
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Neurol. , 18 January 2022
Sec. Neuromuscular Disorders and Peripheral Neuropathies
Volume 12 - 2021 | https://doi.org/10.3389/fneur.2021.783249
 Guoli Wei1,2,3,4†
Guoli Wei1,2,3,4† Jialin Gu1,2,5†
Jialin Gu1,2,5† Zhancheng Gu1,2,5†
Zhancheng Gu1,2,5† Cheng Du6
Cheng Du6 Xiaofei Huang1,2,5
Xiaofei Huang1,2,5 Haiyan Xing1,2
Haiyan Xing1,2 Lingchang Li1,2
Lingchang Li1,2 Aiping Zhang1,2
Aiping Zhang1,2 Xingxing Hu1,2,3,4*
Xingxing Hu1,2,3,4* Jiege Huo1,2*
Jiege Huo1,2*The coronavirus disease 2019 (COVID-19) pandemic is wreaking havoc on public-health and economic systems worldwide. Among the several neurological symptoms of patients with COVID-19 reported in clinical practice, olfactory dysfunction (OD) is the most common. OD occurs as the earliest or the only clinical manifestation in some patients. Increasing research attention has focused on OD, which is listed as one of the main diagnostic symptoms of severe acute respiratory syndrome-coronavirus-2 infection. Multiple clinical and basic-science studies on COVID-19-induced OD are underway to clarify the underlying mechanism of action. In this review, we summarize the clinical characteristics, mechanisms, evaluation methods, prognosis, and treatment options of COVID-19-induced OD. In this way, we hope to improve the understanding of COVID-19-induced OD to aid early identification and precise intervention.
Coronavirus disease 2019 (COVID-19) is caused by severe acute respiratory syndrome-coronavirus-2 (SARS-CoV-2) infection. COVID-19 has wrought havoc on public-health and economic systems worldwide since January 2020. As of September 2021, more than 220 million people have been infected and 4.6 million people in 221 countries have died.
The typical clinical symptoms of COVID-19 are fever, cough, fatigue, headache, myalgia or joint pain, sore throat, olfactory dysfunction (OD), gustatory dysfunction (GD), and diarrhea (1–3). As one of the early and common symptoms of COVID-19, OD (classified as anosmia, hyposmia, or dysosmia) has been a focus of increasing research interest (4–6). Approximately 40–50% of patients report OD as the first or only symptom of COVID-19, which has led to the proposal that OD should be given high priority as a prodromal symptom of COVID-19 (7–12). In contrast to reports on infection by other viruses, OD is associated with a relatively good prognosis in COVID-19, which suggests distinct pathological mechanisms that require further clarification (13, 14).
In this study, we review the clinical characteristics, mechanisms, evaluation methods, prognosis, and treatment options of COVID-19-induced OD.
A recent meta-analysis based on 83 studies provided high-quality evidence of a combined prevalence of OD in 47.9% of COVID-19 patients. Also, the prevalence of anosmia, hyposmia, and dysosmia was documented in 35.39, 36.15, and 2.53% of cases, respectively (15). OD is diagnosed more commonly among female patients and outpatients (16–18).
Following the first report of OD in 13.8% of patients with COVID-19 in China by Mao et al. (19), OD was identified as a widespread symptom in up to 60–90% of patients (20, 21). Following large-scale enrollment of participants, the reported prevalence of OD in patients decreased to 40–50% (22, 23). Due to the global nature of the pandemic, OD prevalence across countries is variable. For instance, studies have reported a prevalence of 13.8–67.2% in Asia (19, 24), 19–68% in North America (21, 25), 19.4–85.6% in Europe (20, 26), and 82.4% in Brazil (18). Interestingly, OD appears to be more prevalent in patients with mild-to-moderate COVID-19 than in those with severe disease (11, 27–30).
Notably, the variable geographic distribution of OD may be due to local differences in the emphasis placed on OD, the study cohort, assessment methods, and study design (9, 31). Moreover, self-reported tests may lead to underestimation of OD prevalence (28, 32–35). The number of patients with OD diagnosed using objective olfactory evaluations has been reported to be 2–3-times higher relative to that using self-reported (survey/questionnaire-reported) tests (23, 36, 37).
Different strains of SARS-CoV-2 can also cause different severities of OD. A systematic review incorporating scientific articles specific to the investigation of post-viral OD found that viral effects on the olfactory system differed according to the viral strain, along with alterations in or damage to components of the olfactory epithelium (OE) and/or olfactory bulb (38). Another systematic evaluation reported that the D614G virus mutation (compared with the D614 strain) increased OD prevalence in COVID-19 (39). In addition, the time of testing, ethnic/racial differences, age or sex, population density, and disease severity may be important contributory factors in prevalence variations among studies (17, 40–42).
Patients with COVID-19 may develop OD suddenly without other respiratory symptoms, such as nasal obstruction, rhinorrhea, and sore throat (1, 43, 44). Lechien et al. examined 1,363 ambulatory and hospitalized patients, 48.5–64.4% of whom presented with a sore throat, nasal obstruction, and rhinorrhea, which was lower than the reported prevalence of OD (81.6%) (27). Significant correlations were not observed between the severity of OD and other nasal symptoms (35).
As a common peripheral neuropathy of COVID-19, OD is closely associated with GD, with several reports of co-accompanying symptoms of OD and GD (45–47). Kaye et al. proposed that GD is a sequela of OD (48). In contrast, Singer-Cornelius et al. suggested, based on a lack of significant associations between the two conditions in objective tests, that GD and OD are two independent symptoms (37). A negative correlation has been demonstrated between OD and post-admission severity and mortality of COVID-19 in several studies (49, 50), in direct contrast to a report on clinical outcomes by Tabari et al. (51). Speth et al. showed that in addition to GD, OD severity was significantly associated with depression and anxiety (52). Interestingly, one study reported rare cases of “phantom OD” after a COVID-19 diagnosis, with a small proportion of cases being associated with objective hyposmia, whereas other patients were normosmic (53). An increasing number of studies have focused on the quantitative evaluation of olfactory function to identify the asymptomatic COVID-19 carriers (54, 55). A standardized quantitative test for an olfactory function could be a cost-effective and high-impact method for widespread screening and monitoring of COVID-19 (56).
Female patients infected with COVID-19 are more likely to develop OD. Jain et al. identified 42 women (49.6%) and 50 men (23.6%) with OD among 410 COVID-19 cases (14). Similar clinical outcomes have been reported in studies by Lechien et al. and Speth et al. (17, 57). In contrast, Meini et al. demonstrated that OD occurred less frequently, but lasted longer, in female patients (58). Interestingly, women were shown to be more susceptible to OD (in particular, hyposmia and anosmia) than men (20). This sex-based difference may be attributed to the different inflammatory processes between men and women.
Olfactory dysfunction occurs frequently in patients with COVID-19, especially in the younger population. A high-quality meta-analysis showed a significant negative association between age and OD, either alone or combined with GD (28). In addition, increasing the mean age of patients with COVID-19 was found to be closely associated with a reduced prevalence of OD (23). However, in a 6-month follow-up study on 169 patients with mild-to-moderate COVID-19, Cristillo et al. showed that patients with long-term OD (identified via objective assessment) were older (68.2 years) compared with those with normal olfactory function (58.2 years) (59). They also found a 2-fold increase in OD risk in individuals aged >65 years and a 3-fold increase in those aged >75 years (59).
The prevalence of OD is higher in Caucasians, with reports of 3–6-times greater prevalence relative to that in Asians and African–Americans (28, 60, 61). In addition to racial differences, the prevalence of chemosensory dysfunction (CD) has been reported to be higher in cohorts from Europe, North America, and the Middle East (19–98%) compared with that in an Asian cohort (11–15%) (5). An international multicenter study reported OD prevalence among Chinese, German, and French patients to be 32, 69, and 49%, respectively (9). The susceptibility of individuals from distinct regions to SARS-CoV-2 infection may explain (at least in part) the racial differences in prevalence (62).
Large-scale clinical samples are required for the identification of the potential risk factors for OD. The most common comorbidities reported in patients with OD are obesity, hypertension, diabetes mellitus, and cardiovascular disease (63–65). Genetic pathways cannot be ignored in COVID-19 and its complications. A study on 3,261 Twins UK volunteers reported 19% heritability of OD (66). Tobacco smoking, asthma, allergic rhinitis, chronic obstructive pulmonary disease, muscle/joint pain, and hypothyroidism are also potential risk factors for OD (22, 67–69). However, large-scale clinical data are lacking, which hampers the drawing of conclusions from studies. Vaira et al. reported an increased likelihood of hyposmia with fever, chest pain, and sputum generation, but not anosmia or an olfactory disturbance (35). Interestingly, studies showed higher viral loads in CD patients with COVID-19 than those in non-CD patients (70), but individual viral loads were not associated with the prevalence, severity, or recovery from OD in subsequent investigations (71, 72). In addition, a study of OD outside of the COVID-19 pandemic found a low educational level, reduced self-perceived olfactory function, and exposure to toxic substances/irritants to be potential risk factors for OD as well, findings that could help to identify the new potential risk factors of OD in patients with COVID-19 (73).
Infections by several types of viruses cause OD, but the high prevalence and relatively short recovery time of OD induced by SARS-CoV-2 infection suggest a distinct mechanism (20, 74). Angiotensin-converting enzyme 2 (ACE2) is a functional receptor for SARS-CoV-2. It facilitates the entry of SARS-CoV-2 using the serine protease TMPRSS2 to “prime” the “spike” protein (75–77). ACE2 is associated significantly with OD under conditions of SARS-CoV-2 infection (77, 78). Although the underlying mechanisms are incompletely understood, data from several studies have provided novel insights. The current hypothesis is that SARS-CoV-2 causes OD through multiple pathways, as depicted in Figure 1.
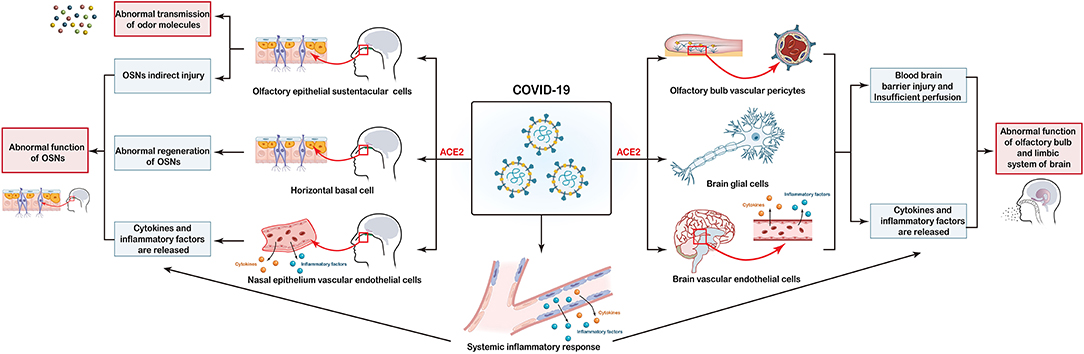
Figure 1. Potential mechanisms of coronavirus disease 2019 (COVID-19)-induced olfactory dysfunction. (i) COVID-19 directly affects sustentacular (SUS) cells through interactions with the ACE2 receptor, thereby leading to abnormal transmission of odor molecules. (ii) Injury to SUS cells, horizontal basal cells (HBCs) as well as the release of cytokines and proinflammatory factors in the systemic and local nasal epithelium lead to abnormal function of olfactory sensory neurons (OSNs). (iii) Olfactory-bulb vascular pericytes, glial-cell injury, and release of cytokines and proinflammatory factors into the systemic and local brain trigger abnormalities in functions of the olfactory bulb and limbic system of the brain.
The olfactory bulb, olfactory field, and limbic areas (e.g., olfactory media and hippocampus) are mainly involved in olfactory formation in the central nervous system (CNS). Lu et al. reported significantly higher bilateral gray-matter volumes in olfactory cortices, hippocampi, insulas, and left rolandic operculum, and the general decline of diffusivity in white matter, in patients with COVID-19 (70). Coronaviruses can invade the CNS via a transneuronal or hematogenous route. One post mortem study demonstrated the presence of SARS-CoV-2-specific antigens and RNA in the cerebrum of infected patients (79). Meinhardt et al. demonstrated the presence of SARS-CoV-2 RNA and protein in anatomically distinct regions of the nasopharynx and brain. They proposed that SARS-CoV-2 could enter the CNS through the neural–mucosal interface of the olfactory mucosa (80). Such new findings add to the understanding of the interactions between SARS-CoV-2 and the brain. However, whether the viral invasion of the CNS via the olfactory system is responsible for COVID-19-induced OD is not clear (81). It was initially hypothesized that SARS-CoV-2 might invade the olfactory neurons of the CNS directly, thereby leading to OD. However, subsequent studies showed that ACE2 was not expressed in olfactory neurons of the olfactory bulb (79, 82). Therefore, SARS-CoV-2 is believed to cause CNS injury through other mechanisms to lead to OD.
Central nervous system injury may be attributable to two main factors. First, ACE2 is expressed in many neurons (excitatory and inhibitory) and some non-neuron cells (mainly astrocytes, oligodendrocytes, endothelial cells, and olfactory-bulb vascular pericytes) in the human middle temporal gyrus and posterior cingulate cortex (78, 79, 82–85). After entry into the body, coronaviruses invade these cells directly and cause damage, produce proinflammatory mediators, and destroy the blood-brain barrier. Injury to blood vessels around the olfactory bulb leads to insufficient perfusion and damage to neurons which, ultimately, affects the function of the olfactory center and results in OD (84, 86–88). Second, SARS-CoV-2 infection triggers dysfunction of the immune system and overproduction of numerous cytokines, such as interleukin-1, interleukin-6, interleukin-12, interferon-γ, and tumor necrosis factor-α (89–91). Cytokine-driven injury and immune-mediated toxicity may destroy the integrity of the blood-brain barrier and cause secondary injury (92). Cytokines may also be directly neurotoxic, mediate, or even inhibit injury to cells of the CNS, either alone or in tandem with other factors (93).
Peripheral tissues related to the olfactory system are distributed mainly in the nasal epithelium, which is classified into the respiratory epithelium (RE) and OE. The primary function of the RE is to humidify air as it enters the nose. The OE contains OSNs, SUS cells, and basal cells. The OE is located in the nasal cavity, where neurons are exposed to the external environment. The OE is responsible for the detection and transmission of odor signals to the brain.
In the early stages of SARS-CoV-2 infection, viral RNA can be detected readily in the upper respiratory tract, which suggests active infection and replication in this region (94). Single-cell RNA sequencing datasets from healthy individuals generated by the Human Cell Atlas Consortium revealed differential expression of ACE2 and TMPRSS2 protease in respiratory and intestinal epithelial cells, with the highest levels being observed in the nasal epithelium (95). Immunostaining of tissues from the human nasal epithelium showed significantly higher expression of ACE2 in the OE relative to that in the RE. However, ACE2 was not detected in olfactory neurons, a finding that was confirmed in a mouse model (82, 96, 97). Nevertheless, recently Cecchini et al., in addition to the clear high expression of ACE2 in the SUS cells of olfactory mucosa samples from humans, showed a sharp dot-like ACE2 expression in olfactory neurons, suggesting possible direct damage to neurons (98). In addition, a recent post mortem study clearly showed SARS-CoV-2 in human olfactory neurons (80).
Peripheral nervous system injury may be attributable to three main mechanisms. The first potential cause is the injury to olfactory epithelial SUS cells. In the physiological state, SUS cells have two main roles. First, SUS cells form the mucus layer of the OE to facilitate the molecular movement of odor molecules and make contact with the cilia of olfactory neurons. Second, SUS cells are close to olfactory neurons and provide structural support for sensory neurons. These cells have specific characteristics (e.g., phagocytosis of potentially harmful substances), a detoxification role, and maintain a local balance of salt and water (99, 100). SARS-CoV-2 affects SUS cells directly through interactions with the ACE2 receptor and TMPRSS2, resulting in impaired or temporary loss of SUS-cell function and, ultimately, abnormal transmission of odor molecules and direct/indirect damage to OSNs (82, 101).
Second, injury to HBCs may have an underlying role. Basal cells of the OE are subdivided into two types: globose basal cells (GBCs) and HBCs. GBCs are primarily responsible for regenerating OSNs during normal epithelial turnover. HBCs can aid the proliferation of epithelial cells and are activated as stem cells upon tissue injury, thereby supplementing GBC activity (102–104). In basal cells, expression of ACE2 and TMPRSS2 is detected only in HBCs, which suggests that SARS-CoV-2 can infect HBCs directly, resulting in abnormal regeneration of OSNs (82).
Third, SARS-CoV-2 infection can lead to vascular endothelial damage and abnormal production of cytokines and proinflammatory mediators. These actions affect CNS function but also cause secondary damage to nasal epithelial cells, thereby resulting in indirect effects upon olfactory function (13, 84, 87, 88, 105).
Two evaluation methods of COVID-19-associated OD are recommended: (i) questionnaire survey for subjective evaluation; (ii) olfactory sensitivity test for objective evaluation. Imaging and invasive endoscopy for OD alone are not advised (106).
The methods employed for subjective evaluation are visual analog scales and questionnaires. Visual analog scales are simple and effective tools to evaluate the presence and degree of olfactory function (9). The short version of the Questionnaire of Olfactory Disorders-Negative Statements (sQOD-NS) is a validated olfactory-specific quality of life (QoL) survey that quantifies patient perception of olfactory function. It is used widely in clinical studies on COVID-19 for people with olfactory dysfunction (10, 20, 107, 108). Despite the wide use of sQOD-NS to evaluate the QoL and mood impacts of OD from a subjective viewpoint, one main drawback is a lack of relevance to COVID-19 characteristics. To improve the specificity for COVID-19, the COVID-19 Task Force of Young Otolaryngologists of the International Federation of Oto-rhino-laryngological Societies (which includes otolaryngologists from North America, Europe, and Asia) has developed a questionnaire composed of the relevant epidemiological and clinical features. This version can be applied to determine the variations, timings, and associated symptoms of OD, thereby providing a reliable instrument for assessing COVID-19-induced OD (20).
Olfactory function is difficult to discriminate subjectively. For a quantitative assessment, also carrying out a validated test (i.e., assessment of hyposmia and anosmia) is beneficial. For qualitative evaluation, questionnaires assessing the frequency of occurrence, intensity, social impact, and visual scales are meaningful (i.e., assessment of phantosmia and parosmia) (109).
The “Sniffin' Sticks” test is a nasal chemosensory assay that utilizes a pen-like odor-distribution device. This test has increased the prevalence of detection of OD in COVID-19 (110, 111). The Sniffin' Sticks test can reflect the level of olfactory function of individuals objectively. However, similar to sQOD-NS, owing to a lack of specificity for COVID-19, stage-specific analysis in conjunction with the disease stage and treatment plan is difficult (111).
Other olfactory sensitivity tests for OD in COVID-19 include the University of Pennsylvania Smell Identification Test, Toyota & Takagi Olfactometer, Cross-Cultural Smell Identification Test, Connecticut Chemosensory Clinical Research Center Test, and Brief Smell Identification Test (37, 112, 113). However, these assays must be developed further and optimized for more specific detection of COVID-19-induced OD.
Coronavirus disease 2019-induced OD is reversible carries a good prognosis and, in general, is characterized by a high chance of recovery. Overall, 48.6–89% of COVID-19 patients with OD experience complete remission or improvement after 4 weeks of follow-up (63, 114, 115). In a recent meta-analysis, the mean recovery period of COVID-19-induced OD was 7.21 ± 12.93 days (22). Significant differences in the prevalence of OD recovery were not observed between the sexes. However, the recovery time for OD was longer in female and older patients (58, 116). Higher susceptibility in a European population was additionally associated with a longer recovery period. A European study involving 2,581 patients with COVID-19 reported a mean OD recovery period of 21.6 ± 17.9 days (27). However, several patients experienced slow recovery or persistent OD, which led to significant negative effects on QoL and morbidity in the form of nutritional disruption, social anxiety, or depression (117–119). Several factors influence rehabilitation from OD: region, ethnicity, sex, age, duration, and method of treatment. Further research is essential for identifying populations with a poor prognosis to facilitate efficacious interventions at early stages.
Olfactory training (OT) is an efficacious method to manage OD caused by various factors. Meta-analyses have shown that OT can significantly improve OD caused by viral infections (120–122). OT is also recommended for improving OD caused by COVID-19 (112, 113, 122). Clinical OT methods include classical olfactory training (COT) and modified olfactory training (MOT). COT involves exposure to four odors (phenyl ethyl alcohol, eucalyptol, citronellal, and eugenol) two times a day for 5 min each time for ≥12 weeks (123). MOT is based on the classic 12-week COT with added exposure to the second group of spices (menthol, thyme, tangerine, and jasmine) for 12 weeks subsequently and a third group of spices (green tea, bergamot, rosemary, and gardenia) for the final 12 weeks, thereby comprising a total treatment cycle of 36 weeks (124). The indications, treatment times, and specific effects of OT for patients with COVID-19-induced OD have yet to be established, and high-quality prospective studies are required to provide more concrete evidence.
Oral or topical corticosteroids have been shown to improve olfactory function in patients. However, early studies included patients with localized nasal inflammation, such as rhinitis and sinusitis (125–128). Moreover, other studies suggested no significant effects of oral or topical corticosteroids on OD (129, 130). A recent systematic review revealed a lack of high-quality studies demonstrating the efficacy of oral or topical corticosteroids on OD unrelated to sinonasal disease (131). In contrast to other types of viral infections, OD in COVID-19 is not correlated significantly with nasal symptoms, such as nasal obstruction or rhinorrhea. Therefore, routine use of oral or topical corticosteroids for OD in COVID-19 is not recommended. For patients with inadequate OT or those participating in clinical studies, oral or topical corticosteroid therapy should be based on knowledge of the underlying disease, existing comorbidities, imaging findings, and adequate risk-benefit assessment, particularly the impact of short-term corticosteroids (112, 113).
Numerous drugs exert potential therapeutic effects on OD: vitamin A, theophylline, intranasal sodium citrate, caroverine, alpha-lipoic acid, minocycline, zinc sulfate, and ginkgo biloba (112, 113, 132, 133). However, except for one case report on vitamin A, clinical studies on their efficacy in COVID-19-induced OD are lacking, so most of these drugs are not recommended for routine use (134).
Olfactory dysfunction prevalence in COVID-19 is high, especially in young and middle-aged individuals, women, patients of European and American Caucasian descent, and those with mild disease. As one of the prodromal clinical symptoms of COVID-19, the inclusion of OD in the screening test is recommended. Current findings suggest that OD in COVID-19 is not caused by direct damage to OSNs but instead is a result of indirect damage to central and peripheral OSNs triggered by multiple pathways, even if the question is still open. Despite the high prevalence of COVID-19-induced OD, efficacious intervention strategies are lacking. OT is considered an efficacious and safe treatment option. Further studies are required to validate its efficacy and indications for oral or topical use of corticosteroids. Considering that COVID-19-induced OD carries a good prognosis and a short recovery period, the focus should be on identifying patients with a poor prognosis who may benefit from early intervention to avoid complications such as OD-induced depression and anxiety.
GW was involved in writing the original draft and methodology. JG and ZG were involved in writing the original draft. XHua performed conceptualization and writing the original draft. HX performed conceptualization. LL and CD were involved in writing the review and editing. JH and XHu supervised the manuscript. All authors contributed to the article and approved the submitted version.
This study was supported by the National Natural Science Foundation of China (82004339), Project of National Clinical Research Base of Traditional Chinese Medicine in Jiangsu Province (JD2019SZXYB02), Jiangsu Province TCM Leading Talent Training project (SLJ0211), and Scientific Research Project of Jiangsu Provincial Health Commission (H2019095).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
COVID-19, Coronavirus disease 2019; SARS-CoV-2, severe acute respiratory syndrome-coronavirus-2; OD, olfactory dysfunction; GD, gustatory dysfunction; ACE2, angiotensin-converting enzyme 2; SUS, sustentacular; CNS, central nervous system; PNS, peripheral nervous system; HBC, horizontal basal cell; OSN, olfactory sensory neuron; RE, respiratory epithelium; OE, olfactory epithelium; CD, chemosensory dysfunction; GBC, globose basal cell; QoL, quality of life; sQOD-NS, short version of the Questionnaire of Olfactory Disorders-Negative Statements; OT, olfactory training; COT, classical olfactory training; MOT, modified olfactory training.
1. Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, et al. 2020 Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. (2020) 382:1708–20. doi: 10.1056/NEJMoa2002032
2. Hu B, Guo H, Zhou P, Shi ZL. Characteristics of SARS-CoV-2 and COVID-19. Nat Rev Microbiol. (2021) 19:141–54. doi: 10.1038/s41579-020-00459-7
3. Zhu J, Ji P, Pang J, Zhong Z, Li H, He C, et al. Clinical characteristics of 3062 COVID-19 patients: a meta-analysis. J Med Virol. (2020) 92:1902–14. doi: 10.1002/jmv.25884
4. Fantozzi PJ, Pampena E, Di Vanna D, Pellegrino E, Corbi D, Mammucari S, et al. Xerostomia, gustatory and olfactory dysfunctions in patients with COVID-19. Am J Otolaryngol. (2020) 41:102721. doi: 10.1016/j.amjoto.2020.102721
5. Wong DKC, Gendeh HS, Thong HK, Lum SG, Gendeh BS, Saim A, et al. A review of smell and taste dysfunction in COVID-19 patients. Med J Malaysia. (2020) 75:574–81.
6. Samaranayake LP, Fakhruddin KS, Panduwawala C. Sudden onset, acute loss of taste and smell in coronavirus disease 2019 (COVID-19): a systematic review. Acta Odontol Scand. (2020) 78:467–73. doi: 10.1080/00016357.2020.1787505
7. Villerabel C, Makinson A, Jaussent A, Picot MC, Negre-Pages L, Rouviere JA, et al. Diagnostic value of patient-reported and clinically tested olfactory dysfunction in a population screened for COVID-19. JAMA Otolaryngol Head Neck Surg. (2021) 147:271–9. doi: 10.1001/jamaoto.2020.5074
8. Ugurlu BN, Akdogan O, Yilmaz YA, Yapar D, Aktar Ugurlu G, Yerlikaya HS, et al. Quantitative evaluation and progress of olfactory dysfunction in COVID-19. Eur Arch Otorhinolaryngol. (2021) 278:2363–9. doi: 10.1007/s00405-020-06516-4
9. Qiu C, Cui C, Hautefort C, Haehner A, Zhao J, Yao Q, et al. Olfactory and gustatory dysfunction as an early identifier of COVID-19 in adults and children: an international multicenter study. Otolaryngol Head Neck Surg. (2020) 163:714–21. doi: 10.1177/0194599820934376
10. Ninchritz-Becerra E, Soriano-Reixach MM, Mayo-Yanez M, Calvo-Henriquez C, Martinez-Ruiz de Apodaca P, Saga-Gutierrez C, et al. Subjective evaluation of smell and taste dysfunction in patients with mild COVID-19 in Spain. Med Clin. (2021) 156:61–4. doi: 10.1016/j.medcle.2020.08.004
11. Spinato G, Fabbris C, Polesel J, Cazzador D, Borsetto D, Hopkins C, et al. alterations in smell or taste in mildly symptomatic outpatients with SARS-CoV-2 infection. JAMA. (2020) 323:2089–90. doi: 10.1001/jama.2020.6771
12. Menni C, Valdes AM, Freidin MB, Sudre CH, Nguyen LH, Drew DA, et al. Real-time tracking of self-reported symptoms to predict potential COVID-19. Nat Med. (2020) 26:1037–40. doi: 10.1038/s41591-020-0916-2
13. Solomon T. Neurological infection with SARS-CoV-2 - the story so far. Nat Rev Neurol. (2021) 17:65–6. doi: 10.1038/s41582-020-00453-w
14. Jain A, Kumar L, Kaur J, Baisla T, Goyal P, Pandey AK, et al. Olfactory and taste dysfunction in coronavirus disease 2019 patients: its prevalence and outcomes. J Laryngol Otol. (2020) 134:1–5. doi: 10.1017/S0022215120002467
15. Saniasiaya J, Islam MA, Abdullah B. Prevalence of olfactory dysfunction in coronavirus disease 2019 (COVID-19): A meta-analysis of 27,492 patients. Laryngoscope. (2021) 131:865–78. doi: 10.1002/lary.29286
16. Nouchi A, Chastang J, Miyara M, Lejeune J, Soares A, Ibanez G, et al. Prevalence of hyposmia and hypogeusia in 390 COVID-19 hospitalized patients and outpatients: a cross-sectional study. Eur J Clin Microbiol Infect Dis. (2021) 40:691–7. doi: 10.1007/s10096-020-04056-7
17. Speth MM, Singer-Cornelius T, Oberle M, Gengler I, Brockmeier SJ, Sedaghat AR. Olfactory dysfunction and sinonasal symptomatology in COVID-19: prevalence, severity, timing, and associated characteristics. Otolaryngol Head Neck Surg. (2020) 163:114–20. doi: 10.1177/0194599820929185
18. Brandao Neto D, Fornazieri MA, Dib C, Di Francesco RC, Doty RL, Voegels RL, et al. Chemosensory dysfunction in COVID-19: prevalences, recovery rates, and clinical associations on a large Brazilian sample. Otolaryngol Head Neck Surg. (2021) 164:512–18. doi: 10.1177/0194599820954825
19. Mao L, Jin H, Wang M, Hu Y, Chen S, He Q, et al. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol. (2020) 77:683–90. doi: 10.1001/jamaneurol.2020.1127
20. Lechien JR, Chiesa-Estomba CM, De Siati DR, Horoi M, Le Bon SD, Rodriguez A, et al. Olfactory and gustatory dysfunctions as a clinical presentation of mild-to-moderate forms of the coronavirus disease (COVID-19): a multicenter European study. Eur Arch Otorhinolaryngol. (2020) 277:2251–61. doi: 10.1007/s00405-020-05965-1
21. Yan CH, Faraji F, Prajapati DP, Boone CE, DeConde AS. Association of chemosensory dysfunction and COVID-19 in patients presenting with influenza-like symptoms. Int Forum Allergy Rhinol. (2020) 10:806–13. doi: 10.1002/alr.22579
22. Wu D, Wang VY, Chen YH, Ku CH, Wang PC. The prevalence of olfactory and gustatory dysfunction in covid-19 - A systematic review. Auris Nasus Larynx. (2021) S0385-8146(21)00200-5. doi: 10.1016/j.anl.2021.07.007
23. Agyeman AA, Chin KL, Landersdorfer CB, Liew D, Ofori-Asenso R. Smell and taste dysfunction in patients with COVID-19: a systematic review and meta-analysis. Mayo Clin Proc. (2020) 95:1621–31. doi: 10.1016/j.mayocp.2020.05.030
24. Sayin I, Yasar KK, Yazici ZM. Taste and smell impairment in COVID-19: an AAO-HNS anosmia reporting tool-based comparative study. Otolaryngol Head Neck Surg. (2020) 163:473–9. doi: 10.1177/0194599820931820
25. Aggarwal S, Garcia-Telles N, Aggarwal G, Lavie C, Lippi G, Henry BM. Clinical features, laboratory characteristics, and outcomes of patients hospitalized with coronavirus disease 2019 (COVID-19): early report from the United States. Diagnosis. (2020) 7:91–6. doi: 10.1515/dx-2020-0046
26. Vaira LA, Deiana G, Fois AG, Pirina P, Madeddu G, De Vito A, et al. Objective evaluation of anosmia and ageusia in COVID-19 patients: Single-center experience on 72 cases. Head Neck. (2020) 42:1252–8. doi: 10.1002/hed.26204
27. Lechien JR, Chiesa-Estomba CM, Beckers E, Mustin V, Ducarme M, Journe F, et al. Prevalence and 6-month recovery of olfactory dysfunction: a multicentre study of 1363 COVID-19 patients. J Intern Med. (2021) 290:451–61. doi: 10.1111/joim.13209
28. von Bartheld CS, Hagen MM, Butowt R. Prevalence of chemosensory dysfunction in COVID-19 patients: a systematic review and meta-analysis reveals significant ethnic differences. ACS Chem Neurosci. (2020) 11:2944–61. doi: 10.1021/acschemneuro.0c00460
29. Cheng MY, Hsih WH, Ho MW, Lai YC, Liao WC, Chen CY, et al. Younger adults with mild-to-moderate COVID-19 exhibited more prevalent olfactory dysfunction in Taiwan. J Microbiol Immunol Infect. (2021) 54:794–800. doi: 10.1016/j.jmii.2021.01.006
30. Mendonca CV, Mendes Neto JA, Suzuki FA, Orth MS, Machado Neto H, Nacif SR. Olfactory dysfunction in COVID-19: a marker of good prognosis? Braz J Otorhinolaryngol. (2021) S1808-8694(20)30240-8. doi: 10.1016/j.bjorl.2020.12.002
31. Kim JW, Han SC, Jo HD, Cho SW, Kim JY. Regional and chronological variation of chemosensory dysfunction in COVID-19: a meta-analysis. J Korean Med Sci. (2021) 36:e40. doi: 10.3346/jkms.2021.36.e40
32. Moein ST, Hashemian SM, Mansourafshar B, Khorram-Tousi A, Tabarsi P, Doty RL. Smell dysfunction: a biomarker for COVID-19. Int Forum Allergy Rhinol. (2020) 10:944–50. doi: 10.1002/alr.22587
33. Vaira LA, Hopkins C, Petrocelli M, Lechien JR, Chiesa-Estomba CM, Salzano G, et al. Smell and taste recovery in coronavirus disease 2019 patients: a 60-day objective and prospective study. J Laryngol Otol. (2020) 134:703–9. doi: 10.1017/S0022215120001826
34. Boscolo-Rizzo P, Menegaldo A, Fabbris C, Spinato G, Borsetto D, Vaira LA, et al. Six-month psychophysical evaluation of olfactory dysfunction in patients with COVID-19. Chem Senses. (2021) 46:bjab006. doi: 10.1093/chemse/bjab006
35. Vaira LA, Lechien JR, Khalife M, Petrocelli M, Hans S, Distinguin L, et al. Psychophysical evaluation of the olfactory function: European multicenter Study on 774 COVID-19 patients. Pathogens. (2021) 10:62. doi: 10.3390/pathogens10010062
36. Tong JY, Wong A, Zhu D, Fastenberg JH, Tham T. The prevalence of olfactory and gustatory dysfunction in COVID-19 patients: a systematic review and meta-analysis. Otolaryngol Head Neck Surg. (2020) 163:3–11. doi: 10.1177/0194599820926473
37. Singer-Cornelius T, Cornelius J, Oberle M, Metternich FU, Brockmeier SJ. Objective gustatory and olfactory dysfunction in COVID-19 patients: a prospective cross-sectional study. Eur Arch Otorhinolaryngol. (2021) 278:3325–32. doi: 10.1007/s00405-020-06590-8
38. Lee JC, Nallani R, Cass L, Bhalla V, Chiu AG, Villwock JA. A systematic review of the neuropathologic findings of post-viral olfactory dysfunction: implications and novel insight for the COVID-19 pandemic. Am J Rhinol Allergy. (2021) 35:323–33. doi: 10.1177/1945892420957853
39. von Bartheld CS, Hagen MM, Butowt R. The D614G virus mutation enhances anosmia in COVID-19 patients: evidence from a systematic review and meta-analysis of studies from South Asia. ACS Chem Neurosci. (2021) 12:3535–49. doi: 10.1021/acschemneuro.1c00542
40. Moein ST, Hashemian SM, Tabarsi P, Doty RL. Prevalence and reversibility of smell dysfunction measured psychophysically in a cohort of COVID-19 patients. Int Forum Allergy Rhinol. (2020) 10:1127–35. doi: 10.1002/alr.22680
41. Mishra P, Gowda V, Dixit S, Kaushik M. Prevalence of new onset anosmia in COVID-19 Patients: is the trend different between European and Indian Population? Indian J Otolaryngol Head Neck Surg. (2020) 72:1–4. doi: 10.1007/s12070-020-01986-8
42. Desiato VM, Levy DA, Byun YJ, Nguyen SA, Soler ZM, Schlosser RJ. The prevalence of olfactory dysfunction in the general population: a systematic review and meta-analysis. Am J Rhinol Allergy. (2021) 35:195–205. doi: 10.1177/1945892420946254
43. Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. (2020) 395:507–13. doi: 10.1016/S0140-6736(20)30211-7
44. Mercante G, Ferreli F, De Virgilio A, Gaino F, Di Bari M, Colombo G, et al. Prevalence of taste and smell dysfunction in coronavirus disease 2019. JAMA Otolaryngol Head Neck Surg. (2020) 146:723–8. doi: 10.1001/jamaoto.2020.1155
45. Bagheri SH, Asghari A, Farhadi M, Shamshiri AR, Kabir A, Kamrava SK, et al. Coincidence of COVID-19 epidemic and olfactory dysfunction outbreak in Iran. Med J Islam Repub Iran. (2020) 34:62. doi: 10.1101/2020.03.23.20041889
46. Klopfenstein T, Kadiane-Oussou NJ, Toko L, Royer PY, Lepiller Q, Gendrin V, et al. Features of anosmia in COVID-19. Med Mal Infect. (2020) 50:436–9. doi: 10.1016/j.medmal.2020.04.006
47. Gupta V, Banavara Rajanna L, Upadhyay K, Bhatia R, Madhav Reddy N, Malik D, et al. Olfactory and gustatory dysfunction in COVID-19 patients from northern India: a cross-sectional observational study. Indian J Otolaryngol Head Neck Surg. (2021) 73:1–8. doi: 10.1007/s12070-021-02391-5
48. Kaye R, Chang CWD, Kazahaya K, Brereton J, Denneny JC. COVID-19 anosmia reporting tool: initial findings. Otolaryngol Head Neck Surg. (2020) 163:132–4. doi: 10.1177/0194599820922992
49. Yagmur AR, Akbal Cufali S, Aypak A, Koksal M, Gunes YC, Ozcan KM. Correlation of olfactory dysfunction with lung involvement and severity of COVID-19. Ir J Med Sci. (2021) 2021:1–6. doi: 10.1007/s11845-021-02732-x
50. Goshtasbi K, Pang J, Lehrich BM, Vasudev M, Birkenbeuel JL, Abiri A, et al. Association between olfactory dysfunction and critical illness and mortality in COVID-19: a meta-analysis. Otolaryngol Head Neck Surg. (2021) 2021:945998211017442. doi: 10.1177/01945998211017442
51. Tabari A, Golpayegani G, Tabari A, Saedi B, Mahdkhah A, Amali A, et al. Olfactory dysfunction is associated with more severe clinical course in COVID-19. Indian J Otolaryngol Head Neck Surg. (2021) 2021:1–6. doi: 10.1007/s12070-021-02507-x
52. Speth MM, Singer-Cornelius T, Oberle M, Gengler I, Brockmeier SJ, Sedaghat AR. Mood, anxiety and olfactory dysfunction in COVID-19: evidence of central nervous system involvement? Laryngoscope. (2020) 130:2520–5. doi: 10.1002/lary.28964
53. Islek A, Balci MK. Phantosmia with COVID-19 related olfactory dysfunction: report of nine case. Indian J Otolaryngol Head Neck Surg. (2021) 2021:1–3. doi: 10.1007/s12070-021-02505-z
54. Bhattacharjee AS, Joshi SV, Naik S, Sangle S, Abraham NM. Quantitative assessment of olfactory dysfunction accurately detects asymptomatic COVID-19 carriers. EClinicalMedicine. (2020) 28:100575. doi: 10.1016/j.eclinm.2020.100575
55. Li J, Wang X, Zhu C, Lin Z, Xiong N. Affected olfaction in COVID-19: re-defining “asymptomatic”. EClinicalMedicine. (2020) 29:100628. doi: 10.1016/j.eclinm.2020.100628
56. Larremore DB, Toomre D, Parker R. Modeling the effectiveness of olfactory testing to limit SARS-CoV-2 transmission. Nat Commun. (2021) 12:3664. doi: 10.1038/s41467-021-23315-5
57. Lechien JR, Cabaraux P, Chiesa-Estomba CM, Khalife M, Hans S, Calvo-Henriquez C, et al. Objective olfactory evaluation of self-reported loss of smell in a case series of 86 COVID-19 patients. Head Neck. (2020) 42:1583–90. doi: 10.1002/hed.26279
58. Meini S, Suardi LR, Busoni M, Roberts AT, Fortini A. Olfactory and gustatory dysfunctions in 100 patients hospitalized for COVID-19: sex differences and recovery time in real-life. Eur Arch Otorhinolaryngol. (2020) 277:3519–23. doi: 10.1007/s00405-020-06102-8
59. Cristillo V, Pilotto A, Cotti Piccinelli S, Zoppi N, Bonzi G, Gipponi S, et al. Age and subtle cognitive impairment are associated with long-term olfactory dysfunction after COVID-19 infection. J Am Geriatr Soc. (2021) 69:2778–80. doi: 10.1111/jgs.17296
60. Garg S, Kim L, Whitaker M, O'Halloran A, Cummings C, Holstein R, et al. Hospitalization rates and characteristics of patients hospitalized with laboratory-confirmed coronavirus disease 2019 - COVID-NET, 14 states, March 1-30, 2020. MMWR Morb Mortal Wkly Rep. (2020) 69:458–64. doi: 10.15585/mmwr.mm6915e3
61. Husain Q, Kokinakos K, Kuo YH, Zaidi F, Houston S, Shargorodsky J. Characteristics of COVID-19 smell and taste dysfunction in hospitalized patients. Am J Otolaryngol. (2021) 42:103068. doi: 10.1016/j.amjoto.2021.103068
62. Forster P, Forster L, Renfrew C, Forster M. Phylogenetic network analysis of SARS-CoV-2 genomes. Proc Natl Acad Sci USA. (2020) 117:9241–3. doi: 10.1073/pnas.2004999117
63. Lv H, Zhang W, Zhu Z, Xiong Q, Xiang R, Wang Y, et al. Prevalence and recovery time of olfactory and gustatory dysfunction in hospitalized patients with COVID19 in Wuhan, China. Int J Infect Dis. (2020) 100:507–12. doi: 10.1016/j.ijid.2020.09.039
64. Samimi Ardestani SH, Mohammadi Ardehali M, Rabbani Anari M, Rahmaty B, Erfanian R, Akbari M, et al. The coronavirus disease 2019: the prevalence, prognosis, and recovery from olfactory dysfunction (OD). Acta Otolaryngol. (2021) 141:171–80. doi: 10.1080/00016489.2020.1836397
65. Morais AHA, Passos TS, de Lima Vale SH, da Silva Maia JK, Maciel BLL. Obesity and the increased risk for COVID-19: mechanisms and nutritional management. Nutr Res Rev. (2021) 34:209–21. doi: 10.1017/S095442242000027X
66. Williams FMK, Freidin MB, Mangino M, Couvreur S, Visconti A, Bowyer RCE, et al. Self-reported symptoms of COVID-19, including symptoms most predictive of SARS-CoV-2 infection, are heritable. Twin Res Hum Genet. (2020) 23:316–21. doi: 10.1017/thg.2020.85
67. Shahrvini B, Prajapati DP, Said M, Liu J, Srinivas S, Jayaraj S, et al. Risk factors and characteristics associated with persistent smell loss in coronavirus disease 2019 (COVID-19) patients. Int Forum Allergy Rhinol. (2021) 11:1280–2. doi: 10.1002/alr.22802
68. Galluzzi F, Rossi V, Bosetti C, Garavello W. Risk factors for olfactory and gustatory dysfunctions in patients with SARS-CoV-2 infection. Neuroepidemiology. (2021) 55:154–61. doi: 10.1159/000514888
69. Tsivgoulis G, Fragkou PC, Karofylakis E, Paneta M, Papathanasiou K, Palaiodimou L, et al. Hypothyroidism is associated with prolonged COVID-19-induced anosmia: a case-control study. J Neurol Neurosurg Psychiatry. (2021) 92:911. doi: 10.1136/jnnp-2021-326587
70. Lu Y, Li X, Geng D, Mei N, Wu PY, Huang CC, et al. Cerebral micro-structural changes in COVID-19 patients - an MRI-based 3-month follow-up study. EClinicalMedicine. (2020) 25:100484. doi: 10.1016/j.eclinm.2020.100484
71. Cho RHW, To ZWH, Yeung ZWC, Tso EYK, Fung KSC, Chau SKY, et al. COVID-19 viral load in the severity of and recovery from olfactory and gustatory dysfunction. Laryngoscope. (2020) 130:2680–5. doi: 10.1002/lary.29056
72. Vaira LA, Deiana G, Lechien JR, De Vito A, Cossu A, Dettori M, et al. Correlations between olfactory psychophysical scores and SARS-CoV-2 viral load in COVID-19 patients. Laryngoscope. (2021) 131:2312–18. doi: 10.1002/lary.29777
73. Castillo-Lopez IY, Govea-Camacho LH, Rodriguez-Torres IA, Recio-Macias DA, Alobid I, Mullol J. Olfactory dysfunction in a Mexican population outside of COVID-19 pandemic: prevalence and associated factors (the OLFAMEX Study). Curr Allergy Asthma Rep. (2020) 20:78. doi: 10.1007/s11882-020-00975-9
74. Cavazzana A, Larsson M, Munch M, Hahner A, Hummel T. Postinfectious olfactory loss: a retrospective study on 791 patients. Laryngoscope. (2018) 128:10–15. doi: 10.1002/lary.26606
75. Wu J, Deng W, Li S, Yang X. Advances in research on ACE2 as a receptor for 2019-nCoV. Cell Mol Life Sci. (2021) 78:531–44. doi: 10.1007/s00018-020-03611-x
76. Hoffmann M, Kleine-Weber H, Schroeder S, Kruger N, Herrler T, Erichsen S, et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. (2020) 181:271–80.e8. doi: 10.1016/j.cell.2020.02.052
77. Ziegler CGK, Allon SJ, Nyquist SK, Mbano IM, Miao VN, Tzouanas CN, et al. SARS-CoV-2 receptor ACE2 is an interferon-stimulated gene in human airway epithelial cells and is detected in specific cell subsets across tissues. Cell. (2020) 181:1016–35.e19.
78. Hamming I, Timens W, Bulthuis ML, Lely AT, Navis G, van Goor H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J Pathol. (2004) 203:631–7. doi: 10.1002/path.1570
79. Solomon IH, Normandin E, Bhattacharyya S, Mukerji SS, Keller K, Ali AS, et al. Neuropathological features of Covid-19. N Engl J Med. (2020) 383:989–92. doi: 10.1056/NEJMc2019373
80. Meinhardt J, Radke J, Dittmayer C, Franz J, Thomas C, Mothes R, et al. Olfactory transmucosal SARS-CoV-2 invasion as a port of central nervous system entry in individuals with COVID-19. Nat Neurosci. (2021) 24:168–75. doi: 10.1101/2020.06.04.135012
81. Lempriere S. SARS-CoV-2 detected in olfactory neurons. Nat Rev Neurol. (2021) 17:63. doi: 10.1038/s41582-020-00449-6
82. Brann DH, Tsukahara T, Weinreb C, Lipovsek M, Van den Berge K, Gong B, et al. Non-neuronal expression of SARS-CoV-2 entry genes in the olfactory system suggests mechanisms underlying COVID-19-associated anosmia. Sci Adv. (2020) 6:eabc5801. doi: 10.1126/sciadv.abc5801
83. Brown LS, Foster CG, Courtney JM, King NE, Howells DW, Sutherland BA. Pericytes and neurovascular function in the healthy and diseased brain. Front Cell Neurosci. (2019) 13:282. doi: 10.3389/fncel.2019.00282
84. Sardu C, Gambardella J, Morelli MB, Wang X, Marfella R, Santulli G. Hypertension, thrombosis, kidney failure, and diabetes: is COVID-19 an endothelial disease? A comprehensive evaluation of clinical and basic evidence. J Clin Med. (2020) 9:1417. doi: 10.3390/jcm9051417
85. Chen R, Wang K, Yu J, Howard D, French L, Chen Z, et al. The spatial and cell-type distribution of SARS-CoV-2 receptor ACE2 in the human and mouse brains. Front Neurol. (2020) 11:573095. doi: 10.3389/fneur.2020.573095
86. Paniz-Mondolfi A, Bryce C, Grimes Z, Gordon RE, Reidy J, Lednicky J, et al. Central nervous system involvement by severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2). J Med Virol. (2020) 92:699–702. doi: 10.1002/jmv.25915
87. Libby P, Luscher T. COVID-19 is, in the end, an endothelial disease. Eur Heart J. (2020) 41:3038–44. doi: 10.1093/eurheartj/ehaa623
88. Siddiqi HK, Libby P, Ridker PM. COVID-19 - a vascular disease. Trends Cardiovasc Med. (2021) 31:1–5. doi: 10.1016/j.tcm.2020.10.005
89. Toor SM, Saleh R, Sasidharan Nair V, Taha RZ, Elkord E. T-cell responses and therapies against SARS-CoV-2 infection. Immunology. (2021) 162:30–43. doi: 10.1111/imm.13262
90. Costela-Ruiz VJ, Illescas-Montes R, Puerta-Puerta JM, Ruiz C, Melguizo-Rodriguez L. SARS-CoV-2 infection: the role of cytokines in COVID-19 disease. Cytokine Growth Factor Rev. (2020) 54:62–75. doi: 10.1016/j.cytogfr.2020.06.001
91. Cazzolla AP, Lovero R, Lo Muzio L, Testa NF, Schirinzi A, Palmieri G, et al. Taste and smell disorders in COVID-19 patients: role of interleukin-6. ACS Chem Neurosci. (2020) 11:2774–81. doi: 10.1021/acschemneuro.0c00447
92. Wang S, Le TQ, Kurihara N, Chida J, Cisse Y, Yano M, et al. Influenza virus-cytokine-protease cycle in the pathogenesis of vascular hyperpermeability in severe influenza. J Infect Dis. (2010) 202:991–1001. doi: 10.1086/656044
93. Allan SM, Rothwell NJ. Cytokines and acute neurodegeneration. Nat Rev Neurosci. (2001) 2:734–44. doi: 10.1038/35094583
94. Wolfel R, Corman VM, Guggemos W, Seilmaier M, Zange S, Muller MA, et al. Virological assessment of hospitalized patients with COVID-2019. Nature. (2020) 581:465–9. doi: 10.1038/s41586-020-2196-x
95. Sungnak W, Huang N, Becavin C, Berg M, Queen R, Litvinukova M, et al. SARS-CoV-2 entry factors are highly expressed in nasal epithelial cells together with innate immune genes. Nat Med. (2020) 26:681–7. doi: 10.1038/s41591-020-0868-6
96. Chen M, Shen W, Rowan NR, Kulaga H, Hillel A, Ramanathan M, et al. Elevated ACE-2 expression in the olfactory neuroepithelium: implications for anosmia and upper respiratory SARS-CoV-2 entry and replication. Eur Respir J. (2020) 56:2001948. doi: 10.1183/13993003.01948-2020
97. Fodoulian L, Tuberosa J, Rossier D, Boillat M, Kan C, Pauli V, et al. SARS-CoV-2 receptors and entry genes are expressed in the human olfactory neuroepithelium and brain. iScience. (2020) 23:101839. doi: 10.1016/j.isci.2020.101839
98. Cecchini MP, Brozzetti L, Cardobi N, Sacchetto L, Gibellini D, Montemezzi S, et al. Persistent chemosensory dysfunction in a young patient with mild COVID-19 with partial recovery 15 months after the onset. Neurol Sci. (2021) 2021:1–6. doi: 10.1007/s10072-021-05635-y
99. Suzuki Y, Takeda M, Farbman AI. Supporting cells as phagocytes in the olfactory epithelium after bulbectomy. J Comp Neurol. (1996) 376:509–17. doi: 10.1002/(SICI)1096-9861(19961223)376:4<509::AID-CNE1>3.0.CO;2-5
100. Vogalis F, Hegg CC, Lucero MT. Ionic conductances in sustentacular cells of the mouse olfactory epithelium. J Physiol. (2005) 562:785–99. doi: 10.1113/jphysiol.2004.079228
101. Chen M, Reed RR, Lane AP. Chronic inflammation directs an olfactory stem cell functional switch from neuroregeneration to immune defense. Cell Stem Cell. (2019) 25:501–13.e505. doi: 10.1016/j.stem.2019.08.011
102. Fletcher RB, Das D, Gadye L, Street KN, Baudhuin A, Wagner A, et al. Deconstructing olfactory stem cell trajectories at single-cell resolution. Cell Stem Cell. (2017) 20:817–30.e8. doi: 10.1016/j.stem.2017.04.003
103. Schwob JE, Jang W, Holbrook EH, Lin B, Herrick DB, Peterson JN, et al. Stem and progenitor cells of the mammalian olfactory epithelium: Taking poietic license. J Comp Neurol. (2017) 525:1034–54. doi: 10.1002/cne.24105
104. Durante MA, Kurtenbach S, Sargi ZB, Harbour JW, Choi R, Kurtenbach S, et al. Single-cell analysis of olfactory neurogenesis and differentiation in adult humans. Nat Neurosci. (2020) 23:323–6. doi: 10.1038/s41593-020-0587-9
105. de Melo GD, Lazarini F, Levallois S, Hautefort C, Michel V, Larrous F, et al. COVID-19-related anosmia is associated with viral persistence and inflammation in human olfactory epithelium and brain infection in hamsters. Sci Transl Med. (2021) 13:eabf8396. doi: 10.1126/scitranslmed.abf8396
106. Lechien JR, Michel J, Radulesco T, Chiesa-Estomba CM, Vaira LA, De Riu G, et al. Clinical and radiological evaluations of COVID-19 patients with anosmia: preliminary report. Laryngoscope. (2020) 130:2526–31. doi: 10.1002/lary.28993
107. Mattos JL, Schlosser RJ, Mace JC, Smith TL, Soler ZM. Establishing the minimal clinically important difference for the Questionnaire of Olfactory Disorders. Int Forum Allergy Rhinol. (2018) 8:1041–6. doi: 10.1002/alr.22135
108. Mattos JL, Edwards C, Schlosser RJ, Hyer M, Mace JC, Smith TL, et al. A brief version of the questionnaire of olfactory disorders in patients with chronic rhinosinusitis. Int Forum Allergy Rhinol. (2019) 9:1144–50. doi: 10.1002/alr.22392
109. Landis BN, Hummel T, Hugentobler M, Giger R, Lacroix JS. Ratings of overall olfactory function. Chem Senses. (2003) 28:691–4. doi: 10.1093/chemse/bjg061
110. Bagnasco D, Passalacqua G, Braido F, Tagliabue E, Cosini F, Filauro M, et al. Quick Olfactory Sniffin' Sticks Test (Q-Sticks) for the detection of smell disorders in COVID-19 patients. World Allergy Organ J. (2021) 14:100497. doi: 10.1016/j.waojou.2020.100497
111. Hummel T, Sekinger B, Wolf SR, Pauli E, Kobal G. “Sniffin' sticks”: olfactory performance assessed by the combined testing of odor identification, odor discrimination and olfactory threshold. Chem Senses. (1997) 22:39–2. doi: 10.1093/chemse/22.1.39
112. Hura N, Xie DX, Choby GW, Schlosser RJ, Orlov CP, Seal SM, et al. Treatment of post-viral olfactory dysfunction: an evidence-based review with recommendations. Int Forum Allergy Rhinol. (2020) 10:1065–86. doi: 10.1002/alr.22624
113. Addison AB, Wong B, Ahmed T, Macchi A, Konstantinidis I, Huart C, et al. Clinical Olfactory Working Group consensus statement on the treatment of postinfectious olfactory dysfunction. J Allergy Clin Immunol. (2021) 147:1704–19. doi: 10.1016/j.jaci.2020.12.641
114. Reiter ER, Coelho DH, Kons ZA, Costanzo RM. Subjective smell and taste changes during the COVID-19 pandemic: Short term recovery. Am J Otolaryngol. (2020) 41:102639. doi: 10.1016/j.amjoto.2020.102639
115. Boscolo-Rizzo P, Borsetto D, Fabbris C, Spinato G, Frezza D, Menegaldo A, et al. Evolution of altered sense of smell or taste in patients with mildly symptomatic COVID-19. JAMA Otolaryngol Head Neck Surg. (2020) 146:729–32. doi: 10.1001/jamaoto.2020.1379
116. Li J, Long X, Zhu C, Wang H, Wang T, Lin Z, et al. Olfactory dysfunction in recovered coronavirus disease 2019 (COVID-19) patients. Mov Disord. (2020) 35:1100–1. doi: 10.1002/mds.28172
117. Croy I, Nordin S, Hummel T. Olfactory disorders and quality of life–an updated review. Chem Senses. (2014) 39:185–94. doi: 10.1093/chemse/bjt072
118. Philpott CM, Boak D. The impact of olfactory disorders in the United kingdom. Chem Senses. (2014) 39:711–18. doi: 10.1093/chemse/bju043
119. Erskine SE, Philpott CM. An unmet need: patients with smell and taste disorders. Clin Otolaryngol. (2020) 45:197–203. doi: 10.1111/coa.13484
120. Pekala K, Chandra RK, Turner JH. Efficacy of olfactory training in patients with olfactory loss: a systematic review and meta-analysis. Int Forum Allergy Rhinol. (2016) 6:299–307. doi: 10.1002/alr.21669
121. Sorokowska A, Drechsler E, Karwowski M, Hummel T. Effects of olfactory training: a meta-analysis. Rhinology. (2017) 55:17–26. doi: 10.4193/Rhin16.195
122. Kattar N, Do TM, Unis GD, Migneron MR, Thomas AJ, McCoul ED. Olfactory Training for postviral olfactory dysfunction: systematic review and meta-analysis. Otolaryngol Head Neck Surg. (2021) 164:244–54. doi: 10.1177/0194599820943550
123. Hummel T, Rissom K, Reden J, Hahner A, Weidenbecher M, Huttenbrink KB. Effects of olfactory training in patients with olfactory loss. Laryngoscope. (2009) 119:496–9. doi: 10.1002/lary.20101
124. Altundag A, Cayonu M, Kayabasoglu G, Salihoglu M, Tekeli H, Saglam O, et al. Modified olfactory training in patients with postinfectious olfactory loss. Laryngoscope. (2015) 125:1763–6. doi: 10.1002/lary.25245
125. Seo BS, Lee HJ, Mo JH, Lee CH, Rhee CS, Kim JW. Treatment of postviral olfactory loss with glucocorticoids, Ginkgo biloba, and mometasone nasal spray. Arch Otolaryngol Head Neck Surg. (2009) 135:1000–4. doi: 10.1001/archoto.2009.141
126. Schriever VA, Merkonidis C, Gupta N, Hummel C, Hummel T. Treatment of smell loss with systemic methylprednisolone. Rhinology. (2012) 50:284–9. doi: 10.4193/Rhino11.207
127. Nguyen TP, Patel ZM. Budesonide irrigation with olfactory training improves outcomes compared with olfactory training alone in patients with olfactory loss. Int Forum Allergy Rhinol. (2018) 8:977–81. doi: 10.1002/alr.22140
128. Kim DH, Kim SW, Hwang SH, Kim BG, Kang JM, Cho JH, et al. Prognosis of olfactory dysfunction according to etiology and timing of treatment. Otolaryngol Head Neck Surg. (2017) 156:371–7. doi: 10.1177/0194599816679952
129. Blomqvist EH, Lundblad L, Bergstedt H, Stjarne P. Placebo-controlled, randomized, double-blind study evaluating the efficacy of fluticasone propionate nasal spray for the treatment of patients with hyposmia/anosmia. Acta Otolaryngol. (2003) 123:862–8. doi: 10.1080/00016480310002140
130. Heilmann S, Huettenbrink KB, Hummel T. Local and systemic administration of corticosteroids in the treatment of olfactory loss. Am J Rhinol. (2004) 18:29–33. doi: 10.1177/194589240401800107
131. Yan CH, Overdevest JB, Patel ZM. Therapeutic use of steroids in non-chronic rhinosinusitis olfactory dysfunction: a systematic evidence-based review with recommendations. Int Forum Allergy Rhinol. (2019) 9:165–76. doi: 10.1002/alr.22240
132. Kanjanaumporn J, Aeumjaturapat S, Snidvongs K, Seresirikachorn K, Chusakul S. Smell and taste dysfunction in patients with SARS-CoV-2 infection: A review of epidemiology, pathogenesis, prognosis, and treatment options. Asian Pac J Allergy Immunol. (2020) 38:69–77. doi: 10.12932/AP-030520-0826
133. Whitcroft KL, Gunder N, Cuevas M, Andrews P, Menzel S, Haehner A, et al. Intranasal sodium citrate in quantitative and qualitative olfactory dysfunction: results from a prospective, controlled trial of prolonged use in 60 patients. Eur Arch Otorhinolaryngol. (2021) 278:2891–7. doi: 10.1007/s00405-020-06567-7
Keywords: COVID-19, olfactory dysfunction, clinical characteristics, mechanism, treatment
Citation: Wei G, Gu J, Gu Z, Du C, Huang X, Xing H, Li L, Zhang A, Hu X and Huo J (2022) Olfactory Dysfunction in Patients With Coronavirus Disease 2019: A Review. Front. Neurol. 12:783249. doi: 10.3389/fneur.2021.783249
Received: 25 September 2021; Accepted: 14 December 2021;
Published: 18 January 2022.
Edited by:
Ghazala Hayat, Saint Louis University, United StatesReviewed by:
Maria Paola Cecchini, University of Verona, ItalyCopyright © 2022 Wei, Gu, Gu, Du, Huang, Xing, Li, Zhang, Hu and Huo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xingxing Hu, aHV4aW5neGluZzgyQDE2My5jb20=; Jiege Huo, aHVvamllZ2VAanNhdGNtLmNvbQ==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.