- 1Department of Neurology, Shenzhen Third People's Hospital, Shenzhen, China
- 2Department of Neurology, The Second Affiliated Hospital, Southern University of Science and Technology, Shenzhen, China
- 3Shantou University Medical College, Shantou University, Shantou, China
- 4Department of Neurology, The First Affiliated Hospital of Shenzhen University, Shenzhen, China
- 5School of Nursing, Anhui Medical University, Hefei, China
The highly lethal cryptogenic brain abscess can be easily misdiagnosed. However, cryptogenic brain abscess caused by Providencia rettgeri is rarely reported. We present the case of a cryptogenic Providencia rettgeri brain abscess and analyze the clinical manifestations, imaging findings, treatment, and outcome to improve the level of awareness, aid in accurate diagnosis, and highlight effective clinical management. A 39-year-old man was admitted to the hospital after experiencing acute speech and consciousness disorder for 1 day. The patient had a medical history of nephrotic syndrome and membranous nephropathy requiring immunosuppressant therapy. Magnetic resonance imaging revealed giant, space-occupying lesions involving the brain stem, basal ganglia, and temporal-parietal lobes without typical ring enhancement, mimicking a tumor. Initial antibiotic treatment was ineffective. Afterward, pathogen detection in cerebrospinal fluid using metagenomic next-generation sequencing revealed Providencia rettgeri. Intravenous maximum-dose ampicillin was administered for 5 weeks, and the patient's symptoms resolved. Cryptogenic Providencia rettgeri brain abscess typically occurs in patients with impaired immunity. Our patient exhibited a sudden onset with non-typical neuroimaging findings, requiring differentiation of the lesion from stroke and brain tumor. Metagenomic next-generation sequencing was important in identifying the pathogen. Rapid diagnosis and appropriate use of antibiotics were key to obtaining a favorable outcome.
Introduction
The brain abscess is a potentially fatal parenchymal brain lesion caused by local infection or remote spread (1). The incidence of brain abscesses is estimated to be 0.3–1.3 per 100,000 persons per year (2). The wide application of antibiotics has contributed to a gradual decline in the prevalence of brain abscesses.
The mortality of brain abscesses decreased from 22.8% in the 1950s to 6.3% in the early 21st century (3). However, cases of cryptogenic brain abscesses with unknown origin and atypical symptoms are increasing (3). The source of infection remains unclear in a significant proportion of brain abscesses, even after thorough investigations, and these are considered cryptogenic brain abscesses (4). Cryptogenic abscesses occur in 4.6–43.4% of cases (4, 5), and they are easily misdiagnosed and have a poor prognosis (1). Early diagnosis and treatment are crucial to minimize complications and reduce mortality (1). Providencia rettgeri is a motile, gram-negative, and facultative anaerobic bacterium widely distributed in the environment (6). It can cause zoonotic opportunistic infections, such as urinary tract infections (7), endocarditis (8), and rare intracranial infections (9).
To our knowledge, cryptogenic giant brain abscess caused by Providencia rettgeri is rare, and its clinical and imaging features are poorly understood. We present such a case with a literature review to improve the diagnosis and management of this disease.
Case description
In October 2021, a 39-year-old man was admitted to our hospital after experiencing speech disorder and right hemiplegia for 1 day with consciousness disorder for over 3 h. Emergency non-contrasted brain computed tomography (CT) scan revealed left basal ganglia and temporoparietal finger-shaped edema. The head and neck CT angiography displayed no obvious abnormality. The patient had presented with edema in both lower limbs 3 months previously and based on renal pathology, nephrologists diagnosed membranous nephropathy (stage II) and nephrotic syndrome. Intravenous (IV) cyclophosphamide, oral prednisone (40 mg per day), and oral valsartan were administered. He had no history of infectious or genetic diseases.
Physical examination on admission showed a body temperature of 36.2°C, blood pressure of 173/103 mmHg, confusion, aphasia, shallow nasolabial sulcus on the right side, decreased muscle strength (0/5) in the right limbs, and decreased muscular tension, with no other abnormal neurological or cardiopulmonary findings. The National Institutes of Health Stroke Scale score was 15.
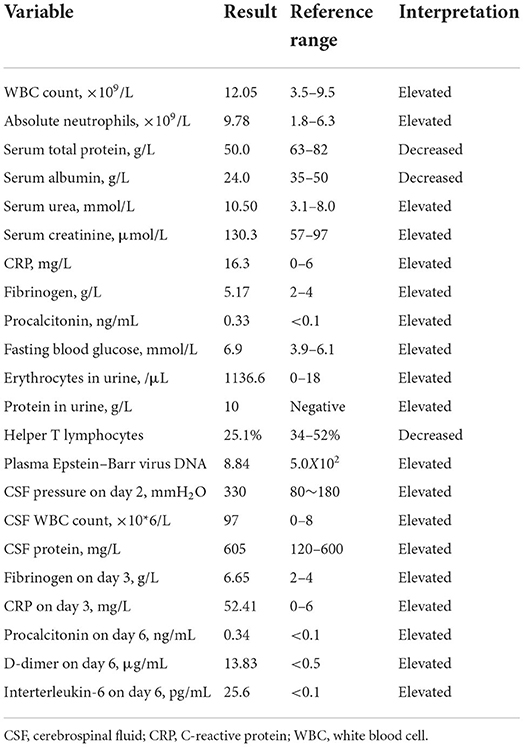
Blood analysis revealed an absence of anti-double-stranded DNA, anti-nucleosome, anti-SM, anti-proliferating cells, Treponema pallidum, human immunodeficiency virus, anti-protease 3, anti-myeloperoxidase, and anti-glomerular basement membrane antibodies. Serum creatine kinase, homocysteine, uric acid, ammonia, troponin I, myoglobin, and N-terminal pro-brain natriuretic peptide levels were within normal limits. Glucose and lactic acid levels in cerebrospinal fluid (CSF) on day 2 were normal. Cytotoxic T lymphocyte count and TCD3+ CD4+/ TCD3+ CD8+ were normal. The gene amplification method for detecting cytomegalovirus DNA in plasma gave a negative result. The CSF smear stained with India ink showed no cryptococcus. The test result for cryptococcal capsular antigen in the CSF was negative. Initial CSF culture yielded no pathogens. Metagenomic next-generation sequencing [mNGS, WillingMed Technology (Beijing) Co., Ltd., Beijing City] of the CSF on day 18 revealed 21 sequences of Providencia rettgeri (reference range < 8 sequences). A repeat mNGS (the Guangzhou Institute for Respiratory Health, Guangzhou City) showed 14 sequences of Providencia rettgeri. Repeat testing of the CSF on day 28 revealed that the white blood cell count and protein level were within normal limits, and the CSF culture yielded no growth. Serum urea and creatinine levels and the glomerular filtration rate on day 34 were normal. The other laboratory results are shown in Table 1.
Chest CT revealed small ground-glass-like nodules in the posterior segment of the upper lobe of the right lung. Ultrasonography of the liver, bile ducts, pancreas, spleen, and urinary system revealed only mild fatty liver. Brain MRI on day 1 (Figures 1A–F) showed extensive foci involving the left frontoparietal-temporal lobe, basal ganglia, and midbrain.
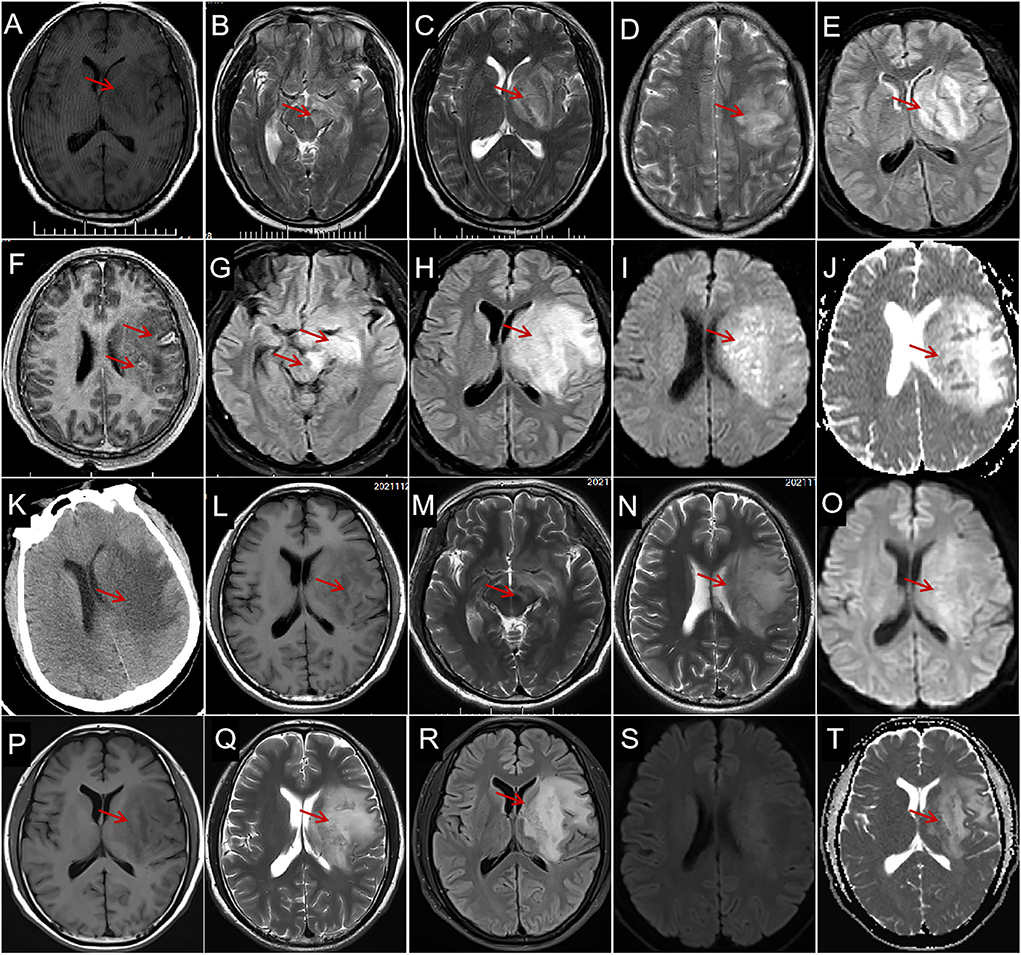
Figure 1. Characteristics and evolution of lesions on brain magnetic resonance imaging (MRI) and computed tomography (CT). (A) Brain MRI on day 1 shows a large hypointensity (arrows) in the left basal ganglia and the temporal lobe on axial T1-weighted imaging (WI); a hyperintense region (arrows) in the left midbrain (B), basal ganglia (C), and temporal-parietal lobe (D) on T2-WI and fluid-attenuated inversion recovery [FLAIR (E)] with space-occupying effect; and individual, small, arc-shaped, ring-shaped lesions are enhanced [(F), arrows]. The MRI on day 5 shows extensive hyperintense lesions enlarged (arrows) in the left midbrain, hippocampus [(G), arrows], basal ganglia, and temporal lobe [(H), arrows] on FLAIR, diffusion-weighted imaging [DWI (I), arrow], and apparent diffusion coefficient [ADC (J), arrow]. CT on day 6 reveals extensive, finger-shaped, low-density areas with space-occupying effects in the left basal ganglia and semioval center [(K), arrow]. MRI on day 34 shows slight hypointensity on T1-WI [(L), arrow], slight hyperintensity on T2-WI [(M,N), arrows], and DWI [(O), arrow], and the space-occupying effect obviously improved. Magnetic resonance imaging on the 25th day after discharge shows hypointensity on T1-WI [(P), arrow], hyperintensity on T2-WI [(Q), arrow], FLAIR [(R), arrow] ADC [(S), arrow], and without diffusion restriction on DWI (T).
The patient was initially suspected to have acute cerebral infarction and was administered clopidogrel, argatroban, rosuvastatin, and symptomatic treatment. The patient's condition deteriorated on day 1, and he presented with fever (temperature: 39.2°C) and light coma, followed by a stress peptic ulcer and gastrointestinal bleeding. Neck rigidity was detected on day 2; IV ceftriaxone (2 g/day × 7 days), IV mannitol, IV albumin, and oral esomeprazole magnesium and prednisone (10 mg/day × 7 days) were administered, followed by IV acyclovir (1.5 g/day × 2 weeks) on day 4. However, the anti-infective treatment provided no obvious effect; a repeat MRI on day 5 (Figures 1G–J) and repeat CT (Figure 1K) showed that the lesions had enlarged. Therefore, IV ampicillin sodium, 12 g/day × 5 days, followed by 13.8 g/day × 30 days, was initiated on day 6.
The disturbance of consciousness, fever, and hemiplegia improved on day 10. Afterward, the patient was aphasic but able to walk and lift the right arm on day 28. The MRI on day 34 (Figures 1L–O) showed that the foci obviously improved. The patient was discharged on day 35 with aphasia but independent ambulation. The MRI (Figures 1P–T) on the 25th day after the discharge shows the lesion remained stable. The patient had no discomfort during the 1-month follow-up after discharge and was satisfied with his treatment and recovery.
Discussion
We report a rare case of a cryptogenic giant brain abscess caused by Providencia rettgeri mimicking stroke with a satisfactory outcome after maximum-dose ampicillin therapy. The atypical symptoms and neuroimaging findings in this patient may be related to autoimmune disease and the use of immunosuppressive agents. Opportunistic infections and differentiation of stroke or brain tumor should be carefully considered in patients such as this. Prompt diagnosis and selection of appropriate antibiotic therapy are key to achieving a favorable outcome.
To our knowledge, only two cases of Providencia stuartii meningitis have been reported, and in both cases, CSF culture yielded pathogenic bacteria biochemically identified as Providencia stuartii; however, no neuroimaging data were provided (9, 10).
Pathogenesis
In older patients, those with reduced immunity, or patients with long-term indwelling catheters, Providencia rettgeri can infect via pathogenic adhesion, colonization, invasion, and then urinary tract (7) and intracranial (9) infections. To invade the host cells, an infectious dose of the bacteria is essential (11). The blood–brain barrier (BBB) of the central nervous system (CNS) is an important physiological barrier. Pathogenic bacteria disrupt tight junction proteins and promote BBB permeability (12), and may thwart the autophagic pathway to destroy BBB cellular defense mechanisms (13). The specific mechanism of entry of Providencia rettgeri through the BBB is unclear.
Clinical features
The majority of patients with brain abscesses have predisposing conditions, with half of the cases originating from severe craniocerebral trauma, neurosurgery, otitis, mastoiditis, or sinusitis (2, 14, 15). Brain abscesses can also originate from lung infections (16) and rare oral infections (17). The exact source of the cryptogenic abscess remains unknown in our patient; however, we speculate that this abscess was most likely caused by occult hematogenous spread from an internal focus. The mean duration from the onset of symptoms to the diagnosis of brain abscess is 8.3 days (2), and definitive diagnosis in patients with cryptogenic brain abscess takes longer. Patients with brain abscesses experienced headaches (69%), fever (53%), and focal neurological deficits (48%) (2). This classic triad is observed in only 20% of patients with typical abscess (2), as in our patient who exhibited a stroke-like onset without fever and headache. This could be due to his limited ability to mount an immune response, thereby obscuring the signs typically associated with infection (4). The presence of autoimmune disease and the use of immunosuppressive agents in our patient caused a weakened response to pathogens, which may be an important reason for the hidden clinical manifestations.
Neuroimaging
The brain MRI in our patient revealed a giant brain abscess in the left temporal-parietal lobe, basal ganglia, and brainstem, with hyperintense lesions on T2-weighted imaging (WI), diffusion-weighted imaging (DWI), apparent diffusion coefficient (ADC), and hypointensity on T1-weighted imaging (T1WI), with space-occupying brain edema, which required differentiation from a brain tumor (18). The appearance of a brain abscess on routine MRI lacks specificity. Diffusion-weighted imaging (DWI) is the most sensitive imaging sequence for brain abscess (19); however, diffusion limitation on MRI can also be seen in high-grade gliomas and primary CNS lymphomas. Diffusion restriction is an uncommon finding in ring-enhancing neoplasms (20, 21). Multimodal MRI is useful in differentiating brain abscesses and tumors.
The most common sites of brain abscesses are in the frontal (5) and temporal lobes (22). An otogenic abscess occurs almost exclusively in the temporal lobe, and sinus infection-related abscess predominantly occurs in the frontal lobe (4). Pyogenic brainstem abscess is rare (23). Abscesses simultaneously involving the brainstem, basal ganglia, and lobes, as in this case, are even more uncommon. The MRI showed no typical intracranial ring-enhancing lesions in this case, which was related either to the absence of abscess capsule formation in the early stage or to the patient's low immune function (4). Enhancing satellite lesions were seen around the abscess (Figure 1F), and multiple lesions of various sizes with annular enhancement have a certain diagnostic value.
Laboratory analysis
Blood cultures should be performed early in all patients with suspected abscesses, although the positivity rate is not high (14–50%) (4). The CSF analysis may reveal pleocytosis, elevated protein level, and decreased glucose level; however, these parameters will be normal in a significant proportion (16%) of patients (2). The CSF culture is infrequently positive (24%) (2). Metagenomic next-generation sequencing (mNGS) testing of CSF has shown higher sensitivity and specificity relative to conventional microbiological testing in identifying the causative pathogen (24). Metagenomic next-generation sequencing (mNGS) for identifying pathogens is very important, as shown in this case.
Treatment
Providencia rettgeri, in this case, was resistant to ceftriaxone. Therefore, ampicillin sodium was administered for 5 weeks based on the drug sensitivity assay. The duration of antibiotic treatment is usually not < 4 weeks (25). For patients with multiple abscesses or impaired immunity, the required course of antibiotic treatment is 6–8 weeks or longer (4, 25). Prolongation of antibiotic treatment is generally advised as long as the abscess focus is visible on the brain MRI (1). Improvements in our patient's symptoms were observed earlier than brain MRI changes. Timely pathogen identification and effective antibiotics are very important in treating brain abscesses. Providencia rettgeri often displays multiple drug resistance (11); therefore, selecting appropriate antibiotics is crucial.
Simple antibiotic therapy is most suitable for patients in good initial clinical condition (Glasgow Coma Scale score >12) and abscesses ≤ 2.5 cm in diameter (25). However, pharmacotherapy alone is rarely effective for abscesses > 3 cm in diameter (26). In our case, the resolution of the abscess—greatly exceeding 3 cm in diameter—was surprising. Although our patient had giant, space-occupying brain lesions, they were deeply situated, involved important functional areas, and did not form an abscess wall. Hence, surgical resection or puncture drainage was not performed.
Neurosurgical treatment of the brain abscess should depend on the location and size of the abscess, the patient's clinical condition, and the chance of achieving successful decompression (15). Surgery should be considered if the condition worsens or there are no clinical and radiological improvements within 1–2 weeks (25). Other surgical indications include cerebellar herniation or periventricular abscess with a risk of rupture (15).
This report is limited in that the origin of the brain abscesses is not clear. The specific process whereby Providencia rettgeri evades immunological defenses in the patient with impaired immunity must be investigated.
Conclusion
Cryptogenic giant Providencia rettgeri brain abscesses are rare. Clinicians should consider this opportunistic infection in patients with impaired immunity. Patients with autoimmune diseases or conditions requiring immunosuppressive agents can present atypical symptoms and neuroimaging results that mimic stroke or giant MRI lesions without typical enhancement that must be differentiated from tumors. Metagenomic next-generation sequencing (mNGS) is important in pathogen identification. Rapid diagnosis and appropriate use of antibiotics are key in obtaining a favorable outcome. This study expands the clinical and imaging spectrum of Providencia rettgeri meningitis; however, the underlying pathophysiological mechanism requires further study.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
This report was approved by the Ethics Review Board of the Shenzhen Third People's Hospital (No: 2022-010). The patients/participants provided their written informed consent to participate in this study.
Author contributions
YZ and DZ were responsible for data collection and writing the first draft. BL and XL provided constructive discussion and translated the manuscript. LC conceived the study, translated the manuscript, and critically revised the manuscript. All authors have read and approved the manuscript.
Funding
This work was supported by the Shenzhen Second People's Hospital Clinical Research Fund of Guangdong Province High-level Hospital Construction Project (No. 20223357021) and the Initializing Fund of Academic Leaders of Shenzhen Third People's Hospital.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Zhou W, Shao X, Jiang X. A clinical report of two cases of cryptogenic brain abscess and a relevant literature review. Front Neurosci. (2019) 12:1054. doi: 10.3389/fnins.2018.01054
2. Brouwer MC, Coutinho JM, van de Beek D. Clinical characteristics and outcome of brain abscess: systematic review and meta-analysis. Neurology. (2014) 82:806–13. doi: 10.1212/WNL.0000000000000172
3. Zhang Z, Cai X, Li J, Kang X, Wang H, Zhang L, et al. Retrospective analysis of 620 cases of brain abscess in Chinese patients in a single center over a 62-year period. Acta Neurochir (Wien). (2016) 158:733–9. doi: 10.1007/s00701-016-2741-4
4. Patel K, Clifford DB. Bacterial brain abscess. Neurohospitalist. (2014) 4:196–204. doi: 10.1177/1941874414540684
5. Roche M, Humphreys H, Smyth E, Phillips J, Cunney R., McNamara E, et al. A twelve-year review of central nervous system bacterial abscesses; presentation and aetiology. Clin Microbiol Infect. (2003) 9:803–9. doi: 10.1046/j.1469-0691.2003.00651.x
6. Shi J, Wang G, Ding M, Zhou Y. A single-center study on antimicrobial resistance and antimicrobial resistance genes of Providencia rettgeri. Chin J Infect Control. (2021) 20:597–601 (Chinese). doi: 10.12138/j.issn.1671-9638.20218118
7. Sagar S, Narasimhaswamy N, D'Souza J. Providencia rettgeri: An emerging nosocomial uropathogen in an indwelling urinary catheterised patient. J Clin Diagn Res. (2017) 11:DD01–2. doi: 10.7860/JCDR/2017/25740.10026
8. Marull JM, De Benedetti ME. Automatic implantable cardioverter defibrillator pocket infection due to Providencia rettgeri: a case report. Cases J. (2009) 2:8607. doi: 10.4076/1757-1626-2-8607
9. Sipahi OR, Bardak-Ozcem S, Ozgiray E, Aydemir S, Yurtseven T, Yamazhan T, et al. Meningitis due to Providencia stuartii. J Clin Microbiol. (2010) 48:4667–8. doi: 10.1128/JCM.01349-10
10. Wu J, Yin R, Wang J. Purulent meningitis caused by Providencia stuartii: a case report. Chin J Lab Med. (1994) 17:200 (Chinese).
11. Kurmasheva N, Vorobiev V, Sharipova M, Efremova T, Mardanova A. The potential virulence factors of Providencia stuartii: motility, adherence, and invasion. Biomed Res Int. (2018) 2018:3589135. doi: 10.1155/2018/3589135
12. Yang R, Liu W., Miao L, Yang X, Fu J, Dou B, et al. Induction of VEGFA and Snail-1 by meningitic Escherichia coli mediates disruption of the blood-brain barrier. Oncotarget. (2016) 7:63839–55. doi: 10.18632/oncotarget.11696
13. Cutting AS, Del Rosario Y, Mu R, Rodriguez A, Till A., Subramani S, et al. The role of autophagy during group B Streptococcus infection of blood-brain barrier endothelium. J Biol Chem. (2014) 289:35711–23. doi: 10.1074/jbc.M114.588657
14. Kangsanarak J, Fooanant S, Ruckphaopunt K, Navacharoen N, Teotrakul S. Extracranial and intracranial complications of suppurative otitis media. Report of 102 cases. J Laryngol Otol. (1993) 107:999–1004. doi: 10.1017/s0022215100125095
15. Brouwer MC, Tunkel AR, van de Beek D. Brain abscess. N Engl J Med. (2014) 371:447–56. doi: 10.1056/NEJMc1410501
16. Browne WD, Lieberson RE, Kabbesh MJ. Nocardia cyriacigeorgica brain and lung abscesses in 77-year-old man with diabetes. Cureus. (2021) 13:e19373. doi: 10.7759/cureus.19373
17. Costa Mendes L, Vaysse F, Maret D. Brain abscess secondary to a dental infection. Case Rep Emerg Med. (2020) 2020:3248174. doi: 10.1155/2020/3248174
18. Xiao D, Wang J, Wang X, Fu P, Zhao H, Yan P, et al. Distinguishing brain abscess from necrotic glioblastoma using MRI-based intranodular radiomic features and peritumoral edema/tumor volume ratio. J Integr Neurosci. (2021) 20:623–34. doi: 10.31083/j.jin2003066
19. Brouwer MC, van de Beek D. Epidemiology, diagnosis, and treatment of brain abscesses. Curr Opin Infect Dis. (2017) 30:129-34. doi: 10.1097/QCO.0000000000000334
20. Chang SC, Lai PH, Chen WL, Weng HH, Ho JT, Wang JS, et al. Diffusion- weighted MRI features of brain abscess and cystic or necrotic brain tumors: comparison with conventional. MRI Clin Imaging. (2002) 26:227–36. doi: 10.1016/s0899-7071(02)00436-9
21. Faehndrich J, Weidauer S, Pilatus U, Oszvald A, Zanella FE, Hattingen E. Neuroradiological viewpoint on the diagnostics of space-occupying brain lesions. Clin Neuroradiol. (2011) 21:123–39. doi: 10.1007/s00062-011-0073-6
22. Slazinski T. Brain abscess. Crit Care Nurs Clin North Am. (2013) 25:381–8. doi: 10.1016/j.ccell.2013.04.001
23. Bulthuis VJ, Gubler FS, Teernstra OP, Temel Y. A case of a brain stem abscess with a favorable outcome. Surg Neurol Int. (2015) 6:161. doi: 10.4103/2152-7806.167087
24. Miller S, Naccache SN, Samayoa E, Messacar K, Arevalo S, Federman S, et al. Laboratory validation of a clinical metagenomic sequencing assay for pathogen detection in cerebrospinal fluid. Genome Res. (2019) 29: 831-42. doi: 10.1101/gr.238170.118
25. Arlotti M, Grossi P, Pea F, Tomei G, Vullo V, De Rosa FG, et al. Consensus document on controversial issues for the treatment of infections of the central nervous system: bacterial brain abscesses. Int J Infect Dis. (2010) 14: S79–S92. doi: 10.1016/j.ijid.2010.05.010
Keywords: Providencia rettgeri, cryptogenic brain abscess, impaired immunity, metagenomic next-generation sequencing, stroke-like onset, brain tumor, case report
Citation: Zhao Y, Lian B, Liu X, Wang Q, Zhang D, Sheng Q and Cao L (2022) Case report: Cryptogenic giant brain abscess caused by Providencia rettgeri mimicking stroke and tumor in a patient with impaired immunity. Front. Neurol. 13:1007435. doi: 10.3389/fneur.2022.1007435
Received: 30 July 2022; Accepted: 01 September 2022;
Published: 23 September 2022.
Edited by:
German Torres, New York Institute of Technology, United StatesReviewed by:
Kenneth Ssebambulidde, Makerere University, UgandaZhigang Liang, Yantai Yuhuangding Hospital, China
Copyright © 2022 Zhao, Lian, Liu, Wang, Zhang, Sheng and Cao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Liming Cao, Y2FvbG0tMjAwN0AxNjMuY29t
†These authors have contributed equally to this work and share first authorship
 Yu Zhao1,2†
Yu Zhao1,2† Baorong Lian
Baorong Lian Daxue Zhang
Daxue Zhang Liming Cao
Liming Cao