
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Neurol. , 14 January 2022
Sec. Stroke
Volume 12 - 2021 | https://doi.org/10.3389/fneur.2021.796046
This article is part of the Research Topic Challenges in Acute Minor Ischemic Stroke View all 14 articles
Introduction: Strategy for the acute management of minor ischemic stroke (IS) with large vessel occlusion (LVO) is under debate, especially the benefits of mechanical thrombectomy. The frequency of minor IS with LVO among overall patients is not well established. This study aimed to assess the proportion of minor IS and to depict characteristics of patients according to the presence of LVO in a comprehensive population-based setting.
Methods: Patients with acute IS were prospectively identified among residents of Dijon, France, using a population-based registry (2013–2017). All arterial imaging exams were reviewed to assess arterial occlusion. Minor stroke was defined as that with a National Institutes of Health Stroke Scale (NIHSS) score of <6. Proportion of patients with LVO was estimated in the minor IS population. The clinical presentation of patients was compared according to the presence of an LVO.
Results: Nine hundred seventy-one patients were registered, including 582 (59.9%) patients with a minor IS. Of these patients, 23 (4.0%) had a LVO. Patients with minor IS and LVO had more severe presentation [median 3 (IQR 2–5) vs. 2 (IQR 1–3), p = 0.001] with decreased consciousness (13.0 vs. 1.6%, p<0.001) and cortical signs (56.5 vs. 30.8%, p = 0.009), especially aphasia (34.8 vs. 15.4%, p = 0.013) and altered item level of consciousness (LOC) questions (26.1 vs. 11.6%, p = 0.037). In multivariable analyses, only NIHSS score (OR = 1.45 per point; 95% CI: 1.11–1.91, p = 0.007) was associated with proximal LVO in patients with minor IS.
Conclusion: Large vessel occlusion (LVO) in minor stroke is non-exceptional, and our findings highlight the need for emergency arterial imaging in any patients suspected of acute stroke, including those with minor symptoms because of the absence of obvious predictors of proximal LVO.
The best strategy for the acute management of minor ischemic stroke (IS) is currently under debate. Indeed, in some cases, patients with minor symptoms at initial presentation of IS, defined as a National Institute of Health Stroke Scale (NIHSS) score of <5, may have a large vessel occlusion (LVO). In such a situation, a strong collateral circulation is usually associated with a relatively preserved cerebral blood perfusion, but there is a subsequent risk of early neurological deterioration when the adaptive process is overtaken if patients are not recanalized (1). Recent guidelines from the European Stroke Organization (ESO) recommended to administer intravenous thrombolysis (IVT) with recombinant tissue-type plasminogen activator (rt-PA) in patients with minor and disabling IS <4.5 h duration (2), in accordance with the results of a meta-analysis of randomized clinical trials showing the effectiveness of rt-PA on the outcome of these patients (3). In addition, the benefits of mechanical thrombectomy (MT) in patients with minor IS and LVO is currently evaluated in dedicated clinical trials (4). However, there is no standardized definition of minor “disabling” stroke, and the evaluation relies on judgement of physicians in clinical practice. Moreover, the frequency of minor IS with LVO among overall patients is not well known.
Therefore, the aim of this study was to assess the proportion of minor IS and to depict characteristics of patients according to the presence of LVO, in a comprehensive population-based setting.
Data were obtained from the Dijon Stroke Registry (5–7), an ongoing prospective population-based study that complies with the criteria for conducting ideal incidence stroke studies (8), and the guidelines for the reporting of incidence and prevalence studies in neuroepidemiology according to Standards of Reporting of Neurological Disorders (9). The methodology of the Dijon Stroke Registry has been described extensively elsewhere (5–7). Briefly, case collection relies on multiple overlapping sources of information to identify hospitalized and not hospitalized cases of stroke among residents of the city of Dijon, France (156,000 inhabitants), including a review of medical records of all patients referred to the Dijon University Hospital where the only stroke unit in the country is located, a review of computerized hospital diagnostic codes using the International Classification of Diseases, Tenth Revision (ICD-10; I61; I62; I63; I64; G45; G46, and G81), a review of medical records from the departments of the private hospitals of the city and its suburbs, a cooperation with local general practitioners and private neurologists to identify stroke patients from home or nursing homes and Dijon residents who had a stroke when outside the city, a review of the medical records of patients identified from a computer-generated list of all requests for imaging to radiology centers in Dijon, and regular reviewing of death certificates to identify fatal strokes that occurred outside the hospital. The final adjudication of cases is systematically made by senior neurologists trained in stroke ascertainment according to the WHO diagnostic criteria (i.e., Rapidly developing clinical signs of focal, at time global, disturbance of cerebral function, lasting >24 h or leading to death with no apparent cause other than that vascular origin) (10).
For this study, analyses were restricted to patients with an acute IS between January 1, 2013 and December 31, 2017 and in whom data about arterial imaging (intracranial computed tomography angiography or magnetic resonance imaging) were available. The etiological classification of patients with IS was derived from the Trial of ORG 10172 in Acute Stroke Treatment (TOAST) classification (11) as follows: large artery atheroma, cardioembolic IS, lacunar IS due to small vessels disease, IS from other identified cause, IS from undetermined cause, and IS from multiple possible causes. The classification was made by a stroke-trained neurologist investigator of the Dijon Stroke Registry based on medical records, including complementary exams performed during the diagnostic workup of IS.
As previously described, vascular risk factors, past medical history, and pre-stroke treatments were collected (7). Pre-stroke cognitive function (no cognitive impairment, mild cognitive impairment, and dementia) and functional status based on the premorbid modified Rankin Scale score were assessed. Pre-existing dependency was defined by a premorbid Rankin Scale score of >2. Stroke severity at onset was quantified using the NIHSS score obtained at the first clinical examination. Minor Stroke was defined as a NIHSS score of <6.
All cervical and intracranial arterial imaging exams were systematically reviewed by stroke-trained investigators to assess the presence and location of arterial occlusion responsible for the acute IS. A proximal LVO was defined as an occlusion site affecting the terminal intracranial internal carotid artery, M1 and M2 segments of the middle cerebral artery (including tandem occlusions), or A1 and A2 segment of the anterior cerebral artery, or the basilar artery. Patients with isolated extracranial internal carotid artery occlusion were not included in this group. In patients with minor IS and proximal occlusion, brain perfusion imaging including CT or MRI were reviewed when performed to assess the presence of a reduced cerebral perfusion in the territory of the occluded artery.
Proportions and mean values of baseline characteristics were compared between groups (patients with minor stroke vs. patients with non-minor stroke; minor stroke patients with vs. without proximal LVO) using the Chi-2 test and the Mann-Withney test. A multivariate logistic regression analysis was performed to evaluate factors associated with minor stroke. In models, we introduced age, sex, and variables with a p < 0.20 in unadjusted models. Another multivariate logistic regression analysis was performed to evaluate factors associated with proximal LVO among patients with minor IS. In models, variables with a p < 0.20 were introduced. Statistical analysis was performed with STATA 13 software (StataCorp LP, College Station, TX)
The Dijon Stroke Registry was approved using the following national ethics boards: The Comité d'Evaluation des Registres (French National Committee of Registers), Santé Publique France (French Institute for Public Health Surveillance), and the Commission Nationale Informatique et Liberté (French data protection authority). In accordance with the French legislation boards, the need for written patient consent was waived.
From January 1, 2013 to December 31, 2017, among the 1,060 recorded IS patients, 989 cases had available arterial imaging. In detail, 836 patients had a CT angiography, 456 had an MRI, and 683 patients had a US Doppler of cervical arteries, among whom 453 had a transcranial Doppler. The NIHSS score was available in 971 patients.
Among these patients, 582 (59.9%) suffered a minor stroke. Compared with non-minor stroke, minor stroke patients were younger (median age 78 vs. 82 years old, p < 0.001), had less frequent hypertension (69.2 vs. 76%, p = 0.02), atrial fibrillation (22.2 vs. 40.8%, p < 0.01), and history of coronary disease (13.0 vs. 18.1%, p = 0.03) (Table 1). In addition, pre-existing mild cognitive impairment (MCI) (10.2 vs. 16.4%) and dementia (9.5 vs. 18.2%) were less frequently observed in patients with minor stroke (p < 0.001) who were also less frequently functionally dependent (21.0 vs. 31.8%, p < 0.001) or institutionalized (7.1 vs. 16.0%, p < 0.001) before their stroke. IS etiology differed between patients with or without minor-stroke, with a greater proportion of cardioembolic IS observed among patients with non-minor stroke (45 vs. 24.6%). In multivariable analyses, past myocardial infarction (OR = 0.62; 95% CI:0.40−0.97, p = 0.035), MCI (OR = 0.58; 95% CI:0.36–0.95, p = 0.029), small vessel disease etiology (OR = 3.69; 95% CI: 1.76–7.74, p = 0.001), undetermined etiology (OR = 2.49; 95% CI: 1.52–4.06, p < 0.001), and IS with multiple causes (OR = 5.50; 95% CI: 1.97–15.34, p = 0.001) were associated with minor stroke.
A total of 174 cases of IS with a proximal LVO were recorded in our study population. Among the 389 patients with non-minor stroke, 149 (38.3%) had a proximal LVO. In contrast, among the 582 patients with minor stroke, 23 (4.0%) had a proximal LVO (Figure 1). In these patients, the M1 or M2 segment of the MCA was occluded in 6 and 14 cases, respectively, whereas in 3 cases, the site of occlusion was the basilar artery. Three patients had an NIHSS score of 0, one patient scored 1, four patients scored 2, five patients scored 3, three patients scored 4, and seven patients scored 5. Among the 20 patients with a proximal occlusion of the MCA, 13 had perfusion imaging with an onset-to-imaging time ranging from 36 at 330 min. In all cases, perfusion imaging showed a hypoperfusion corresponding to the territory of the occluded artery. Ten patients received acute recanalization therapy (7 IV thrombolysis, 1 mechanical thrombectomy, and 2 bridging therapy). Among the 13 patients who did not receive acute recanalization therapy, three had a clinical deterioration with an increase in NIHSS score of 4 points per patient, among whom 2 had beneficiated from a brain perfusion imaging.
In patients with minor IS, those with a proximal LVO more often had atrial fibrillation (43.5 vs. 21.4%, p = 0.012), a higher NIHSS score [median 3 (IQR 2–5) vs. 2 (IQR 1–3), p = 0.001], and a greater proportion of cardioembolic IS mechanism (Table 2). In multivariable analyses, only NIHSS score (OR = 1.45 per point; 95% CI: 1.11–1.91, p = 0.007) was associated with proximal LVO in patients with minor stroke.
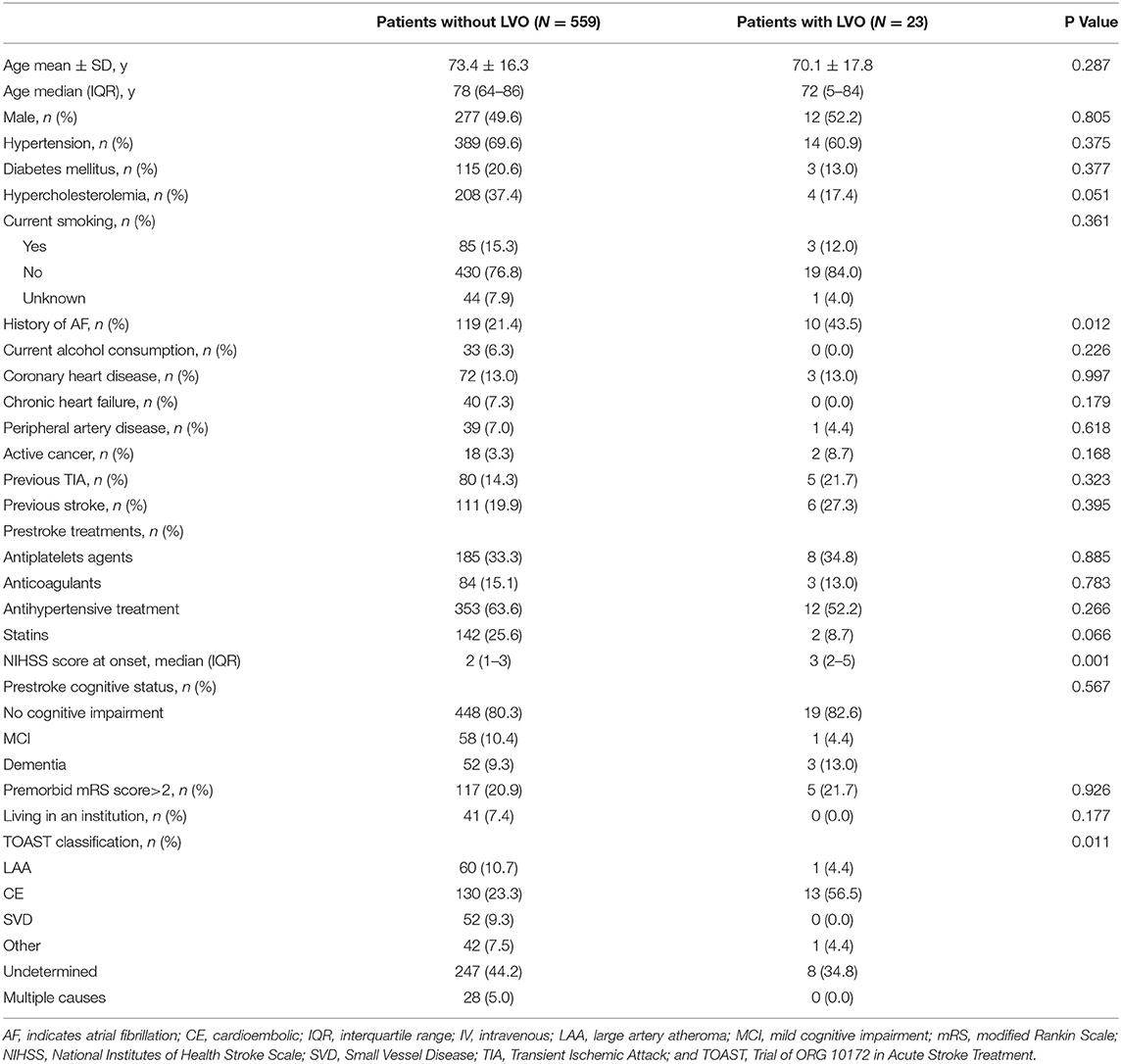
Table 2. Characteristics of minor ischemic stroke patients presenting with (n = 23) or without large vessel occlusion (LVO; n = 557).
By studying the different items of the NIHSS, patients with minor IS and proximal LVO more often had decreased consciousness (13 vs. 1.6%, p < 0.001) and more often had cortical signs (56.5 vs. 30.8%, p = 0.009), especially aphasia (34.8 vs. 15.4%, p = 0.013) and altered item level of consciousness (LOC) Questions (26.1 vs. 11.6%, p = 0.037) (Figure 2).

Figure 2. Proportion of patients with minor ischemic stroke who have impairment in each item of National Institutes of Health Stroke Scale NIHSS score according to the presence of proximal large vessel occlusion (LVO). *p < 0.05.
In a sensitivity analysis, 441 patients (45.4%) had a minor IS, defined as that with an NIHSS score of ≤ 3. Among these patients, 13 (2.9%) had a proximal LVO. Of note, 3 out of 110 patients with a NIHSS score of 0 had a proximal LVO.
This study provided original data about the prevalence of proximal LVO in patients presenting with minor IS in a large population-based setting. We observed that ~4% of patients with a mild clinical presentation had an LVO, thus representing those potentially eligible for mechanical thrombectomy. Although LVO in patients with minor IS was more frequently noticed in patients with atrial fibrillation and/or a cardioembolic etiology, only a greater NIHSS score was independently associated with LVO, which was explained by more frequent decreased consciousness, aphasia, or altered item LOCquestions.
Different definitions are used to define the minor stroke in the literature. Fischer et al. suggested several definitions of minor stroke and concluded that a maximum score of 1 on every baseline NIHSS score, except level consciousness items, or a total NIHSS score of ≤3 could be the best definition (12). In the Oxford Vascular Study (OXVASC), a NIHSS score of <3 was used to define minor stroke (13). Regarding recent randomized clinical trials focusing on dual antiplatelet therapy in secondary prevention of IS patients, both Platelet-oriented Inhibition in New TIA and Minor Ischemic Stroke (POINT) (14) and Clopidogrel in High-risk Patients with Acute Non-disabling Cerebrovascular Events (CHANCE) (15) trials considered minor stroke if the NIHSS score was ≤ 3. Conversely, in Acute Stroke Or Transient IsChaemic Attack TReated With Aspirin or Ticagrelor and Patient OutcomES (SOCRATES) (16) and The Acute Stroke or Transient Ischaemic Attack treated with Tricagelor and Tricagelor and Acetylsalicylic Acid for Prevention of Stroke and Death (THALES) (17) trials, minor stroke was defined as that with a NIHSS score of ≤ 5. Some authors suggested a definition of mild severity as a NIHSS score <5 (18, 19). Recent guidelines from the ESO on the use of dual antiplatelets therapy in minor IS used a NIHSS score ≤ 3 as a threshold (20, 21). However, for the management of IS with endovascular therapy, current recommendations used a NIHSS score of <6 for defining IS with mild symptoms (22, 23). Whether these patients should benefit from mechanical thrombectomy is a challenging issue. Therefore, we used this definition to assess the true prevalence of LVO in minor IS.
Minor IS, defined as a NIHSS score of <6, accounted for ~60% of overall IS patients in our population. So as to compare with the Oxford Vascular (OXVASC) study, we found a similar proportion of minor IS when considering a definition with a NIHSS score <3 (45% in our study vs. 47% in the Oxford Vascular Study). This high rate of minor IS reflects the fact that, in both studies, we used a population-based setting rather than a hospital-based recruitment that would have led to higher clinical severity of included patients (24).
Our study provides new information on the prevalence of LVO in patients with minor IS. Although this prevalence was relatively low (4%), we did not find any factor associated with the presence of LVO in these patients, except the NIHSS score. However, the difference was very small, and, therefore, it is not useful for the discrimination between patients with vs. without occlusion. Consequently, our findings suggest that in a patient presenting with a clinical picture of minor stroke, it is impossible to easily predict the existence of a proximal LVO. This is important in the current context of discussion about the best therapeutic strategy in this patient, i.e., whether to administer IV thrombolysis and/or mechanical thrombectomy, and it should be considered that until proven otherwise, these patients may have a LVO and may therefore benefit from urgent brain and arterial imaging even if the neurological symptoms are mild. In addition, we noticed that all patients with minor IS and a proximal LVO of the anterior circulation had a hypoperfusion in the corresponding arterial territory when perfusion imaging was performed. A majority of these patients received IV thrombolysis despite the fact that the guidelines regarding the indication of this therapy were not established at the time this study was conducted. Of note, 2 out of 3 patients with proximal occlusion and a hypoperfusion and who did not receive IV thrombolysis had an early neurological deterioration. This suggests that brain perfusion imaging could be useful for the selection of minor IS patients eligible to acute revascularization therapy.
The major strength of our study is the use of a population-based registry and a relatively large sample size of patients. The reliability of the classification of patients as having or not having an LVO was ensured by a systematic review of all arterial imaging exams by stroke-trained investigators. However, our study was limited by a small number of cases with LVO, thus limiting the study power and additional subgroup analyses.
To conclude, LVO in minor stroke is non-exceptional, and our findings highlight the need for emergency arterial imaging in any patients suspected of acute stroke, including those with minor symptoms, because of the absence of obvious predictors of proximal LVO.
The datasets presented in this article are not readily available because of restrictions due to national legislation. Requests to access the datasets should be directed to eWFubmljay5iZWpvdEBjaHUtZGlqb24uZnI=.
GD: study concept and design, acquisition, analysis and interpretation of data, reviewing arterial imaging, and drafting and revising the manuscript for content. VC, PJ, MG, and CV: acquisition of data and critical revision of manuscript for intellectual content. YB: study concept and design, acquisition, analysis and interpretation of data, study supervision, obtaining funding, and drafting and revising the manuscript for content. All authors contributed to the article and approved the submitted version.
The Dijon Stroke Registry was supported by Santé Publique France, Institut national de la santé et de la recherche médicale (INSERM), and Dijon University Hospital.
YB reports personal fees from BMS, Pfizer, Medtronic, Amgen, Servier, NovoNordisk, and Boehringer-Ingelheim, outside the submitted work.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Seners P, Ben Hassen W, Lapergue B, Arquizan C, Heldner MR, Henon H, et al. Prediction of early neurological deterioration in individuals with minor stroke and large vessel occlusion intended for intravenous thrombolysis alone. JAMA Neurol. (2021) 78:321–8. doi: 10.1001/jamaneurol.2020.4557
2. Berge E, Whiteley W, Audebert H, De Marchis G, Fonseca AC, Padiglioni C, et al. European Stroke Organisation (ESO) guidelines on intravenous thrombolysis for acute ischaemic stroke. Euro Stroke J. (2021) 6:I–LXII. doi: 10.1177/2396987321989865
3. Emberson J, Lees KR, Lyden P, Blackwell L, Albers G, Bluhmki E, et al. Effect of treatment delay, age, and stroke severity on the effects of intravenous thrombolysis with alteplase for acute ischaemic stroke: a meta-analysis of individual patient data from randomised trials. Lancet. (2014) 384:1929–35. doi: 10.1016/S0140-6736(14)60584-5
4. University Hospital Montpellier. Evaluation of Acute Mechanical Revascularization in Large Vessel Occlusion Stroke With Minor Symptoms (NIHSS<6) in Patients Last Seen Well<24 Hours. clinicaltrials.gov. (2020). Available online at: https://clinicaltrials.gov/ct2/show/NCT03796468 (accessed October 14, 2021).
5. Béjot Y, Bailly H, Graber M, Garnier L, Laville A, Dubourget L, et al. Impact of the Ageing Population on the Burden of Stroke: The Dijon Stroke Registry. Neuroepidemiology. (2019) 52:78–85. doi: 10.1159/000492820
6. Giroud M, Delpont B, Daubail B, Blanc C, Durier J, Giroud M, et al. Temporal trends in sex differences with regard to stroke incidence: the dijon stroke registry (1987–2012). Stroke. (2017) 48:846–9. doi: 10.1161/STROKEAHA.116.015913
7. Graber M, Garnier L, Mohr S, Delpont B, Blanc-Labarre C, Vergely C, et al. Influence of Pre-Existing mild cognitive impairment and dementia on post-stroke mortality. the dijon stroke registry. Neuroepidemiology. (2019) 19:1–8. doi: 10.1159/000497614
8. Feigin V, Norrving B, Sudlow CLM, Sacco RL. Updated criteria for population-based stroke and transient ischemic attack incidence studies for the 21st Century. Stroke. (2018) 49:2248–55. doi: 10.1161/STROKEAHA.118.022161
9. Bennett DA, Brayne C, Feigin VL, Barker-Collo S, Brainin M, Davis D, et al. Development of the standards of reporting of neurological disorders (STROND) checklist: a guideline for the reporting of incidence and prevalence studies in neuroepidemiology. Eur J Epidemiol. (2015) 30:569–76. doi: 10.1007/s10654-015-0034-5
10. The World Health Organization MONICA. Project (monitoring trends and determinants in cardiovascular disease): a major international collaboration. WHO MONICA project principal investigators. J Clin Epidemiol. (1988) 41:105–14. doi: 10.1016/0895-4356(88)90084-4
11. Adams HP, Bendixen BH, Kappelle LJ, Biller J, Love BB, Gordon DL, et al. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke. (1993) 24:35–41. doi: 10.1161/01.str.24.1.35
12. Fischer U, Baumgartner A, Arnold M, Nedeltchev K, Gralla J, De Marchis GM, et al. What is a minor stroke. (2010) Stroke. 41:661–6. doi: 10.1161/STROKEAHA.109.572883
13. Pendlebury ST, Rothwell PM. Incidence and prevalence of dementia associated with transient ischaemic attack and stroke: analysis of the population-based Oxford Vascular Study. Lancet Neurol. (2019) 18:248–58. doi: 10.1016/S1474-4422(18)30442-3
14. Johnston SC, Easton JD, Farrant M, Barsan W, Battenhouse H, Conwit R, et al. Platelet-oriented inhibition in new TIA and minor ischemic stroke (POINT) trial: rationale and design. Int J Stroke. (2013) 8:479–83. doi: 10.1111/ijs.12129
15. Wang Y, Wang Y, Zhao X, Liu L, Wang D, Wang C, et al. Clopidogrel with aspirin in acute minor stroke or transient ischemic attack. n Engl J Med. (2013) 369:11–9. doi: 10.1056/NEJMoa1215340
16. Johnston SC, Amarenco P, Albers GW, Denison H, Easton JD, Evans SR, et al. Ticagrelor vs. aspirin in acute stroke or transient ischemic attack. New Engl J Med. (2016) 375:35–43. doi: 10.1056/NEJMoa1603060
17. Johnston SC, Amarenco P, Denison H, Evans SR, Himmelmann A, James S, et al. Ticagrelor and aspirin or aspirin alone in acute ischemic stroke or TIA. New Engl J Med. (2020) 383:207–17. doi: 10.1056/NEJMoa1916870
18. Dhamoon MS, Moon YP, Paik MC, Boden-Albala B, Rundek T, Sacco RL, et al. Long-term functional recovery after first ischemic stroke: the Northern Manhattan study. Stroke. (2009) 40:2805–11. doi: 10.1161/STROKEAHA.109.549576
19. Yakhkind A, McTaggart RA, Jayaraman MV, Siket MS, Silver B, Yaghi S. Minor stroke and transient ischemic attack: research and practice. Front Neurol. (2016) 7:86. doi: 10.3389/fneur.2016.00086
20. Kleindorfer DO, Towfighi A, Chaturvedi S, Cockroft KM, Gutierrez J, Lombardi-Hill D, et al. 2021 Guideline for the prevention of stroke in patients with stroke and transient ischemic attack: a guideline from the American heart association/American stroke association. Stroke. (2021) 52:e364–467. doi: 10.1161/STR.0000000000000375
21. Fonseca AC, Merwick Á, Dennis M, Ferrari J, Ferro JM, Kelly P, et al. European Stroke Organisation (ESO) guidelines on management of transient ischaemic attack. Euro Stroke J. (2021) 239698:7321992905. doi: 10.1177/2396987321992905
22. Turc G, Bhogal P, Fischer U, Khatri P, Lobotesis K, Mazighi M, et al. European Stroke Organisation (ESO) - European society for minimally invasive neurological therapy (ESMINT) guidelines on mechanical thrombectomy in acute ischemic stroke. J NeuroIntervent Surg. (2019) 19:14569 doi: 10.1136/neurintsurg-2018-014569
23. Powers WJ, Rabinstein AA, Ackerson T, Adeoye OM, Bambakidis NC, Becker K, et al. Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 guidelines for the early management of acute ischemic stroke: a guideline for healthcare professionals from the American heart Association/American stroke association. Stroke. (2019) 50:e344–418. doi: 10.1161/STR.0000000000000211
Keywords: stroke, ischemic stroke, registry, epidemiology, minor stroke, large vessel occlusion, population-based studies
Citation: Duloquin G, Crespy V, Jakubina P, Giroud M, Vergely C and Béjot Y (2022) Large Vessel Occlusion in Patients With Minor Ischemic Stroke in a Population-Based Study. The Dijon Stroke Registry. Front. Neurol. 12:796046. doi: 10.3389/fneur.2021.796046
Received: 15 October 2021; Accepted: 16 December 2021;
Published: 14 January 2022.
Edited by:
Linxin Li, University of Oxford, United KingdomCopyright © 2022 Duloquin, Crespy, Jakubina, Giroud, Vergely and Béjot. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yannick Béjot, eWFubmljay5iZWpvdEBjaHUtZGlqb24uZnI=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.