- Department of Neurology, The First Affiliated Hospital of Chongqing Medical University, Chongqing, China
Ischemic stroke is a common cerebrovascular disease that seriously affects human health. However, most patients do not practice self-care and cannot rely on the current clinical treatment for guaranteed functional recovery. Stem cell transplantation is an emerging treatment studied in various central nervous system diseases. More importantly, animal studies show that transplantation of mesenchymal stem cells (MSCs) can alleviate neurological deficits and bring hope to patients suffering from ischemic stroke. This paper reviews the biological characteristics of MSCs and discusses the mechanism and progression of MSC transplantation to provide new therapeutic directions for ischemic stroke.
Introduction
Stroke is a common neurological disease affecting human survival and health; it is characterized by high morbidity, mortality, disability, and a high recurrence rate (1). Statistically, more than 13.7 million people suffer from strokes worldwide annually, and 5.8 million die (2). More remarkable, ischemic stroke incidence is increasing yearly due to the aging population and other reasons. Therefore, ischemic stroke has received increasing attention as the most common type (accounting for ~70% of strokes) (3).
Ischemic stroke is a pathological process caused by a blood circulatory disorder in the brain that leads to neuronal cell death or softening of the brain tissue. As a terminally differentiated cell, the death of a large number of neuronal cells leads to irreversible damage to brain tissue. Early recovery of blood volume in the ischemic area and reduction of nerve cell death are the key points in the treatment of ischemic stroke. However, treatments such as thrombolysis and mechanical thrombectomy benefit only 5% of patients because of narrow therapeutic windows and severe treatment complications (4–6). Thus, further research for safer and more effective ways is still warranted (7).
Stem cells are primitive and unspecialized cells that can develop into diverse specialized cells through mitosis and differentiation and have the potential to regenerate a variety of tissues and organs (8). Extensive preclinical evidence suggests that stem cell transplantation therapy can alleviate brain tissue damage by directional proliferation and differentiation of nerve cells and other pathways. A large number of abnormal nerve cell deaths can occur after an ischemic stroke, and stem cell transplantation will be a viable treatment to relieve neurological deficits in the future (9).
Various types of stem cells have been studied in animal models or clinical studies, such as neural progenitor cells (NPC), mesenchymal stem cells (MSC), endothelial progenitor cells (EPC), and human umbilical cord blood cells (HUCBCs) (10). However, these kinds of stem cells all have limitations in therapeutic effects. For example, EPC therapy faces ethical problems (11). NPCs are tricky to harvest and have a low proliferation rate (12). The treatment of engineered cells, such as induced pluripotent stem cells (iPSC), NT2N cells, CTX0E3, and SB623, is hampered by technology (13, 14). Nevertheless, it is worth noting that MSC cells have become the preferred cells for treating ischemic stroke due to their characteristics, such as high availability, efficient isolating and culturing, high immune tolerance, and fewer treatment complications. Furthermore, MSC cell therapy is not contrary to social ethics (15, 16).
In this paper, we analyze the biological characteristics of MSCs and the neuroprotective mechanism in treating ischemic stroke with the hope of providing new therapeutic directions for ischemic stroke.
Overview of MSCs
MSCs were first described as spindle-bone marrow stromal cells adhered to plastic by Friedenstein and his colleagues in 1970 (17). Four years later, they found that MSCs can form colonies outside the body that adhere to the wall like fibroblasts. Hence, MSCs are also known as cluster unit fibroblasts (CFU-Fs) (18). In 1991, Caplan coined the term “mesenchymal stem cells” and predicted that these mesodermally derived cells would represent the main arsenal of autologous therapies for regenerative purposes (19). With their development in recent decades, MSCs have become the most widely studied stem cell population. They are widely used in clinical trials and/or the treatment of various diseases, especially neurological diseases (20, 21).
MSCs were isolated from bone marrow for the first time. MSCs have previously been isolated from a variety of tissues, such as the lung, liver, kidney, placenta, fallopian tubes, endometrial polyps, adipose tissue, dental pulp, salivary glands, inferior turbinate, umbilical cord blood, menstrual blood, and other tissues (22, 23). They are plastic-adherent and can express mesenchymal markers, including CD90, CD105, CD73, and others, but cannot express CD11b, CD14, CD19, CD34, CD45, and human leukocyte antigen (HLA)-DR (24). MSCs can be harvested from different tissues, and various donor characteristics restrict the surface markers, quality, and isolated numbers of MSCs. Currently, the most frequently reported sources of MSCs utilized in clinical trials are the bone marrow, adipose tissue, and umbilical cord. MSCs obtained from adipose tissue (AD-MSCs) can express CD49d and produce more HGF and VEGF than bone marrow-derived stem cells (BM-MSCs) (25). Compared with bone marrow-derived stem cells, the number of cells obtained from 1 g of fat tissue may be 500 times greater than that of the same weight of bone marrow (26). However, BM-MSCs are safer than AD-MSCs because they can promote the proliferation of existing cancer cells, especially breast cancer (27). Both BM-MSCs and AD-MSCs have significant neurotrophic potential to stimulate neurite growth in DRG-neurons despite different growth factors, which further supports the feasibility of MSC-based stroke treatment (28). Recent investigations into the transplantation of human umbilical cord mesenchymal stem cells (hUC-MSCs)in stroke models have displayed favorable results, including a reduction in infarct size, improved functional recovery, and increased expression of several neuroprotective factors (including VEGF and BDNF) (29). Yet, their isolation can be difficult (30). MSCs from other sources, such as canine-derived MSCs (cMSCs), have not obtained sufficient clinical evidence and cannot be directly applied (31) (Table 1).
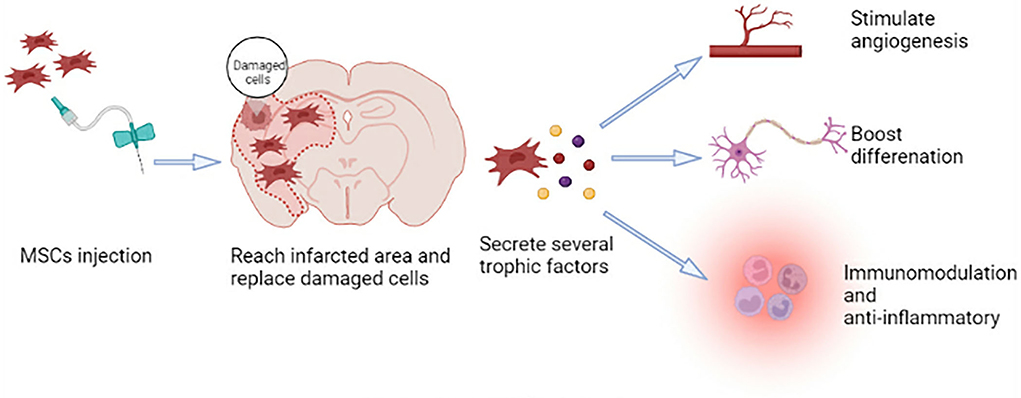
MSCs can self-renew and show polymorphic differentiation (41). They can differentiate into mesoderm cells (described above), endoderm (smooth muscle cells), and ectoderm (neurons) cells under certain conditions (42), which can promote the repair of various damaged tissues (41). Neural regeneration, including neurogenesis, angiogenesis, and synaptic plasticity, is crucial for functional recovery after a stroke. Because MSCs have the characteristics of plasticity, multidirectional differentiation, immunomodulation, and anti-inflammatory, they have the potential to participate in brain regeneration, which can promote tissue repair after ischemic stroke (Figure 1) (43).
The role and research progress of MSC transplantation in the treatment of ischemic stroke
Since Azizi et al. published the first report on the transplantation of human BM-MSCs into the rat brain in 1998, an increasing number of studies on treating neurological diseases by MSC transplantation have been conducted. Moreover, MSC transplantation therapy is gradually shifting from laboratory to clinical therapy. Successful clinical studies demonstrate the clinical transformation of MSC transplantation in treating ischemic stroke. Researchers have adopted various methods (intravenous, artery, and intrathecal injection) to administer MSCs to patients with ischemic stroke. They have focused on the safety, feasibility, and short-term effectiveness of MSCs in treating ischemic stroke. This section summarizes several clinical trials to explore the feasibility of MSCs in treating patients with ischemic stroke.
Plentiful preclinical studies of MSC transplantation therapy in ischemic stroke provide a theoretical basis for clinical practice
Medication, rehabilitation training, and physical union therapy have not been effective as experimental treatments for ischemic stroke. Except for thrombolysis and mechanical thrombectomy, no effective medications or procedures have yet been developed. In this situation, new therapeutic strategies using multiple mechanisms are sought, with MSC transplantation being one of them.
In the preclinical studies, researchers explore different sources of MSCs, feasibility, security, and specific mechanisms of MSC-based therapy. First, using in vitro models, they isolate MSCs from various tissues and demonstrate cell differentiation, neuroprotection, neurogenesis, and angiogenesis. In in vivo models, MSCs are injected into animals by different pathways. Researchers demonstrate that MSC transplantation has a potential therapeutic activity that can repair damaged brain tissue, and it seems feasible and secure (summarized in Table 2).
Model in vitro
The in vitro propagation of MSCs is a three-step process: Extracted from various tissues, MSCs are separated and obtained using density gradient centrifugation digesting culture before being cultured in a plastic cell tissue culture bottle for 3–5 days for further expansion. These steps are then repeated to expand adhered MSCs (63). The following are detailed procedures: To begin, isolate MSCs from multiple tissues such as bone, adipose tissue, tooth tissue, and others using Percoll or Ficoll density gradient centrifugation. Second, rinse MSCs with buffer once to eliminate contaminants before cultivation in 10% fetal bovine serum (FBS) or FBS substitutes, and incubate them in flasks at 37 °C in a humidified 5% CO2 incubator for 2 days. Non-adherent cells are removed by replacing the medium with a fresh one. Subsequently, the attached MSCs proliferate for 2–3 weeks with regular medium change. When the cells have grown to cover about 80% of the flask, it is critical to separate them and allow them to proliferate continually (63).
At present, researchers agree on the multi-lineage differentiation and transplantation potential of MSCs to replace lost tissue after ischemic stroke. When these isolated MSCs are treated with corresponding growth factors or induction medium, they can differentiate into various cell types from different blastoderms (64, 65). These differentiated neuron cells also have functional activity. For example, electrophysiological measurements show that the differentiated cells have voltage-gated sodium and potassium currents, which can be reversibly blocked by tetrodotoxin and tetraethylammonium, respectively (6, 9). However, we need more evidence to prove whether the differentiated cells can fire repetitive action potentials (64).
In addition, MSCs have great neuroprotective effects and promote neurite outgrowth in vitro. Liu et al. claim that BM-MSCs can promote the survival of oxygen-glucose deprivation (OGD) injured neurons, promote axonal outgrowth, and upregulate the expression of GAP-43 when they are cocultured for 48 h with neurons following OGD injury (66). BM-MSCs can also stimulate neurite outgrowth of DRG neurons (67). When hippocampal slices or cortical neurons are cocultured with hMSCs or MSC-derived SB623 cells separated by a semi-porous membrane or with MSC- or SB623 cell-conditioned medium following OGD, neural cell death or damage is decreased. Moreover, 11 neurotrophic factors are identified as secreted by MSCs and/or SB623 cells, and most of them are potentially beneficial to neural tissue following an ischemic insult (68). Furthermore, BM-MSCs from normal healthy and cerebral ischemia rats increase neuronal survival and connectivity in glial-neuron mixed cultures (69). These reports support the fact that MSCs have neuroprotective effects and stimulate neurite development in vitro.
Lastly, the ability of MSCs to stimulate angiogenesis, participate in vascularization, and re-establish a blood supply is evaluated in in vitro models. It is also the fundamental process of tissue repair (70). In in vitro models, MSCs can differentiate into endothelial lineage cells to protect ECs against hypoxia-induced cell death (71), promote the formation of endothelial rings (65), improve the paracrine activity of angiogenic growth factors and EC migration, and form mature vascular tissue (70). Hypoxic preconditioning enhances the pro-angiogenic effects of MSCs by increasing the expression of angiogenic growth factors and boosting the proliferation and migration of ECs (72).
Thus, MSCs show great promise in vitro, including the potential for cell differentiation, neuroprotection, neurogenesis, and pro-angiogenesis. Therefore, several studies have ulteriorly transplanted MSCs into animal models of ischemic stroke and evaluated the outcomes as discussed below.
Model in vivo
In in vivo studies, researchers focus more on the effectiveness, including the neuroprotection, immune regulation, angiogenesis, neurogenesis, and other potential of MSC transplantation, as well as the feasibility and security. The commonly used transplantation pathways are stereotactic administration (intrastriatum and intraventricular), systemic administration (intra-vein IV, intra-arterial IA), and other routes used, such as the intranasal route (73). Peripheral transplantation may be more appropriate for acute stroke patients because the BBB is permeable in this period, and stem cells are easy to pass through, whereas direct stereotaxic cell administration seems appropriate for fixed, chronic stroke patients (74).
Stereotactic transplantation (intracerebral and intraventricular), also called intracranial transplantation, is used to transplant MSCs into various brain parts. In particular, MSCs can be precisely administered to a selected location by intracerebral (IC), and intraventricular (IV) techniques, which are the earliest transplantation means used in experiments. Moreover, more MSCs can reach the target sites of brain injury in this way without going through the whole body's metabolic cycle (75). Zhao et al. pioneer intracranial transplantation of MSCs and find that MSCs migrate toward the infarct region of the brain and can survive in the host brain and promote functional recovery (45). Transplanted hMSCs can differentiate into mature neurons and gliacytes, which are observed at the grafting site and along the migration pathways (47). Rats are given two injections of grafted HMSCs into the infarct cortex within 24 h after MCAO, which is shown to substantially enhance neuronal metabolic activity, facilitate repair of the infarct cortex, and improve functional outcomes in rats (46). By expressing neuroprotective and growth-associated cytokines, intracerebral transplanted MSCs also increase neuronal activity, decrease cell death, and promote angiogenesis in the infarct cortex (47). They also modulate the immune response and produce TGF-, which inhibits MCP-1 secretion and restricts the infiltration of CD68 + cells into the damaged tissue (48). When MSCs are injected into the ventricles and cisterns, they are distributed throughout the brain and spinal cord with cerebrospinal fluid. It can effectively improve the survival rate of transplanted MSCs without going through general metabolism. It is proven that hADSCs can migrate safely into damaged areas and survive when injected into subarachnoid space through the superior orbital fissure (76). However, it may have higher risks due to the invasive procedures and complexity of stereotaxic procedures, which are unbearable for most clinical patients (54).
Systemic administration (Intra-vein IV, Intra-arterial IA): To overcome the invasiveness and complexity of stereotaxic transplantation, researchers find that a microtrauma method can be achieved. Bone marrow MSCs injected intravenously or arterially can migrate into the infarct area to promote neurogenesis and angiogenesis (77), facilitate axonal sprouting and remyelination (55), attenuate microglia/macrophage infiltration (69, 78), inhibit gliosis and apoptosis in the ischemic brain, and reduce infarct volume and improve functional outcomes in ischemic stroke rats (59). The intravenous route has advantages such as simplicity, safety, high feasibility, repeatable operation, high patient acceptance, and small adverse reactions. Furthermore, the IV MSCs will preferentially migrate to the spleen (50) and abrogate the systematic inflammation-plagued secondary cell death. However, IV delivery has the “first-pass effect” after transplantation; that is, the transplanted cells are distributed in the liver, lung, spleen, kidney, bone marrow, thymus, and even skin and tumors on the way. Thus, only a few transplanted cells reach the lesion site (77). Therefore, IV delivery needs a large number of cells to be injected into patients, which may increase potential side effects and the cost of the treatment (79). As the number of IA catheter-based interventions depending on stroke therapy characteristics (such as mechanical thrombectomy and catheter-directed thrombolysis for patients with penumbra) is constantly increasing, it seems that IA cell injection is ideally suited in the specific setting (80, 81). The arterial route has a high transplantation rate, does not pass through other organs, reaches the cerebral cortex and peripheries of the lesion directly and quickly, expresses glial and neuronal markers, and induces faster improvement of neurological function in animals (56). However, it may form microemboli and cause new infarctions. Intra-arterial cell administration at low doses may reduce the risk of embolic complications and promote functional recovery. However, more research is still needed to determine the most effective dosage (82).
Another route: Intranasal administration is a simple, convenient, and non-invasive delivery method that bypasses the BBB, directly guides therapeutics to the CNS (83). reaches the frontal part of the brain within 30 mins after administration, and distributes throughout the whole brain after 3 h (84). Wei et al. first provided information on intranasal cell delivery for treating ischemic stroke (60). In the study, hypoxic-preconditioned BM-MSCs were intranasally delivered 24 h after stroke. The result shows that BM-MSCs reach the ischemic cortex and deposit outside vasculatures as early as one and a half hours after administration, upregulating expressions of MMP-2, MMP-9, and the SDF-1 receptor CXCR4, reducing cell death and infarct volume and improving functional outcomes (60). This method is effective and minimally invasive and has been used in the treatment of neonatal stroke. In the neonatal HI model, intranasal delivery of MSC- and MSC-BDNF significantly reduces infarct size and gray matter loss, increases Ki67-positive cell number in the SVZ, enhances endogenous repair processes, and effectively reduces long-term functional impairment (61). In the perinatal brain injury (PBI) model, MSC-exosomes reduce gray and white matter injuries and improve functional recovery (84).
Additionally, intranasally administered BM-MSCs improve local cerebral blood flow in the ischemic cortex after a neonatal stroke and significantly decrease infarct size and BBB disruption. They also promote angiogenesis and neurogenesis (62). These reports indicate that intranasal-delivered BM-MSCs are feasible and effective, but more research and exploration are still needed.
In order to make cell therapy more viable, it is necessary to clarify cell migration, viability, and efficient delivery to target locations after transplantation. In a study by Archana Mukherjee, the researcher labeled umbilical cord-derived MSCs with 51Cr (85). After 96 h of being injected into healthy Swiss mice via the tail vein, retention of activity in the blood and high uptake of 51Cr in the kidneys were still observed. The rate and proportion of cells reaching the damaged site vary with administration. Factors determining cell distribution have not been fully elucidated. A study by Ilya et al. demonstrated a high coefficient of determination of up to 30% correlation between the distribution of IA transplanted MSCs and brain perfusion (86). The apoptosis of MSCs is the same as normal cells, which are cleared via the hepatobiliary and renal routes with time. Biodistribution and imaging studies are desired in animal models to disclose more mechanisms. A study by Seungho Lim and colleagues provides dual-modal stem cell imaging probes as a new method of labeling human AD-MSCs to realize non-invasive and precise tracking after transplantation in living subjects, which will be a forceful tool in the future (87).
Increasingly successful clinical studies provide evidence for the possibility of clinical transformation of MSC transplantation in the treatment of ischemic stroke
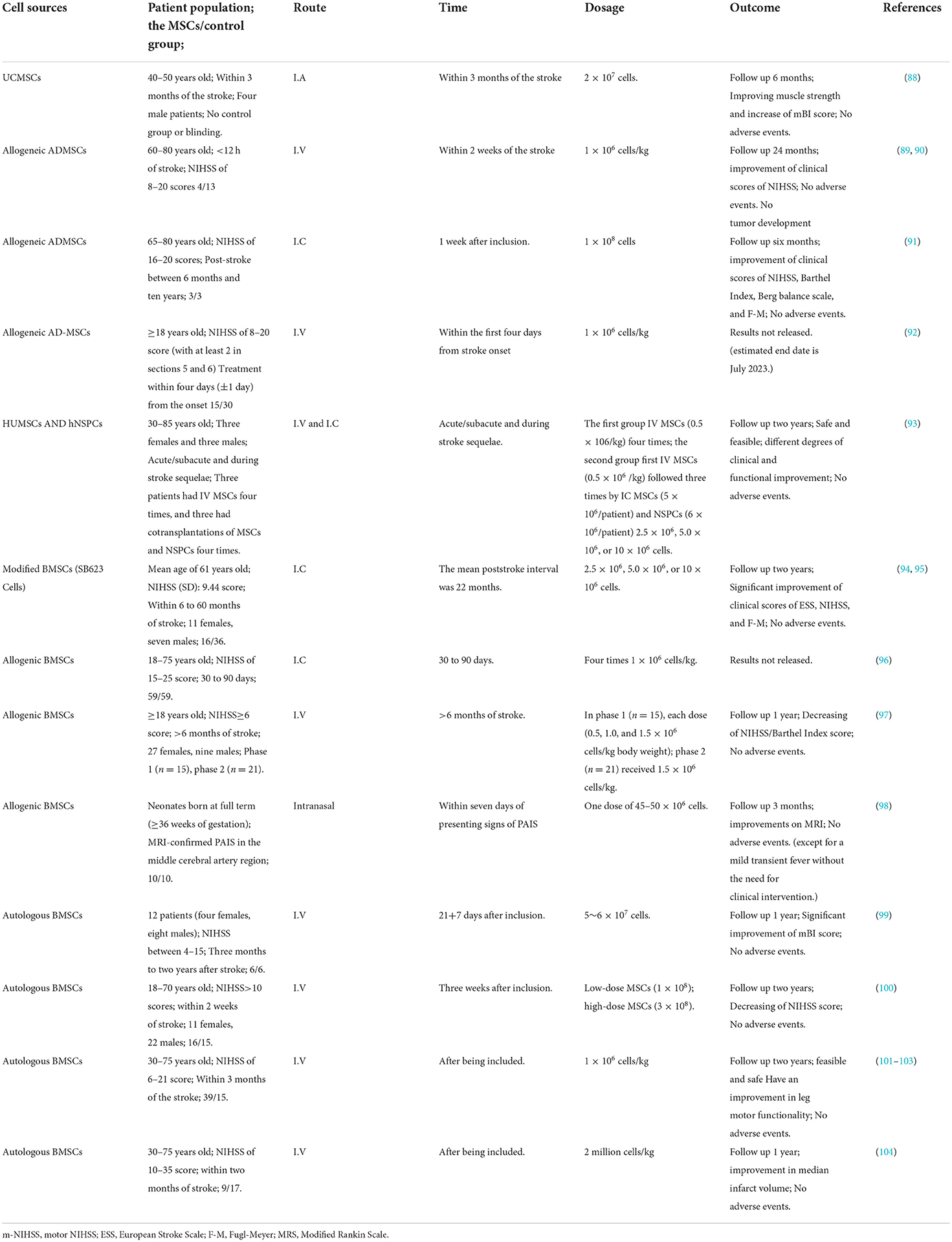
In recent years, clinical trials have focused on the safety, feasibility, and short-term effectiveness of MSCs in treating ischemic stroke (9) (summarized in Table 3). Transplantations of MSCs in human patients began in 1995, with most early trials focusing on the potential benefits of autologous MSCs in promoting the engraftment of hematopoietic stem cells in the setting of hematological malignancy (105). In 2005, the first randomized case-control clinical trial was conducted to evaluate the effects of intravenous autologous MSCs on patients with middle cerebral artery infarction (106). Thirty patients from 30 to 75 years (n = 5 in the MSCs group and n = 25 in the control group) were randomized to receive 1 × 108 BM-MSCs intravenously within 7 days of the stroke. It showed that BM-MSCs could improve functional recovery, and no adverse events were associated with transplantation during the 1-year follow-up period. Three cases of ischemic stroke and one case of hemorrhagic stroke in the territory of the middle cerebral artery were treated with 2 × 107 umbilical cord-derived MSCs by a catheter to a near lesion site in 2012 by Jiang et al. (88), and the patients were followed up for 6 months after the procedure. Muscle strength was improved in two ischemic stroke patients, and there were no adverse events. Subsequently, in 2014, 8 patients with large cerebral infarction or anterior arterial infarction were treated with MSCs alone (intravenous injection of MSCs at 0.5 × 106/kg body weight four times) or MSCs combined with neural stem/progenitor cells (NSPCs) (intravenous injection of MSCs at 0.5 × 106/kg body weight the first time, and then injected three times at 5 × 106/patient MSCs and 6 × 106/patient neural NSPCs) through the cerebellar bulbar cistern. Except for the occurrence of slight dizziness and low fever within 2–24 h, there were no other serious adverse reactions, and levels of disability and daily living ability improved. The result indicates that the combined transplantation of NSPCs and MSCs is a safe and feasible method to improve neural function (93). In 2018, Deng et al. (96) included 108 patients with IS within 30 to 90 days of onset and were randomized into an experimental group (n = 59) and a control group (n = 59). Then allogeneic BM-MSCs (1 × 106 cells/kg body weight) were injected intrathecally four times a week. All patients underwent a detailed functional assessment and magnetic resonance imaging before the cell infusion and at 1-year intervals, then assess its safety and feasibility. Currently, it is in the phase II experiment. In 2020, a single-center, open-label, randomized controlled trial enrolled patients aged 18–70 following moderate-severe ischemic carotid stroke (National Institutes of Health Stroke Scale, NIHSS>10) for < 2 weeks was conducted. Patients were randomized 2:1 to receive intravenous 1–3 × 108 BM-MSCs (n = 31) or not (n = 16), with a 2-year follow-up. Researchers found that the intravenous injection of autologous MSCs was safe and feasible for treating moderate to severe stroke (100). In 2021, Chung et al. confirmed that intravenous application of preconditioned autologous MSCs with autologous serum was feasible and safe for patients with chronic major stroke (101). A neuroimaging study also showed positive changes in network reorganization to facilitate motor recovery after stroke (102). A phase 2, single-center, assessor-blinded randomized controlled study by Zhe Kang Law and colleagues estimated the safety, tolerability, and efficacy of intravenous infusion of autologous MSCs (104). The treatment group (received culture-expanded autologous BM-MSCs intravenously) and the control group (received standard medical care) had recovery effects, but there was no significant difference between them. The 17 patients were all safe and welltolerated. Consistent with these results, intravenous injection of autologous MSC administration is safe and feasible for treating stroke. In 2022, a Phase I open-label study by Tsung-Lang Chiu adopted autologous adipose-derived stem cells to treat three chronic stroke patients by stereotactic implantation, which improved patients in many aspects without any adverse effects observed (91). Another study by Elena de Celis-Ruiz also demonstrated the safety of intravenous adipose tissue-derived mesenchymal stem cell therapy from the first 2 weeks after ischemic stroke to 24 months of follow-up (89, 91). A first-in-human, open-label intervention study by Lisanne M. Baak and team used intranasally delivered bone marrow-derived allogeneic MSCs for neonates with perinatal arterial ischaemic stroke (98). All ten enrolled neonates were welltolerated with the therapy, and no serious adverse events were observed until 3 months of age follow-up.
These clinical trials have tried all of the common modes of drug delivery, including stereotactic administration, intravenous injection, and intranasal routes, and patient ages range from newborn to middle-aged and elderly. The existing results provide confirmed data for the safety and feasibility of MSC therapy. However, given the differences in patient population, cell origins, time of administration, and drug delivery systems, as well as small sample sizes and lack of randomization and double-blind control, the interpretation of these results is limited. Therefore, higher quality randomized clinical trials, including better phenotypes and larger series, are still necessary to provide more reliable data to further clarify the safety and efficacy of MSC-based therapy in cerebrovascular disease.
More new ideas for MSC-related therapies
SB623 cells are a gene-modified derivate of MSCs using gene transfection with Notch intracellular domain (NICD) and are subsequently followed by the administration of certain trophic factors (107). Though the transfected plasmid gets lost because of the expansion and split of cells, patterns of DNA methylation and protein expression have been altered. Compared to hMSCs from the same donors, preclinical studies showed that SB623 cells had higher secretion of angiogenin, angiopoietin-2, HB-EGF, and VEGF levels in the conditioned medium (108). SB623 cells also secrete higher-level factors that protect cells and enhance MSC migration after a hypoxic injury, such as DKK-1, IL-6, IL-8, MCP-1, and GM-CSF (68, 109). These trophic factors play a pivotal role in anti-inflammatory and immunosuppressive effects.
They are intended for use as an allogeneic cell therapy for chronic motor deficiency, particularly after a stable stroke, due to their ability to produce trophic factors, encouraging neuronal cells' reconstructive approach. In the completed 2-year phase 1/2a, open-label, a single-arm study by Gary K. Steinberg and his colleagues, 18 patients with stable, chronic stroke received SB623 cell implantation therapy (94). At 12 months after treatment, there were many meaningful developments in measures assessed for acute and long-term outcomes, like the mean scale scores of ESS, NIHSS, F-M total score, and F-M motor function total score. During the 2-year follow-up of treatment-emergent adverse events (TEAE), all patients experienced at least one TEAE, although no evidence suggested that it was probably related to SB623 cell treatment. All patients were generally safe and welltolerated at 2 years (94, 95).
Some researchers considered that combination cell therapy for MSCs might interact well with other types of stem cells. In a study by Seyed et al. BMSCs and neural stem cells (NSCs) were used together to treat MCAO rats (49). From a theory basis, MSCs could provide an appropriate microenvironment for NSCs after stroke via immunomodulation, and anti-inflammatory and NSCs have a great capacity to differentiate into neural lineage cells. Luckily, they got satisfactory results: In the group receiving combination therapy, neurological function was improved, infarct area was reduced, and they had the lowest amount of apoptosis. This therapy is effective in the prevention of strokes as well (110). A study published by Li-yan Qiao in 2004 also proved its effectiveness and safety (mentioned on page 11, line 294) (93). We believe these new therapies can lead to better post-stroke recovery for patients.
Concluding remarks
The potential of MSCs in the treatment of ischemic stroke is huge. In this article, we review MSCs in IS therapy. Although a large number of preclinical and clinical studies support their safety and restorative effects, each patient with ischemic stroke has different symptoms and physiological conditions. Thus, many key issues must be resolved before the clinical application, like optimal cell source, dosage, transplantation time window and pathway, and adverse event monitoring and management. Therefore, a better understanding of the mechanism of stem cell treatment of stroke will be required to help solve the above problems. The data regarding their exact mechanisms of action remain incompletely clear. The paracrine effects of MSCs, a crucial part of their therapeutic potential, have powerful neurotrophic, neuroprotective, angiogenesis, and neurogenesis activity. However, exploring the interaction between various soluble cytokines in the ischemic brain is necessary. In addition, their distinctive immunological profile supports their clinical application, especially as a product with the use of allogeneic MSCs. Moreover, the cell replacement and modulating multicellular fate of MSCs have a significant impact on the repair of brain tissue and are worth further study in the future. In conclusion, it seems that MSCs can be utilized as therapeutic candidates in stroke therapy and may pave the way for new treatments in the near future to improve neurologic function, survival, and quality of life for patients with ischemic stroke.
Author contributions
QY designed and approved the final manuscript for publication. LZ and JW wrote the manuscript. JH, XS, YW, XC, and YT collected the references and modified the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This study was supported by the National Natural Science Foundation of China (Grant No. 82171456 and 81971229, to QY).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Wang J, Gao L, Yang Y, Li Y, Chang T, Man M, et al. Low serum levels of brain-derived neurotrophic factor were associated with poor short-term functional outcome and mortality in acute ischemic stroke. Mol Neurobiol. (2017) 54:7335–42. doi: 10.1007/s12035-016-0236-1
2. Feigin VL, Stark BA, Johnson CO, Roth GA, Bisignano C, et al. Global, regional, and national burden of stroke and its risk factors, 1990-2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet Neurol. (2021) 20:795–820.
3. Phipps MS, Cronin CA. Management of acute ischemic stroke. BMJ (Clinical research ed). (2020) 368:l6983. doi: 10.1136/bmj.l6983
4. Nogueira RG, Jadhav AP, Haussen DC, Bonafe A, Budzik RF, Bhuva P, et al. Thrombectomy 6 to 24 Hours after Stroke with a Mismatch between Deficit and Infarct. N Engl J Med. (2018) 378:11–21. doi: 10.1056/NEJMoa1706442
5. Primiani CT, Vicente AC, Brannick MT, Turk AS, Mocco J, Levy EI, et al. Direct aspiration versus stent retriever thrombectomy for acute stroke: a systematic review and meta-analysis in 9127 patients. J Stroke Cerebrovasc Dis. (2019) 28:1329–37. doi: 10.1016/j.jstrokecerebrovasdis.2019.01.034
6. Pianta S, Lee JY, Tuazon JP, Castelli V, Mantohac LM, Tajiri N, et al. A short bout of exercise prior to stroke improves functional outcomes by enhancing angiogenesis. Neuromolecular Med. (2019) 21:517–28. doi: 10.1007/s12017-019-08533-x
7. Powers WJ, Rabinstein AA, Ackerson T, Adeoye OM, Bambakidis NC, Becker K, et al. Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 guidelines for the early management of acute ischemic stroke: a guideline for healthcare professionals from the American heart association/American stroke association. Stroke. (2019) 50:e344–418. doi: 10.1161/STR.0000000000000211
8. Abou-El-Enein M, Elsanhoury A, Reinke P. Overcoming challenges facing advanced therapies in the eu market. Cell Stem Cell. (2016) 19:293–7. doi: 10.1016/j.stem.2016.08.012
9. Toman NG, Grande AW, Low WC. Neural repair in stroke. Cell Transplant. (2019) 28:1123–6. doi: 10.1177/0963689719863784
10. Kawabori M, Shichinohe H, Kuroda S, Houkin K. Stem cell therapy in stroke. Cell Mol Life Sci. (2009) 66:757–72. doi: 10.1007/s00018-008-8346-1
11. Johnson TC, Siegel D. Directing stem cell fate: the synthetic natural product connection. Chem Rev. (2017) 117:12052–86. doi: 10.1021/acs.chemrev.7b00015
12. Shinozuka K, Dailey T, Tajiri N, Ishikawa H, Kim DW, Pabon M, et al. Stem cells for neurovascular repair in stroke. J Stem Cell Res Ther. (2013) 4:12912. doi: 10.4172/2157-7633.S4-004
13. Yu SP, Tung JK, Wei ZZ, Chen D, Berglund K, Zhong W, et al. Optochemogenetic stimulation of transplanted iPS-NPCs enhances neuronal repair and functional recovery after ischemic stroke. J Neurosci. (2019) 39:6571–94. doi: 10.1523/JNEUROSCI.2010-18.2019
14. Stonesifer C, Corey S, Ghanekar S, Diamandis Z, Acosta SA, Borlongan CV. Stem cell therapy for abrogating stroke-induced neuroinflammation and relevant secondary cell death mechanisms. Prog Neurobiol. (2017) 158:94–131. doi: 10.1016/j.pneurobio.2017.07.004
15. Ferri ALM, Bersano A, Lisini D, Boncoraglio G, Frigerio S, Parati E. Mesenchymal stem cells for ischemic stroke: progress and possibilities. Curr Med Chem. (2016) 23:1598–608. doi: 10.2174/0929867323666160222113702
16. Ha BC, Jung J, Kwak BK. Susceptibility-weighted imaging for stem cell visualization in a rat photothrombotic cerebral infarction model. Acta Radiologica. (2015) 56:219–27. doi: 10.1177/0284185114525605
17. Friedenstein AJ, Chailakhjan RK, Lalykina KS. The development of fibroblast colonies in monolayer cultures of guinea-pig bone marrow and spleen cells. Cell Tissue Kinetics. (1970) 3:393–403. doi: 10.1111/j.1365-2184.1970.tb00347.x
18. Friedenstein AJ, Chailakhyan RK, Latsinik NV, Panasyuk AF, Keiliss-Borok IV. Stromal cells responsible for transferring the microenvironment of the hemopoietic tissues. Cloning in vitro and retransplantation in vivo. Transplantation. (1974) 17:331–40. doi: 10.1097/00007890-197404000-00001
20. Erpicum P, Weekers L, Detry O, Bonvoisin C, Delbouille M, Grégoire C, et al. Infusion of third-party mesenchymal stromal cells after kidney transplantation: a phase I-II, open-label, clinical study. Kidney Int. (2019) 95:693–707. doi: 10.1016/j.kint.2018.08.046
21. Fathollahi A, Gabalou NB, Aslani S. Mesenchymal stem cell transplantation in systemic lupus erythematous, a mesenchymal stem cell disorder. Lupus. (2018) 27:1053–64. doi: 10.1177/0961203318768889
22. Shi Y, Wang Y, Li Q, Liu K, Hou J, Shao C, et al. Immunoregulatory mechanisms of mesenchymal stem and stromal cells in inflammatory diseases. Nat Rev Nephrol. (2018) 14:493–507. doi: 10.1038/s41581-018-0023-5
23. Lim H, Park SH, Kim SW, Cho K. Therapeutic potential of human turbinate-derived mesenchymal stem cells in experimental acute ischemic stroke. Int Neurourol J. (2018) 22:S131–138. doi: 10.5213/inj.1836220.110
24. Ullah I, Subbarao RB, Rho GJ. Human mesenchymal stem cells—current trends and future prospective. Biosci Rep. (2015) 35:2. doi: 10.1042/BSR20150025
25. Ikegame Y, Yamashita K, Hayashi S, Mizuno H, Tawada M, You F, et al. Comparison of mesenchymal stem cells from adipose tissue and bone marrow for ischemic stroke therapy. Cytotherapy. (2011) 13:675–85. doi: 10.3109/14653249.2010.549122
26. Gimble JM, Bunnell BA, Guilak F. Human adipose-derived cells: an update on the transition to clinical translation. Regen Med. (2012) 7:225–35. doi: 10.2217/rme.11.119
27. Vincenzo Eterno AZ. 1,2 Lorenzo Pavesi,1,2 Laura Villani,4 Vittorio Zanini,3 Gianfranco Petrolo,3 Stefania Manera,1 Antonella Tuscano,1 and Angela Amato1, Adipose-derived mesenchymal stem cells (ASCs) may favour breast cancer recurrence via HGF/c-Met signaling. Oncotarget. (2014) 5:613–33. doi: 10.18632/oncotarget.1359
28. Petrenko Y, Vackova I, Kekulova K, Chudickova M, Koci Z, Turnovcova K, et al. A comparative analysis of multipotent mesenchymal stromal cells derived from different sources, with a focus on neuroregenerative potential. Sci Rep. (2020) 10:4290. doi: 10.1038/s41598-020-61167-z
29. Liang C, Liu H, Chang S, Chen S, Lee T. The protective effect of human umbilical cord blood CD34+ cells and estradiol against focal cerebral ischemia in female ovariectomized rat: cerebral MR imaging and immunohistochemical study. PLoS One. (2016) 11:e0147133. doi: 10.1371/journal.pone.0147133
30. Bieback K, Kern S, Klüter H, Eichler H. Critical parameters for the isolation of mesenchymal stem cells from umbilical cord blood. Stem cells (Dayton, Ohio). (2004) 22:625–34. doi: 10.1634/stemcells.22-4-625
31. Bearden RN, Huggins SS, Cummings KJ, Smith R, Gregory CA, Saunders WB. In-vitro characterization of canine multipotent stromal cells isolated from synovium, bone marrow, and adipose tissue: a donor-matched comparative study. Stem Cell Res Ther. (2017) 8:218. doi: 10.1186/s13287-017-0639-6
32. Heo JS, Choi Y, Kim H, Kim HO. Comparison of molecular profiles of human mesenchymal stem cells derived from bone marrow, umbilical cord blood, placenta and adipose tissue. Int J Mol Med. (2016) 37:115–25. doi: 10.3892/ijmm.2015.2413
33. Kim M, Bae YK, Um S, Kwon JH, Kim G, Choi SJ, et al. A small-sized population of human umbilical cord blood-derived mesenchymal stem cells shows high stemness properties and therapeutic benefit. Stem Cells Int. (2020) 2020:5924983. doi: 10.1155/2020/5924983
34. Sultana T, Lee S, Yoon H, Lee JI. Current status of canine umbilical cord blood-derived mesenchymal stem cells in veterinary medicine. Stem Cells Int. (2018) 2018:8329174. doi: 10.1155/2018/8329174
35. Baker N, Boyette L, Tuan R. Characterization of bone marrow-derived mesenchymal stem cells in aging. Bone. (2015) 70:37–47. doi: 10.1016/j.bone.2014.10.014
36. Li W, Xu H, Qian C. c-Kit-positive adipose tissue-derived mesenchymal stem cells promote the growth and angiogenesis of breast cancer. BioMed Res Int. (2017) 2017:7407168. doi: 10.1155/2017/7407168
37. Amati E, Sella S, Perbellini O, Alghisi A, Bernardi M, Chieregato K, et al. Generation of mesenchymal stromal cells from cord blood: evaluation of in vitro quality parameters prior to clinical use. Stem Cell Res Ther. (2017) 8:14. doi: 10.1186/s13287-016-0465-2
38. Um S, Ha J, Choi SJ, Oh W, Jin HJ. Prospects for the therapeutic development of umbilical cord blood-derived mesenchymal stem cells. World J Stem Cells. (2020) 12:1511–28. doi: 10.4252/wjsc.v12.i12.1511
39. Mazini L, Rochette L, Amine M, Malka G. Regenerative capacity of adipose derived stem cells (ADSCs), comparison with mesenchymal stem cells (MSCs). Int J Mol Sci. (2019) 20:10. doi: 10.3390/ijms20102523
40. Dominici M, Blanc KL, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. Int Soc Cellular Therapy Posit State Cytotherap. (2006) 8:315–7. doi: 10.1080/14653240600855905
41. Bianco P. “Mesenchymal” stem cells. Annu Rev Cell Dev Biol. (2014) 30:677–704. doi: 10.1146/annurev-cellbio-100913-013132
42. Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, et al. Multilineage potential of adult human mesenchymal stem cells. Science. (1999) 284:143–7. doi: 10.1126/science.284.5411.143
43. Caprnda M, Kubatka P, Gazdikova K, Gasparova I, Valentova V, Stollarova N, et al. Immunomodulatory effects of stem cells: Therapeutic option for neurodegenerative disorders. Biomed Pharmacother. (2017) 91:60–9. doi: 10.1016/j.biopha.2017.04.034
44. Kang SK, Lee DH, Bae YC, Kim HK, Baik SY, Jung JS. Improvement of neurological deficits by intracerebral transplantation of human adipose tissue-derived stromal cells after cerebral ischemia in rats. Exp Neurol. (2003) 183:355–66. doi: 10.1016/S0014-4886(03)00089-X
45. Zhao L, Duan W, Reyes M, Keene CD, Verfaillie CM, Low WC. Human bone marrow stem cells exhibit neural phenotypes and ameliorate neurological deficits after grafting into the ischemic brain of rats. Exp Neurol. (2002) 174:11–20. doi: 10.1006/exnr.2001.7853
46. Lin Y, Ko T, Shih Y, Lin MA, Fu T, Hsiao H, et al. Human umbilical mesenchymal stem cells promote recovery after ischemic stroke. Stroke. (2011) 42:2045–53. doi: 10.1161/STROKEAHA.110.603621
47. Liao W, Xie J, Zhong J, Liu Y, Du L, Zhou B, et al. Therapeutic effect of human umbilical cord multipotent mesenchymal stromal cells in a rat model of stroke. Transplantation. (2009) 87:350–9. doi: 10.1097/TP.0b013e318195742e
48. Yoo S, Chang D, Lee H, Kim G, Park J, Ryu B, et al. Immune following suppression mesenchymal stem cell transplantation in the ischemic brain is mediated by TGF-β. Neurobiol Dis. (2013) 58:249–57. doi: 10.1016/j.nbd.2013.06.001
49. Hosseini SM, Farahmandnia M, Razi Z, Delavari S, Shakibajahromi B, Sarvestani FS, et al. Combination cell therapy with mesenchymal stem cells and neural stem cells for brain stroke in rats. Int J Stem Cells. (2015) 8:99–105. doi: 10.15283/ijsc.2015.8.1.99
50. Acosta SA, Tajiri N, Hoover J, Kaneko Y, Borlongan CV. Intravenous bone marrow stem cell grafts preferentially migrate to spleen and abrogate chronic inflammation in stroke. Stroke. (2015) 46:2616–27. doi: 10.1161/STROKEAHA.115.009854
51. Liu Y, Seçkin H, Izci Y, Du ZW, Yan Y, Başkaya MK. Neuroprotective effects of mesenchymal stem cells derived from human embryonic stem cells in transient focal cerebral ischemia in rats. J Cereb Blood Flow Metab. (2009) 29:780–91. doi: 10.1038/jcbfm.2009.1
52. Wakabayashi K, Nagai A, Sheikh AM, Shiota Y, Narantuya D, Watanabe T, et al. Transplantation of human mesenchymal stem cells promotes functional improvement and increased expression of neurotrophic factors in a rat focal cerebral ischemia model. J Neurosci Res. (2010) 88:1017–25. doi: 10.1002/jnr.22279
53. Tanaka E, Ogawa Y, Mukai T, Sato Y, Hamazaki T, Nagamura-Inoue T, et al. Dose-dependent effect of intravenous administration of human umbilical cord-derived mesenchymal stem cells in neonatal stroke mice. Front Neurol. (2018) 9:133. doi: 10.3389/fneur.2018.00133
54. Lemmens R, Steinberg GK. Stem cell therapy for acute cerebral injury: what do we know and what will the future bring? Curr Opinion Neurol. (2013) 26:617–25. doi: 10.1097/WCO.0000000000000023
55. Shen LH Li Y, Chen J, Zhang J, Vanguri P, Borneman J, et al. Intracarotid transplantation of bone marrow stromal cells increases axon-myelin remodeling after stroke. Neuroscience. (2006) 137:393–9. doi: 10.1016/j.neuroscience.2005.08.092
56. Toyoshima A, Yasuhara T, Kameda M, Morimoto J, Takeuchi H, Wang F, et al. Intra-arterial transplantation of allogeneic mesenchymal stem cells mounts neuroprotective effects in a transient ischemic stroke model in rats: analyses of therapeutic time window and its mechanisms. PLoS One. (2015) 10:e0127302. doi: 10.1371/journal.pone.0127302
57. Vibhuti, Khan R, Sharma A, Jain S, Mohanty S, Prasad K. Intra-arterial transplantation of human bone marrow mesenchymal stem cells (hBMMSCs) improves behavioral deficits and alters gene expression in rodent stroke model. J Neurochemistr. (2017) 143:722–35. doi: 10.1111/jnc.14241
58. Ishizaka S, Horie N, Satoh K, Fukuda Y, Nishida N, Nagata I. Intra-arterial cell transplantation provides timing-dependent cell distribution and functional recovery after stroke. Stroke. (2013) 44:720–6. doi: 10.1161/STROKEAHA.112.677328
59. Jiang W, Liang G, Li X, Li Z, Gao X, Feng S, et al. Intracarotid transplantation of autologous adipose-derived mesenchymal stem cells significantly improves neurological deficits in rats after MCAo. J Mater Sci Mater Med. (2014) 25:1357–66. doi: 10.1007/s10856-014-5157-9
60. Wei N, Yu SP, Gu X, Taylor TM, Song D, Liu X, et al. Delayed intranasal delivery of hypoxic-preconditioned bone marrow mesenchymal stem cells enhanced cell homing and therapeutic benefits after ischemic stroke in mice. Cell Transplant. (2013) 22:977–91. doi: 10.3727/096368912X657251
61. Velthoven CTJ, Kavelaars A, Derugin N, Vexler ZS, Willemen HLDM, et al. Mesenchymal stem cell transplantation attenuates brain injury after neonatal stroke. Stroke. (2013) 44:1426–32. doi: 10.1161/STROKEAHA.111.000326
62. Wei ZZ, Gu X, Ferdinand A, Lee JH Ji X, Ji XM, et al. Intranasal delivery of bone marrow mesenchymal stem cells improved neurovascular regeneration and rescued neuropsychiatric deficits after neonatal stroke in rats. Cell Transplant. (2015) 24:391–402. doi: 10.3727/096368915X686887
63. Mushahary D, Spittler A, Kasper C, Weber V, Charwat V. Isolation, cultivation, and characterization of human mesenchymal stem cells. Cytometry Part A : J Int Soc Analytic Cytol. (2018) 93:19–31. doi: 10.1002/cyto.a.23242
64. Gervois P, Struys T, Hilkens P, Bronckaers A, Ratajczak J, Politis C, et al. Neurogenic maturation of human dental pulp stem cells following neurosphere generation induces morphological and electrophysiological characteristics of functional neurons. Stem Cells Dev. (2015) 24:296–311. doi: 10.1089/scd.2014.0117
65. Warrier S, Haridas N, Bhonde R. Inherent propensity of amnion-derived mesenchymal stem cells towards endothelial lineage: vascularization from an avascular tissue. Placenta. (2012) 33:850–8. doi: 10.1016/j.placenta.2012.07.001
66. Liu Y, Zhang Y, Lin L, Lin F, Li T, Du H, et al. Effects of bone marrow-derived mesenchymal stem cells on the axonal outgrowth through activation of PI3K/AKT signaling in primary cortical neurons followed oxygen-glucose deprivation injury. PLoS One. (2013) 8:e78514. doi: 10.1371/journal.pone.0078514
67. Gu W, Zhang F, Xue Q, Ma Z, Lu P, Yu B. Bone mesenchymal stromal cells stimulate neurite outgrowth of spinal neurons by secreting neurotrophic factors. Neurol Res. (2012) 34:172–80. doi: 10.1179/1743132811Y.0000000068
68. Tate CC, Fonck C, McGrogan M, Case CC. Human mesenchymal stromal cells and their derivative, SB623 cells, rescue neural cells via trophic support following in vitro ischemia. Cell Transplant. (2010) 19:973–84. doi: 10.3727/096368910X494885
69. Tsai M, Tsai S, Hu B, Liou D, Huang S, Huang M, et al. Recovery of neurological function of ischemic stroke by application of conditioned medium of bone marrow mesenchymal stem cells derived from normal and cerebral ischemia rats. J Biomed Sci. (2014) 21:5. doi: 10.1186/1423-0127-21-5
70. Miceli V, Pampalone M, Vella S, Carreca AP, Amico G, Conaldi PG. Comparison of immunosuppressive and angiogenic properties of human amnion-derived mesenchymal stem cells between 2D and 3D culture systems. Stem Cells Int. (2019) 2019:7486279. doi: 10.1155/2019/7486279
71. Delfi IRA, Sheard JJ, Wood CR, Vernallis A, Innes JF, Myint P, et al. Canine mesenchymal stem cells are neurotrophic and angiogenic: an in vitro assessment of their paracrine activity. Veterinary J. (2016) 217:10–17. doi: 10.1016/j.tvjl.2016.09.003
72. Oses C, Olivares B, Ezquer M, Acosta C, Bosch P, Donoso M, et al. Preconditioning of adipose tissue-derived mesenchymal stem cells with deferoxamine increases the production of pro-angiogenic, neuroprotective and anti-inflammatory factors: Potential application in the treatment of diabetic neuropathy. PLoS One. (2017) 12:e0178011. doi: 10.1371/journal.pone.0178011
73. Sarmah D, Agrawal V, Rane P, Bhute S, Watanabe M, Kalia K, et al. Mesenchymal stem cell therapy in ischemic stroke: a meta-analysis of preclinical studies. Clin Pharmacol Ther. (2018) 103:990–8. doi: 10.1002/cpt.927
74. Yasuhara T, Matsukawa N, Hara K, Maki M, Ali MM Yu SJ, et al. Notch-induced rat and human bone marrow stromal cell grafts reduce ischemic cell loss and ameliorate behavioral deficits in chronic stroke animals. Stem Cells Dev. (2009) 18:1501–14. doi: 10.1089/scd.2009.0011
75. Ohta M, Suzuki Y, Noda T, Ejiri Y, Dezawa M, Kataoka K, et al. Bone marrow stromal cells infused into the cerebrospinal fluid promote functional recovery of the injured rat spinal cord with reduced cavity formation. Exp Neurol. (2004) 187:266–78. doi: 10.1016/j.expneurol.2004.01.021
76. Amini N, Vousooghi N, Alizade A, Ramezani S, Joghataei MT, Milan PB, et al. Transplantation of adipose tissue-derived stem cells into brain through cerebrospinal fluid in rat models: protocol development and initial outcome data. Curr Stem Cell Res Ther. (2019) 14:191–5. doi: 10.2174/1574888X13666180720112322
77. Lalu MM, Foster M, Presseau J, Dowlatshahi D, Castillo G, Cardenas A, et al. What are potential barriers and enablers to patient and physician participation in Canadian cell therapy trials for stroke? a stakeholder interview study. BMJ open. (2020) 10:e034354. doi: 10.1136/bmjopen-2019-034354
78. Li X, Huang M, Zhao R, Zhao C, Liu Y, Zou H, et al. Intravenously delivered allogeneic mesenchymal stem cells bidirectionally regulate inflammation and induce neurotrophic effects in distal middle cerebral artery occlusion rats within the First 7 days after stroke. Cell Physiol Biochem. (2018) 46:1951–70. doi: 10.1159/000489384
79. Wagner J, Kean T, Young R, Dennis JE, Caplan AI. Optimizing mesenchymal stem cell-based therapeutics. Curr Opin Biotechnol. (2009) 20:531–6. doi: 10.1016/j.copbio.2009.08.009
80. Spiliopoulos S, Festas G, Reppas L, Brountzos E. Intra-arterial administration of cell-based biological agents for ischemic stroke therapy. Expert Opin Biol Ther. (2019) 19:249–59. doi: 10.1080/14712598.2019.1566454
81. Guzman R, Janowski M, Walczak P. Intra-arterial delivery of cell therapies for stroke. Stroke. (2018) 49:1075–82. doi: 10.1161/STROKEAHA.117.018288
82. Fukuda Y, Horie N, Satoh K, Yamaguchi S, Morofuji Y, Hiu T, et al. Intra-arterial transplantation of low-dose stem cells provides functional recovery without adverse effects after stroke. Cell Mol Neurobiol. (2015) 35:399–406. doi: 10.1007/s10571-014-0135-9
83. Dhuria SV, Hanson LR. Intranasal delivery to the central nervous system: mechanisms and experimental considerations. J Pharmaceutic Sci. (2010) 99:1654–73. doi: 10.1002/jps.21924
84. Thomi G, Joerger-Messerli M, Haesler V, Muri L, Surbek D, Schoeberlein A. Intranasally administered exosomes from umbilical cord stem cells have preventive neuroprotective effects and contribute to functional recovery after perinatal brain injury. Cells. (2019) 8:8. doi: 10.3390/cells8080855
85. Mukherjee A, Tipnis S, Sarma HD, Ravindran G, Samuel G, Viswanathan C, et al. Radiolabeling of umbilical cord-derived mesenchymal stem cells for in vivo tracking. Cancer Biother Radiopharm. (2012) 27:614–9. doi: 10.1089/cbr.2011.1146
86. Gubskiy IL, Namestnikova DD, Revkova VA, Cherkashova EA, Sukhinich KK, Beregov MM, et al. The impact of cerebral perfusion on mesenchymal stem cells distribution after intra-arterial transplantation: a quantitative MR study. Biomedicines. (2022) 10:2. doi: 10.3390/biomedicines10020353
87. Lim S, Yoon HY, Jang HJ, Song S, Kim W, Park J, et al. Dual-modal imaging-guided precise tracking of bioorthogonally labeled mesenchymal stem cells in mouse brain stroke. ACS Nano. (2019) 13:10991–1007. doi: 10.1021/acsnano.9b02173
88. Jiang Y, Zhu W, Zhu J, Wu L, Xu G, Liu X. Feasibility of delivering mesenchymal stem cells via catheter to the proximal end of the lesion artery in patients with stroke in the territory of the middle cerebral artery. Cell Transplant. (2013) 22:2291–8. doi: 10.3727/096368912X658818
89. de Celis-Ruiz E, Fuentes B, Alonso de Leciñana M, Gutiérrez-Fernández M, Borobia AM, Gutiérrez-Zúñiga R, et al. Phase II. double-blind, placebo-controlled, single-center, pilot clinical trial. Cell Transplant. (2022) 31:9636897221083863. doi: 10.1177/09636897221083863
90. Díez-Tejedor E, Gutiérrez-Fernández M, Martínez-Sánchez P, Rodríguez-Frutos B, Ruiz-Ares G, Lara ML, et al. Reparative therapy for acute ischemic stroke with allogeneic mesenchymal stem cells from adipose tissue: a safety assessment: a phase II randomized, double-blind, placebo-controlled, single-center, pilot clinical trial. J Stroke Cerebrovasc Dis. (2014) 23:2694–700. doi: 10.1016/j.jstrokecerebrovasdis.2014.06.011
91. Chiu T, Baskaran R, Tsai S, Huang C, Chuang M, Syu W, et al. Intracerebral transplantation of autologous adipose-derived stem cells for chronic ischemic stroke: A phase I study. J Tissue Eng Regen Med. (2022) 16:3–13. doi: 10.1002/term.3256
92. Celis-Ruiz Ed, Fuentes B, Moniche F, Montaner J, Borobia AM, Gutiérrez-Fernández M, et al. Allogeneic adipose tissue-derived mesenchymal stem cells in ischaemic stroke (AMASCIS-02): a phase IIb, multicentre, double-blind, placebo-controlled clinical trial protocol. BMJ Open. (2021) 11:e051790. doi: 10.1136/bmjopen-2021-051790
93. Qiao L, Huang F, Zhao M, Xie J, Shi J, Wang J, et al. A two-year follow-up study of cotransplantation with neural stem/progenitor cells and mesenchymal stromal cells in ischemic stroke patients. Cell Transplant. (2014) null(undefined):S65–72. doi: 10.3727/096368914X684961
94. Steinberg GK, Kondziolka D, Wechsler LR, Lunsford LD, Coburn ML, Billigen JB, et al. Clinical outcomes of transplanted modified bone marrow-derived mesenchymal stem cells in stroke: A Phase 1/2a Study. Stroke. (2016) 47:1817–24. doi: 10.1161/STROKEAHA.116.012995
95. Steinberg GK, Kondziolka D, Wechsler LR, Lunsford LD, Kim AS, Johnson JN, et al. Two-year safety and clinical outcomes in chronic ischemic stroke patients after implantation of modified bone marrow-derived mesenchymal stem cells (SB623): a phase 1/2a study. J Neurosurg. (2018) 2018:1–11. doi: 10.3171/2018.5.JNS173147
96. Deng L, Peng Q, Wang H, Pan J, Zhou Y, Pan K, et al. Intrathecal injection of allogenic bone marrow-derived mesenchymal stromal cells in treatment of patients with severe ischemic stroke: study protocol for a randomized controlled observer-blinded trial. Transl Stroke Res. (2019) 10:170–7. doi: 10.1007/s12975-018-0634-y
97. Levy ML, Crawford JR, Dib N, Verkh L, Tankovich N, Cramer SC. Phase I/II study of safety and preliminary efficacy of intravenous allogeneic mesenchymal stem cells in chronic stroke. Stroke. (2019) 50:2835–41. doi: 10.1161/STROKEAHA.119.026318
98. Baak LM, Wagenaar N., Aa NEvd, Groenendaal F, Dudink J, Tataranno ML, et al. Feasibility and safety of intranasally administered mesenchymal stromal cells after perinatal arterial ischaemic stroke in the Netherlands (PASSIoN): a first-in-human, open-label intervention study. Lancet Neurol. (2022) 21:528–36. doi: 10.1016/S1474-4422(22)00117-X
99. Bhasin A, Kumaran SS, Bhatia R, Mohanty S, Srivastava MVP. Safety and feasibility of autologous mesenchymal stem cell transplantation in chronic stroke in indian patients. a four-year follow up. J Stem Cells & Regenerat Med. (2017) 13:14–9. doi: 10.46582/jsrm.1301003
100. Jaillard A, Hommel M, Moisan A, Zeffiro TA, Favre-Wiki IM, Barbieux-Guillot M, et al. Autologous mesenchymal stem cells improve motor recovery in subacute ischemic stroke: a randomized clinical trial. Transl Stroke Res. (2020). doi: 10.1007/s12975-020-00787-z
101. Chung J, Chang WH, Bang OY, Moon GJ, Kim SJ, Kim S, et al. Efficacy and safety of intravenous mesenchymal stem cells for ischemic stroke. Neurology. (2021) 96:e1012–23. doi: 10.1212/WNL.0000000000011440
102. Lee J, Chang WH, Chung J, Kim SJ, Kim S, Lee JS, et al. Efficacy of intravenous mesenchymal stem cells for motor recovery after ischemic stroke: a neuroimaging study. Stroke. (2022) 53:20–8. doi: 10.1161/STROKEAHA.121.034505
103. Kim SJ, Moon GJ, Chang WH, Kim Y, Bang OY. collaborators S(cARaTIN. Intravenous transplantation of mesenchymal stem cells preconditioned with early phase stroke serum: current evidence and study protocol for a randomized trial. Trials. (2013) 14:317. doi: 10.1186/1745-6215-14-317
104. Law ZK, Tan HJ, Chin SP, Wong CY, Yahya WNNW, Muda AS, et al. The effects of intravenous infusion of autologous mesenchymal stromal cells in patients with subacute middle cerebral artery infarct: a phase 2 randomized controlled trial on safety, tolerability and efficacy. Cytotherapy. (2021) 23:833–40. doi: 10.1016/j.jcyt.2021.03.005
105. Lazarus HM, Haynesworth SE, Gerson SL, Rosenthal NS, Caplan AI. Ex vivo expansion and subsequent infusion of human bone marrow-derived stromal progenitor cells (mesenchymal progenitor cells): implications for therapeutic use. Bone Marrow Transplant. (1995) 16:557–64.
106. De Keyser J. Autologous mesenchymal stem cell transplantation in stroke patients. Annal Neurol. (2005) 58:653–4; author reply 654-5. doi: 10.1002/ana.20612
107. Dezawa M, Kanno H, Hoshino M, Cho H, Matsumoto N, Itokazu Y, et al. Specific induction of neuronal cells from bone marrow stromal cells and application for autologous transplantation. J Clin Invest. (2004) 113:1701–10. doi: 10.1172/JCI200420935
108. Dao M, Tate CC, McGrogan M, Case CC. Comparing the angiogenic potency of naïve marrow stromal cells and Notch-transfected marrow stromal cells. J Transl Med. (2013) 11:81. doi: 10.1186/1479-5876-11-81
109. Dao MA, Tate CC, Aizman I, McGrogan M, Case CC. Comparing the immunosuppressive potency of naïve marrow stromal cells and Notch-transfected marrow stromal cells. J Neuroinflammation. (2011) 8:133. doi: 10.1186/1742-2094-8-133
Keywords: mesenchymal stem cells (MeSH ID: D059630), stem cell transplantation (HSCT), ischemic stroke (IS), stem-cell based therapy, experimental treatment
Citation: Zhou L, Wang J, Huang J, Song X, Wu Y, Chen X, Tan Y and Yang Q (2022) The role of mesenchymal stem cell transplantation for ischemic stroke and recent research developments. Front. Neurol. 13:1000777. doi: 10.3389/fneur.2022.1000777
Received: 22 July 2022; Accepted: 03 October 2022;
Published: 16 November 2022.
Edited by:
Ashfaq Shuaib, University of Alberta, CanadaCopyright © 2022 Zhou, Wang, Huang, Song, Wu, Chen, Tan and Yang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qin Yang, eHlxaDIwMEAxMjYuY29t
†These authors have contributed equally to this work and share first authorship
 Li Zhou†
Li Zhou† Jiani Wang
Jiani Wang Jiagui Huang
Jiagui Huang Qin Yang
Qin Yang