
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Neurol. , 01 September 2022
Sec. Stroke
Volume 13 - 2022 | https://doi.org/10.3389/fneur.2022.1001609
Recurrent stroke risk secondary to intracranial atherosclerotic disease remains high despite aggressive medical treatment. This risk is further amplified in subgroups possessing biomarkers of hemodynamic insufficiency and potential for embolization, which have been shown to be independently and synergistically predictive of recurrent stroke. Luminal stenosis was predominantly used as entry criteria in major treatment trials, discounting the potential role of hemodynamics from primary analyses, limiting the strength of evidence and conclusions of these biomarkers to post-hoc analyses and other natural history studies. Future treatment trials should consider stratifying patients using a combination of these high-risk biomarkers. In the absence of trials, risk stratifying patients based on the presence of these markers may lend to more individualized clinical decisions. We aimed to summarize the studies that have investigated the relationship between biomarkers and their role in predicting recurrent stroke risk in intracranial atherosclerotic disease.
Intracranial atherosclerotic disease (ICAD) is the most common cause of ischemic stroke globally with the highest prevalence in Asians, Africans, and Hispanics (1, 2). The disease carries a poor natural history with high recurrent stroke rates in both medical and endovascular treated patients (3–5). The Comparison of Warfarin and Aspirin for Symptomatic Intracranial Arterial Stenosis (WASID) trial showed that warfarin was associated with significantly higher rates of intracranial hemorrhage and provided no benefit over aspirin in preventing ischemic strokes, with two-year cerebrovascular event rates over 20% in both arms (4). The results of this trial encouraged the exploration of endovascular treatments in this population at a time of rapid advancement in endovascular technologies. In a multicenter study of prospectively enrolled patients into the US Wingspan Registry, investigators reported periprocedural stroke rates as low as 5%, suggesting an acceptable rate of periprocedural morbidity in this population (6). These findings were challenged with the results of the SAMMPRIS and VISSIT trials which showed significantly higher rates of stroke with angioplasty and stenting compared to dual antiplatelet therapy, shaping national guidelines which endorse aggressive medical therapy and advise against endovascular treatment in this population (5, 7, 8). Furthermore, the more recent CASSISS trial showed no benefit of angioplasty and stenting compared to aggressive medical management, further validating current recommendations (9). None of these trials, however, risk-stratified patients based on hemodynamics or thromboembolic potential, relying exclusively on luminal stenosis as major entry criteria.
Although these studies targeted higher risk patients by including patients with at least moderate-to-high-grade stenosis, there is likely a high degree of heterogeneity within this group. For example, patients with a dominant hemodynamic mechanism may be at a greater risk of recurrent stroke compared to patients with thromboembolic mechanisms (10–12). Several prospective and retrospective studies have attempted to risk stratify these patients using various clinical and radiographic biomarkers, such as infarction patterns, hemodynamics, embolic signaling, plaque morphology, and vessel wall analysis (11, 13). Our aim was to review the current literature of the various biomarkers that have been used to stratify recurrent stroke risk. Future randomized studies of endovascular versus medical treatment of patients with ICAD may benefit by stratifying patients using these proven biomarkers.
We searched the terms: “Intracranial atherosclerotic disease”; “ICAS”; ICAD”; “hemodynamics in intracranial atherosclerotic disease”; “risk stratification of intracranial atherosclerotic disease”; WASID; SAMMPRIS; MyRIAD; TOSS-2; “embolic signaling in intracranial atherosclerotic disease”; “hypoperfusion in intracranial atherosclerotic disease” in Pubmed.
Ischemic events that localize to a downstream atherosclerotic intracranial large artery narrowed to at least 50% of its vessel diameter can be classified under the stroke mechanism of ICAD (4). Although the degree of stenosis may correlate loosely with hemodynamics and (14) has been used in landmark randomized controlled trials (4), defining a heterogenous disease based exclusively on luminal narrowing discounts other potentially risk-defining features, such as regional hemodynamics and plaque instability. Since the presence of collaterals and variability of vascular anatomy strongly influence upstream blood flow, the degree of focal stenosis may not correlate with regional hemodynamics (11). Several imaging modalities and methods have been used to measure hemodynamics, plaque stability, and potential for distal embolism, such as quantitative magnetic resonance angiography (QMRA) (11), CT and MR perfusion (15–18), standardized digital subtraction angiography (DSA) collateral scores (19), vasomotor reactivity and embolic detection using transcranial dopplers (TCDs), and high-resolution MRI. Prospective studies have used these methods to delineate the specific mechanisms of stroke and to identify high-risk patients (11, 13), but have largely been left out of treatment trials which relied exclusively on luminal size for inclusion (4, 5). The index events and primary outcomes used in treatment trials have also limited inclusion to transient ischemic attacks or clinical strokes, while other known sequelae of ICAD are disregarded such as subclinical infarction and cognitive function (20, 21). Indeed, this is a complex disease with a heterogenous patient population and should not be simplified to a strict definition based exclusively on lumen size.
Ischemic strokes attributable to ICAD can occur due to various mechanisms, including hemodynamic ischemia, artery-to-artery embolism, branch atheromatous disease, and a combination of these mechanisms. There is likely a complementary relationship between these mechanisms, since hypoperfusion likely increases thrombosis and decrease the distal washout of thromboemboli (22–24). Infarction patterns are often used to infer stroke mechanism such that a borderzone pattern implies hemodynamic insufficiency, territorial pattern implies thromboembolism, and a perforator pattern implies branch atheromatous disease. The relationships between luminal stenosis, infarction patterns, hemodynamics, and recurrent stroke risk in intracranial atherosclerotic disease have been investigated in prospective studies and post-hoc analyses of the major trials below:
The first comprehensive study that systematically evaluated angiographic collaterals in patients with intracranial atherosclerotic disease was conducted by Liebeskind et. al in a WASID post-hoc analysis which found a strong relationship between the degree of stenosis and angiographic collaterals, and an inverse relationship between angiographic collaterals and anterograde flow across a stenosis (TIMI score) and into the downstream territory (TICI score) (25). This conceptualized angiographic collaterals as novel biomarkers in subclassifying patients with intracranial atherosclerotic disease. In a subsequent post-hoc analysis, the extent of collaterals was found to be independent predictors of recurrent stroke in a symptomatic arterial territory in the total population, however, two divergent patterns emerged depending on the degree of stenosis: collaterals were protective in preventing recurrent stroke in patients with severe stenosis but predicted an increased risk of recurrent stroke in patients with moderate stenosis (10). Although mechanisms that explain these apparent paradoxical findings remain uncertain and speculative, this study suggested that collaterals may identify more unstable milder stenoses. A subsequent study performed to evaluate the relationship between infarction pattern and collateral status did not find a statistically significant relationship between angiographic collaterals and baseline infarction patterns, including a relationship between borderzone infarctions and poor collaterals (26). Artery-to-artery embolism was thought to be the predominant stroke mechanism in this population, since 51% of the baseline infarctions and 62% of recurrent infarctions were in the territory of a single artery. In summary, these studies showed that angiographic collaterals correlate strongly with the degree of stenosis and are predictive of recurrent infarction but were unable to draw a relationship between infarction pattern and collateral status.
In a SAMMPRIS post-hoc analysis of anterior circulation infarctions, patients with qualifying events attributable to internal and cortical borderzones were at significantly higher rates of recurrent infarction compared to non-borderzone infarctions at a median follow-up of 31 months (26.4 vs. 10.4%, p = 0.054) (12). Impaired DSA collaterals were significantly associated with recurrent infarction compared to complete collaterals (27 vs. 6%, p = 0.014) and were found in 70% of patients with borderzone infarctions. The presence of a borderzone pattern coupled with impaired collaterals had the highest rate of recurrent infarction at 37%. In addition, the rate of recurrent infarction continued to increase beyond 1 year in patients with either borderzone patterns or impaired collaterals while rates remained steady in patients with non-borderzone patterns or complete collaterals. Among patients with borderzone infarctions as the qualifying event, the primary endpoint was lower in the stenting (18%) vs. medically managed group (26%), and a Kaplan-Maier curve of primary endpoints using this subgroup favored endovascular treatment, although this was not statistically significant (p-value 0.30). This study confirmed the strong relationship between angiographic collaterals and recurrent stroke as previously seen in the WASID post-hoc analyses above (10), challenged the results of previous WASID post-hoc analysis by establishing a relationship between infarction pattern and angiographic collaterals (26), and most importantly, found that the coexistence of borderzone infarction with impaired collateral flow substantially increased the risk of recurrent stroke. These differences in findings of the relationship between collaterals and infarction patterns that were not found in WASID were thought to be due to the inclusion of moderate grade stenosis in WASID and differences in the grouping of collaterals despite use of the same grading system (12).
The Vertebrobasilar Flow Evaluation and Risk of Transient Ischemic Attack and Stroke (VERiTAS) study was the first prospective study to evaluate the relationship between hemodynamics and recurrent stroke risk in patients with ICAD (11). Vertebrobasilar hemodynamics were measured using QMRA to dichotomize patients into low-flow and normal-flow groups based on prespecified algorithms that intrinsically accounted for collaterals by incorporating basilar and non-fetal posterior cerebral artery flow. Low distal flow status was associated with a three times higher rate of recurrent stroke compared to normal distal flow status (28% vs. 9%, p = 0.04) with a hazard ratio of 11.55 (95% CI, 1.88–71.00; p = 0.008) in a risk factor-adjusted multivariate analysis which was resistant to the effects of disease severity and location. A related study was performed by the study group that found a correlation between vessel-specific flow and the severity of stenosis, however, distal flow status, incorporating collateral capacity, was not predicted by the severity or location of disease (27). The VERiTAS studies supported hypoperfusion as a key mechanism of stroke in patients with posterior circulation ICAD, validated QMRA-defined distal-flow status as a possible biomarker of recurrent posterior circulation stroke and emphasized the importance of regional hemodynamics and collaterals in preventing stroke.
MyRIAD was a prospective study that used various hemodynamic and plaque instability biomarkers to determine the mechanisms of recurrent ischemia in patients with ICAD (13). Anterograde flow was measured using QMRA, distal perfusion using perfusion MR (PWI), and vasomotor reactivity (VMR) and microemboli signals (MES) using transcranial dopplers. The primary outcome of ischemic stroke at 1 year was reached in 8.8% while secondary outcomes of TIA in 5.9%. Interestingly, 24.7% of patients were found to have subclinical infarctions in the territory of the symptomatic artery at 6–8 weeks follow-up. There was no significant association between abnormal imaging biomarkers and recurrent stroke, TIA, or new infarctions. Combining abnormal imaging biomarkers—such as QMRA low-flow and PWI delays—did not show clear synergistic effects in predicting recurrent infarction, although a trend toward significance was appreciated when comparing the presence of two biomarkers with one or fewer (33 vs. 17%, p = 0.07). A post-hoc analysis showed that the baseline number of diffusion-weighted imaging lesions (>1: 40.0%, 1: 26.9% vs. 0: 4.4%, p < 0.01) and borderzone infarction patterns were significantly associated with new or recurrent infarction (63.6 vs. 25.0%, p = 0.01), implying that hypoperfusion and artery-to-artery embolism likely contribute to early subclinical infarction (28). In summary, MyRIAD investigators were unable to prospectively identify a subgroup of ICAD at high-risk of recurrent ischemic stroke using various imaging biomarkers, however, baseline borderzone or multifocal infarction patterns were retrospectively found to be strong predictors of recurrent subclinical infarction.
The Trial of Cilostazol in Symptomatic Intracranial Arterial Stenosis (TOSS-2) failed to show a difference in recurrent stroke between patients treated with dual-antiplatelet therapy vs. monotherapy (29). A subanalysis evaluating patients that underwent baseline imaging and imaging at 7 months found a 12.5% rate of subclinical infarction and 3.7% rate of clinical recurrent stroke in the territory of the initial symptomatic intracranial artery (21). After classifying initial infarction patterns by location (subcortical vs. cortical vs. subcortico-cortical) and multiplicity (single vs. multiple), subcortico-cortical patterns and multiple lesions were found to be independent predictors of new ischemic lesions (OR, 3.01; 95% CI, 1.33–7.01; p = 0.03; OR, 2.81; 95% CI, 1.34–5.9; p = 0.006) and clinical recurrent stroke. Severe stenosis was associated with subcorti-cocortical pattern and multiple lesions on baseline imaging (p <0.001) but was not predictive of recurrent infarction.
Several retrospective studies have associated perfusion parameters and recurrent stroke risk in patients with intracranial atherosclerotic disease; Tmax >6s delays with mismatch volumes of at least 15 mL in the territory of a symptomatic intracranial artery were shown to be associated with recurrent cerebrovascular events (15, 17, 18) and increased lengths of hospital stay (30), while Tmax >4s delays were not (15, 30). Patients with anterior circulation internal borderzone infarction patterns were more likely to have recurrent cerebrovascular events and a target mismatch profile using Tmax >6s delays compared to non-internal borderzone infarctions (16), greater volumes of Tmax >4s and Tmax>6s delay compared to perforator patterns (31), and greater volume difference between Tmax >4s and Tmax>6s delay when compared to thromboembolic patterns (31). Although the MyRIAD study was unable to prospectively find an association between Tmax >6s delays and recurrent stroke risk, a trend toward significance was appreciated when Tmax >6s delays were coupled with QMRA low-flow states (13). The utility of perfusion imaging in predicting recurrent cerebrovascular events is less well established in the posterior circulation. In a prespecified sub-analysis of VERiTAS patients, rCBV and MTT ratios did not differ between posterior circulation QMRA low flow vs. normal flow states, inferring that MR perfusion may not be a reliable metric in evaluating regional hypoperfusion in the posterior circulation (32). Probable biomarkers of stroke recurrence are displayed in Figure 1 and a proposed workflow diagram is shown in Figure 2.
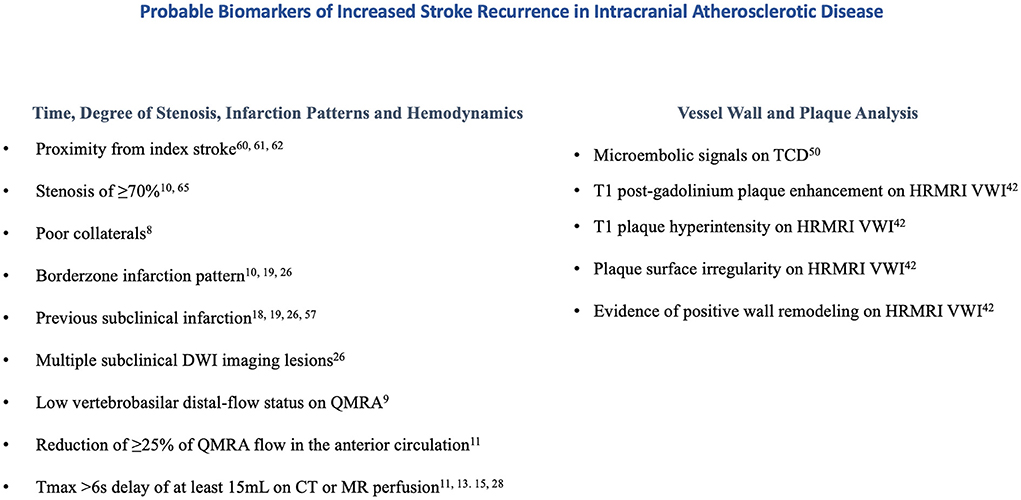
Figure 1. Probable biomarkers of increased stroke recurrence in intracranial atherosclerotic disease.
High resolution vessel wall imaging by MRI is a novel non-invasive imaging tool that has been used to distinguish ICAD from alternate stroke etiologies and to stratify stroke risk (33, 34). Our pathophysiological understanding of the radiographic findings is based largely on extracranial carotid studies that collectively suggested that plaque enhancement likely occurs due to neovascularization, inflammation, and endothelial dysfunction resulting in gadolinium leakage, while T1 hyperintensity and surface irregularity presumably reflect intraplaque hemorrhage and rupture of the fibrous cap (35–39). Several meta-analyses have shown that intracranial plaque enhancement, positive remodeling, T1 hyperintensity, and surface irregularity are strong imaging biomarkers of symptomatic plaques in patients with ischemic events (40, 41) may be even more important than luminal changes in predicting stroke occurrence (42). In fact, intracranial plaque enhancement was associated with a four-times higher rate of recurrent stroke at 1 year compared to non-enhancing plaques in a prospective longitudinal study (43). Plaque enhancement has also been shown to correlate with multifocal infarction patterns perhaps implicating fragile plaques (44) and has been inversely correlated with time from stroke onset (45).
Microembolic signals (MES) detected by transcranial doppler were first used as markers of plaque instability in extracranial symptomatic and asymptomatic carotid stenosis as MES correlated with several biomarkers of plaque morphology, including plaque ulceration (46) and neovascularization (47) and were independently associated with an increased risk of stroke (48–50). In symptomatic intracranial atherosclerotic stenosis, MES occurs in over a quarter of patients (13, 51, 52), with increased frequency with degree of stenosis (51) and multifocal infarction patterns (53). In a prospective study of acute stroke patients with middle cerebral artery stenosis, MES were predictive of future cerebral ischemia (51), while the more recent MYRIAD study failed to show this association in a population of anterior and posterior circulation atherosclerotic stenosis (13). Combination therapy with clopidogrel and aspirin was more effective than aspirin alone in reducing MES in a randomized study of predominantly symptomatic intracranial atherosclerotic stenosis in the anterior circulation (52), in line with previous randomized studies of MES in extracranial carotid stenosis (54).
Subclinical infarctions can occur due various stroke mechanisms (20) and have been associated with future stroke risk and cognitive decline (55–57). The primary endpoint of the major ICAD treatment trials predominantly focused on clinical strokes or TIAs, while subclinical infarction was often disregarded from primary analyses (4, 5). Recent evidence has shown that subclinical infarction occurs frequently in the early stages of an ischemic stroke and is predictive of future stroke (20, 21, 28, 58). In a SAMMPRIS sub-analysis, previous infarction more than doubled the risk of recurrent ischemic stroke (58). Subclinical infarction was found in a quarter of patients with ICAD at 6–8 weeks following an index stroke (28), and in more than half of patients in an Asian population (20), rates 3–5 times higher than that of recurrent clinical ischemic strokes (4, 5). Comparable to previous studies that have reported associations between infarction patterns and recurrent clinical stroke (12, 26), multiple DWI lesions (20, 21, 28, 59) and subcorticocortical infarction (21) patterns were shown to be independent predictors of subclinical infarction, implying similar hemodynamic and thromboembolic mechanisms. Interestingly, while antiplatelets have been shown to significantly reduce the rate of recurrent clinical stroke, neither antiplatelets nor anticoagulants influenced the occurrence of subclinical infarction in a retrospective study (20). Although subclinical infarction and white matter hyperintensities have been strongly associated with cognitive decline and future risk of neurocognitive disorders, most of these studies have focused on small vessel etiologies (60). Recent studies have shown this relationship in patients with asymptomatic extracranial large artery atherosclerosis (55–57), encouraging future studies in the intracranial vasculature. The inclusion of these radiographic and clinical biomarkers as entry or endpoint markers in future treatment trails may help capture a wider scope of pathology related to intracranial atherosclerotic disease.
Ischemic strokes due to ICAD recur more frequently in the early period after the index stroke (61, 62), with drastically higher rates within 1 week of the event (63). Similarly, the risk of periprocedural stroke after intracranial stenting increases within this early time window, likely due to unstable plaques (hot plaques) and potential for embolization (5). ICAD treatment trials must be viewed in this context, since early enrollment may overestimate the risk of periprocedural complications and late enrollment may underestimate the risk in both endovascular and medically managed groups. For example, recurrent ischemic stroke risk was significantly higher in WASID patients that were randomized within the median enrollment time of 17 days compared to patients randomized after 17 days (62). Likewise, the periprocedural stroke risk in SAMMPRIS was more than five times that of WEAVE, with median enrollment times of 7 and 22 days from index event, respectively (5, 64). A SAMMPRIS analysis of periprocedural strokes, however, found no relationship between time from qualifying event and periprocedural ischemic stroke risk, and the benefit of medical therapy over endovascular treatment was similar in patients enrolled within 7 days of their qualifying event compared to patients enrolled beyond 7 days (65). The variability in other factors, such as operator experience (66), likely partially account for the large differences in periprocedural stroke risk among treatment trials, making it difficult to assert firm conclusions based exclusively on enrollment time alone. The recently published CASSISS trial limited enrollment of patients to beyond 3 weeks after their index event and found no difference in stroke recurrence between endovascular and medically treated patients. Both groups, however, had significantly lower rates of stroke recurrence compared to historical controls (5, 7), likely in part due to later enrollment (9). In summary, both the risk of recurrent ischemic stroke and periprocedural stroke appears to be highest in the early period after an index stroke, and therefore the risks of each must be weighed carefully in real-world treatment decisions and in the design of future prospective studies.
Recurrent stroke risk in intracranial atherosclerotic disease remains high despite aggressive medical and endovascular therapies. Defining a vastly heterogenous disease exclusively based on luminal size discounts the role of other high-risk biomarkers, such as hemodynamics and potential for embolization, limiting the generalizability of current treatment trials. Incorporating these biomarkers in isolation as entry criteria into future trials may pose a challenge given the uncertain thresholds of hemodynamic insufficiency and thromboembolic potential. Perhaps the integration of biomarkers, given the probable synergism, may better target a hemodynamically insufficient subgroup most resistant to antithrombotic therapies, justifying endovascular flow augmentation, despite its current risks. While randomized controlled trials should remain the gold standard in guiding treatment, biomarkers of stroke recurrence may perhaps be used as an adjunct in clinical decision-making to better estimate recurrent stroke risk, allowing for more targeted and individualized treatment. The myriad of stroke mechanisms in intracranial atherosclerotic disease may be too complex and multidimensional to be managed by simplified and universal treatment strategies.
AB and DL contributed to conceptualization, literature search, writing—original draft, and writing—review and editing. Both authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Wong LKS. Global burden of intracranial atherosclerosis. Int J Stroke. (2006) 1:158–9. doi: 10.1111/j.1747-4949.2006.00045.x
2. Gorelick PB, Wong KS, Bae HJ, Pandey DK. Large artery intracranial occlusive disease: A large worldwide burden but a relatively neglected frontier. Stroke. (2008) 39:2396–9. doi: 10.1161/STROKEAHA.107.505776
3. Mazighi M, Tanasescu R, Ducrocq X, Vicaut E, Bracard S, Houdart E, et al. Prospective study of symptomatic atherothrombotic intracranial stenoses. Neurology. (2006) 66:1187–91. doi: 10.1212/01.wnl.0000208404.94585.b2
4. Chimowitz M, Lynn M, Howlett-Smith H, Stern B, Hertzberg V, Frankel M, et al. Warfarin-Aspirin symptomatic intracranial disease trial investigators. Comparison of warfarin and aspirin for symptomatic intracranial arterial stenosis. N Engl J Med. (2005) 352:1305–16. doi: 10.1056/NEJMoa043033
5. Chimowitz M, Lynn M, Derdeyn C, Turan T, Fiorella D, Lane B, et al. SAMMPRIS Trial Investigators. Stenting versus aggressive medical therapy for intracranial arterial stenosis. N Engl J Med. (2011) 365:993–1003. doi: 10.1056/NEJMoa1105335
6. Fiorella D, Levy EI, Turk AS, Albuquerque FC, Niemann DB, Aagaard-Kienitz B, et al. US multicenter experience with the Wingspan stent system for the treatment of intracranial atheromatous disease: periprocedural results. Stroke. (2007) 38:881–7. doi: 10.1161/01.STR.0000257963.65728.e8
7. Zaidat OO, Fitzsimmons BF, Woodward BK, Wang Z, Killer-Oberpfalzer M, Wakhloo A, et al. Effect of a balloon-expandable intracranial stent vs medical therapy on risk of stroke in patients with symptomatic intracranial stenosis: the VISSIT randomized clinical trial. JAMA. (2015) 313:1240–8. doi: 10.1001/jama.2015.1693
8. Kleindorfer DO, Towfighi A, Chaturvedi S, Cockroft KM, Gutierrez J, Lombardi-Hill D, et al. 2021 guideline for the prevention of stroke in patients with stroke and transient ischemic attack: A Guideline From the American Heart Association/American Stroke Association. Stroke. (2021) 52:E364–467. doi: 10.1161/STR.0000000000000375
9. Gao P, Wang T, Wang D, Liebeskind DS, Shi H, Li T, et al. Effect of stenting plus medical therapy vs medical therapy alone on risk of stroke and death in patients with symptomatic intracranial stenosis. JAMA. (2022) 328:534. doi: 10.1001/jama.2022.12000
10. Liebeskind DS, Cotsonis GA, Saver JL, Lynn MJ, Turan TN, Cloft HJ, et al. Collaterals dramatically alter stroke risk in intracranial atherosclerosis. Ann Neurol. (2011) 69:963–74. doi: 10.1002/ana.22354
11. Amin-Hanjani S, Pandey DK, Rose-Finnell L, Du X, Richardson DJ, Thulborn KR, et al. Effect of hemodynamics on stroke risk in symptomatic atherosclerotic vertebrobasilar occlusive disease. JAMA Neurol. (2016) 73:178–85. doi: 10.1001/jamaneurol.2015.3772
12. Wabnitz AM, Derdeyn CP, Fiorella DJ, Lynn MJ, Cotsonis GA, Liebeskind DS, et al. Hemodynamic markers in the anterior circulation as predictors of recurrent stroke in patients with intracranial stenosis. Stroke. (2019) 50:143–7. doi: 10.1161/STROKEAHA.118.020840
13. Romano JG, Prabhakaran S, Nizam A, Feldmann E, Sangha R, Cotsonis G, et al. Infarct recurrence in intracranial atherosclerosis: results from the MyRIAD study. J Stroke Cerebrovasc Dis. (2021) 30:105504. doi: 10.1016/j.jstrokecerebrovasdis.2020.105504
14. Spencer MP, Reid JM. Quantitation of carotid stenosis with continuous-wave (C-W) Doppler ultrasound. Stroke. (1979) 10:326–30. doi: 10.1161/01.STR.10.3.326
15. Yaghi S, Khatri P, Prabhakaran S, Yeatts SD, Cutting S, Jayaraman M, et al. What threshold defines penumbral brain tissue in patients with symptomatic anterior circulation intracranial stenosis: an exploratory analysis. J Neuroimaging. (2019) 29:203–5. doi: 10.1111/jon.12577
16. Yaghi S, Grory B, mac Prabhakaran S, Yeatts SD, Cutting S, Jayaraman M, et al. Infarct pattern, perfusion mismatch thresholds, and recurrent cerebrovascular events in symptomatic intracranial stenosis. J Neuroimaging. (2019) 29:640–4. doi: 10.1111/jon.12630
17. de Havenon A, Khatri P, Prabhakaran S, Yeatts SD, Peterson C, Sacchetti D, et al. Hypoperfusion distal to anterior circulation intracranial atherosclerosis is associated with recurrent stroke. J Neuroimaging. (2020) 30:468–70. doi: 10.1111/jon.12710
18. Sacchetti DC, Cutting SM, McTaggart RA, Chang AD, Hemendinger M, mac Grory B, et al. Perfusion imaging and recurrent cerebrovascular events in intracranial atherosclerotic disease or carotid occlusion. Int J Stroke. (2018) 13:592–9. doi: 10.1177/1747493018764075
19. Higashida RT, Furlan AJ, Roberts H, Tomsick T, Connors B, Barr J, et al. Trial design and reporting standards for intra-arterial cerebral thrombolysis for acute ischemic Stroke. Stroke. (2003) 34:e109–37. doi: 10.1161/01.STR.0000082721.62796.09
20. Kang DW, Kwon SU, Yoo SH, Kwon KY, Choong GC, Sang JK, et al. Early recurrent ischemic lesions on diffusion-weighted imaging in symptomatic intracranial atherosclerosis. Arch Neurol. (2007) 64:50–4. doi: 10.1001/archneur.64.1.50
21. Jung JM, Kang DW Yu KH, Koo JS, Lee JH, Park JM, Hong KS, et al. Predictors of recurrent stroke in patients with symptomatic intracranial arterial stenosis. Stroke. (2012) 43:2785–7. doi: 10.1161/STROKEAHA.112.659185
22. Sedlaczek O, Caplan L, Hennerici M. Impaired washout – embolism and ischemic stroke: further examples and proof of concept. Cerebrovasc Dis. (2005) 19:396–401. doi: 10.1159/000085831
23. Caplan LR, Ka SW, Gao S, Hennerici MG. Is hypoperfusion an important cause of strokes? If so, how? Cerebrovasc Dis. (2006) 21:145–53. doi: 10.1159/000090791
24. Caplan LR, Hennerici M. Impaired clearance of emboli (washout) is an important link between hypoperfusion, embolism, and ischemic stroke. Arch Neurol. (1998) 55:1475–82. doi: 10.1001/archneur.55.11.1475
25. Liebeskind DS, Cotsonis GA, Saver JL, Lynn MJ, Cloft HJ, Chimowitz MI. Collateral circulation in symptomatic intracranial atherosclerosis. J Cereb Blood Flow Metab. (2011) 31:1293–301. doi: 10.1038/jcbfm.2010.224
26. López-Cancio E, Matheus MG, Romano JG, Liebeskind DS, Prabhakaran S, Turan TN, et al. Infarct patterns, collaterals and likely causative mechanisms of stroke in symptomatic intracranial atherosclerosis. Cerebrovasc Dis. (2014) 37:417–22. doi: 10.1159/000362922
27. Amin-Hanjani S, Du X, Rose-Finnell L, Pandey DK, Richardson D, Thulborn KR, et al. Hemodynamic features of symptomatic vertebrobasilar disease. Stroke. (2015) 46:1850–6. doi: 10.1161/STROKEAHA.115.009215
28. Prabhakaran S, Liebeskind DS, Cotsonis G, Nizam A, Feldmann E, Sangha RS, et al. Predictors of early infarct recurrence in patients with symptomatic intracranial atherosclerotic disease. Stroke. (2021) 52:1961–6. doi: 10.1161/STROKEAHA.120.032676
29. Kwon SU, Cho YJ, Koo JS, Bae HJ, Lee YS, Hong KS, et al. Cilostazol prevents the progression of the symptomatic intracranial arterial stenosis: the multicenter double-blind placebo-controlled trial of cilostazol in symptomatic intracranial arterial stenosis. Stroke. (2005) 36:782–6. doi: 10.1161/01.STR.0000157667.06542.b7
30. Yaghi S, Havenon A de, Honda T, Hinman JD, Raychev R, Sharma LK, et al. Impaired distal perfusion predicts length of hospital stay in patients with symptomatic middle cerebral artery stenosis. J Neuroimaging. (2021) 31:475–9. doi: 10.1111/jon.12839
31. Kim SJ, Morales JM, Yaghi S, Honda T, Scalzo F, Hinman JD, et al. Intracranial atherosclerotic disease mechanistic subtypes drive hypoperfusion patterns. J Neuroimaging. (2021) 31:686–90. doi: 10.1111/jon.12863
32. See AP, Stapleton CJ, Du X, Charbel FT, Amin-Hanjani S. Perfusion-MRI is a poor indicator of hemodynamic compromise in vertebrobasilar disease in the VERiTAS study. J Neuroimaging. (2021) 31:151–4. doi: 10.1111/jon.12802
33. Bodle JD, Feldmann E, Swartz RH, Rumboldt Z, Brown T, Turan TN. High-resolution magnetic resonance imaging: an emerging tool for evaluating intracranial arterial disease. Stroke. (2013) 44:287–92. doi: 10.1161/STROKEAHA.112.664680
34. Mandell DM, Mossa-Basha M, Qiao Y, Hess CP, Hui F, Matouk C, et al. Intracranial vessel wall MRI: principles and expert consensus recommendations of the American Society of Neuroradiology. Am J Neuroradiol. (2017) 38:218–29. doi: 10.3174/ajnr.A4893
35. Derksen WJM, Peeters W, van Lammeren GW, Tersteeg C, de Vries JPPM, de Kleijn DPV, et al. Different stages of intraplaque hemorrhage are associated with different plaque phenotypes: A large histopathological study in 794 carotid and 276 femoral endarterectomy specimens. Atherosclerosis. (2011) 218:369–77. doi: 10.1016/j.atherosclerosis.2011.07.104
36. Cai J, Hatsukami TS, Ferguson MS, Kerwin WS, Saam T, Chu B, et al. In vivo quantitative measurement of intact fibrous cap and lipid-rich necrotic core size in atherosclerotic carotid plaque: comparison of high-resolution, contrast-enhanced magnetic resonance imaging and histology. Circulation. (2005) 112:3437–44. doi: 10.1161/CIRCULATIONAHA.104.528174
37. Turan TN, Rumboldt Z, Granholm AC, Columbo L, Welsh CT, Lopes-Virella MF, et al. Intracranial atherosclerosis: correlation between in-vivo 3T high resolution MRI and pathology. Atherosclerosis. (2014) 237:460–3. doi: 10.1016/j.atherosclerosis.2014.10.007
38. Millon A, Boussel L, Brevet M, Mathevet JL, Canet-Soulas E, Mory C, et al. Clinical and histological significance of gadolinium enhancement in carotid atherosclerotic plaque. Stroke. (2012) 43:3023–8. doi: 10.1161/STROKEAHA.112.662692
39. Xu WH Li ML, Gao S, Ni J, Yao M, Zhou LX, Peng B, et al. Middle cerebral artery intraplaque hemorrhage: prevalence and clinical relevance. Ann Neurol. (2012) 71:195–8. doi: 10.1002/ana.22626
40. Song JW, Pavlou A, Xiao J, Kasner SE, Fan Z, Messé SR. Vessel wall magnetic resonance imaging biomarkers of symptomatic intracranial atherosclerosis: a meta-analysis. Stroke. (2020) 193–202. doi: 10.1161/STROKEAHA.120.031480
41. Gupta A, Baradaran H, Al-Dasuqi K, Knight-Greenfield A, Giambrone AE, Delgado D, et al. Gadolinium enhancement in intracranial atherosclerotic plaque and ischemic stroke: a systematic review and meta-analysis. J Am Heart Assoc. (2016) 5:e003816. doi: 10.1161/JAHA.116.003816
42. Kim HJ, Choi EH, Chung JW, Kim JH, Kim YS, Seo WK, et al. Luminal and wall changes in intracranial arterial lesions for predicting stroke occurrence. Stroke. (2020) 51:2495–504. doi: 10.1161/STROKEAHA.120.030012
43. Kim JM, Jung KH, Sohn CH, Moon J, Shin JH, Park J, et al. Intracranial plaque enhancement from high resolution vessel wall magnetic resonance imaging predicts stroke recurrence. Int J Stroke. (2016) 11:171–9. doi: 10.1177/1747493015609775
44. Kim JM, Jung KH, Sohn CH, Moon J, Han MH, Roh JK. Middle cerebral artery plaque and prediction of the infarction pattern. Arch Neurol. (2012) 69:1470–5. doi: 10.1001/archneurol.2012.1018
45. Skarpathiotakis M, Mandell DM, Swartz RH, Tomlinson G, Mikulis DJ. Intracranial atherosclerotic plaque enhancement in patients with ischemic stroke. AJNR Am J Neuroradiol. (2013) 34:299–304. doi: 10.3174/ajnr.A3209
46. Mayor I, Comelli M, Vassileva E, Burkhard P, Sztajzel R. Microembolic signals and carotid plaque morphology: a study of 71 patients with moderate or high grade carotid stenosis. Acta Neurol Scand. (2003) 108:114–7. doi: 10.1034/j.1600-0404.2003.00099.x
47. Ritter MA, Theismann K, Schmiedel M, Ringelstein EB, Dittrich R. Vascularization of carotid plaque in recently symptomatic patients is associated with the occurrence of transcranial microembolic signals. Eur J Neurol. (2013) 20:1218–21. doi: 10.1111/ene.12030
48. King A, Markus HS. Doppler embolic signals in cerebrovascular disease and prediction of stroke risk: A systematic review and meta-analysis. Stroke. (2009) 40:3711–7. doi: 10.1161/STROKEAHA.109.563056
49. Markus HS, MacKinnon A. Asymptomatic embolization detected by Doppler ultrasound predicts stroke risk in symptomatic carotid artery stenosis. Stroke. (2005) 36:971–5. doi: 10.1161/01.STR.0000162717.62684.40
50. Altaf N, Kandiyil N, Hosseini A, Mehta R, MacSweeney S, Auer D. Risk factors associated with cerebrovascular recurrence in symptomatic carotid disease: a comparative study of carotid plaque morphology, microemboli assessment and the European Carotid Surgery Trial risk model. J Am Heart Assoc. (2014) 3:e000173. doi: 10.1161/JAHA.113.000173
51. Gao S, Ka SW, Hansberg T, Lam WWM, Droste DW, Ringelstein EB. Microembolic signal predicts recurrent cerebral ischemic events in acute stroke patients with middle cerebral artery stenosis. Stroke. (2004) 35:2832–6. doi: 10.1161/01.STR.0000147035.31297.b6
52. Wong KSL, Chen C, Fu J, Chang HM, Suwanwela NC, Huang YN, et al. Clopidogrel plus aspirin versus aspirin alone for reducing embolisation in patients with acute symptomatic cerebral or carotid artery stenosis (CLAIR study): a randomised, open-label, blinded-endpoint trial. Lancet Neurol. (2010) 9:489–97. doi: 10.1016/S1474-4422(10)70060-0
53. Wong KS, Gao S, Chan YL, Hansberg T, Lam WWM, Droste DW, et al. Mechanisms of acute cerebral infarctions in patients with middle cerebral artery stenosis: a diffusion-weighted imaging and microemboli monitoring study. Ann Neurol. (2002) 52:74–81. doi: 10.1002/ana.10250
54. Markus HS, Droste DW, Kaps M, Larrue V, Lees KR, Siebler M, et al. Dual antiplatelet therapy with clopidogrel and aspirin in symptomatic carotid stenosis evaluated using doppler embolic signal detection: The clopidogrel and aspirin for reduction of emboli in symptomatic carotid stenosis (CARESS) trial. Circulation. (2005) 111:2233–40. doi: 10.1161/01.CIR.0000163561.90680.1C
55. Marshall RS, Festa JR, Cheung YK, Chen R, Pavol MA, Derdeyn CP, et al. Cerebral hemodynamics and cognitive impairment: Baseline data from the RECON trial. Neurology. (2012) 78:250–5. doi: 10.1212/WNL.0b013e31824365d3
56. Silvestrini M, Paolino I, Vernieri F, Pedone C, Baruffaldi R, Gobbi B, et al. Cerebral hemodynamics and cognitive performance in patients with asymptomatic carotid stenosis. Neurology. (2009) 72:1062–8. doi: 10.1212/01.wnl.0000345015.35520.52
57. Lazar RM, Wadley VG, Myers T, Jones MR, Heck DV, Clark WM, et al. Baseline cognitive impairment in patients with asymptomatic carotid stenosis in the CREST-2 trial. Stroke. (2021) 52:3855–63. doi: 10.1161/STROKEAHA.120.032972
58. Waters MF, Hoh BL, Lynn MJ, Kwon HM, Turan TN, Derdeyn CP, et al. Factors associated with recurrent ischemic stroke in the medical group of the SAMMPRIS trial. JAMA Neurol. (2016) 73:308–15. doi: 10.1001/jamaneurol.2015.4315
59. Amarenco P, Lavallée PC, Labreuche J, Albers GW, Bornstein NM, Canhão P, et al. One-year risk of stroke after transient ischemic attack or minor stroke. N Engl J Med. (2016) 374:1533–42. doi: 10.1056/NEJMoa1412981
60. Azeem F, Durrani R, Zerna C, Smith EE. Silent brain infarctions and cognition decline: systematic review and meta-analysis. J Neurol. (2020) 267:502–12. doi: 10.1007/s00415-019-09534-3
61. Ovbiagele B, Cruz-Flores S, Lynn MJ, Chimowitz MI. Early stroke risk after transient ischemic attack among individuals with symptomatic intracranial artery stenosis. Arch Neurol. (2008) 65:733–7. doi: 10.1001/archneur.65.6.733
62. Kasner SE, Chimowitz MI, Lynn MJ, Howlett-Smith H, Stern BJ, Hertzberg VS, et al. Predictors of ischemic stroke in the territory of a symptomatic intracranial arterial stenosis. Circulation. (2006) 113:555–63. doi: 10.1161/CIRCULATIONAHA.105.578229
63. Sangha RS, Naidech AM, Corado C, Ansari SA, Prabhakaran S. Challenges in the medical management of symptomatic intracranial stenosis in an Urban setting. Stroke. (2017) 48:2158–63. doi: 10.1161/STROKEAHA.116.016254
64. Alexander MJ, Zauner A, Chaloupka JC, Baxter B, Callison RC, Gupta R, et al. Trial. Stroke. (2019) 50:889–94. doi: 10.1161/STROKEAHA.118.023996
65. Fiorella D, Derdeyn CP, Lynn MJ, Barnwell SL, Hoh BL, Levy EI, et al. Detailed analysis of periprocedural strokes in patients undergoing intracranial stenting in stenting and aggressive medical management for preventing recurrent stroke in intracranial stenosis (SAMMPRIS). Stroke. (2012) 43:2682–8. doi: 10.1161/STROKEAHA.112.661173
Keywords: intracranial atherosclerosis (ICAS), hemodynamics, perfusion, intracranial atherosclerotic disease (ICAD), quantitative magnetic resonance angiography
Citation: Ballout AA and Liebeskind DS (2022) Recurrent stroke risk in intracranial atherosclerotic disease. Front. Neurol. 13:1001609. doi: 10.3389/fneur.2022.1001609
Received: 23 July 2022; Accepted: 18 August 2022;
Published: 01 September 2022.
Edited by:
Majaz Moonis, UMass Memorial Medical Center, United StatesReviewed by:
Yuan Wang, Xuanwu Hospital, Capital Medical University, ChinaCopyright © 2022 Ballout and Liebeskind. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: David S. Liebeskind, ZGF2aWRsaWViZXNraW5kQHlhaG9vLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.