleCIMT. Therefore, understanding of acceptance and feasibility is essential to explore if TeleCIMT can be delivered successfully, or if further adjustments need to be made to support therapists and participants with remote delivery of CIMT. If this study shows that TeleCIMT is acceptable and feasible, this group will seek to perform a larger, randomized controlled trial. This pilot feasibility study is not sufficiently powered to report on efficacy. We also acknowledge that the varied time post-stroke of participants may increase the variability of outcomes.
Conclusions
The ReCITE study will explore the feasibility of TeleCIMT delivery to stroke survivors in routine practice. The study will also evaluate the safety and acceptability of TeleCIMT from the perspectives of therapists delivering TeleCIMT and stroke survivors receiving the intervention. These results will be used to evaluate if a larger randomized controlled trial is feasible, and help determine sample size calculations.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.
Ethics statement
The studies involving human participants were reviewed and approved by St Vincent's Hospital Human Research Ethics Committee. The patients/participants provided their written informed consent to participate in this study.
Author contributions
LJC, AMc, and SM conceptualized the study with input from NL regarding the study design. LJC, AMc, CS, AK, JB, AMe, SF, AD, and SM received funding to conduct this study. LJC and EH designed the multifaceted therapist support package with NF, NL, AMc, CS, AK, and SM. JB, AMe, AK, and LJC designed the TeleCIMT intervention. LC contributed to the design of the data analysis plan. All authors contributed to the design of the study protocol, manuscript draft, and provide consent for the final manuscript submission. All authors contributed to the article and approved the submitted version.
Funding
This study was supported by a Stroke Foundation Early Career Seed Grant and St Vincent's Clinic Foundation Multidisciplinary Research Grant. NL was supported by a fellowship from the Heart Foundation (Australia), GNT102055. AD was supported by a University of La Frontera Research Project grant (Chile), DIUFRO 21/077.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
ARAT, Action Research Arm Test; BBT, Box and Block Test; COM-B, Capability, Opportunity, Motivation-Behaviour model; CIMT, Constraint induced movement therapy; EQ 5D 5L, EuroQOL 5 dimensions, 5 level; MCID, minimal clinically important difference; MAL-30, Motor Activity Log; NHPT, Nine Hole Peg Test; SIS-16, Stroke Impact Scale-16; TDF, Theoretical Domains Framework; TFA, Theoretical Framework of Acceptability; POS, Participant Opinion Survey; ReCITE, Remote Constraint Induced Therapy of the upper Extremity.
References
2. Taub E, Uswatte G, Pidikiti R. Constraint-Induced Movement Therapy: a new family of techniques with broad application to physical rehabilitation–a clinical review. J Rehabil Res Dev. (1999) 36:237–51.
3. Corbetta D, Sirtori V, Castellini G, Moja L, Gatti R. Constraint-induced movement therapy for upper extremities in people with stroke. Cochrane Database Syst Rev. (2015) 2015:Cd004433. doi: 10.1002/14651858.CD004433.pub3
4. Taub E, Uswatte G, King DK, Morris D, Crago JE, Chatterjee A, et al. A placebo-controlled trial of constraint-induced movement therapy for upper extremity after stroke. Stroke. (2006) 37:1045–9. doi: 10.1161/01.STR.0000206463.66461.97
5. Taub E, Miller NE, Novack TA, Cook EW, Fleming WC, Nepomuceno CS, et al. Technique to improve chronic motor deficit after stroke. Arch Phys Med Rehabil. (1993) 74:347–54.
6. Kwakkel G, Veerbeek JM, van Wegen EE, Wolf SL. Constraint-induced movement therapy after stroke. Lancet Neurol. (2015) 14:224–34. doi: 10.1016/S1474-4422(14)70160-7
7. Intercollegiate Stroke Working Party. National Clinical Guideline for Stroke. 5 ed. London: Royal College of Physicians (2016).
8. Teasell R, Salbach NM, Foley N, Mountain A, Cameron JI, Jong Ad, et al. Canadian stroke best practice recommendations: Rehabilitation, recovery, and community participation following stroke. Part one: Rehabilitation and recovery following stroke. 6th Ed. Int J Stroke. (2020) 15:763–88. doi: 10.1177/1747493019897843
9. Wolf SL, Winstein CJ, Miller JP, Taub E, Uswatte G, Morris D, et al. effect of constraint-induced movement therapy on upper extremity function 3 to 9 months after strokethe EXCITE randomized clinical trial. JAMA. (2006) 296:2095–104. doi: 10.1001/jama.296.17.2095
10. Page SJ, Levine P, Leonard AC. Modified constraint-induced therapy in acute stroke: a randomized controlled pilot study. Neurorehabil Neural Repair. (2005) 19:27–32. doi: 10.1177/1545968304272701
11. Kwakkel G, Winters C, Van Wegen EE, Nijland RH, Van Kuijk AA, Visser-Meily A, et al. Effects of unilateral upper limb training in two distinct prognostic groups early after stroke: the EXPLICIT-stroke randomized clinical trial. Neurorehabil Neural Repair. (2016) 30:804–16. doi: 10.1177/1545968315624784
12. Barzel A, Ketels G, Tetzlaff B, Krüger H, Haevernick K, Daubmann A, et al. Enhancing activities of daily living of chronic stroke patients in primary health care by modified constraint-induced movement therapy (HOMECIMT): study protocol for a cluster randomized controlled trial. Trials. (2013) 14:334. doi: 10.1186/1745-6215-14-334
13. Doussoulin A, Rivas C, Rivas R, Saiz J. Effects of modified constraint-induced movement therapy in the recovery of upper extremity function affected by a stroke: a single-blind randomized parallel trial-comparing group versus individual intervention. Int J Rehabil Res. (2018) 41:35–40. doi: 10.1097/MRR.0000000000000257
14. Wolf SL, Thompson PA, Winstein CJ, Miller JP, Blanton SR, Nichols-Larsen DS, et al. The EXCITE stroke trial: comparing early and delayed constraint-induced movement therapy. Stroke. (2010) 41:2309–15. doi: 10.1161/STROKEAHA.110.588723
15. Fleet A, Che M, MacKay-Lyons M, MacKenzie D, Page S, Eskes G, et al. Examining the use of constraint-induced movement therapy in canadian neurological occupational and physical therapy. Physiother Can. (2014) 66:60–71. doi: 10.3138/ptc.2012-61
16. Pedlow K, Lennon S, Wilson C. Application of constraint-induced movement therapy in clinical practice: an online survey. Arch Phys Med Rehabil. (2014) 95:276–82. doi: 10.1016/j.apmr.2013.08.240
17. Sweeney G, Barber M, Kerr A. Exploration of barriers and enablers for evidence-based interventions for upper limb rehabilitation following a stroke: use of constraint induced movement therapy and robot assisted therapy in NHS Scotland. Br J Occup Ther. (2020) 83:690–700. doi: 10.1177/0308022620909023
18. Page SJ, Levine P, Sisto S, Bond Q, Johnston MV. Stroke patients' and therapists' opinions of constraint-induced movement therapy. Clin Rehabil. (2002) 16:55–60. doi: 10.1191/0269215502cr473oa
19. Christie LJ, Rendell R, McCluskey A, Fearn N, Hunter A, Lovarini M. Adult experiences of constraint-induced movement therapy programmes: a qualitative study using the Theoretical Domains Framework and Capability, Opportunity, Motivation – Behaviour system. Brain Impair. (2022) 1–16. doi: 10.1017/BrImp.2022.18
20. Andrabi M, Taub E, Mckay Bishop S, Morris D, Uswatte G. Acceptability of constraint induced movement therapy: influence of perceived difficulty and expected treatment outcome. Top Stroke Rehabil. (2021) 29:507–15. doi: 10.1080/10749357.2021.1956046
21. Laver KE, Adey-Wakeling Z, Crotty M, Lannin NA, George S, Sherrington C. Telerehabilitation services for stroke. Cochrane Database Syst Rev. (2020) 1:Cd010255. doi: 10.1002/14651858.CD010255.pub3
22. Appleby E, Gill ST, Hayes LK, Walker TL, Walsh M, Kumar S. Effectiveness of telerehabilitation in the management of adults with stroke: a systematic review. PLoS ONE. (2019) 14:e0225150. doi: 10.1371/journal.pone.0225150
23. Sarfo FS, Ulasavets U, Opare-Sem OK, Ovbiagele B. Tele-rehabilitation after stroke: an updated systematic review of the literature. J Stroke Cerebrovasc Dis. (2018) 27:2306–18. doi: 10.1016/j.jstrokecerebrovasdis.2018.05.013
24. Caughlin S, Mehta S, Corriveau H, Eng JJ, Eskes G, Kairy D, et al. Implementing telerehabilitation after stroke: lessons learned from Canadian trials. Telemed J E Health. (2020) 26:710–19. doi: 10.1089/tmj.2019.0097
25. Barzel A, Ketels G, Stark A, Tetzlaff B, Daubmann A, Wegscheider K, et al. Home-based constraint-induced movement therapy for patients with upper limb dysfunction after stroke (HOMECIMT): a cluster-randomised, controlled trial. Lancet Neurol. (2015) 14:893–902. doi: 10.1016/S1474-4422(15)00147-7
26. Uswatte G, Taub E, Lum P, Brennan D, Barman J, Bowman MH, et al. Tele-rehabilitation of upper-extremity hemiparesis after stroke: Proof-of-concept randomized controlled trial of in-home Constraint-Induced Movement therapy. Restor Neurol Neurosci. (2021) 39:303–18. doi: 10.3233/RNN-201100
27. Lum PS, Taub E, Schwandt D, Postman M, Hardin P, Uswatte G. Automated Constraint-Induced Therapy Extension (AutoCITE) for movement deficits after stroke. J Rehabil Res Dev. (2004) 41:249–58. doi: 10.1682/JRRD.2003.06.0092
28. Lum PS, Uswatte G, Taub E, Hardin P, Mark VW. A telerehabilitation approach to delivery of constraint-induced movement therapy. J Rehabil Res Dev. (2006) 43:391–400. doi: 10.1682/JRRD.2005.02.0042
29. Gauthier LV, Nichols-Larsen DS, Uswatte G, Strahl N, Simeo M, Proffitt R, et al. Video game rehabilitation for outpatient stroke (VIGoROUS): a multi-site randomized controlled trial of in-home, self-managed, upper-extremity therapy. EClinicalMedicine. (2022) 43:101239. doi: 10.1016/j.eclinm.2021.101239
30. Borstad AL, Crawfis R, Phillips K, Lowes LP, Maung D, McPherson R, et al. In-home delivery of constraint-induced movement therapy via virtual reality gaming. J Patient Cent Res Rev. (2018) 5:6–17. doi: 10.17294/2330-0698.1550
31. Laver K, Walker M, Ward N. Telerehabilitation for stroke is here to stay. But at what cost? Neurorehabil Neural Repair. (2022) 36:331–4. doi: 10.1177/15459683221100492
32. Cox NS, Scrivener K, Holland AE, Jolliffe L, Wighton A, Nelson S, et al. A brief intervention to support implementation of telerehabilitation by community rehabilitation services during COVID-19: a feasibility study. Arch Phys Med Rehabil. (2021) 102:789–95. doi: 10.1016/j.apmr.2020.12.007
33. Skivington K, Matthews L, Simpson SA, Craig P, Baird J, Blazeby JM, et al. A new framework for developing and evaluating complex interventions: update of Medical Research Council guidance. BMJ. (2021) 374:n2061. doi: 10.1136/bmj.n2061
34. Christie LJ. Factors influencing the implementation and sustainability of upper limb constraint-induced movement therapy (CIMT) programs for people with stroke and traumatic brain injury [Published thesis]. The University of Sydney, Camperdown, NSW, Australia (2021)
35. Boydell J, Meharg A, Kilkenny A, Christie L. TeleCIMT Program. (2021). Available online at: www.telecimt.com
36. Schulz KF, Altman DG, Moher D. CONSORT 2010 statement: updated guidelines for reporting parallel group randomized trials. Ann Intern Med. (2010) 152:726–32. doi: 10.7326/0003-4819-152-11-201006010-00232
37. Chan AW, Tetzlaff JM, Altman DG, Laupacis A, Gøtzsche PC, KrleŽa-Jerić K, et al. SPIRIT 2013 statement: defining standard protocol items for clinical trials. Ann Intern Med. (2013) 158:200–7. doi: 10.7326/0003-4819-158-3-201302050-00583
38. Pinnock H, Barwick M, Carpenter CR, Eldridge S, Grandes G, Griffiths CJ, et al. Standards for Reporting Implementation Studies (StaRI) statement. BMJ. (2017) 356:i6795. doi: 10.1136/bmj.i6795
39. Hoffmann TC, Glasziou PP, Boutron I, Milne R, Perera R, Moher D, et al. Better reporting of interventions: template for intervention description and replication (TIDieR) checklist and guide. BMJ. (2014) 348:g1687. doi: 10.1136/bmj.g1687
40. Lannin N. Reliability, validity and factor structure of the upper limb subscale of the Motor Assessment Scale (UL-MAS) in adults following stroke. Disabil Rehabil. (2004) 26:109–16. doi: 10.1080/0963828032000157970
41. Wolf SL, Binder-MacLeod SA. Electromyographic biofeedback applications to the hemiplegic patient. Changes in upper extremity neuromuscular and functional status. Phys Ther. (1983) 63:1393–403. doi: 10.1093/ptj/63.9.1393
42. Simmons-Mackie N, Kagan A, Shumway E. Aphasia Severity Rating. (2018). Available online at: https://www.aphasia.ca/health-care-providers/resources-and-tools/rating-scales/#ASR
43. Bruijnen C, Dijkstra BA, Walvoort SJ, Budy MJ, Beurmanjer H, De Jong CA, et al. Psychometric properties of the Montreal Cognitive Assessment (MoCA) in healthy participants aged 18-70. Int J Psychiatry Clin Pract. (2020) 24:293–300. doi: 10.1080/13651501.2020.1746348
44. Cooke DM, McKenna K, Fleming J, Darnell R. The reliability of the Occupational Therapy Adult Perceptual Screening Test (OT-APST). Br J Occup Ther. (2005) 68:509–17. doi: 10.1177/030802260506801105
45. Atkins L, Francis J, Islam R, O'Connor D, Patey A, Ivers N, et al. A guide to using the Theoretical Domains Framework of behaviour change to investigate implementation problems. Implement Sci. (2017) 12:77. doi: 10.1186/s13012-017-0605-9
46. Michie S, Atkins L, West R. The Behaviour Change Wheel: A Guide To Designing Interventions, London: Silverback Publishing (2014).
47. Taub E, Uswatte G, Mark VW, Morris DM, Barman J, Bowman MH, et al. Method for enhancing real-world use of a more affected arm in chronic stroke: transfer package of constraint-induced movement therapy. Stroke. (2013) 44:1383–8. doi: 10.1161/STROKEAHA.111.000559
48. Morris D, Taub E, Mark V. Constraint-induced movement therapy: characterizing the intervention protocol. Eur Medicophys. (2006) 42:257–68.
49. Smith JD, Li DH, Rafferty MR. The Implementation Research Logic Model: a method for planning, executing, reporting, and synthesizing implementation projects. Implement Sci. (2020) 15:84. doi: 10.1186/s13012-020-01041-8
50. Keyworth C, Epton T, Goldthorpe J, Calam R, Armitage CJ. Acceptability, reliability, and validity of a brief measure of capabilities, opportunities, motivations (“COM-B”). Br J Health Psychol. (2020) 25:474–501. doi: 10.1111/bjhp.12417
51. Glasgow RE, Estabrooks PE. Pragmatic applications of RE-AIM for health care initiatives in community and clinical settings. Prev Chronic Dis. (2018) 15:E02. doi: 10.5888/pcd15.170271
52. Yozbatiran N, Der-Yeghiaian L, Cramer SC. A standardized approach to performing the action research arm test. Neurorehabil Neural Repair. (2008) 22:78–90. doi: 10.1177/1545968307305353
53. Mathiowetz V, Volland G, Kashman N, Weber K. Adult norms for the Box and Block Test of manual dexterity. Am J Occup Ther. (1985) 39:386–91. doi: 10.5014/ajot.39.6.386
54. Mathiowetz V, Weber K, Kashman N, Volland G. Adult norms for the nine hole peg test of finger dexterity. Occup Ther J Res. (1985) 5:24–38. doi: 10.1177/153944928500500102
55. Bertrand AM, Mercier C, Bourbonnais D, Desrosiers J, Gravel D. Reliability of maximal static strength measurements of the arms in subjects with hemiparesis. Clin Rehabil. (2007) 21:248–257. doi: 10.1177/0269215506070792
56. Hammer AM, Lindmark B. Responsiveness and validity of the Motor Activity Log in patients during the subacute phase after stroke. Disabil Rehabil. (2010) 32:1184–93. doi: 10.3109/09638280903437253
57. Taub E, McCulloch K, Uswatte G, Morris DM, Bowman M, Crago J. Motor Activity Log (MAL) Manual. Birmingham, AL: Univeristy of Birmingham (2011).
58. Janssen MF, Pickard AS, Golicki D, Gudex C, Niewada M, Scalone L, et al. Measurement properties of the EQ-5D-5L compared to the EQ-5D-3L across eight patient groups: a multi-country study. Qual Life Res. (2013) 22:1717–27. doi: 10.1007/s11136-012-0322-4
59. Duncan PW, Lai SM, Bode RK, Perera S, DeRosa J, GAIN Americas Investigators. Stroke Impact Scale-16: A brief assessment of physical function. Neurology. (2003) 60:291–6. doi: 10.1212/01.WNL.0000041493.65665.D6
60. Sekhon M, Cartwright M, Francis JJ. Development of a theory-informed questionnaire to assess the acceptability of healthcare interventions. BMC Health Serv Res. (2022) 22:279. doi: 10.1186/s12913-022-07577-3
61. Thabane L, Ma J, Chu R, Cheng J, Ismaila A, Rios LP, et al. A tutorial on pilot studies: the what, why and how. BMC Med Res Methodol. (2010) 10:1. doi: 10.1186/1471-2288-10-1
62. Lang CE, Edwards DF, Birkenmeier RL, Dromerick AW. Estimating minimal clinically important differences of upper-extremity measures early after stroke. Arch Phys Med Rehabil. (2008) 89:1693–700. doi: 10.1016/j.apmr.2008.02.022
63. Simpson LA, Eng JJ. Functional recovery following stroke: capturing changes in upper-extremity function. Neurorehabil Neural Repair. (2012) 27:240–50. doi: 10.1177/1545968312461719
64. van der Lee JH, Beckerman H, Lankhorst GJ, Bouter LM. The responsiveness of the Action Research Arm test and the Fugl-Meyer Assessment scale in chronic stroke patients. J Rehabil Med. (2001) 33:110–3. doi: 10.1080/165019701750165916
65. McCaffrey N, Kaambwa B, Currow DC, Ratcliffe J. Health-related quality of life measured using the EQ-5D-5L: South Australian population norms. Health Qual Life Outcomes. (2016) 14:133. doi: 10.1186/s12955-016-0537-0
66. Fulk GD, Ludwig M, Dunning K, Golden S, Boyne P, West T. How much change in the stroke impact scale-16 is important to people who have experienced a stroke? Top Stroke Rehabil. (2010) 17:477–83. doi: 10.1310/tsr1706-477
67. Krefting L. Rigor in qualitative research: the assessment of trustworthiness. Am J Occup Ther. (1991) 45:214–22. doi: 10.5014/ajot.45.3.214
68. Deloitte Access Economics. No Postcode Untouched Stroke in Australia. Sydney Deloitte Access Economics Pty Ltd (2020).
Keywords: telerehabilitation, stroke, behavior change, implementation, upper extremity (arm), occupational therapy, physiotherapy
Citation: Christie LJ, Fearn N, McCluskey A, Lannin NA, Shiner CT, Kilkenny A, Boydell J, Meharg A, Howes E, Churilov L, Faux S, Doussoulin A and Middleton S (2022) Remote constraint induced therapy of the upper extremity (ReCITE): A feasibility study protocol. Front. Neurol. 13:1010449. doi: 10.3389/fneur.2022.1010449
Received: 03 August 2022; Accepted: 24 October 2022;
Published: 18 November 2022.
Edited by:
Margit Alt Murphy, University of Gothenburg, SwedenReviewed by:
Gitendra Uswatte, University of Alabama at Birmingham, United StatesUshotanefe Useh, North West University, South Africa
Copyright © 2022 Christie, Fearn, McCluskey, Lannin, Shiner, Kilkenny, Boydell, Meharg, Howes, Churilov, Faux, Doussoulin and Middleton. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lauren J. Christie, bGF1cmVuLmNocmlzdGllQHN2aGEub3JnLmF1
 Lauren J. Christie
Lauren J. Christie Nicola Fearn1
Nicola Fearn1 Natasha A. Lannin
Natasha A. Lannin Christine T. Shiner
Christine T. Shiner Leonid Churilov
Leonid Churilov Steven Faux
Steven Faux Arlette Doussoulin
Arlette Doussoulin Sandy Middleton
Sandy Middleton