
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Neurol. , 26 January 2022
Sec. Endovascular and Interventional Neurology
Volume 12 - 2021 | https://doi.org/10.3389/fneur.2021.817989
 Wei Li1,2†
Wei Li1,2† Ming Ye3†
Ming Ye3† Alexandru Cimpoca4
Alexandru Cimpoca4 Hans Henkes4,5
Hans Henkes4,5 Honglei Wang6
Honglei Wang6 Xiang Xu7
Xiang Xu7 Yuxiang Gu8
Yuxiang Gu8 Huaizhang Shi9
Huaizhang Shi9 Hongming Ji10
Hongming Ji10 Feng Wang11
Feng Wang11 Yuanli Zhao12
Yuanli Zhao12 Geng Guo13
Geng Guo13 Hongqi Zhang3*
Hongqi Zhang3* Youxiang Li1,14*
Youxiang Li1,14*Purpose: Endovascular coil occlusion is a successful and rapidly evolving strategy used to treat patients who present with intracranial aneurysms. This study aimed to compare the safety and efficacy of the Avenir® and AxiumTM passive mechanically detachable coil systems.
Methods: A prospective, multicenter, randomized controlled study was carried out at ten medical centers from March 2018 to December 2019. A series of consecutive patients diagnosed with intracranial aneurysms were randomly assigned to undergo endovascular treatment with either the Avenir® or the AxiumTM mechanically detachable coil systems. The short-term outcomes from the two groups were compared with a focus on treatment efficacy and safety.
Results: A total of 162 and 161 patients were enrolled in the Avenir and Axium groups, respectively. The rate of successful coil detachment was 100% for the Avenir group and 99.38% for the Axium group. At the six-month follow-up visit, the overall aneurysm occlusion rate was 94.66% for the Avenir group and 96.95% for the Axium group (p > 0.05). We observed no statistically significant differences in clinical condition (as per the modified Rankin Scale) or the degree of aneurysm occlusion (as determined by digital subtraction angiography [DSA] and Raymond-Roy Occlusion Classification). Surgical complications were reported in 27 subjects in the Avenir group and 22 in the Axium group (p > 0.05). DSA performed at 6 months revealed complete aneurysm occlusion in 84 and 86% of patients in the Avenir and Axium groups, respectively.
Conclusion: We observed no significant short-term differences with respect to efficacy or safety when using either Avenir® or AxiumTM coils for the treatment of intracranial aneurysms.
The incidence of intracranial aneurysms was reported to be at least 1–2% in an unselected adult population (1). Unruptured intracranial aneurysms can be found incidentally on imaging studies, or they may be heralded by symptoms that include headaches, hemiparesis, visual field defects, and seizures. As the aneurysms enlarge, they may compress adjacent neuronal structures. While only a small proportion of intracranial aneurysms will ultimately rupture, this complication can result in subarachnoid hemorrhage and a 30% risk of death or permanent disability (2, 3). There are currently three strategies that can be used to treat intracranial aneurysms that have ruptured or are at risk of doing so, including conservative management, endovascular treatment, or craniotomy with microsurgical clipping (4, 5). Endovascular techniques are gradually becoming the preferred first-line option as they are significantly less invasive than open surgery. When compared to microsurgical clipping, the use of endovascular techniques to treat incidental, symptomatic, or ruptured intracranial aneurysms has resulted in equivalent or improved clinical outcomes (6). Other studies confirmed that the use of endovascular strategies resulted in improved long-term outcomes for patients with ruptured lesions (7), although the clinical outcomes for coil occlusion and microsurgical clipping of unruptured aneurysms were virtually identical (8–10).
The two main complications of endovascular coil occlusion for intracranial aneurysms are peri-procedural perforation of the aneurysm and thromboembolic events. The standard endovascular procedure involves the insertion of a microcatheter and microguidewire to target the aneurysm followed by the insertion of a suitable coil which then undergoes controlled detachment. This procedure has evolved rapidly over the past 30 years (11). The long period that was initially required for detachment of the coil spurred several medical device manufacturers to develop novel methods to facilitate its rapid detachment under controlled conditions. The Target® electrolytically detachable coil system was first released in 1995, followed in 2002 by the Hydrolink hydraulic detachable coil device (MicroVention, Inc.) In 2005, MICRUS (now Codman Neurovascular, Inc.) introduced a thermoelectric detachable coil system. Medtronic PLC released a mechanically detachable coil system (AxiumTM) in 2007 (12).
To date, there are only a few prospective studies that focus on the safety and efficacy of these endovascular coils. Among the most significant concerns, aneurysms may recur due to coil compaction. Likewise, a coil may migrate into an intra-aneurysmal thrombus and trigger modifications to its surface. Likewise, there is no evidence to suggest that the use of modified coils can significantly improve the long-term outcomes of endovascular procedures compared to the use of conventional platinum coils (13–15). Although bare platinum coils are currently in wide use, there remains considerable room for improvement. Coil occlusion of an aneurysm is a complex procedure, and the coils used must have reliable and consistent physical properties. Among these properties, coils must be easy to insert and be clearly visible, with consistent filling and a straightforward detachment mechanism to facilitate complete removal of the pushrod after the procedure (16, 17).
Coil systems that are currently available use different modes of energy transmission to achieve detachment, including electrical, hydraulic, and mechanical impact (12). Mechanically detachable coil systems are passive in nature, undergo rapid detachment, and require only a few supporting devices. The Avenir® coil system (Wallaby Medical, Shanghai, China) is a new, noose-based, passive mechanically-detachable coil system that needs no detachment tools.
This study aimed to evaluate the safety and efficacy of the Avenir® mechanically detachable coil system for the treatment of intracranial aneurysms in clinical practice.
This study was a prospective, multicenter, randomized controlled validation trial with a non-inferiority trial design. The study protocol was approved by the ethics committees of Beijing Tiantan Hospital of Capital Medical University (Institutional Review Board of Beijing Tiantan Hospital, Capital Medical University/ID approval number QX2017-006-03), Xuanwu Hospital of Capital Medical University (Institutional Review Board of Xuanwu Hospital Capital Medical University/ID approval number 2018-003), Huashan Hospital of Fudan University (Huashan Hospital Institutional Review Board/ID approval number 2018-474), the First Hospital of Jilin University (Institutional Review Board of First Hospital of Jilin University/ID approval number 19Q012-001), the First Affiliated Hospital of Dalian Medical University [Institutional Review Board of First Affiliated Hospital of Dalian Medical University/ID approval number PJ-JG-QX-2018-06(X)], the First Affiliated Hospital of Harbin Medical University (The Ethics Committee of First Affiliated Hospital of Harbin Medical University/ID approval number 201803), Shanxi Provincial People's Hospital (Institutional Review Board of Shanxi Provincial People's Hospital/ID approval number 2018-001), Peking University International Hospital (Institutional Review Board of Peking University International Hospital/ID approval number E2018-003), the First Hospital of Shanxi Medical University (Ethics Committee of First Hospital of Shanxi Medical University/ID approval number 2018-Q11), and Tangshan Worker's Hospital (Institutional Review Board of Tangshan Worker's Hospital/ID approval number 2018-06).
This clinical trial was conducted under Good Clinical Practice regulations. All patients were informed of the nature, purpose, and potential risks associated with the trial and each signed informed consent forms before participation. The trial passed the Shanghai Food and Drug Administration (FDA) inspection with no significant findings and also passed the clinical trial quality check for each participating hospital. The clinical trial registration number is ChiCTR2100046506.
Patients with intracranial aneurysms who presented at one of the ten participating hospitals between March 2018 and December 2019 were enrolled prospectively. Study institutions competed with one another to enroll patients from each of the different hospitals. Patient inclusion criteria included (1) males and females 18–80 years of age, (2) diagnosis of at least one intracranial aneurysm based on computed tomography angiography (CTA), magnetic resonance angiography (MRA), or digital subtraction angiography (DSA) imaging performed during the previous 6 months, (3) clinical condition rated as Hunt-Hess grade 0, I, II, or III (18), and (4) a clear understanding of the trial information and capacity to sign the informed consent form. Patients were excluded if (1) their condition precluded endovascular treatment of the aneurysm (e.g., due to a space-occupying effect of an intracranial hematoma, giant aneurysm), (2) they were diagnosed with liver or kidney dysfunction (defined as alanine aminotransferase [ALT] or aspartate transaminase [AST] levels that were more than two times greater than the upper limit of normal), (3) they had participated in other clinical trials in the past 3 months, (4) they were diagnosed with concomitant diseases that would preclude effective treatment or evaluation (e.g., cancer, infection, severe metabolic diseases, and/or mental disorders), (4) they were pregnant, or (5) they were allergic or had contraindications to aspirin, heparin, or local or general anesthesia.
Patients were assigned at random to one of the two groups. To avoid bias, each patient was assigned to the Avenir or Axium group based on information they received after scratching off the silver coating on a random card that was provided in the order in which they were enrolled in the study. Complementary treatment was based on the group assignment determined by the random card distribution.
The patients assigned to the Avenir and Axium groups were evaluated using the same criteria. All imaging findings were assessed in a blinded manner by neurosurgeons or radiologists with an intermediate professional title or higher who were not directly involved in the clinical study or the data collection. Assessments were made several times for each patient. Means and medians were used to generate the final results.
Routine preoperative and postoperative assessments included coagulation tests (prothrombin time [PT] and activated partial thromboplastin time [APTT]) and platelet counts were performed for all patients. Results from a thromboelastography (TEG) procedure were assessed in patients with a bleeding tendency.
Quality assurance measures included: (1) the interventional procedure was performed by a high-level associate chief or neuro-interventionalist physician with more than 5 years of experience with independent aneurysm coil treatments in large tertiary care hospitals, and (2) all individuals performing the surgery had been trained with standardized procedures and methods before the initiation of the study.
All procedures were performed with the patient under general anesthesia. The procedure involved a femoral artery puncture followed by placement of a sheath using the Seldinger technique. All patients were administered systemic heparinization via intravenous injection of 45 IU unfractionated heparin per kg body weight. Diagnostic cerebral angiography and rotational angiography with three-dimensional (3D) reconstruction of the aneurysm was performed at the beginning of the procedure to determine a suitable working projection that clearly revealed the parent artery together with the aneurysm neck and sac without foreshortening or over-projection. The diameters of the neck and fundus of the target aneurysm were measured after calibrating the DSA system.
Patients were treated with either coil occlusion alone or with stent-assisted coiling based on the anatomy of the parent vessel and aneurysm. The individual treatment strategy and the selection of access products and procedures were at the discretion of the operator.
In our study, an Echelon 10 microcatheter (Medtronic PLC, Dublin, Ireland) was used most frequently for coil embolization of cerebral aneurysms. The Avenir® coil system includes numerous coil models that can be selected based on clinical needs (see Supplementary Material). For stent-assisted coil embolization, most operators used an Enterprise (Codman Neurovascular, Raynham, MA, USA) or LVISTM stent (MicroVention Inc., Tustin, CA, USA).
A microcatheter supported by a shaped microguidewire was inserted into the aneurysm or the parent artery under roadmap guidance. After determining the appropriate coil diameter and length based on the angiographic measurements, a suitable coil was placed into the aneurysm or the parent artery. This process was repeated until the interventionist deemed that sufficient occlusion was achieved. After coil occlusion, angiography was repeated at the previously identified working projection and at standard posterior-anterior and lateral projections to determine the extent of aneurysm occlusion as well as the patency of the parent artery.
The patients assigned to the Avenir group underwent a procedure in which Avenir® mechanically detachable coils (Wallaby Medical, Shanghai, China) were used. This device includes 3D framing coils, two-dimensional (2D) filling coils, 2D finishing coils, and 3D finishing coils. The 3D framing coils were used to form the initial basket within the target aneurysm. Avenir® filling coils were then used to fill the space within the framing coils and Avenir® finishing coils were used to complete the final occlusion at the aneurysm neck. The diameter and length of the Avenir® coils used for each procedure were chosen by the interventionist.
Patients assigned to the Axium group were treated with mechanically detachable AxiumTM coils (Medtronic PLC). The coil occlusion procedure and the decision-making criteria, including all technical details, were identical for both patient groups.
The primary outcome parameter was the degree of aneurysm occlusion as determined by a follow-up DSA that was performed 6 months after coil treatment. The quantitative analysis was based on the amount of contrast medium filling the parent artery and entering into the aneurysm. The Raymond-Roy Occlusion Classification (RROC) system was used to evaluate coiled aneurysms; the ratings included Class I, complete obliteration; Class II, residual neck; and Class III, residual aneurysm (19). The extent of aneurysm occlusion was evaluated by three qualified investigators who performed their assessments independently and who were not involved in the clinical trial. One investigator was based in China and two were from Germany.
Surgical complications of these procedures included hematoma at the puncture site, intracranial vessel perforation or dissection, aneurysm rupture, parent artery occlusion, incomplete filling of the aneurysm caused by the inability to reposition the microcatheter after it has been dislodged from the aneurysm, distal vessel occlusion due to emboli, local vasospasm, migration or dislocation of coils, coils that detached too early or failed to detach, and ischemic stroke.
All patients underwent a final DSA during the procedure immediately after coiling. Patients were then observed for the following 6 months. The patients were first evaluated 3 months post-procedure at the outpatient clinic or by telephone. Scheduled follow-up angiography was performed during the second follow-up 6 months after treatment. Any additional follow-up examinations that were required as clinical routine and/or specific patient needs were not included in the framework of this clinical trial.
This study was designed as a non-inferiority trial. The aneurysm occlusion rate at 6 months follow-up based on angiography findings was assessed according to the RROC scheme (19, 20).
The clinically recognized threshold for non-inferiority was −10% (absolute value) based on equal numbers of patients in both the Avenir and Axium groups. With α set at 0.025 (unilateral) and the power of the test (1-β) set at 0.8, the sample size for the non-inferiority trial of each group was calculated at n = 129 (PASS 13 software; NCSS LLC, Kaysville, UT, USA). Taking into account a potential dropout rate of 20% and the need for randomization, we enrolled 324 patients for the entire study, with 162 subjects assigned randomly to each group. The clinical trial institutions were requested to pursue active patient enrollment; each was asked to enroll a maximum of 162 patients (50% of the total sample size) and a minimum of 24 patients (7% of the total sample size).
An independent data management center (Peking University First Hospital) was engaged to evaluate the clinical and angiography data and to perform the calculations and statistical analysis. SAS9.4 software (SAS Institute Inc., Cary, NC, USA) was used for the statistical analysis. Two-sided tests were used for all statistical comparisons unless specified. Differences between groups were deemed statistically significant for p < 0.05. Quantitative indicators were expressed as means, standard deviations (SDs), medians, minimums, maximums, and quartiles. Numerical data were expressed as frequencies (proportions). A paired t-test (in the event of homogeneous variance and normal distribution) or Wilcoxon rank-sum test was used to compare quantitative data between groups based on the distribution of data. A chi-square test or Fisher's exact test (if chi-square test was not applicable) was used to evaluate classified data. The Cochran–Mantel–Haenszel (CMH) test was used for ranked data.
Three hundred and twenty-four patients were initially enrolled in the study. One patient in the Axium group was excluded because this coil was not used in the procedure. This resulted in a final total of 323 patients (Figure 1).
General and demographic information for the patients in each of the two groups is included in Table 1. A full set analysis (FAS) was performed on 162 patients assigned to the Avenir group and 161 patients assigned to the Axium group. At the 6-month follow-up visit, imaging studies were completed in 139 patients, which included 140 aneurysms diagnosed in patients assigned to the Avenir group and 142 aneurysms in patients assigned to the Axium group. The two groups were generally comparable; we observed no statistically significant differences in age, gender, ethnicity, pretreatment Hunt-Hess grade, or clinical data (p > 0.05). However, we did identify significant differences between the two groups with respect to past medical and surgical history (p < 0.05).
A total of 1,210 coils were successfully implanted into patients assigned to the Avenir group, resulting in an average of seven coils used for each aneurysm. By contrast, 973 coils, or an average of six coils per aneurysm, were used in procedures performed on patients in the Axium group.
There was also no significant difference between the two groups with respect to stent-assisted coiling. One hundred and nineteen patients (73.46%) assigned to the Avenir group were treated with stent-assisted therapy, compared to 117 (72.67%) in the Axium group.
The aneurysm occlusion rates at 6 months post-procedure are shown in Table 2. One hundred and twenty-four of 131 aneurysms treated in patients in the Avenir group (94.66%) achieved an RROC classification of I or II; 127 of the 131 aneurysms treated in patients in the Axium group (96.95%) achieved this result. As indicated by these results, we identified no statistically significant differences in the occlusion rate between the two groups (p = 0.356). A representative case from the Avenir group is shown in Figure 2.
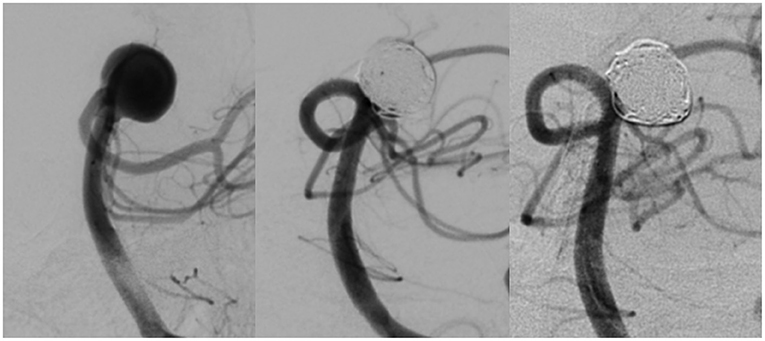
Figure 2. DSA images of an aneurysm at the basilar artery bifurcation before treatment (left), immediately after coil occlusion with Avenir coils (middle), and at 6-month follow-up (right).
We detected no significant differences in modified Rankin Scale scores 6 months after the endovascular coil procedure.
Surgical complications were recorded up until the end of the 6-month follow-up period (Table 3). There were no procedure-associated patient deaths in our study. Among the patients assigned to the Avenir group, one had a ruptured aneurysm, 22 had ischemic strokes, three exhibited incomplete aneurysm filling, and one developed a hematoma at the puncture site. In the Axium group, two patients had a ruptured aneurysm, 14 had ischemic strokes, two exhibited incomplete aneurysm filling, three developed hematomata at the puncture site, and one had difficulties with coil detachment. There were no statistically significant differences between the two groups with respect to these complications.
This study aimed to compare the efficacy and safety of the Avenir® and AxiumTM coils for the treatment of intracranial aneurysms. As this was a preliminary investigation, our study was designed to evaluate non-inferiority. We found that the Avenir® coil system was both safe and effective for the treatment of intracranial aneurysms and that it was not inferior to the AxiumTM coil system when used to promote occlusion of intracranial aneurysms.
The AxiumTM system used in this study was previously evaluated by Kim et al. (21) and presented promising results in terms of its efficacy and safety. This device used here is a bare platinum coil, which is generally preferred by many interventionists. Recent developments include several advanced coil materials, including polymer-coated matrix coils and hydrocoils with a hydrophilic gel surface. However, only a few large-scale studies have been performed to evaluate these coils. The general consensus is that there appears to be little difference in the outcomes compared to the use of bare platinum coils (22). However, Broeders et al. (23) reported that the rate of complete occlusion may be higher in procedures performed using coils made from these materials.
The mechanisms underlying recurrent aneurysm perfusion following coil occlusion have not been fully clarified. Various factors have been implicated in this complication including the precise location and size of the aneurysm, blood flow, blood pressure, previous incidences of aneurysm rupture, and the volume of coils used to fill the aneurysm (24–26). The coils must fill the aneurysm in a uniform and dense fashion in order to avoid recurrence (25, 27).
Several clinical studies and multicenter trials with long-term follow-up reported an aneurysm occlusion rate of 90–96% (28–30). In the present study, the 6-month follow-up DSA after coil treatment showed an aneurysm occlusion rate of up to 94.66% (i.e., RROC class I and class II) for patients assigned to the Avenir group and 96.95% for those in the Axium group. These results suggest that comparable rates of recurrence may also be expected over the long term.
Coil occlusion endovascular procedures carry the risk of both device-related and device-unrelated surgical complications. The former group includes hematoma at the puncture site, intracranial vessel perforation, aneurysm rupture, parent artery occlusion, embolic vessel occlusion, vasospasm, migration or dislocation of coils, ischemic stroke, early coil detachment or detachment failure, and neurological deficits resulting in disability or death. Procedure-related (but not device-related) complications were reported for 27 patients assigned to the Avenir group and 22 in the Axium group. These complications were primarily ischemic strokes that were diagnosed based on the symptoms and diffusion-weighted imaging (DWI) studies performed 24 h after embolization therapy. It is important to note that the ischemia that develops in response to vasospasm of a ruptured aneurysm may be difficult to distinguish from ischemia as a complication of intervention. Thus, all incidents of cerebral ischemia are counted as postoperative complications.
The main technological advance used by the Avenir® coil system is a novel detachment mechanism. This new mechanism is a noose-based and passive mechanically detachable coil system that does not require the use of any detachment tools. The forward-directed force of the coil system is based on the softness of the coils and the friction that develops between the pushrod and the microcatheter (11). The push rod of the Avenir® coil system has a tapered design that maintains stability at the proximal end and permits the distal end to turn smoothly. The detachment mechanism influences the microscopic movements of the distal ends of the conveyor rod and microcatheter, thereby reducing the pressure of the coils against the aneurysm wall. The Avenir® coil system is detached through a noose by manual breakage of a proximal push rod. We experienced a 100% success rate for coil detachment using the Avenir® mechanism. This mechanism ensures safety and limits the amount of time needed to manage peri-procedural aneurysm ruptures. If a coil does not undergo successful detachment, the distal end can become trapped or locked, and the coil may stretch, unwind, or even break. Once a coil becomes loose, it is difficult if not impossible to push it back into the aneurysm or to withdraw it completely into the microcatheter. Therefore, controlled coil detachment is critical for a successful endovascular procedure.
This study was designed as a preliminary, non-inferiority trial and therefore has some limitations. Further research will be needed to determine whether the new detachment method used in the Avenir® system improves the delivery of the coils. Longer follow-up will be needed to determine the risk of recurrence at 1 year and beyond.
Our study revealed no apparent differences in short-term occlusion rates and safety in a comparison of endovascular procedures performed using the Avenir® vs. the AxiumTM detachable coil systems.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The study was approved by the Ethics Committees of Beijing Tiantan Hospital of Capital Medical University, Xuanwu Hospital of Capital Medical University, Huashan Hospital of Fudan University, The First Hospital of Jilin University, The First Affiliated Hospital of Dalian Medical University, The First Affiliated Hospital of Harbin Medical University, Shanxi Provincial People's Hospital, Peking University International Hospital, The First Hospital of Shanxi Medical University, and Tangshan Worker's Hospital. The trial passed the Shanghai FDA inspection with no major findings and the trial also passed each sites' quality check according to the hospitals' clinical trial quality check. The clinical trial registration number is ChiCTR2100046506. The patients/participants provided their written informed consent to participate in this study.
WL and MY: conceptualization and design of the study, data collection, statistical analysis, organization and presentation of the data, review, and critique of the manuscript. AC and HH: data curation, evaluation of the rate of aneurysm occlusion, review, and editing of the manuscript. HW: organization and execution of the study, data collection, and critique of the manuscript. XX: analyzing data and critique of the manuscript. YG: data interpretation and critical review of the manuscript. HS: clinical study design and patient recruitment. HJ: patient management, follow-up design, and conceptualization of the study. FW: manuscript editing and critique of the manuscript. YZ: acquisition, analysis and interpretation of data, and technical and administrative support. GG: concept, design, and drafting of the manuscript. HZ and YL: design of the study, writing of the first draft, review, and critique of the manuscript. All authors have read and agreed to the published version of the manuscript.
HH is co-founder and share-holder of phenox GmbH, which is selling Avenir coils in Europe and the US.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2021.817989/full#supplementary-material
1. Brown RD, Broderick JP. Unruptured intracranial aneurysms: epidemiology, natural history, management options, and familial screening. Lancet Neurol. (2014) 13:393–404. doi: 10.1016/S1474-4422(14)70015-8
2. Etminan N, Brown RD, Beseoglu K, Juvela S, Raymond J, Morita A, et al. The unruptured intracranial aneurysm treatment score: a multidisciplinary consensus. Neurology. (2015) 85:881–9. doi: 10.1212/WNL.0000000000001891
3. Findlay JM, Deagle GM. Causes of morbidity and mortality following intracranial aneurysm rupture. Can J Neurol Sci. (1998) 25:209–15. doi: 10.1017/S031716710003403X
4. Molyneux AJ, Kerr RSC, Yu L-M, Clarke M, Sneade M, Yarnold JA, et al. International Subarachnoid Aneurysm Trial (ISAT) Collaborative Group. International subarachnoid aneurysm trial (ISAT) of neurosurgical clipping versus endovascular coiling in 2143 patients with ruptured intracranial aneurysms: a randomised comparison of effects on survival, dependency, seizures, rebleeding, subgroups, and aneurysm occlusion. Lancet. (2005) 366:809–17. doi: 10.1016/S0140-6736(05)67214-5
5. Ogilvy CS, Jordan NJ, Ascanio LC, Enriquez-Marulanda AA, Salem MM, Moore JM, et al. Surgical and endovascular comprehensive treatment outcomes of unruptured intracranial aneurysms: reduction of treatment bias. World Neurosurg. (2019) 126:e878–87. doi: 10.1016/j.wneu.2019.03.005
6. Ruan C, Long H, Sun H, He M, Yang K, Zhang H, et al. Endovascular coiling vs. surgical clipping for unruptured intracranial aneurysm: a meta-analysis. Br J Neurosurg. (2015) 29:485–92. doi: 10.3109/02688697.2015.1023771
7. Spetzler RF, McDougall CG, Zabramski JM, Albuquerque FC, Hills NK, Russin JJ, et al. The Barrow Ruptured Aneurysm Trial: 6-year results. J Neurosurg. (2015) 123:609–17. doi: 10.3171/2014.9.JNS141749
8. Molyneux AJ, Birks J, Clarke A, Sneade M, Kerr RSC. The durability of endovascular coiling versus neurosurgical clipping of ruptured cerebral aneurysms: 18 year follow-up of the UK cohort of the International Subarachnoid Aneurysm Trial (ISAT). Lancet. (2015) 385:691–7. doi: 10.1016/S0140-6736(14)60975-2
9. Lozano CS, Lozano AM, Spears J. The Changing Landscape of Treatment for Intracranial Aneurysm. Can J Neurol Sci. (2019) 46:159–65. doi: 10.1017/cjn.2019.7
10. Darsaut TE, Findlay JM, Magro E, Kotowski M, Roy D, Weill A, et al. Surgical clipping or endovascular coiling for unruptured intracranial aneurysms: a pragmatic randomised trial. J Neurol Neurosurg Psychiatry. (2017) 88:663–8. doi: 10.1136/jnnp-2016-315433
11. Shintai K, Matsubara N, Izumi T, Miyachi S, Yamada H, Marui N, et al. Experimental study of coil delivery wire insertion force in intracranial aneurysm embolization: force discrepancy generated inside the microcatheter through that coil delivery wire passes. Nagoya J Med Sci. (2019) 81:217–25. doi: 10.18999/nagjms.81.2.217
12. Hui FK, Fiorella D, Masaryk TJ, Rasmussen PA, Dion JE, A. history of detachable coils: 1987-2012. J Neurointerv Surg. (2014) 6:134–8. doi: 10.1136/neurintsurg-2013-010670
13. Raymond J, Klink R, Chagnon M, Barnwell SL, Evans AJ, Mocco J, et al. Hydrogel versus bare platinum coils in patients with large or recurrent aneurysms prone to recurrence after endovascular treatment: a randomized controlled trial. AJNR Am J Neuroradiol. (2017) 38:432–41. doi: 10.3174/ajnr.A5101
14. White PM, Lewis SC, Gholkar A, Sellar RJ, Nahser H, Cognard C, et al. HELPS trial collaborators. Hydrogel-coated coils versus bare platinum coils for the endovascular treatment of intracranial aneurysms (HELPS): a randomised controlled trial. Lancet. (2011) 377:1655–62. doi: 10.1016/S0140-6736(11)60408-X
15. Piotin M, Pistocchi S, Bartolini B, Blanc R. Intracranial aneurysm coiling with PGLA-coated coils versus bare platinum coils: long-term anatomic follow-up. Neuroradiology. (2012) 54:345–8. doi: 10.1007/s00234-011-0870-2
16. Konishi Y, Takeuchi M, Fukasaku K. Optimum coil insertion speed of various coils in brain aneurysm embolization in vitro. Interv Neuroradiol. (2016) 22:506–11. doi: 10.1177/1591019916653250
17. Khatri R, Chaudhry SA, Rodriguez GJ, Suri MFK, Cordina SM, Qureshi AI. Frequency and factors associated with unsuccessful lead (first) coil placement in patients undergoing coil embolization of intracranial aneurysms. Neurosurgery. (2013) 72:452–8. doi: 10.1227/NEU.0b013e3182804ad1
18. Hunt WE, Hess RM. Surgical risk as related to time of intervention in the repair of intracranial aneurysms. J Neurosurg. (1968) 28:14–20. doi: 10.3171/jns.1968.28.1.0014
19. Mascitelli JR, Moyle H, Oermann EK, Polykarpou MF, Patel AA, Doshi AH, et al. An update to the Raymond-Roy Occlusion Classification of intracranial aneurysms treated with coil embolization. J Neurointerv Surg. (2015) 7:496–502. doi: 10.1136/neurintsurg-2014-011258
20. Murayama Y, Nien YL, Duckwiler G, Gobin YP, Jahan R, Frazee J, et al. Guglielmi detachable coil embolization of cerebral aneurysms: 11 years' experience. J Neurosurg. (2003) 98:959–66. doi: 10.3171/jns.2003.98.5.0959
21. Kim BM, Kim DJ, Jeon P, Yoon PH, Lee BH, Lee MS, et al. Endovascular embolization of intracranial aneurysms using bare platinum axiumTM detachable coils: immediate and short-term follow-up results from a multicenter registry. Neurointervention. (2012) 7:85–92. doi: 10.5469/neuroint.2012.7.2.85
22. Khan S-NH, Nichols C, Depowell JJ, Abruzzo TA, Ringer AJ. Comparison of coil types in aneurysm recurrence. Clin Neurol Neurosurg. (2012) 114:12–6. doi: 10.1016/j.clineuro.2011.07.017
23. Broeders JA, Ahmed Ali U, Molyneux AJ, Poncyljusz W, Raymond J, White PM, et al. Bioactive versus bare platinum coils for the endovascular treatment of intracranial aneurysms: systematic review and meta-analysis of randomized clinical trials. J Neurointerv Surg. (2016) 8:898–908. doi: 10.1136/neurintsurg-2015-011881
24. Johnston SC, Dowd CF, Higashida RT, Lawton MT, Duckwiler GR, Gress DR. CARAT Investigators. Predictors of rehemorrhage after treatment of ruptured intracranial aneurysms: the Cerebral Aneurysm Rerupture After Treatment (CARAT) study. Stroke. (2008) 39:120–5. doi: 10.1161/STROKEAHA.107.495747
25. Grunwald IQ, Papanagiotou P, Struffert T, Politi M, Krick C, Gül G, et al. Recanalization after endovascular treatment of intracerebral aneurysms. Neuroradiology. (2007) 49:41–7. doi: 10.1007/s00234-006-0153-5
26. Slob MJ, van Rooij WJ, Sluzewski M. Coil thickness and packing of cerebral aneurysms: a comparative study of two types of coils. AJNR Am J Neuroradiol. (2005) 26:901–3.
27. Tian Z, Liu J, Zhang Y, Zhang Y, Zhang X, Zhang H, et al. Risk factors of angiographic recurrence after endovascular coil embolization of intracranial saccular aneurysms: a retrospective study using a multicenter database. Front Neurol. (2020) 11:1026. doi: 10.3389/fneur.2020.01026
28. Fargen KM, Blackburn S, Deshaies EM, Carpenter JS, Jabbour P, Mack WJ, et al. Final results of the multicenter, prospective Axium MicroFX for Endovascular Repair of IntraCranial Aneurysm Study (AMERICA). J Neurointerv Surg. (2015) 7:40–3. doi: 10.1136/neurintsurg-2013-011049
29. Gallas S, Januel AC, Pasco A, Drouineau J, Gabrillargues J, Gaston A, et al. Long-term follow-up of 1036 cerebral aneurysms treated by bare coils: a multicentric cohort treated between 1998 and 2003. AJNR Am J Neuroradiol. (2009) 30:1986–92. doi: 10.3174/ajnr.A1744
Keywords: intracranial aneurysm, endovascular procedures, coil occlusion, safety, comparative effectiveness research
Citation: Li W, Ye M, Cimpoca A, Henkes H, Wang H, Xu X, Gu Y, Shi H, Ji H, Wang F, Zhao Y, Guo G, Zhang H and Li Y (2022) Avenir® vs. AxiumTM Coils for the Treatment of Intracranial Aneurysms: Results of a Multicenter Randomized Controlled Trial With Short-Term Follow-Up. Front. Neurol. 12:817989. doi: 10.3389/fneur.2021.817989
Received: 18 November 2021; Accepted: 30 December 2021;
Published: 26 January 2022.
Edited by:
Christian Senft, University Hospital Jena, GermanyReviewed by:
Stefan Schob, University Hospital in Halle, GermanyCopyright © 2022 Li, Ye, Cimpoca, Henkes, Wang, Xu, Gu, Shi, Ji, Wang, Zhao, Guo, Zhang and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hongqi Zhang, eHd6aGFuZ2hxQDE2My5jb20=; Youxiang Li, bGl5b3V4aWFuZ0BianR0aC5vcmc=
†These authors share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.