
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
MINI REVIEW article
Front. Neurol. , 06 January 2023
Sec. Neurocritical and Neurohospitalist Care
Volume 13 - 2022 | https://doi.org/10.3389/fneur.2022.1008544
This article is part of the Research Topic Healthcare Disparities in Neurocritical Care View all 4 articles
Health disparities in the obstetric population affect maternal morbidity and mortality. In the past years, there has been no significant improvement in disparities in care in the obstetric population. Patients who are pregnant are known to have a higher risk of pregnancy-associated neurologic conditions such as stroke and intracerebral hemorrhage. They can also experience concomitant neurocritical care disease states such as status epilepticus and traumatic brain injury. Studies exploring the disparities of care among pregnant patients who are neurotically ill are lacking. We aim to provide the landscape of disparities of care among the obstetric neurocritically-ill population and provide potential actionable opportunities to address these disparities in care.
A health disparity is defined as an increased burden of an adverse health outcome or health determinant within a specific subset of the population (1). These are directly related to inequality of race, ethnicity, economical and social status, and political and environmental aspects of the healthcare system. Racial and ethnic minority populations are projected to grow; thus, the impact of these disparities would become more evident and can affect outcomes (1). The cost of the race and ethnic health disparity in the United States (US) is about $245 M (2). Women face increased challenges in the setting of multi-level disparity in care due to societal and healthcare demands unique to them such as childbearing, child-rearing, and household responsibilities.
Several studies have demonstrated that severe maternal morbidity and maternal deaths are preventable by addressing racial and ethnic disparities (1). While it has also been shown that poverty, lack of education, poor nutritional status, smoking, alcohol abuse, and neighborhood have been associated with poor maternal and infant outcomes (3).
Pregnancy is known to increase the risk of cerebrovascular conditions such as ischemic stroke (IS), intracerebral hemorrhage (ICH), subarachnoid hemorrhage (SAH), and cerebral venous sinus thrombosis (CVST) (4). Patients who are pregnant can also be predisposed to seizures and status epilepticus (SE) (5). Due to the increased demands on the heart, pregnancy can also result in sudden cardiac arrest (6). Independent conditions can also cause traumatic brain injury (TBI) that can result in potential complications for the obstetric patient. The disparities in obstetric care and neurocritical care (NCC) and their effect on patient outcomes have not been widely explored. The aim of this review was to describe the current state of the disparities in obstetric and neurocritical care and to propose future directions to decrease the disparities in care.
Maternal mortality rates (MMRs) have been increasing over the last three decades in the US despite recent advances in healthcare technology and infrastructure. MMR is highest in the US among all the developed countries (7). From 2011 to 2015, the national pregnancy-related mortality rate (PRMR), defined as (while pregnant or within 1 year) was 17.2 per 100,000 live births. Black women and American Indian/Alaska Native women had the highest PRMRs (42.8 and 32.5, respectively), 3.3 and 2.5 times as high, respectively, compared to the PRMR for non-Hispanic White women (13.0). Hypertension in pregnancy, cardiomyopathy, and thromboembolic complications are significantly more common in Black women as compared to their White patient counterparts, and this disparity is worse in Black women aged 30–34 years. Hispanic women on other hand are more likely to die due to hemorrhage (8). Positive predictors of severe morbidity were also age < 20 and ≥30 years, self-pay or Medicaid coverage for delivery, low socioeconomic status, and presence of chronic medical conditions, but these factors do not completely explain the racial and ethnic disparities seen in maternal morbidity and mortality (8). Racial differences in the impact of inflammatory markers such as neutrophil-to-lymphocyte ratio (NLR), on the outcomes of neurologic, obstetric, and gynecologic patients, have been found as well (9–13). Among patients with stroke, it has been found that a higher NLR is seen among African Americans with both COVID-19 and stroke (9) while in women with adnexal torsion (AT), NLR is higher among Caucasians with AT vs. those with benign ovarian cysts without torsion, while for East Asian patients, there was no difference detected. Among patients with preeclampsia, a higher NLR ratio has been noted in severe cases (14). Another consideration in studying disparities in care is the genetic predisposition to malignancies that may occur concomitantly while pregnant. While there is increasing detection of brain metastasis among patients with breast cancer (15, 16), endocrine therapy responsiveness is becoming challenging, especially with resistance development reported up to 40% (15). The variability of therapy response according to race has been analyzed among hormone receptor-positive patients, and it has been shown that African American patients had a significantly higher risk of death than White patients (17). This disparity can be extrapolated toward obstetric patients with concomitant breast cancer and brain metastases and should be considered in treatment planning (18, 19). Overall, the multifactorial interplay of other factors such as educational background, access to clinical care (20), and pregnancy intent plays a role in disparity (21).
The divide in obstetric care also exists in patients in rural vs. urban populations, those with substance use disorder (SUD) and those who identify as LGBTQ (lesbian, gay, bisexual, trans, and queer). The PRMR analysis by urban–rural classifications shows that from 2016 to 2018, the PRMRs were lower in larger metro counties compared to the rural noncore counties (13.8 deaths per 100,000 live births for persons living in large fringe metro counties which were lower than 24.4 deaths per 100,000 live births for persons living in noncore counties) (22). Women who are uninsured or have Medicaid coverage living in rural areas, and living in counties with lower income and educational attainment, had to travel farther to the nearest hospital with obstetric services or neonatal care than their counterparts (23). This disparity of access to care leads to insufficient care in high-risk pregnancies (24) likely leading to worsened perinatal outcomes. Compared to heterosexual women, bisexual and lesbian women were more likely to report miscarriages (OR = 1.77, 95% CI = 1.34, 2.35) and pregnancies ending in stillbirth (OR = 2.85, 95% CI = 1.40, 5.83). Lesbian women were also more likely to report low birth weight infants (OR = 2.64, 95% CI = 1.38, 5.07), and bisexual and lesbian women were more likely to report very preterm births (OR = 1.84, 95% CI = 1.11, 3.04) compared to heterosexual women (25). Patients with a SUD history also face implicit bias in their treatment. A study explored potential stigma and attitudes among medical providers within a maternal/fetal healthcare setting toward maternal SUD (26), and it was found that providers who consider drug abuse as a disease rather than a stigma have more favorable interactions with obstetric patients with a drug abuse history.
Stroke is a leading cause of morbidity and mortality around the world, as well as in the US. It is usually grouped into categories of ischemic stroke (IS) and hemorrhagic stroke (HS), with cerebral venous sinus thrombosis (CVST) variably discussed separately in the literature. In the US, data from 2015 to 2018 report a total of 7.6 million patients over age 20 with stroke over the course of those 3 years (27). Of those, 4.1 million were female patients, compared to 3.5 million male patients. The lifetime risk of stroke for women is one in five, and one in six for men. A study in the Netherlands showed an increase in the incidence of stroke from 1998 to 2010 (28). According to their data in 2010 for women aged 18–49, within the childbearing age, the incidence of stroke was 18.86 per 100,000 person-years, compared to 15.64 in their male counterparts. When broken down for type of stroke (IS vs. hemorrhagic vs. any type which included CVST), the difference in incidence between women and men was statistically significant in any type and IS categories but not significant in HS. They referenced the possibility that female-specific factors could play a role in this difference, pregnancy being one of those factors.
For women of childbearing age, the overall incidence of stroke of all types is reported as 11 per 100,000 (29). In a meta-analysis reviewing the incidence of stroke in pregnancy, the pooled data from studies across the world showed an all-type stroke incidence of 30 per 100,000 live births (30). These data ranged from regions with higher incidence such as North America, Taiwan, and India, to lower incidence countries such as Israel. In the US, an incidence of 38.6 per 100,000 live births has been reported (31). The data suggest an increase of three times the stroke incidence in pregnancy compared to all strokes during childbearing age with concern that this is still an underestimated number.
Maternal stroke and maternal stroke-associated in-hospital mortality have been found to be higher among Black women (32). In a study analyzing the National Inpatient Sample (NIS) 2000–2001 (33), pregnant Black women were found to have the highest risk of stroke (52.5 per 100,000 deliveries) compared to Hispanic women or White women. It has been shown that maternal morbidity and mortality are higher among Black and Hispanic mothers compared to White mothers when it comes to hypertensive disorders of pregnancy (34–36), while among those with chronic hypertension, all women who were in the minority population including Asian and Pacific Islanders had higher stroke risk compared to White women (36). Globally, the geographic disparity in stroke event rate was reported (21), and it showed variability among studies from North America, Taiwan, India, and Israel. This geographic disparity can be attributed to variability in stroke risk factors, access, and quality of obstetric care, the consequence of which can translate to a higher prevalence of cerebrovascular diseases.
Hemorrhagic stroke is the most common stroke subtype in patients who are pregnant, compared to the general population where IS is more common (37). It is also responsible for 5%−12% of all maternal deaths with high mortality rates of 35%−83% (38). The hypertensive disorders of pregnancy are reported to be responsible for the highest risk of maternal HS. The most common reason for preeclampsia-associated deaths was ICH (36–40). These disorders occur with co-existent racial disparities and a continued lack of improvement, as the rate of these disorders continues to climb (40–43). In terms of HS, a study conducted in Japan found a high HS rate (73.5%) among patients with pregnancy-associated strokes (41) which is consistent with reports that Asians have an increased risk of the hemorrhagic type of stroke compared with Caucasians and African Americans (42). However, in a study analyzing the NIS for the years 1993 through 2002, it was found that African American race was an independent risk factor for ICH in pregnancy (39). In addition to this, a study utilizing administrative data from New York, California, and Florida showed consistent findings that non-White race including African Americans, Asians, and Hispanics, was an independent predictor of ICH among patients who are pregnant (43). When analyzing the trends in spontaneous SAH in pregnancy in a 12-year period, it was found that the greatest increase in the incidence of SAH was among African American women (13.4–16.39 per 100,000 births) (44). In this population, it was also shown that pregnant women who were admitted to hospitals with larger bed sizes or urban teaching hospitals had lower odds of discharge to long-term facilities.
The above represents alarming data, and with stroke incidence on the rise overall, there should be a call to action to address this special population of patients, with attention to higher incidence countries and regions and modifiable factors, as well as research to determine causes for this increased risk and possible treatment mechanisms. It is also important to specify ways to acknowledge and improve the racial/ethnic and other disparities that exist within this subset of the population.
Cardiac arrest is a rare but feared complication in pregnancy. The rates of out-of-hospital cardiac arrest in the general US population is 95/100,000, 67–170/100,000 in Europe, and 60/100,000 in South Korea, with poor outcomes. In-hospital cardiac arrest rates are 6–7/1,000 in US and 1.5–2.8/1,000 admissions in Europe (45). Taking a closer look at younger patients, the rate of sudden cardiac arrest in one study was reported as 4.4/100,000 in patients 25–35 and 1.44/100,000 in patients 14–24, of all genders combined (46). The prevalence of maternal cardiac arrest suggested in one review (47) was one in 12,000 in the US and one in 16,000 in the UK, comparable to 8.3 and 6.25 per 100,000, respectively, significantly higher than rates of the general population around childbearing age. Only minimal data are available to give information on specific disparities of care among patients with maternal post-cardiac arrest neurologic morbidity. In a study in California analyzing 64 cardiovascular pregnancy-related deaths, they found that women were likely to be African American if they died from cardiovascular disease (48). The implications of this for post-cardiac arrest care cannot be understated. Guidelines that involve neurologic resuscitation post-cardiac arrest should include patients who are pregnant (49–51).
Traumatic brain injury is a global health crisis, with over 27 M people worldwide experiencing TBI in 2016 alone, along with over 55 M people living with disability related to prior TBI. There are numerous literature reviews on TBI in the general population but few focused on TBI in pregnancy. The rate of pregnancy-associated trauma has been reported to be 6%−46% (52), creating a high risk for morbidity and mortality. A study on women who had moderate–severe TBI showed that fewer patients with TBI had one or more live births and had more post-partum issues compared to non-TBI controls (53). It was noted that in TBI across a woman's lifespan, women have the highest incidence of TBI in ages 21–50, encompassing the childbearing age (54). The difference in mechanisms was noted where TBI in women was from falls, collisions, and domestic violence unlike in men, where it is from motor vehicle accidents or combat. TBI is also associated with pregnancy difficulties (55). Intimate partner violence (IPV) that can result in TBI significantly affects ethnic minorities (Black/African American, Hispanic/Latina, Native American/Alaska Native, and Asian American) (56), and TBI from IPV has been found to result in chronic psychiatric disorders such as posttraumatic stress disorder (57) and post-partum depression (58). This knowledge is important when treating a patient who is pregnant in the minority population with severe TBI in neurocritical care.
Status epilepticus has limited literature in regard to disparities in care among the obstetric population. It is generally known that seizures are well-controlled even during pregnancy (59). However, 30%−40% of patients with epilepsy can still have drug-resistant epilepsy and pregnancy may complicate their management (60). A study analyzing only pregnant patients with refractory epilepsy found that 8.5% of the patients developed SE over the course of the pregnancy which is higher than 0.6%−2.1%, which has been reported prior (61, 62). Low compliance is one of the factors that was associated with SE (59). Poor compliance has been shown to be associated with lower socioeconomic status and inadequate insurance (63). Few studies on pregnancy outcomes and seizures in terms of race/ethnicity have not been conclusive due to the small sample and lower prevalence in this specific population (64, 65). Studies on SE associated with pregnancy and disparities in care are significantly lacking. There is a need for continued focus on this patient population when it comes to SE as well.
Disparities in maternal healthcare have been well reported; however, there is little sign that the disparities are dissipating. The summary of the disparities found in obstetric and neurocritical care is shown (Table 1). These factors may be considered in the care of neurocritically-ill pregnant patients since these explore the overlapping risk factors for neurocritically-ill pregnant patients that can affect acute neurologic disease states and their outcomes.
Figure 1 shows how clinicians, policymakers, organizational leaders, and patients can potentially approach the challenge caused by the disparities in obstetric and neurocritical care along the continuum of care. Table 2 shows potential actionable solutions that can promote the decrease in disparities of care at the institutional, regional, national, and global levels. Leveraging organizational resources that can provide sets of bundled guidance to provide standardization for maternal healthcare services can be helpful (16). Such standardization can likely help address structural racism (66) that is prevalent in the healthcare industry due to pre-existing economic and social barriers (67). Collaboration among national and international societies should also be done to broaden the outreach for research, education, and information sharing among clinicians. One example of this is the United Kingdom registry of high-risk obstetric anesthesia with a focus on neurologic disease which collected data from 1997 to 2002 (68). Such a model entailed organization members to report, via a data collection form, any neurologic cases that they encountered in their obstetric units.
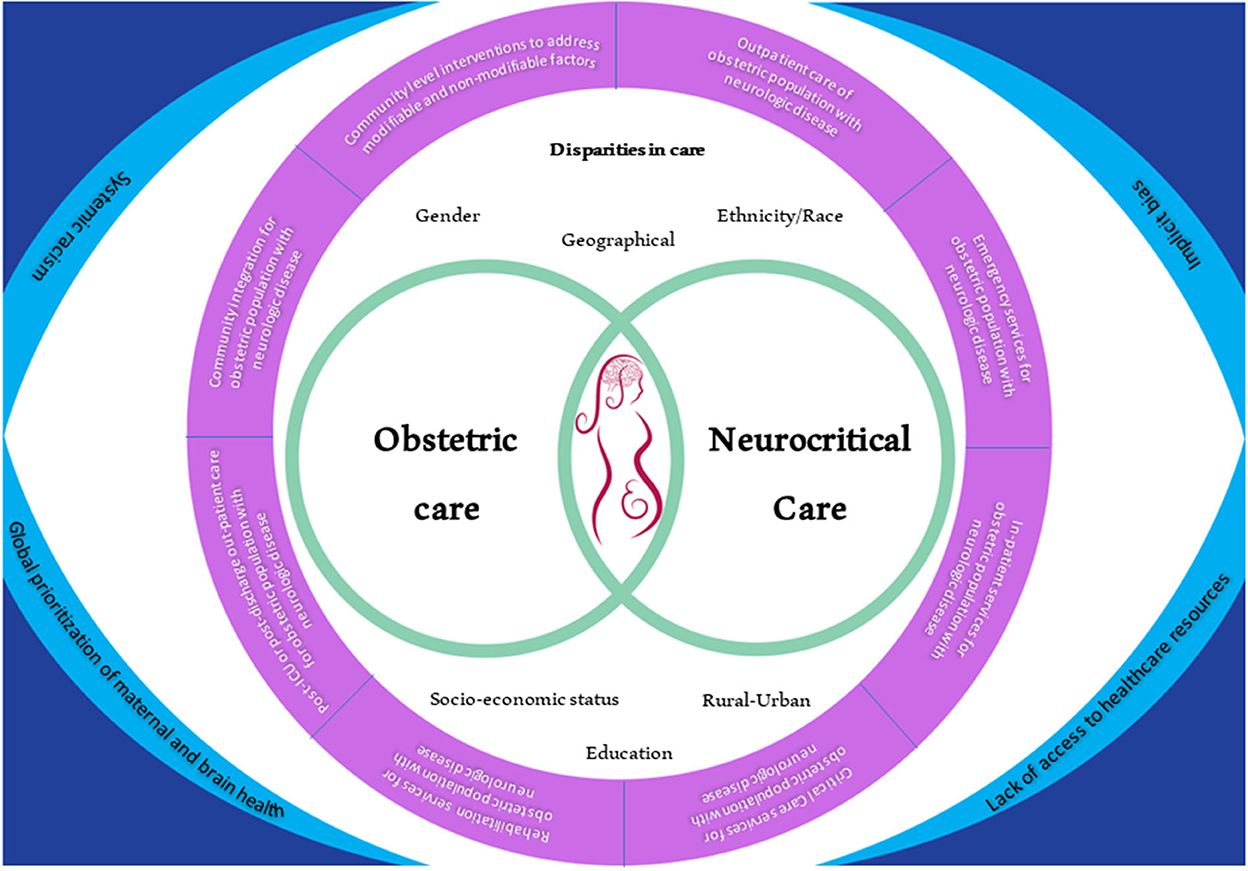
Figure 1. A new lens into looking at the landscape of disparities in obstetric neurocritical care and its integration into the patient continuum of care. On the corners are the main issues that are prevalent and that have to be integrated across the continuum of care. All of these issues also affect both obstetric care and neurocritical care and ultimately affect the outcome of the mother and the fetus. The areas of disparities in care that should be considered through the care of these patients are also shown.
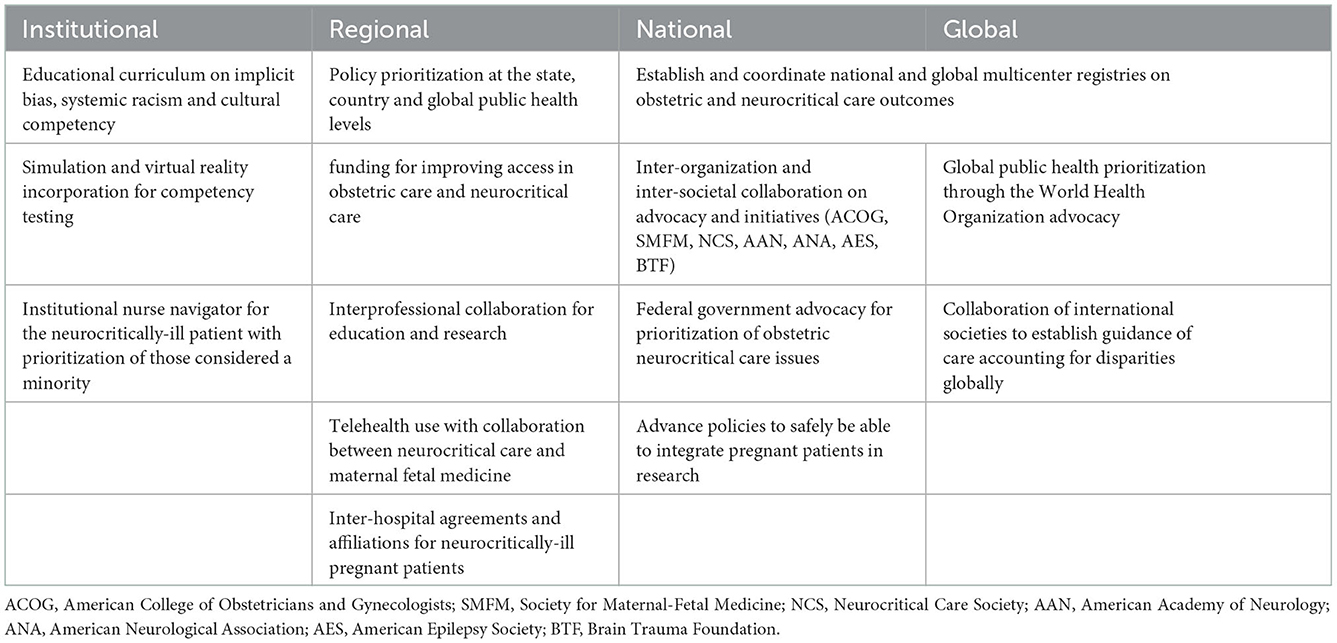
Table 2. Potential targeted and actionable interventions to address disparities in obstetric care and neurocritical care of pregnant patients who are neurotically ill.
Research has shown that nearly half of these maternal deaths and morbidity events are preventable by good quality healthcare services from preconception up until delivery. Attempts should be made to improve healthcare delivery to the underserved population possibly utilizing telehealth in collaboration with NCC and obstetric services (69–71). Obstetric tele-neurology has been explored globally as a means to bridge the gap to access; however, more robust data on metrics as well as optimization of available technological devices are needed (70). There are those with no access to the Internet that may benefit from telehealth services by collaborating with their local medical team closer to their home, an example of which is a telemedicine program in rural Panama (71). This type of undertaking requires not only healthcare support but also government support as well to be able to initiate and sustain this program.
The conceptual model approach to disparities in obstetric care has been explored before (1). The patient's socioeconomic status, age, ethnicity, race, education, marital status, insurance, language, employment, psychosocial support, and literacy all play a role in patient disparity analysis in addition to community, provider, and system factors.
There is a paucity of data on the best way to approach disparities of care in obstetric neurocritical care. Novel to this review, the major gaps in research in disparities of care among the obstetric neurocritically-ill population were delineated. The possible directions to take to move toward decreasing disparities in care for this population were also described.
Disparities in the care of the obstetric population affect maternal outcomes. Due to the increased risk of acute neurologic comorbidities and the risk of concomitant acute neurologic conditions among the obstetric population, the effect of these disparities on the management and outcomes of the patient who is neurocritically-ill should be studied. There is a large gap in information regarding disparities in the care of obstetric neurocritically-ill patients. Further research should be done to delineate this as part of the multidimensional approach to eliminate the disparities in obstetric and neurocritical care.
SM, DA, RO, and FI: manuscript writing. CN: conceptualization, manuscript writing, and final editing. All authors contributed to the article and approved the submitted version.
The authors would like to thank the woman who said: “Real change, enduring change, happens one step at a time”, for inspiring everyone.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Howell EA. Reducing disparities in severe maternal morbidity and mortality. Clin Obstet Gynecol. (2018) 61:387–99. doi: 10.1097/GRF.0000000000000349
2. Small MJ, Allen TK, Brown HL. Global disparities in maternal morbidity and mortality. Semin Perinatol. (2017) 41:318–22. doi: 10.1053/j.semperi.2017.04.009
3. Howell EA, Zeitlin J. Improving hospital quality to reduce disparities in severe maternal morbidity and mortality. Semin Perinatol. (2017) 41:266–72. doi: 10.1053/j.semperi.2017.04.002
4. Ijäs P. Trends in the incidence and risk factors of pregnancy-associated stroke. Front Neurol. (2022) 13:833215. doi: 10.3389/fneur.2022.833215
5. Miller EC, Vollbracht S. Neurology of preeclampsia and related disorders: an update in neuro-obstetrics. Curr Pain Headache Rep. (2021) 25:40. doi: 10.1007/s11916-021-00958-z
6. Keepanasseril A, Pfaller B, Metcalfe A, Siu SC, Davis MB, Silversides CK. Cardiovascular deaths in pregnancy: growing concerns and preventive strategies. Can J Cardiol. (2021) 37:1969–78. doi: 10.1016/j.cjca.2021.09.022
7. Howell EA, Zeitlin J. Quality of care and disparities in obstetrics. Obstet Gynecol Clin North Am. (2017) 44:13–25. doi: 10.1016/j.ogc.2016.10.002
8. Creanga AA, Bateman BT, Kuklina EV, Callaghan WM. Racial and ethnic disparities in severe maternal morbidity: a multistate analysis, 2008-2010. Am J Obstet Gynecol. (2014) 210:435. doi: 10.1016/j.ajog.2013.11.039
9. Lin C, Arevalo YA, Nanavati HD, Lin DM. Racial differences and an increased systemic inflammatory response are seen in patients with COVID-19 and ischemic stroke. Brain Behav Immun Health. (2020) 8:100137. doi: 10.1016/j.bbih.2020.100137
10. Sisti G, Faraci A, Silva J, Upadhyay R. Neutrophil-to-lymphocyte ratio, platelet-to-lymphocyte ratio, and routine complete blood count components in HELLP syndrome: a matched case control study. Medicina. (2019) 55:123. doi: 10.3390/medicina55050123
11. Khanzadeh S, Tahernia H, Hernandez J, Sarcone C, Lucke-Wold B, Salimi A, et al. Predictive role of neutrophil to lymphocyte ratio in adnexal torsion: a systematic review and meta-analysis. Mediators Inflamm. (2022) 2022:1–8. doi: 10.1155/2022/9680591
12. Hai L, Hu ZD. The clinical utility of neutrophil to lymphocyte ratio in pregnancy related complications: a mini-review. J Lab Precis Med. (2020) 5:1–9. doi: 10.21037/jlpm.2019.10.03
13. Azab B, Camacho-Rivera M, Taioli E. Average values and racial differences of neutrophil lymphocyte ratio among a nationally representative sample of United States subjects. PLoS ONE. (2014) 9:e112361. doi: 10.1371/journal.pone.0112361
14. Taşkömür AT, Erten Ö. The role of cystatin C, neutrophil-lymphocyte ratio and platelet-lymphocyte ratio in the evaluation of kidney function in women with preeclampsia. Taiwan J Obstet Gynecol. (2021) 60:615–20. doi: 10.1016/j.tjog.2021.05.007
15. Willman M, Willman J, Lucke-Wold B. Endocrine resistant breast cancer: brain metastasis. Explor Target Antitumor Ther. (2022) 3:240–51. doi: 10.37349/etat.2022.00081
16. Frisk G, Svensson T, Bäcklund LM, Lidbrink E, Blomqvist P, Smedby KE. Incidence and time trends of brain metastases admissions among breast cancer patients in Sweden. Br J Cancer. (2012) 106:1850–3. doi: 10.1038/bjc.2012.163
17. Rauscher GH, Silva A, Pauls H, Frasor J, Bonini MG, Hoskins K. Racial disparity in survival from estrogen and progesterone receptor-positive breast cancer: implications for reducing breast cancer mortality disparities. Breast Cancer Res Treat. (2017) 163:321–30. doi: 10.1007/s10549-017-4166-z
18. Proskynitopoulos PJ, Lam FC, Sharma S, Young BC, Laviv Y, Kasper EM. A review of the neurosurgical management of brain metastases during pregnancy. Can J Neurol Sci. (2021) 48:698–707. doi: 10.1017/cjn.2020.254
19. Sharma A, Nguyen HS, Lozen A, Sharma A, Mueller W. Brain metastases from breast cancer during pregnancy. Surg Neurol Int. (2016) 7:S603–6. doi: 10.4103/2152-7806.189730
20. Howell EA, Ahmed ZN. Eight steps for narrowing the maternal health disparity gap. Contemp Ob Gyn. (2019) 64:30–6.
21. Hall JA, Benton L, Copas A, Stephenson J. Pregnancy intention and pregnancy outcome: systematic review and meta-analysis. Matern Child Health J. (2017) 21:1051–63. doi: 10.1007/s10995-016-2237-0
22. Centers for Disease Control and Prevention. Pregnancy Mortality Surveillance System. Maternal and Infant Health | CDC. Atlanta, GA: Reproductive Health. (2019).
23. Hung P, Casey MM, Kozhimannil KB, Karaca-Mandic P, Moscovice IS. Rural-urban differences in access to hospital obstetric and neonatal care: how far is the closest one? J Perinatol. (2018) 38:645–52. doi: 10.1038/s41372-018-0063-5
24. Phiri SNA, Fylkesnes K, Moland KM, Byskov J, Kiserud T. Rural-urban inequity in unmet obstetric needs and functionality of emergency obstetric care services in a Zambian district. PLoS ONE. (2016) 11:e0145196. doi: 10.1371/journal.pone.0145196
25. Everett BG, Kominiarek MA, Mollborn S, Adkins DE, Hughes TL. Sexual orientation disparities in pregnancy and infant outcomes. Matern Child Health J. (2019) 23:72–81. doi: 10.1007/s10995-018-2595-x
26. Weber A, Miskle B, Lynch A, Arndt S, Acion L. Substance use in pregnancy: identifying stigma and improving care. Subst Abuse Rehabil. (2021) 12:105–21. doi: 10.2147/SAR.S319180
27. CDC. Cdc.Gov Stroke Facts. Atlanta, GA: CDC (2020).
28. Ekker MS, Verhoeven JI, Vaartjes I, van Nieuwenhuizen KM, Klijn CJM, de Leeuw FE. Stroke incidence in young adults according to age, subtype, sex, and time trends. Neurology. (2019) 92:e2444–54. doi: 10.1212/WNL.0000000000007533
29. Bushnell CD. Stroke in women: risk and prevention throughout the lifespan. Neurol Clin. (2008) 26:1161–76. doi: 10.1016/j.ncl.2008.05.009
30. Swartz RH, Cayley ML, Foley N, Ladhani NNN, Leffert L, Bushnell C, et al. The incidence of pregnancy-related stroke: a systematic review and meta-analysis. Int J Stroke. (2017) 12:687–97. doi: 10.1177/1747493017723271
31. Leffert LR, Clancy CR, Bateman BT, Bryant AS, Kuklina EV. Hypertensive disorders and pregnancy-related stroke : frequency, trends, risk factors, and outcomes. Obstet Gynecol. (2015) 125:124–31. doi: 10.1097/AOG.0000000000000590
32. Elgendy IY, Gad MM, Mahmoud AN, Keeley EC, Pepine CJ. Acute stroke during pregnancy and puerperium. J Am Coll Cardiol. (2020) 75:180–90. doi: 10.1016/j.jacc.2019.10.056
33. James AH, Bushnell CD, Jamison MG, Myers ER. Incidence and risk factors for stroke in pregnancy and the puerperium. Obstet Gynecol. (2005) 106:509–16. doi: 10.1097/01.AOG.0000172428.78411.b0
34. Creanga AA, Berg CJ, Syverson C, Seed K, Bruce FC, Callaghan WM. Race, ethnicity, and nativity differentials in pregnancy-related mortality in the united states: 1993-2006. Obstet Gynecol. (2012) 120:261–8. doi: 10.1097/AOG.0b013e31825cb87a
35. Persky RW, Turtzo LC, McCullough LD. Stroke in women: disparities and outcomes. Curr Cardiol Rep. (2010) 12:6–13. doi: 10.1007/s11886-009-0080-2
36. Miller EC, Zambrano Espinoza MD, Huang Y, Friedman AM, Boehme AK, Bello NA, et al. Maternal race/ethnicity, hypertension, and risk for stroke during delivery Admission. J Am Heart Assoc. (2020) 9:e014775. doi: 10.1161/JAHA.119.014775
37. Liang ZW, Lin L, Gao WL, Feng LM. A clinical characteristic analysis of pregnancy-associated intracranial haemorrhage in China. Sci Rep. (2015) 5:9509. doi: 10.1038/srep09509
38. Foo L, Bewley S, Rudd A. Maternal death from stroke: a thirty year national retrospective review. Eur J Obstet Gynecol Reprod Biol. (2013) 171:266–70. doi: 10.1016/j.ejogrb.2013.09.021
39. Bateman BT, Schumacher HC, Bushnell CD, Pile-Spellman J, Simpson LL, Sacco RL, et al. Intracerebral hemorrhage in pregnancy: frequency, risk factors, and outcome. Neurology. (2006) 67:424–9. doi: 10.1212/01.wnl.0000228277.84760.a2
40. Cordonnier C, Sprigg N, Sandset EC, Pavlovic A, Sunnerhagen KS, Caso V, et al. Stroke in women-from evidence to inequalities. Nat Rev Neurol. (2017) 13:521–32. doi: 10.1038/nrneurol.2017.95
41. Yoshida K, Takahashi JC, Takenobu Y, Suzuki N, Ogawa A, Miyamoto S. Strokes associated with pregnancy and puerperium: a Nationwide Study by the Japan stroke society. Stroke. (2017) 48:276–82. doi: 10.1161/STROKEAHA.116.014406
42. Sells CM, Feske SK. Stroke in pregnancy. Semin Neurol. (2017) 37:669–78. doi: 10.1055/s-0037-1608940
43. Meeks JR, Bambhroliya AB, Alex KM, Sheth SA, Savitz SI, Miller EC, et al. Association of primary intracerebral hemorrhage with pregnancy and the postpartum period. JAMA Netw Open. (2020) 3:e202769. doi: 10.1001/jamanetworkopen.2020.2769
44. Limaye K, Patel A, Dave M, Kenmuir C, Lahoti S, Jadhav AP, et al. Secular increases in spontaneous subarachnoid hemorrhage during pregnancy: a nationwide sample analysis. J Stroke Cerebrovasc Dis. (2019) 28:1141–8. doi: 10.1016/j.jstrokecerebrovasdis.2019.01.025
45. Lee S, Lee SW, Han KS Ki M, Ko YH, Kim SJ. Analysis of characteristics and mortality in cardiac arrest patients by hospital level: a nationwide population-based study. J Korean Med Sci. (2021) 36:e173. doi: 10.3346/jkms.2021.36.e173
46. Meyer L, Stubbs B, Fahrenbruch C, Maeda C, Harmon K, Eisenberg M, et al. Incidence, causes, and survival trends from cardiovascular-related sudden cardiac arrest in children and young adults 0 to 35 years of age: a 30-year review. Circulation. (2012) 126:1363–72. doi: 10.1161/CIRCULATIONAHA.111.076810
47. Zelop CM, Einav S, Mhyre JM, Martin S. Cardiac arrest during pregnancy: ongoing clinical conundrum. Am J Obstet Gynecol. (2018) 219:52–61. doi: 10.1016/j.ajog.2017.12.232
48. Hameed AB, Lawton ES, McCain CL, Morton CH, Mitchell C, Main EK, et al. Pregnancy-related cardiovascular deaths in California: beyond peripartum cardiomyopathy. Am J Obstet Gynecol. (2015) 213:379. doi: 10.1016/j.ajog.2015.05.008
49. Geocadin RG, Wijdicks E, Armstrong MJ, Damian M, Mayer SA, Ornato JP, et al. Practice guideline summary: reducing brain injury following cardiopulmonary resuscitation. Neurology. (2017) 88:2141–9. doi: 10.1212/WNL.0000000000003966
50. Berg KM, Cheng A, Panchal AR, Topjian AA, Aziz K, Bhanji F, et al. Part 7: systems of care 2020 American heart association guidelines for cardiopulmonary resuscitation and emergency cardiovascular care. Circulation. (2020) 142:S580–604. doi: 10.1161/CIR.0000000000000918
51. Nolan JP, Sandroni C, Böttiger BW, Cariou A, Cronberg T, Friberg H, et al. European Resuscitation Council and European Society of Intensive Care Medicine guidelines 2021: post-resuscitation care. Intensive Care Med. (2021) 47:369–421. doi: 10.1007/s00134-021-06368-4
52. Chames MC, Pearlman MD. Trauma during pregnancy: outcomes and clinical management. Clin Obstet Gynecol. (2008) 51:398–408. doi: 10.1097/GRF.0b013e31816f2aa7
53. Colantonio A, Mar W, Escobar M, Yoshida K, Velikonja D, Rizoli S, et al. Women's health outcomes after traumatic brain injury. J Womens Health. (2010) 19:1109–16. doi: 10.1089/jwh.2009.1740
54. Blaya MO, Raval AP, Bramlett HM. Traumatic brain injury in women across lifespan. Neurobiol Dis. (2022) 164:105613. doi: 10.1016/j.nbd.2022.105613
55. Vaajala M, Kuitunen I, Nyrhi L, Ponkilainen V, Kekki M, Luoto T, et al. Pregnancy and delivery after traumatic brain injury: a nationwide population-based cohort study in Finland. J Matern Fetal Neonatal Med. (2022) 25:9709–16. doi: 10.1080/14767058.2022.2050899
56. Stockman JK, Hayashi H, Campbell JC. Intimate partner violence and its health impact on ethnic minority women. J Womens Health. (2015) 24:62–79. doi: 10.1089/jwh.2014.4879
57. Hunnicutt G, Murray C, Lundgren K, Crowe A, Olson L. Exploring correlates of probable traumatic brain injury among intimate partner violence survivors. J Aggress Maltreat Trauma. (2019) 28:677–94. doi: 10.1080/10926771.2019.1587656
58. Silveira MF, Mesenburg MA, Bertoldi AD, de Mola CL, Bassani DG, Domingues MR, et al. The association between disrespect and abuse of women during childbirth and postpartum depression: findings from the 2015 Pelotas birth cohort study. J Affect Disord. (2019) 256:441–7. doi: 10.1016/j.jad.2019.06.016
59. Kusznir Vitturi B, Barreto Cabral F, Mella Cukiert C. Outcomes of pregnant women with refractory epilepsy. Seizure. (2019) 69:251–7. doi: 10.1016/j.seizure.2019.05.009
60. Battino D, Tomson T, Bonizzoni E, Craig J, Lindhout D, Sabers A, et al. Seizure control and treatment changes in pregnancy: observations from the EURAP epilepsy pregnancy registry. Epilepsia. (2013) 54:1621–7. doi: 10.1111/epi.12302
61. Lu YT, Hsu CW, Tsai WC, Cheng MY, Shih FY, Fu TY, et al. Status epilepticus associated with pregnancy: a cohort study. Epilepsy Behav. (2016) 59:92–7. doi: 10.1016/j.yebeh.2016.03.034
62. Rajiv KR, Radhakrishnan A. Status epilepticus in pregnancy: etiology, management, and clinical outcomes. Epilepsy Behav. (2017) 76:114–9. doi: 10.1016/j.yebeh.2017.07.002
63. Burneo JG, Jette N, Theodore W, Begley C, Parko K, Thurman DJ, et al. Disparities in epilepsy: report of a systematic review by the North American Commission of the international league against epilepsy. Epilepsia. (2009) 50:2285–95. doi: 10.1111/j.1528-1167.2009.02282.x
64. Lawn N, Laich E, Ho S, Martin R, Faught E, Knowlton R, et al. Eclampsia, hippocampal sclerosis, and temporal lobe epilepsy: accident or association? Neurology. (2004) 62:1352–6. doi: 10.1212/01.WNL.0000120544.64972.10
65. Yerby M, Koepsell T, Daling J. Pregnancy complications and outcomes in a cohort of women with epilepsy. Epilepsia. (1985) 26:631–5. doi: 10.1111/j.1528-1157.1985.tb05703.x
66. Linnander EL, Ayedun A, Boatright D, Ackerman-Barger K, Morgenthaler TI, Ray N, et al. Mitigating structural racism to reduce inequities in sepsis outcomes: a mixed methods, longitudinal intervention study. BMC Health Serv Res. (2022) 22:975. doi: 10.1186/s12913-022-08331-5
67. Johnson-Agbakwu CE, Ali NS, Oxford CM, Wingo S, Manin E, Coonrod DV. Racism, COVID-19, and Health Inequity in the USA: a call to action. J Racial Ethn Health Disparities. (2022) 9:52–8. doi: 10.1007/s40615-020-00928-y
68. May AE, Fombon FN, Francis S. UK registry of high-risk obstetric anaesthesia: report on neurological disease. Int J Obstet Anesth. (2008) 17:31–6. doi: 10.1016/j.ijoa.2007.03.016
69. Haranath S, Ganapathy K, Kesavarapu S, Kuragayala S. eNeuroIntensive care in India: the need of the hour. Neurol India. (2021) 69:245–51. doi: 10.4103/0028-3886.314591
70. Alves DS, Times VC, da Silva ÉMA, Melo PSA, Novaes MA. Advances in obstetric telemonitoring: a systematic review. Int J Med Inform. (2020) 134:104004. doi: 10.1016/j.ijmedinf.2019.104004
71. Vega S, Marciscano I, Holcomb M, Erps KA, Major J, Lopez AM, et al. Testing a top-down strategy for establishing a sustainable telemedicine program in a developing country: the Arizona telemedicine program-US army-republic of panama initiative. Telemed J E Health. (2013) 19:746–53. doi: 10.1089/tmj.2013.0025
Keywords: obstetric care, neurocritical care, disparities health, pregnancy, health care
Citation: Mittal S, Alsbrook D, Okwechime RT, Iqbal F and Nobleza CO'HS (2023) The landscape of disparities in obstetric neurocritical care and a path forward. Front. Neurol. 13:1008544. doi: 10.3389/fneur.2022.1008544
Received: 31 July 2022; Accepted: 05 December 2022;
Published: 06 January 2023.
Edited by:
Brandon Peter Lucke-Wold, University of Florida, United StatesReviewed by:
Ritvij Bowry, University of Texas Health Science Center at Houston, United StatesCopyright © 2023 Mittal, Alsbrook, Okwechime, Iqbal and Nobleza. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Christa O'Hana S. Nobleza,  Y2hyaXN0YW9oYW5hMTRtZEB5YWhvby5jb20=
Y2hyaXN0YW9oYW5hMTRtZEB5YWhvby5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.