- 1Dongzhimen Hospital, Beijing University of Chinese Medicine, Beijing, China
- 2Department of Neurology, Dongzhimen Hospital, Beijing University of Chinese Medicine, Beijing, China
Background: Clinical presentations and treatment programs about anti-leucine-rich glioma inactivated 1 (LGI1) encephalitis still remain incompletely understood.
Objective: This study analyzed the clinical features and therapeutic effects of anti-LGI1 encephalitis.
Methods: PubMed, EMBASE, and the Cochrane Library were searched to identify published English and Chinese articles until April 2021. Data were extracted, analyzed, and recorded in accordance with the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) guidelines.
Results: A total of 80 publications detailing 485 subjects matched our inclusion criteria. Short-term memory loss (75.22%), faciobrachial dystonic seizures (FBDS) (52.53%), other seizures excluding FBDS (68.48%), psychiatric symptoms (57.67%), and sleep disturbances (34.30%) were the most frequently described symptoms in anti-LGI1 encephalitis. Hyponatremia (54.90%) was the most common hematologic examination change. The risk of incidence rate of malignant tumors was higher than in healthy people. The positive rate of anti-LGI1 in serum (99.79%) was higher than CSF (77.38%). Steroids (93.02%), IVIG (87.50%), and combined use (96.67%) all had a high remission rate in the initial visit. A total of 35 of 215 cases relapsed, of which 6/35 (17.14%) did not use first-line treatment, and 21 (60.00%) did not maintain long-term treatment. Plasma exchange (PE) could be combined in severe patients, immunosuppressant could be used for refractory patients or for recurrence and using an anti-epileptic drug to control seizures may benefit cognition.
Conclusions: Short-term memory loss, FBDS, psychiatric symptoms, and hyponatremia were key features in identifying anti-LGI1 encephalitis. Serum and CSF antibody tests should be considered in diagnosis criteria. Steroids with IVIG should be recommended, PE was combined for use in severe patients, immunosuppressant therapy might improve outcomes if recurrence or progression occurred, and control seizures might benefit cognition. The useful ways to reduce relapse rate were early identification, clear diagnosis, rapid treatment, and maintaining long-term treatment. The follow-up advice was suggested according to the research of paraneoplastic syndrome, and concern about tumors was vital as well.
Introduction
Anti-leucine-rich glioma inactivated 1 (LGI1) encephalitis is an autoimmune encephalitis (AE), whose clinical presentations are memory disturbances, faciobrachial dystonic seizures (FBDS), confusion or psychiatric disorders, and hyponatremia (1, 2). Anti-LGI1 encephalitis can be diagnosed through clinical features, magnetic resonance imaging (MRI), serum or cerebrospinal fluid (CSF) tests, and electroencephalogram (EEG) (3). The gold standard for diagnosis is a positive LGI1 antibody in serum or CSF. Most articles about clinical presentations and treatment programs of anti-LGI1 encephalitis are case reports or case series, thus, overall understanding and an especially comprehensive treatment program of the disease are needed. As a result, the main objective of this study is to analyze clinical features and therapeutic effects of anti-LGI1 encephalitis by reviewing relevant literature systematically.
Methods
This systematic review was conducted according to the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) guidelines (4).
Criteria for Considering Studies for Review
Studies were included with the following designs: case reports/series, case–control studies, cross-sectional studies, cohort studies, or clinical trials, if available. Studies reporting clinical features and/or treatment programs, involving patients diagnosed with confirmed anti-LGI1 encephalitis according to clinical criteria and presence of antibodies in serum and/or CSF were included. There was no restriction on age, sex, ethnicity of patients, or year of publication in this review. Other types of articles such as short communications, animal studies, unavailable full-text articles, and articles not published in Chinese or English were excluded.
Search Strategy
We searched PubMed, EMBASE, and the Cochrane Library for literature published in Chinese or English up until April 2021. General and MeSH search terms were “LGI1 protein, human (Supplementary Concept) AND encephalitis (MeSH).” Up to date articles were traced for supplementary searching.
We assessed the titles and abstracts of identified records based on the screening criteria above. Studies meeting the inclusion criteria were retrieved as full-text articles and subjected to predefined eligibility criteria.
Data Extraction and Analysis
Data were independently extracted by two authors. Demographic figures of characteristics, clinical presentation, neuroimaging, serum and CSF analysis findings, descriptive findings in the EEG, treatment programs, therapeutic effects, and other clinical information of subjects in each study were extracted. Categorical variables were summarized by counts and percentages, while continuous variables were pooled by median and range.
Results
Included Studies
We identified 185 articles from the initial search. After removal of 11 duplications, 87 out of 174 articles met the inclusion criteria. A total of 80 articles were eligible for the review, consisting of 65 case reports and 15 case series (Figure 1).
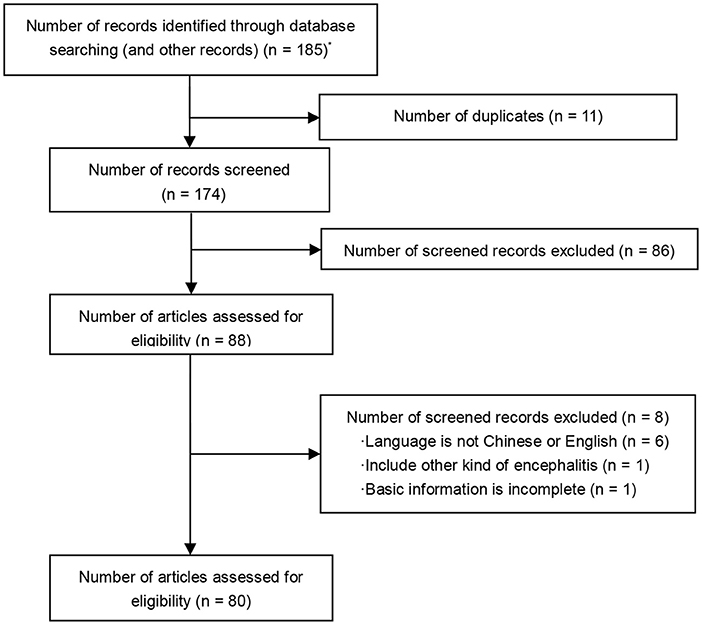
Figure 1. PRISMA flow diagram. *A total of 185 articles were identified through database searching: PubMed (n = 155), The Cochrane Library (n = 7), EMBASE (n = 22), supplementary reference (n = 1).
Population Characteristics
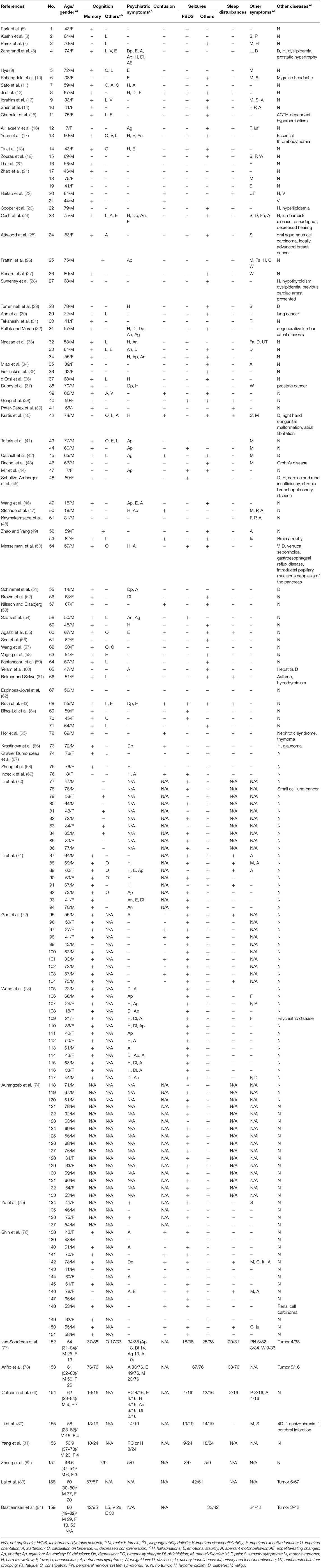
A total of 485 cases with confirmed positive LGI1 antibody in serum and/or CSF were included. The demographic and clinical information of the included cases are summarized in Table 1.
There were 281/431 (65.20%) men, 150/431 (34.80%) women, and 54 patients with unknown gender. Age ranged from 7 to 92 years (mean age 59.61 years), including four pediatric patients (44, 51, 69, 78). Fifty-three participants were not included due to unclear demographic details.
Clinical Features
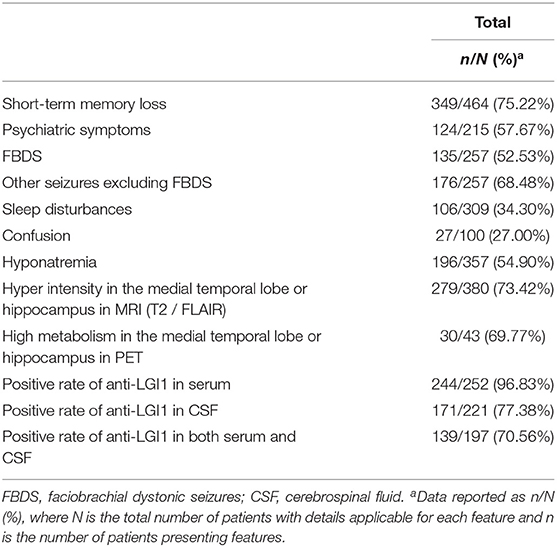
The main clinical features in anti-LGI1 encephalitis are summarized in Table 2. A total of 412 out of 485 cases showed cognitive impairments. Apart from 21 patients, 464 reported certain categories, in which 349 (75.22%) had short-term memory loss, 30 (17.96%) had impaired orientation, 27 (16.17%) had language ability deficits, 37 (22.16%) had impaired executive function, 5 (2.99%) had inattention, 32 (19.16%) had impaired visuospatial ability, 2 (1.20%) had calculation disturbance, and 1 (0.60%) had decreased comprehension.
There were 307 patients who reported the occurrence of psychiatric symptoms. Overall, 124/215 (57.67%) were abnormal [two articles (78, 79) which lacked the total number of patients with psychiatric symptoms were excluded]. Apart from 21/30 cases, 277 reported the classification of psychiatric symptoms. A total of 77 (27.80%) had emotionalist ability deficits, 62 (22.38%) had aberrant motor behaviors, 35 (12.64%) had apathy, 29 (10.47%) had hallucinations, 23 (8.30%) had mental disorders, 17 (6.14%) had agitation, 16 (5.78%) had delusions, 14 (5.05%) had disinhibition, 12 (4.33%) had anxiety, 8 (2.89%) had depression, 8 (2.89%) had personality changes or hallucinations, 4 (1.44%) had personality changes, and 1 (0.36%) had appetite/eating changes.
Seizures were also reported. In total, 27/100 (27.00%) had confusion, 135/257 (52.53%) had FBDS, and 176/257 (68.48%) had other seizures. Other symptoms, such as sleep disturbances (106/309, 34.30%), autonomic symptoms (16/169, 9.47%), motor symptoms (15/169, 8.88%), weight loss (13/164, 7.93%), fever (8/169, 4.73%), peripheral nervous system symptoms (5/163, 3.07%), dizziness (5/169, 2.96%) were reported as well.
Combined Diseases
For comorbidities, 24/430 (5.58%) reported tumor incidence, 3/430 (0.70%) reported vitiligo, and there were other comorbidities reported as well, such as diabetes, hypothyroidism, dyslipidemia, hypothyroidism, etc.
Laboratory Examination
Data of neuroimaging, assay systems for the LGI1 antibody test, EEG, and treatments are summarized in Supplementary Table 1. The therapeutic effects and other clinical information are summarized in Supplementary Table 2.
For laboratory examination, 196/357 (54.90%) reported hyponatremia. For antibody detection, 241/249 (96.78%) reported anti-LGI1 in serum, while 171/221 (77.38%) reported anti-LGI1 in CSF. There were also other antibodies reported, such as VGKC (19/76, 25.00%), NMDAR (2/76, 2.63%), CASPR2 (1/76, 1.32%), and AMPAR (1/76, 1.32%).
Auxiliary Examinations
For neuroimaging, 279/380 (73.42%) reported hyper intensity in the medial temporal lobe or hippocampus in MRI (T2/FLAIR), while 30/43 (69.77%) reported high metabolism in the medial temporal lobe or hippocampus in PET. Of 288 cases with EEG outcomes, 101 (35.07%) reported epileptiform discharge, comparatively, 100 (34.72%) reported abnormalities but no epileptiform discharge in EEGs, and the other 87 (30.21%) reported no abnormal EEGs. After comparing the syndrome of seizures and the results of EEG in 126 cases, 26 (20.63%) FBDS and 31 (24.60%) other seizures showed epileptiform discharge in EEG, 30 (23.81%) FBDS and 35 (27.78%) other seizures showed abnormalities but no epileptiform discharge in EEG, and 24 (19.05%) FBDS and 22 (17.46%) other seizures showed no abnormal EEGs.
Treatments and Outcomes
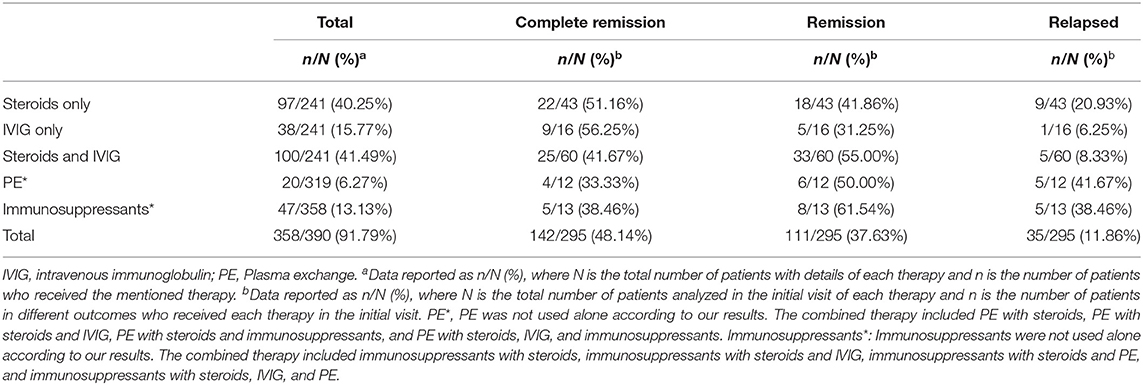
Treatments and outcomes in anti-LGI1 encephalitis are summarized in Table 3. Among the 390 cases, 358 documented the processes of treatment. As a result, 285/358 (79.61%) received steroids, and 106/285 (37.19%) received steroid pulse therapy. Aside from 38 cases which reported on the combination of intravenous immunoglobulin (IVIG) and plasma exchange (PE), 166/320 (51.88%) received IVIG, and 12 cases received this treatment more than once. For other treatments, 20/320 (6.25%) received PE, and 47/358 (13.13%) received immunosuppressants including rituximab (17/47), azathioprine (15/47), mycophenolate mofetil (8/47), cyclophosphamide (6/47), tacrolimus (2/47), and cyclosporine (1/47). For anti-epileptic treatment, 122 of 390 cases recorded the use of anti-epileptic drugs, and 86/122 (55.74%) received drug therapy, in which 26 cases reported the reactions but only 5/26 (19.23%) reported that it helped. Combined therapy from cases with details of treatment is summarized in Supplementary Table 3.
Overall, 295 of 390 cases reported outcomes of treatments. A total of 137/295 (46.44%) achieved complete remission, 109/295 (36.95%) achieved remission, 46/295 (15.59%) relapsed, 14/295 (4.75%) did not reach remission, 1/295 (0.34%) rejected further treatment, and 15/295 (5.08%) died.
For the initial visit, 241 of 390 cases kept detailed records of combined therapy, and 100/241 (41.49%) received both steroids and IVIG. Among the cases reported on outcomes, 25/60 (41.67%) who received a combination of steroids and IVIG achieved complete remission, 33/60 (55.00%) achieved remission, 2/60 (3.33%) did not achieve remission, and 5/60 (8.33%) relapsed. Comparatively, in 97/241 (40.25%) cases receiving steroids only, 22/43 (51.16%) achieved complete remission, 18/43 (41.86%) achieved remission, 3/43 (6.98%) did not achieve remission, and 9/43 (20.93%) relapsed among the recorded cases. In total, 38/241 (15.77%) received IVIG only, and it turned out that 9/16 (56.25%) achieved complete remission, 5/16 (31.25%) achieved remission, 1/16 (6.25%) did not achieve remission, 1/16 (6.25%) rejected further treatment, and 1/16 (6.25%) relapsed. Overall, 17/269 (6.32%) used PE, and 4/12 (33.33%) achieved complete remission, 6/12 (50.00%) achieved remission, 2/12 (16.67%) did not achieve remission, and 5/12 (41.67%) relapsed.
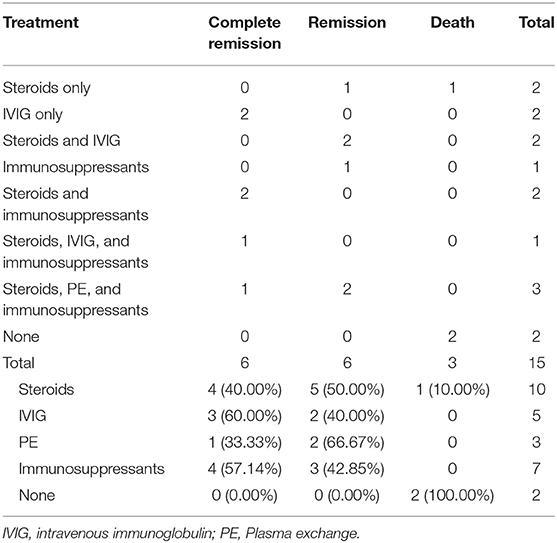
For the visit after recurrence, 35 of 215 cases relapsed, of which 6/35 (17.14%) did not use first-line treatment, and 21 (60.00%) did not maintain long-term treatment. A total of 15 of 35 cases kept detailed records of therapy, of which 10/15 (66.67%) used steroids, 5/15 (33.33%) used IVIG, 3/15 (20.00%) used PE, 7/15 (46.67%) used immunosuppressants, and 2/15 (13.33%) were not treated (Table 4). All the above 12 cases achieved remission or complete remission in the end, but 3 patients died, possibly attributed to leukemia, myocardial infarction, and unknown causes.
Discussion
This review described clinical features and therapeutic effects of anti-LGI1 encephalitis comprehensively. According to our results, the most common symptom of anti-LGI1 encephalitis was short-term memory loss, which is a common characteristic in other AE (84). A quarter of patients with anti-LGI1 encephalitis suffered from cognitive decline in orientation, while fewer patients had impairment in visuospatial skills and executive function. Contrary to our results, Bastiaansen et al. (84) discovered that patients with anti-LGI1 encephalitis showed similarities in frequency and severity of visuospatial and executive function impairment as those with anti-GABABR encephalitis (~70% in anti-LGI1 encephalitis and 55% in anti-GABABR encephalitis). We drew the controversial conclusion that this was possibly because some cases we included did not contain complete information on cognitive disorders, which could serve as a reminder for clinicians to pay more attention to cognitive impairments in patients with anti-LGI1 encephalitis.
The frequency occurrence of other seizure types was higher than FBDS, likely due to the fact that too many case reports were included in our study. According to previous research (74), FBDS was considered as pathognomonic for anti-LGI1 encephalitis, in which EEG typically showed prominent muscle artifacts (lasting 0.5–1.6 s). Meanwhile, FBDS was also reported to be the most common seizure type in anti-LGI1 encephalitis, as well as a distinction among anti-LGI1 encephalitis and other AE (84).
As AE can affect any brain network involving initiating and regulating sleep, all types of sleep disorders can occur, with distinct association, frequency, and intensity (85). Compared to other research (84), the rate of sleep disorders in anti-LGI1 encephalitis was lower based on our results, thus it reminded us to pay more attention to patients' sleep problems especially for clinicians.
A multiple-center study (86) demonstrated that in 379 patients, anti-NMDAR-AE patients had the highest incidence of tumors, accounting for 8.79% from analysis. As a kind of AE, anti-LGI1 encephalitis might be associated with paraneoplastic syndrome (PNS). According to previous case series, PNS has a 0–31% chance of revealing tumors (77–83, 87), among which thymoma and lung cancer were considered the most common ones (1). Nonetheless, 5.58% of our included cases showed carcinogenesis, including oral squamous cell carcinoma and locally advanced lung cancer (30, 70), breast cancer (25), prostate cancer (37), thymoma (65), renal cell carcinoma (76), etc., which are inconsistent with the former results. It is likely the tumor types mentioned above were not included, so further investigations are needed to gather more complete information. As the lack of a specific suggestion of tumor screening for AE, the tumor screening routine of PNS should provide a valuable reference (88), suggesting a repeated second screening after 3–6 months, followed by regular screening every 6 months for 4 years if the initial screening is negative in patients with PNS. For immune disorder in anti-LGI1 encephalitis and PNS, the incidence rate of malignant tumors seems to be significantly higher in anti-LGI1 encephalitis patients (89). According to the follow-up regulation in PNS, subsequent specialty consultations are suggested in anti-LGI1 encephalitis regardless of negative tumor markers or imaging examinations.
In our study, hyponatremia was regarded as the most common electrolyte disturbance. Muhr et al. (90) concluded that the underlying mechanisms leading to hyponatremia might be inadequate ADH secretion. Additionally, severe hyponatremia could be regarded as a precursor of anti-LGI1 encephalitis.
In our study, the positive rate of LGI1 antibodies in CSF was 77.38%, similar to a cohort study (with a positive rate of 78%). The positive rate in serum was 96.83%, suggesting a higher sensitivity in diagnosing anti-LGI1 encephalitis. Despite the relatively lower positive rate of LGI1 antibodies in CSF, there were still advantages in distinguishing different forms of encephalitis from CSF antibody tests.
As for neuroimaging, our results showed that MRI (T2/FLAIR) and PET both had a relatively high positive rate in diagnosis. Additionally, according to a meta-analysis (91), the detection sensitivity of PET in anti-LGI1 encephalitis was 87% (79–92%), I2 of 0% (p = 0.89), suggesting that PET, as a new medical technology, was of high value in diagnosis of anti-LGI1 encephalitis. As for EEG, epileptiform discharge and abnormal EEG with no epileptiform discharge were two key features, in line with another study (92).
Interestingly, three patients from two articles (22, 50) reported vitiligo, as the authors hypothesized that vitiligo might work as an inducer for anti-LGI1 encephalitis.
As anti-LGI1 encephalitis was mostly reported in adults (44), we did a comparison of symptoms for three anti-LGI1 encephalitis pediatric patients, and found all patients had psychiatric symptoms and different types of seizures, but none had cognitive disturbance (16, 44, 69), highlighting the necessity to be suspicious of AE when new onset seizures and psychiatric symptoms occur in children.
First-line immunotherapy of AE included corticosteroids, IVIG, and PE (93). According to our research, steroids (93.02%, 40/43), IVIG (87.50%, 14/16), and combined use (96.67%, 58/60) all had a high remission rate. However, in some previous studies, the remission rate of using steroids alone was 100% (1/1, 4/4) (70, 71), using IVIG alone was 87.5% (7/8) (72), and combined using was 100% (8/8, 4/4) (70, 71). These differences may be due to the increase in the representativeness of the population after the expansion of the sample size. Among 189 cases with follow-up over 6 months, only 1 case (43) reported adverse events after using steroids. Accepting steroid intravenous impulse therapy was considered relatively safe. In a recent retrospective study (94), the combined treatment with PE and IVIG was found to be more effective than IVIG alone. Steroids combined with IVIG was reported to have good responses and few adverse events. So, more research on the efficacy of other combined therapies in relapsed patients or those with bad responses are needed in the future.
Since recovery and symptom remission were accompanied by a decline of antibody titers in other AE (95), it was hypothesized that aiming to get a decrease of LGI1 antibody titers might be a primary therapy approach. PE and immunoadsorption (IA) both provide an opportunity for the extracorporeal elimination of circulating antibodies (96). Zhang et al. (97) demonstrated that therapeutic PE might be an effective rescue therapy for rapid functional improvement in patients with severe steroid/IVIG refractory antibody-associated AE, including anti-LGI1 encephalitis, and with no fatal adverse events. Another pilot study (96) with 21 AE cases including 4 anti-LGI1 encephalitis cases illustrated that both IA and PE resulted in a moderate to marked clinical improvement, also with a relatively low adverse event risk. As a result, on account of its high cost and invasive damage, PE might be a suitable therapy for emergent treatment in critically ill patients to achieve more rapid remission. Due to the limited numbers of anti-LGI1 encephalitis patients included, more research is needed to further test the safety and long-term efficacy of PE. Additionally, since PE can only eliminate antibodies that already exist rather than intervening in their production, how to combine PE with another therapy to prevent recurrence and achieve complete remission should also be taken into consideration.
Second-line immunotherapy of AE included rituximab and cyclophosphamide (93), and was suggested to be immediately started in those who failed to respond or deteriorated during first-line immunotherapy (98). Nepal et al. (99) found rituximab was effective for treatment of AE with an acceptable toxicity profile, while Lee et al. (100) found that high doses of rituximab showed benefits in refractory AE patients. The international consensus (101) recommended rituximab for cases refractory to the first-line agent in both anti-NMDAR AE children and adults, while cyclophosphamide was suggested 1–3 months after second-line initiation. As the cases included in these three studies above were mostly anti-NMDAR AE, the results did not exactly match our research. We found that, after adding immunosuppressants, 100% of relapsed patients reached remission (42.85%) or complete remission (57.14%), among which rituximab alone had efficacy against anti-LGI1 encephalitis. Despite the relatively high rate of remission, adding rituximab also led to the occurrence of adverse incidence such as infusion-related reactions (IRRs) (15.7%), pneumonia (6.0%) and severe sepsis (1.1%), which we cannot afford to neglect.
A systematic review including 87 anti-NMDAR AE children showed that only 7% of patients relapsed on mycophenolate mofetil, azathioprine, or methotrexate (102), though there was little evidence supporting their importance in refractory or relapsed anti-LGI1 encephalitis. Our research found that 7 out of 15 cases had used these 4 agents after relapse, and all of them achieved complete remission or remission afterwards.
Cognition might be related to FBDS. Thompson et al. (103) found that FBDS showed significant time-sensitive responses to immunotherapy, and the development of cognitive impairment could be prevented with their surcease. Overall, 10% showed cessation of FBDS with anti-epileptic drugs alone, while 51% showed cessation of FBDS 30 days after addition of first-line immunotherapy. Our result showed that only 19.23% of epilepsy symptoms were controlled after using anti-epileptic drugs. The choice to use anti-epileptic drugs depends on the physicians' assessment, and the efficacy needs further research.
Inadequate dosage and duration of first-line agents were possibly responsible for recurrence (77). Our results showed that 17.14% of relapsed patients did not initiate first-line treatment, and 60.00% did not maintain treatment. So, in order to prevent relapse, early recognition, definite diagnosis, rapid treatment, and first-line treatment with adequate dosage and duration are all necessary in the process.
In view of the fact that our included studies were mostly case reports, this systematic review has a number of limitations, such as increased risks of reporting and selection biases. The integrity of clinical features, test results, and treatment effects from included articles might limit the conclusions as well. And the lack of follow-up details affected the final judgment of therapeutic effects. Though there is not any result from the randomized controlled trial, the result of this study could be referred. We are looking forward to more high-quality studies about efficacy and safety of anti-LGI1 encephalitis treatment.
Conclusion
In this review, according to our results, it is suggested that clinicians should suspect or consider anti-LGI1 encephalitis when the following symptoms appear: short-term memory loss, psychiatric symptoms, hyponatremia, seizures, or FBDS, especially in patients aged over 40. Brain MRI scanning and serum and CSF antibody tests should be done when considering diagnosis. EEG is necessary when suspicious seizures occur, and using anti-epileptic drugs to control seizures may benefit cognition. As for treatment, the statistics of our study suggest the combination of steroids with IVIG at the onset; gradually decreasing oral steroids and regular follow-up afterwards are also necessary. If anti-LGI1 encephalitis becomes severe, PE could be introduced. If anti-LGI1 encephalitis is refractory or recurs, immunosuppressant therapy such as rituximab, cyclophosphamide, mycophenolate mofetil, azathioprine, and methotrexate may provide potential benefits. Due to the high risk of incidence rate of malignant tumors in the population of anti-LGI1 encephalitis, a follow-up advice reference to PNS is suggested, which requires a repeated second screening after 3–6 months, followed by regular screening every 6 months for 4 years.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
Author Contributions
JT, JS, MW, and TL contributed to conceive and design this systematic review. YT and TL conducted the study selection and extracted the data from the selected articles. YT ran the data analysis. YT, ZY, and MS drafted the manuscript with supervision from TL and JN.
Funding
This work was supported by the Fundamental Research Funds for the Central Universities (No. 2019-JYB-TD-007) and Qihuang Scholar Foundation (China).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We thank all the members from our research group who made helpful comments.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2021.791014/full#supplementary-material
References
1. van Sonderen A, Schreurs MW, Wirtz PW, Sillevis Smitt PA, Titulaer MJ. From VGKC to LGI1 and Caspr2 encephalitis: the evolution of a disease entity over time. Autoimmun Rev. (2016) 15:970–4. doi: 10.1016/j.autrev.2016.07.018
2. Dalmau J, Graus F. Antibody-mediated encephalitis. N Engl J Med. (2018) 378:840–51. doi: 10.1056/NEJMra1708712
3. Graus F, Titulaer MJ, Balu R, Benseler S, Bien CG, Cellucci T, et al. A clinical approach to diagnosis of autoimmune encephalitis. Lancet Neurol. (2016) 15:391–404. doi: 10.1016/S1474-4422(15)00401-9
4. Moher D, Shamseer L, Clarke M, Ghersi D, Liberati A, Petticrew M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev. (2015) 4:1. doi: 10.1186/2046-4053-4-1
5. Park S, Choi H, Cheon GJ, Wook Kang K, Lee DS. 18F-FDG PET/CT in anti-LGI1 encephalitis: initial and follow-up findings. Clin Nucl Med. (2015) 40:156–8. doi: 10.1097/RLU.0000000000000546
6. Kuehn JC, Scheuerle A, Bauer J, Becker AJ, Wirtz R, Lewerenz J. A 64-year-old patient with a mesiotemporal mass and symptomatic epilepsy. Brain Pathol. (2020) 30:413–4. doi: 10.1111/bpa.12818
7. Perez A, Roman GC, Powell SZ, Fisher R, Rivera AL, Masdeu JC, et al. A 70-year old man with dystonic and choreiform movements. Brain Pathol. (2020) 30:415–6. doi: 10.1111/bpa.12819
8. Zangrandi A, Gasparini F, Marti A, Bhalla R, Napoli M, Angelini D, et al. A 9-year neuropsychological report of a patient with LGI1-associated limbic encephalitis. J Clin Exp Neuropsychol. (2019) 41:749–59. doi: 10.1080/13803395.2019.1617836
9. Hye Y. A case of anti-leucine-rich glioma-inactivated 1 antibody-mediated limbic encephalitis. Intern Med J. (2019) 49:932–3. doi: 10.1111/imj.14338
10. Rahangdale R, Scott T, Leichliter T, Baser S, Valeriano J. A case of paroxysmal dystonia associated with LGI-1 antibody encephalitis. Clin Neurol Neurosurg. (2019) 186:105508. doi: 10.1016/j.clineuro.2019.105508
11. Sato M, Kishida D, Miyazaki D, Sekijima Y. A patient with limbic encephalitis associated with anti-leucine-rich glioma-inactivated 1 (LGI1) antibody presenting with slowly progressive cognitive impairment and fluctuating striatal lesions. Intern Med. (2019) 58:287–91. doi: 10.2169/internalmedicine.1082-18
12. Ji T, Huang Z, Lian Y, Wang C, Zhang Q, Li J. A rare case of anti-LGI1 limbic encephalitis with concomitant positive NMDAR antibodies. BMC Neurol. (2020) 20:336. doi: 10.1186/s12883-020-01918-7
13. Ibrahim H, Al Jasser AN, Khan SA, Tlili KG. A rare case of autoimmune limbic encephalitis: an uncharted territory! Neurosciences. (2017) 22:292–7. doi: 10.17712/nsj.2017.4.20170150
14. Shen N, Ren H, Wu J, Feng J, Cui L, Fang S. A rare case of limbic encephalitis with anti leucine-rich glioma inactivated-1 (LGI1) antibodies. Neuro Endocrinol Lett. (2014) 35:95–7. doi: 10.1016/j.enbuild.2015.06.029
15. Chapelet G, Baguenier-Desormeaux C, Lejeune P, Boureau AS, Berrut G, de Decker L. A reversible rapidly progressive cognitive disorder: limbic encephalitis with leucine-rich glioma inactivated-1 protein antibody and an ectopic adrenocorticotropic hormone syndrome. J Am Geriatr Soc. (2015) 63:1486–7. doi: 10.1111/jgs.13516
16. AlHakeem AS, Mekki MS, AlShahwan SM, Tabarki BM. Acute psychosis in children: do not miss immune-mediated causes. Neurosciences. (2016) 21:252–5. doi: 10.17712/nsj.2016.3.20150760
17. Yuan X, Man X, Zhang J, Sun J, Liang J, Ma H, et al. Anti-leucine-rich glioma inactivated-1 encephalitis associated with essential thrombocythemia. Intern Med. (2020) 59:271–5. doi: 10.2169/internalmedicine.2963-19
18. Tu TH, Chan YE, Bai YM. Anti-leucine-rich glioma-inactivated 1 encephalitis with manic symptoms as the initial manifestation. Aust N Z J Psychiatry. (2018) 52:714–5. doi: 10.1177/0004867417742522
19. Zouras S, Stephens JW, Abburu SR, Emelle C. Anti-LGI1 encephalitis causing faciobrachial dystonic seizures. BMJ Case Rep. (2017) 2017:bcr2017221089. doi: 10.1136/bcr-2017-221089
20. Li X, Yuan J, Liu L, Hu W. Antibody-LGI 1 autoimmune encephalitis manifesting as rapidly progressive dementia and hyponatremia: a case report and literature review. BMC Neurol. (2019) 19:19. doi: 10.1186/s12883-019-1251-4
21. Zhao PP, Zhang Y, Gao L, Sun L. Assessing the clinical features of LGI1 antibody encephalitis. Acta Neurol Belg. (2016) 116:109–12. doi: 10.1007/s13760-015-0517-x
22. Haitao R, Huiqin L, Tao Q, Xunzhe Y, Xiaoqiu S, Wei L, et al. Autoimmune encephalitis associated with vitiligo? J Neuroimmunol. (2017) 310:14–6. doi: 10.1016/j.jneuroim.2017.05.019
23. Cooper CM, Cheung PW, Penney EB, Linnoila JJ. Case 15-2020: a 79-year-old man with hyponatremia and involuntary movements of the arm and face. N Engl J Med. (2020) 382:1943–50. doi: 10.1056/NEJMcpc1913477
24. Cash SS, Larvie M, Dalmau J. Case records of the massachusetts general hospital. Case 34-2011: A 75-year-old man with memory loss and partial seizures. N Engl J Med. (2011) 365:1825–33. doi: 10.1056/NEJMcpc1100924
25. Attwood JE, Naseer S, Michael S, Riley J. Clinical diagnosis of LGI1 antibody encephalitis in an 83-year-old woman. BMJ Case Rep. (2021) 14:e237398. doi: 10.1136/bcr-2020-237398
26. Frattini E, Monfrini E, Bitetto G, Ferrari B, Arcudi S, Bresolin N, et al. Clinical reasoning: a 75-year-old man with parkinsonism, mood depression, weight loss. Neurology. (2018) 90:572–5. doi: 10.1212/WNL.0000000000005177
27. Renard D, Collombier L, Lippi A, Honnorat J, Thouvenot E. Cyclophosphamide-responsive Lgi1-related limbic encephalitis with basal ganglia hypermetabolism. Acta Neurol Belg. (2016) 116:379–81. doi: 10.1007/s13760-015-0567-0
28. Sweeney M, Galli J, McNally S, Tebo A, Haven T, Thulin P, et al. Delayed LGI1 seropositivity in voltage-gated potassium channel (VGKC)-complex antibody limbic encephalitis. BMJ Case Rep. (2017) 2017:bcr2016218893. doi: 10.1136/bcr-2016-218893
29. Tumminelli G, Battisti C, Cioni C, Mignarri A, Annunziata P, Federico A. Demyelinating polyneuropathy in a case of anti-LGI1 encephalitis. Muscle Nerve. (2017) 56:E2–3. doi: 10.1002/mus.25572
30. Ahn SW, Kim JM, Kim JE, Lee ST, Ahn DW, Sung JJ. Development of LGI1 antibody encephalitis after treatment of lung cancer. Can J Neurol Sci. (2014) 41:669–71. doi: 10.1017/cjn.2014.17
31. Takahashi Y, Mikami T, Suzuki H, Komatsu K, Yamamoto D, Shimohama S, et al. Development of moyamoya disease after non-herpetic acute limbic encephalitis: a case report. J Clin Neurosci. (2018) 53:250–3. doi: 10.1016/j.jocn.2018.04.042
32. Pollak TA, Moran N. Emergence of new-onset psychotic disorder following recovery from LGI1 antibody-associated limbic encephalitis. BMJ Case Rep. (2017) 2017:bcr2016218328. doi: 10.1136/bcr-2016-218328
33. Naasan G, Irani SR, Bettcher BM, Geschwind MD, Gelfand JM. Episodic bradycardia as neurocardiac prodrome to voltage-gated potassium channel complex/leucine-rich, glioma inactivated 1 antibody encephalitis. JAMA Neurol. (2014) 71:1300–4. doi: 10.1001/jamaneurol.2014.1234
34. Miao A, Wang X, Wang L. Facial dystonic seizures-plus associated with anti-LGI1 antibody encephalitis. Epileptic Disord. (2019) 21:493–4. doi: 10.1684/epd.2019.1089
35. Fidzinski P, Jarius S, Gaebler C, Boegner F, Nohr R, Ruprecht K. Faciobrachial dystonic seizures and antibodies to Lgi1 in a 92-year-old patient: a case report. J Neurol Sci. (2014) 347:404–5. doi: 10.1016/j.jns.2014.10.026
36. d'Orsi G, Martino T, Lalla A, Claudio MTD, Carapelle E, Avolio C. Faciobrachial dystonic seizures expressed as epileptic spasms, followed by focal seizures in anti-LGI1 encephalitis: a video-polygraphic study. Epileptic Disord. (2018) 20:525–9. doi: 10.1684/epd.2018.1010
37. Dubey D, Alqallaf A, Warnack W, Gupta P, Harvey J, Vernino S. Faciobrachial dystonic spells: presenting feature of autoimmune encephalopathy. Neurol India. (2017) 65:1149–51. doi: 10.4103/neuroindia.NI_452_16
38. Gong J, Zhang Y, Wang F, Huang Y, Zhang W. Frequent hemianesthesia as initial symptom of limbic encephalitis associated with LGI1 antibodies. Neurol Sci. (2015) 36:1953–5. doi: 10.1007/s10072-015-2296-9
39. Peter-Derex L, Devic P, Rogemond V, Rheims S, Mauguière F, Honnorat J. Full recovery of agrypnia associated with anti-Lgi1 antibodies encephalitis under immunomodulatory treatment: a case report with sequential polysomnographic assessment. Sleep Med. (2012) 13:554–6. doi: 10.1016/j.sleep.2012.01.002
40. Kurtis MM, Toledano R, García-Morales I, Gil-Nagel A. Immunomodulated parkinsonism as a presenting symptom of LGI1 antibody encephalitis. Parkinsonism Relat Disord. (2015) 21:1286–7. doi: 10.1016/j.parkreldis.2015.08.014
41. Tofaris GK, Irani SR, Cheeran BJ, Baker IW, Cader ZM, Vincent A. Immunotherapy-responsive chorea as the presenting feature of LGI1-antibody encephalitis. Neurology. (2012) 79:195–6. doi: 10.1212/WNL.0b013e31825f0522
42. Casault C, Alikhani K, Pillay N, Koch M. Jerking & confused: leucine-rich glioma inactivated 1 receptor encephalitis. J Neuroimmunol. (2015) 289:84–6. doi: 10.1016/j.jneuroim.2015.10.010
43. Rachdi A, Dupouy J, Benaiteau M, Bost C, Moreau MS, Courbon CB, et al. Leucine-rich glioma-inactivated 1 encephalitis: broadening the sphere. Tremor Other Hyperkinet Mov. (2019) 9. doi: 10.5334/tohm.477
44. Mir A, Thani Z, Bashir S, Ayed H, Albaradie R. LGI-1 antibody encephalitis in a seven-year-old girl. Epileptic Disord. (2019) 21:591–7. doi: 10.1684/epd.2019.1117
45. Schultze-Amberger J, Pehl D, Stenzel W. LGI-1-positive limbic encephalitis: a clinicopathological study. J Neurol. (2012) 259:2478–80. doi: 10.1007/s00415-012-6559-6
46. Wang D, Hao Q, He L, Wang Q. LGI1 antibody encephalitis and psychosis. Australas Psychiatry. (2018) 26:612–4. doi: 10.1177/1039856218771513
47. Steriade C, Day GS, Lee L, Murray BJ, Fritzler MJ, Keith J. LGI1 autoantibodies associated with cerebellar degeneration. Neuropathol Appl Neurobiol. (2014) 40:645–9. doi: 10.1111/nan.12132
48. Kaymakamzade B, Kansu T, Tan E, Dericioglu N. LGI1 related limbic encephalitis and response to immunosuppressive therapy. J Neurol. (2011) 258:2075–7. doi: 10.1007/s00415-011-6044-7
49. Zhao JJ, Yang YH. [Leucine-rich glioma-inactivated protein 1 antibody-associated encephalitis:report of two cases]. Zhongguo Yi Xue Ke Xue Yuan Xue Bao. (2019) 41:714–8. doi: 10.3881/j.issn.1000-503X.11197
50. Messelmani M, Fekih-Mrissa N, Zaouali J, Mrissa R. Limbic encephalitis associated with leucine-rich glioma-inactivated 1 antibodies. Ann Saudi Med. (2015) 35:76–9. doi: 10.5144/0256-4947.2015.76
51. Schimmel M, Frühwald MC, Bien CG. Limbic encephalitis with LGI1 antibodies in a 14-year-old boy. Eur J Paediatr Neurol. (2018) 22:190–3. doi: 10.1016/j.ejpn.2017.08.004
52. Brown JW, Martin PJ, Thorpe JW, Michell AW, Coles AJ, Cox AL, et al. Long-term remission with rituximab in refractory leucine-rich glioma inactivated 1 antibody encephalitis. J Neuroimmunol. (2014) 271:66–8. doi: 10.1016/j.jneuroim.2014.03.012
53. Nilsson AC, Blaabjerg M. More evidence of a neurocardiac prodrome in anti-LGI1 encephalitis. J Neurol Sci. (2015) 357:310–1. doi: 10.1016/j.jns.2015.07.015
54. Szots M, Marton A, Kover F, Kiss T, Berki T, Nagy F, et al. Natural course of LGI1 encephalitis: 3-5 years of follow-up without immunotherapy. J Neurol Sci. (2014) 343:198–202. doi: 10.1016/j.jns.2014.05.048
55. Agazzi P, Bien CG, Staedler C, Biglio V, Gobbi C. Over 10-year follow-up of limbic encephalitis associated with anti-LGI1 antibodies. J Neurol. (2015) 262:469–70. doi: 10.1007/s00415-014-7540-3
56. Sen A, Wang J, Laue-Gizzi H, Lee T, Ghougassian D, Somerville ER. Pathognomonic seizures in limbic encephalitis associated with anti-LGI1 antibodies. Lancet. (2014) 383:2018. doi: 10.1016/S0140-6736(14)60684-X
57. Wang SJ, Zhao YY, Wang QZ, Guo B, Liu YM, Yan CZ. Pearls & Oy-sters: limbic encephalitis associated with positive anti-LGI1 and antithyroid antibodies. Neurology. (2016) 86:e16–8. doi: 10.1212/WNL.0000000000002259
58. Vogrig A, Pauletto G, Lettieri C, Valente M, Gigli GL. Peculiar EEG signatures, ictal drinking and long-term follow-up in anti-LGI1 encephalitis. Neurol Sci. (2019) 40:1503–05. doi: 10.1007/s10072-019-3729-7
59. Fantaneanu TA, Bhattacharyya S, Milligan TA, Pennell PB. Rapidly cycling auras and episodic focal dystonia in anti-lgi1 autoimmune encephalitis. JAMA Neurol. (2016) 73:1150. doi: 10.1001/jamaneurol.2016.1085
60. Yelam A, Nagarajan E, Bollu PC. Rapidly progressive global cerebral atrophy in the setting of anti-LGI1 encephalitis. BMJ Case Rep. (2019) 12:e228428. doi: 10.1136/bcr-2018-228428
61. Beimer NJ, Selwa LM. Seizure semiology of anti-LGI1 antibody encephalitis. Epileptic Disord. (2017) 19:461–4. doi: 10.1684/epd.2017.0936
62. Espinosa-Jovel C, Toledano R, García-Morales I, Álvarez-Linera J, Gil-Nagel A. Serial arterial spin labeling MRI in autonomic status epilepticus due to anti-LGI1 encephalitis. Neurology. (2016) 87:443–4. doi: 10.1212/WNL.0000000000002903
63. Rizzi R, Fisicaro F, Zangrandi A, Ghidoni E, Baiardi S, Ragazzi M, et al. Sudden cardiac death in a patient with LGI1 antibody-associated encephalitis. Seizure. (2019) 65:148–50. doi: 10.1016/j.seizure.2019.01.013
64. Bing-Lei W, Jia-Hua Z, Yan L, Zan Y, Xin B, Jian-Hua S, et al. Three cases of antibody-LGI1 limbic encephalitis and review of literature. Int J Neurosci. (2019) 129:642–8. doi: 10.1080/00207454.2018.1512985
65. Hor JY, Lim TT, Cheng MC, Chia YK, Wong CK, Lim SM, et al. Thymoma-associated myasthenia gravis and LGI1-encephalitis, with nephrotic syndrome post-thymectomy. J Neuroimmunol. (2018) 317:100–2. doi: 10.1016/j.jneuroim.2018.01.011
66. Krastinova E, Vigneron M, Le Bras P, Gasnault J, Goujard C. Treatment of limbic encephalitis with anti-glioma-inactivated 1 (LGI1) antibodies. J Clin Neurosci. (2012) 19:1580–2. doi: 10.1016/j.jocn.2011.12.025
67. Gravier Dumonceau A, Jeannin-Mayer S, Roche P, Honnorat J, Joubert B, Thobois S, et al. Unilateral clinical manifestations in LGI1-antibody encephalitis. Rev Neurol. (2019) 175:481–3. doi: 10.1016/j.neurol.2018.09.022
68. Zheng YM, Sun W, Wang ZX, Zhang W, Yuan Y. [Leucine-rich glioma inactivated-1 protein antibody associated limbic encephalitis: one case report]. Beijing Da Xue Xue Bao Yi Xue Ban. (2014) 46:646–9. doi: 10.3969/j.issn.1671-167X.2014.04.032
69. Incecik F, Hergüner OM, Besen S, Yilmaz M, Altunbasak S. Limbic encephalitis associated with anti-leucine-rich glioma-inactivated-1 protein antibodies in a child. Neurol India. (2016) 64:1321–3. doi: 10.4103/0028-3886.193776
70. Li Z, Cui T, Shi W, Wang Q. Clinical analysis of leucine-rich glioma inactivated-1 protein antibody associated with limbic encephalitis onset with seizures. Medicine. (2016) 95:e4244. doi: 10.1097/MD.0000000000004244
71. Li W, Wu S, Meng Q, Zhang X, Guo Y, Cong L, et al. Clinical characteristics and short-term prognosis of LGI1 antibody encephalitis: a retrospective case study. BMC Neurol. (2018) 18:96. doi: 10.1186/s12883-018-1099-z
72. Gao L, Liu A, Zhan S, Wang L, Li L, Guan L, et al. Clinical characterization of autoimmune LGI1 antibody limbic encephalitis. Epilepsy Behav. (2016) 56:165–9. doi: 10.1016/j.yebeh.2015.12.041
73. Wang D, Hao Q, He L, He L, Wang Q. LGI1 antibody encephalitis - detailed clinical, laboratory and radiological description of 13 cases in China. Compr Psychiatry. (2018) 81:18–21. doi: 10.1016/j.comppsych.2017.11.002
74. Aurangzeb S, Symmonds M, Knight RK, Kennett R, Wehner T, Irani SR. LGI1-antibody encephalitis is characterised by frequent, multifocal clinical and subclinical seizures. Seizure. (2017) 50:14–7. doi: 10.1016/j.seizure.2017.05.017
75. Yu J, Yu X, Fang S, Zhang Y, Lin W. The treatment and follow-up of anti-LGI1 limbic encephalitis. Eur Neurol. (2016) 75:5–11. doi: 10.1159/000441944
76. Shin YW, Lee ST, Shin JW, Moon J, Lim JA, Byun JI, et al. VGKC-complex/LGI1-antibody encephalitis: clinical manifestations and response to immunotherapy. J Neuroimmunol. (2013) 265:75–81. doi: 10.1016/j.jneuroim.2013.10.005
77. van Sonderen A, Thijs RD, Coenders EC, Jiskoot LC, Sanchez E, de Bruijn MA, et al. Anti-LGI1 encephalitis: clinical syndrome and long-term follow-up. Neurology. (2016) 87:1449–56. doi: 10.1212/WNL.0000000000003173
78. Ariño H, Armangu, é T, Petit-Pedrol M, Sabater L, Martinez-Hernandez E, Hara M, et al. Anti-LGI1-associated cognitive impairment: presentation and long-term outcome. Neurology. (2016) 87:759–65. doi: 10.1212/WNL.0000000000003009
79. Celicanin M, Blaabjerg M, Maersk-Moller C, Beniczky S, Marner L, Thomsen C, et al. Autoimmune encephalitis associated with voltage-gated potassium channels-complex and leucine-rich glioma-inactivated 1 antibodies - a national cohort study. Eur J Neurol. (2017) 24:999–1005. doi: 10.1111/ene.13324
80. Li LH, Ma CC, Zhang HF, Lian YJ. Clinical and electrographic characteristics of seizures in LGI1-antibody encephalitis. Epilepsy Behav. (2018) 88:277–82. doi: 10.1016/j.yebeh.2018.08.019
81. Yang X, Li AN, Zhao XH, Liu XW, Wang SJ. Clinical features of patients with anti-leucine-rich glioma inactivated-1 protein associated encephalitis: a Chinese case series. Int J Neurosci. (2019) 129:754–61. doi: 10.1080/00207454.2019.1567507
82. Zhang YX, Yang HL, Wu YY, Wang CC, Gao XY, Shi YY, et al. [Clinical analysis of 9 cases with Anti-leucine-rich glioma inactivated 1 protein antibody associated limbic encephalitis]. Zhonghua Yi Xue Za Zhi. (2017) 97:1295–8. doi: 10.3760/cma.j.issn.0376-2491.2017.17.004
83. Lai M, Huijbers MG, Lancaster E, Graus F, Bataller L, Balice-Gordon R, et al. Investigation of LGI1 as the antigen in limbic encephalitis previously attributed to potassium channels: a case series. Lancet Neurol. (2010) 9:776–85. doi: 10.1016/S1474-4422(10)70137-X
84. Bastiaansen AEM, van Steenhoven RW, de Bruijn M, Crijnen YS, van Sonderen A, van Coevorden-Hameete MH, et al. Autoimmune encephalitis resembling dementia syndromes. Neurol Neuroimmunol Neuroinflamm. (2021) 8:e1039. doi: 10.1212/NXI.0000000000001039
85. Muñoz-Lopetegi A, Graus F, Dalmau J, Santamaria J. Sleep disorders in autoimmune encephalitis. Lancet Neurol. (2020) 19:1010–22. doi: 10.1016/S1474-4422(20)30341-0
86. Shan W, Yang H, Wang Q. Neuronal surface antibody-medicated autoimmune encephalitis (Limbic Encephalitis) in China: a multiple-center, retrospective study. Front Immunol. (2021) 12:621599. doi: 10.3389/fimmu.2021.621599
87. Irani SR, Alexander S, Waters P, Kleopa KA, Pettingill P, Zuliani L, et al. Antibodies to Kv1 potassium channel-complex proteins leucine-rich, glioma inactivated 1 protein and contactin-associated protein-2 in limbic encephalitis, morvan's syndrome and acquired neuromyotonia. Brain. (2010) 133:2734–48. doi: 10.1093/brain/awq213
88. Titulaer MJ, Soffietti R, Dalmau J, Gilhus NE, Giometto B, Graus F, et al. Screening for tumours in paraneoplastic syndromes: report of an EFNS task force. Eur J Neurol. (2011) 18:19–3. doi: 10.1111/j.1468-1331.2010.03220.x
89. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2018) 68:394–424. doi: 10.3322/caac.21492
90. Muhr P, Goldammer U, Bien CG, Bien C, Sindern E. [Severe hyponatremia as precursor of LGI1 autoimmune encephalitis]. Nervenarzt. (2018) 89:942–4. doi: 10.1007/s00115-017-0471-3
91. Bordonne M, Chawki MB, Doyen M, Kas A, Guedj E, Tyvaert L, et al. Brain (18)F-FDG PET for the diagnosis of autoimmune encephalitis: a systematic review and a meta-analysis. Eur J Nucl Med Mol Imag. (2021) 48:3847–58. doi: 10.1007/s00259-021-05299-y
92. Roberto KT, Espiritu AI, Fernandez MLL, Gutierrez JC. Electroencephalographic findings in antileucine-rich glioma-inactivated 1 (LGI1) autoimmune encephalitis: a systematic review. Epilepsy Behav. (2020) 112:107462. doi: 10.1016/j.yebeh.2020.107462
93. Neurology Branch of Chinese Medical Association. Expert Consensus on Diagnosis and Treatment of Autoimmune Encephalitis in China. Chin J Neurol. (2017) 50:91–8. doi: 10.3760/cma.j.issn.1006-7876.2017.02.004
94. DeSena AD, Noland DK, Matevosyan K, King K, Phillips L, Qureshi SS, et al. Intravenous methylprednisolone versus therapeutic plasma exchange for treatment of anti-N-methyl-D-aspartate receptor antibody encephalitis: a retrospective review. J Clin Apher. (2015) 30:212–6. doi: 10.1002/jca.21363
95. Gresa-Arribas N, Titulaer MJ, Torrents A, Aguilar E, McCracken L, Leypoldt F, et al. Antibody titres at diagnosis and during follow-up of anti-NMDA receptor encephalitis: a retrospective study. Lancet Neurol. (2014) 13:167–77. doi: 10.1016/S1474-4422(13)70282-5
96. Heine J, Ly LT, Lieker I, Slowinski T, Finke C, Prüss H, et al. Immunoadsorption or plasma exchange in the treatment of autoimmune encephalitis: a pilot study. J Neurol. (2016) 263:2395–402. doi: 10.1007/s00415-016-8277-y
97. Zhang Y, Huang HJ, Chen WB, Liu G, Liu F, Su YY. Clinical efficacy of plasma exchange in patients with autoimmune encephalitis. Ann Clin Transl Neurol. (2021) 8:763–73. doi: 10.1002/acn3.51313
98. Ghimire P, Khanal UP, Gajurel BP, Karn R, Rajbhandari R, Paudel S, et al. Anti-LGI1, anti-GABABR, and Anti-CASPR2 encephalitides in Asia: a systematic review. Brain Behav. (2020) 10:e01793. doi: 10.1002/brb3.1793
99. Nepal G, Shing YK, Yadav JK, Rehrig JH, Ojha R, Huang DY, et al. Efficacy and safety of rituximab in autoimmune encephalitis: a meta-analysis. Acta Neurol Scand. (2020) 142:449–59. doi: 10.1111/ane.13291
100. Lee WJ, Lee ST, Moon J, Sunwoo JS, Byun JI, Lim JA, et al. Tocilizumab in autoimmune encephalitis refractory to rituximab: an institutional cohort study. Neurotherapeutics. (2016) 13:824–32. doi: 10.1007/s13311-016-0442-6
101. Nosadini M, Thomas T, Eyre M, Anlar B, Armangue T, Benseler SM, et al. International consensus recommendations for the treatment of pediatric NMDAR antibody encephalitis. Neurol Neuroimmunol Neuroinflamm. (2021) 8:e1052. doi: 10.1212/NXI.0000000000001052
102. Nosadini M, Mohammad SS, Toldo I, Sartori S, Dale RC. Mycophenolate mofetil, azathioprine and methotrexate usage in paediatric anti-NMDAR encephalitis: a systematic literature review. Eur J Paediatr Neurol. (2019) 23:7–18. doi: 10.1016/j.ejpn.2018.09.008
Keywords: anti-leucine rich glioma inactivated 1 encephalitis, LGI1, clinical features, diagnosis, treatment
Citation: Teng Y, Li T, Yang Z, Su M, Ni J, Wei M, Shi J and Tian J (2022) Clinical Features and Therapeutic Effects of Anti-leucine-rich Glioma Inactivated 1 Encephalitis: A Systematic Review. Front. Neurol. 12:791014. doi: 10.3389/fneur.2021.791014
Received: 07 October 2021; Accepted: 02 December 2021;
Published: 12 January 2022.
Edited by:
Hideyuki Takeuchi, Yokohama City University, JapanReviewed by:
Fumitaka Shimizu, Yamaguchi University School of Medicine, JapanNobuaki Yoshikura, Gifu University Hospital, Japan
Copyright © 2022 Teng, Li, Yang, Su, Ni, Wei, Shi and Tian. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jing Shi, c2hpamluZzg3QGhvdG1haWwuY29t; Jinzhou Tian, anp0aWFuQGhvdG1haWwuY29t
†These authors have contributed equally to this work and share first authorship
 Yuou Teng
Yuou Teng Ting Li
Ting Li Zhizhong Yang
Zhizhong Yang Mingwan Su
Mingwan Su Jingnian Ni
Jingnian Ni Mingqing Wei
Mingqing Wei Jing Shi
Jing Shi Jinzhou Tian
Jinzhou Tian