
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Neurol. , 01 February 2022
Sec. Stroke
Volume 12 - 2021 | https://doi.org/10.3389/fneur.2021.780080
 Viviënne Huppertz1*
Viviënne Huppertz1* Sonia Guida2
Sonia Guida2 Anne Holdoway3
Anne Holdoway3 Stefan Strilciuc4,5
Stefan Strilciuc4,5 Laura Baijens6
Laura Baijens6 Jos M. G. A. Schols7
Jos M. G. A. Schols7 Ardy van Helvoort1,2
Ardy van Helvoort1,2 Mirian Lansink2
Mirian Lansink2 Dafin F. Muresanu4,5
Dafin F. Muresanu4,5Background: Malnutrition is common after stroke and can affect rehabilitation and healthcare costs. A comprehensive overview of stroke patients' nutritional condition from the hyperacute to the chronic phase is lacking. This systematic review aimed to investigate the prevalence of impaired nutritional condition (INC) across the continuum of care in specific phases after stroke.
Methods: CAB ABSTRACTS, Embase, MEDLINE, were used to collect studies published between 01-01-1999 and 26-08-2020. Primary and secondary outcomes were prevalence of INC and prevalence of malnutrition, respectively. Exploratory outcomes were prevalence of INC at follow-up, nutritional examination methods, prevalence of dysphagia, stroke severity, adverse events, and continent-specific prevalence of INC. A random-effects meta-analysis model was used to estimate the phase-specific pooled prevalence of INC and malnutrition.
Results: The dataset consisted of 78 study groups selected over a total of 1,244 identified records. The pooled prevalence of INC and malnutrition were 19% (95%CI:7–31) (N = 4) and 19% (95%CI:9–29) (N = 3), 34% (95%CI:25–43) (N = 34) and 26% (95%CI:18–35) (N = 29), 52% (95%CI:43–61) (N = 34) and 37% (95%CI:28–45) (N = 31), 21% (95%CI:12–31) (N = 3) and 11% (95%CI:0–24) (N = 3) and 72% (95%CI:41–100) (N = 3) and 30% (95%CI:0–76) (N = 2) in the hyperacute, acute, early subacute, late subacute, and chronic phase, respectively.
Conclusion: INC and malnutrition are highly prevalent in all stages of stroke care. Since malnutrition has been shown to negatively affect clinical outcomes, mortality, and overall healthcare expenditure in stroke survivors, it is essential to examine and monitor the nutritional status of stroke patients throughout their care journey to guide and plan, timely nutritional support and dietary modification.
Malnutrition is common after stroke (1) and relates to poor outcomes as assessed with the modified Ranking Scale, increased prevalence of complications, length of hospital stay, mortality, and hospitalization costs (2, 3). Several factors that occur after stroke, including dysphagia (4), hemiparesis, decreased mobility, depression (5) and post-stroke dementia (6) compound the risk of malnutrition. Multiple studies in stroke patients have consistently demonstrated that the recommended nutritional intake is not achieved after stroke (7–11). Over the past decade, stroke patient outcomes have continuously improved through thrombectomy, recombinant tissue plasminogen activator treatments and case management in stroke units (12). As stroke mortality declines, rehabilitation's importance is growing due to high disability rates among survivors, leading to a high overall burden on global healthcare. In Europe, the total cost of stroke was estimated at €60.0 billion in 2017 (13); almost half of this budget was spent on direct healthcare. The remaining costs were related to informal care, social care systems, non-health or social care areas and productivity losses. Multidisciplinary and structured stroke rehabilitation reduces disability related to stroke regardless of age, sex, and stroke severity (14). Combining neurorehabilitation strategies, such as early mobilization and pharmacological intervention (15, 16), also offers the potential to improve outcomes and reduce costs after stroke. The clinical stroke pathway begins immediately after onset (hyperacute phase), ultimately reaching a chronic phase around six months post-stroke (17). The optimal time window for rehabilitation therapies is considered to be before the observed peak of recovery, between stroke onset and three months after the stroke event (18). Stroke care guidelines recommend using a multidisciplinary approach (5, 14) including nutritional screening and treatment of malnutrition (19, 20). As indicated, malnutrition is common after a stroke. Foley (21) reviewed studies on the prevalence of malnutrition after stroke and possible causes for heterogeneity of its prevalence. They observed a prevalence of malnutrition ranging from 6.1 to 62.0%, but a comprehensive overview of stroke patients' nutritional status from the hyperacute to the chronic phase is lacking. Considering the relevance of nutritional status in the recovery process, this systematic review aimed to investigate the prevalence of impaired nutritional condition (INC), defined as the percentage of not well-nourished patients, across the continuum of care in specific phases after stroke. The term “nutritional condition” is used to describe the results of this review.
This systematic review was executed following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) checklist (22) and registered in the international Prospective Register of Systematic Reviews (PROSPERO) (23) (registration number: CRD42020205891).
The literature search was performed in ProQuest® by a librarian specialist. ProQuest® was used to inspect three databases (CAB ABSTRACTS, Embase, MEDLINE) for a conceptual string composed of “stroke” (OR synonyms) AND “malnutrition” (OR synonyms). The search was performed for literature published in English between 01-01-1999 to 26-08-2020. Document types excluded from the search were conference abstracts, conference papers, conference reviews, case reports, book chapters, short surveys, retracted publications, letters, editorials, clinical trial protocols, and technical reports. The full search strategy is available in the Supplemental Material (Supplemental Table 1).
Meta-analyses, reviews, intended trials, case reports, pharmaceutical clinical trials, and studies including a re-analysis of a study sample were excluded. The population's inclusion criteria were met if the age was ≥ 18 years, and patients were examined for nutritional status within 0 h up to two years after stroke onset. Studies where the nutritional status was used as an eligibility criterion to recruit patients with a specific nutritional status were excluded. Studies were excluded when the entire population was in a comatose/vegetative state or on parenteral nutrition at admission to the study. Selection of the data required that the prevalence of INC was reported in the study as a percentage value or absolute number. The study was excluded if the nutritional status was examined using body mass index (BMI) only. BMI categories might be difficult to interpret considering that both underweight and obese patients can suffer from malnutrition (24). Studies where no indications were provided on the method used for the nutritional examination were excluded. Follow-up data were not included when interventions with an impact on the nutritional status were investigated. If a study reported the prevalence of INC or malnutrition in completely independent groups, the data were treated separately. For example, in studies where the study population was separated into two groups that received the nutritional examination in different time periods, the data on prevalence were treated separately.
Duplicates were removed manually. Screening of titles and abstracts was performed by one reviewer (VH or SG). Two reviewers (VH and SG) performed the screening of full-text articles and data extraction for primary and secondary parameters. A third reviewer (CvdB) was consulted in case of a disagreement. One reviewer performed the data extraction of the exploratory parameters (VH), and in case of ambiguity, the second reviewer (SG) was consulted. Percentage values were recalculated for accuracy when needed. Reasons for exclusion of the full-text articles were classified according to the Population, Intervention, Comparison, and Outcome (PICO) framework (25). The PICO framework can be used to systematically identify and document clinical evidence. Studies were excluded if the inclusion criteria related to the “population” (e.g., age) and/or to the “outcome” (e.g., missing prevalence data) were not met, or if there was any other reason for exclusion (e.g., language) which was defined “non-PICO”. The current systematic review does not aim to address research questions related to treatments or differences between intervention and control groups; therefore, the categories “intervention” and “comparison” were not used.
The primary outcome is the prevalence of INC in each phase after stroke by using the definition of timing described by Bernhardt (17) and limiting the chronic phase to two years after stroke: hyperacute (≤ 24 h), acute (> 24 h– ≤ 7 days), early subacute (> 7 days– <3 months), late subacute (≥ 3 months– <6 months), and chronic (≥ 6 months−2 years). Prevalence of INC at baseline was reported for each study included in the analysis and comprised the full dataset. The secondary outcome is the prevalence of malnutrition in the phases mentioned above. A phase-specific pooled prevalence was estimated for the primary and secondary outcomes. Exploratory outcomes are the prevalence of INC at follow-up, methods used for the nutritional examination (percentage of study groups reporting on screening/assessment tools and anthropometrical/biochemical measurements, and description of the methods), the prevalence of dysphagia, stroke severity evaluated with the National Institutes of Health Stroke Scale (NIHSS), adverse events, and continent-specific prevalence of INC.
The prevalence data, as shown in this paper, were based on the method found in the respective study. In case a study reported multiple methods to examine the nutritional status, only one method was selected based on whether it was a method used to generate the primary results or a method largely used in the literature. The methods found in the studies were distinguished in screening/assessment tools or anthropometrical/biochemical measurements. Screening/assessment tools included methods whose outcomes were expressed in pre-defined categories (e.g., no malnutrition, at risk of malnutrition or malnourished). Examples of these tools are, among others, the Malnutrition Universal Screening Tool (“MUST”), and the Mini Nutritional Assessment (MNA). Anthropometrical/biochemical measurements included methods that used measurements of anthropometrical or biochemical parameters. Examples of these measurements are bodyweight and serum albumin levels. The extracted data from the nutritional screening/assessment tools needed to be harmonized according to the definition of INC and malnutrition in this systematic review. There was no need to harmonize data from the Global Leadership Initiative on Malnutrition (GLIM) and the European Society for Clinical Nutrition and Metabolism (ESPEN) diagnostic criteria for malnutrition because in this case the outcomes are not expressed in pre-defined categories but rather on the diagnosis of malnutrition after screening. The criteria used for harmonization of data derived from the remaining screening/assessment tools are shown in the Supplemental Table 2.
In case the time of nutritional examination after stroke (TNE-S-E) was not reported in the study it was estimated according to the following conditions: (i) time of admission after stroke (TA-S) and time of nutritional examination after admission (TNE-A) were available, (ii) TA-S was missing, but information on the phase after stroke was available. The criteria used for the estimation of TNE-S-E are reported in the Supplemental Figure 1.
Risk of bias was evaluated for each study using a self-developed checklist including seven questions related to selection, performance, detection, and reporting bias: (1) Is there a reason to believe that the study population is not representative for the stroke population in the assigned phase after stroke? (selection bias). The answer to this question evaluated whether the setting in which the patients were recruited was representative for the phase to which the study group was assigned. All acute care settings were considered representative for study groups assigned to the hyperacute, or acute phase after stroke. Hospitals, rehabilitation centers, long term care facilities and home (care) were considered representative settings for study groups assigned to the early subacute, late subacute and chronic phase after stroke; however, in case the study was performed in only one of these settings a risk of bias was detected (2) Was the stroke diagnosis confirmed using a CT scan / MRI? (performance bias I) (3) Was a validated screening/assessment tool used for nutritional examination? (performance bias II) (4) Was the method used for the nutritional examination clearly defined in the study? (detection bias I) (5) Was the method used for the nutritional examination consistently used in the study? (detection bias II) (6) Where the prevalence data for all stroke patients who received the nutritional examination available in the study? (reporting bias I) (7) Where the prevalence data complete according to the criteria applied to the screening/assessment tools? (reporting bias II). Question 1, 2, 4, 5, and 6 were scored dichotomously (risk of bias/no risk of bias) and question 3 and 7 were scored trichotomously (risk of bias/no risk of bias/not applicable). Question 3 was not applicable in case the nutritional status was examined using anthropometrical/biochemical measurements. Question 7 was not applicable in case the nutritional status was examined using anthropometrical/biochemical measurements or if GLIM or ESPEN criteria were used. A relative risk of bias [relative risk (%)] was calculated as a percentage of the total number of items that were scored.
TNE-S-E and TA-S were used as initially reported in the study either as mean (SD), median [range, interquartile range (IQR)] or as a value described in the text. When the mean (SD) was not available, the median (range, IQR) was used. This approach is in line with Hozo (26), who showed that, for sample sizes larger than 25, replacing the sample mean with the reported median is the best estimator for the sample mean. The pooled prevalence of INC and malnutrition was estimated using random-effects (RE) (27) and fixed-effect (FE) (28) meta-analysis models. A RE meta-analysis model was preferred over a FE (29, 30) and used for the interpretation of the results. The between-study variance of the RE model, τ2, was estimated via the restricted maximum likelihood approach (31). A meta-analysis of prevalence estimated a weighted average prevalence of the observed proportions, accompanied by a 95% confidence interval (95% CI). NIHSS scores were collected as originally reported in the study, either as a mean or as a median, and used to define the category of stroke severity according to the NIHS Scale. The statistical analyses were carried out in RStudio (R, version 4.0.0; R Project), using the function “rma.uni” from the package metafor to pool the raw proportions and package meta to create the forest plots.
A total of 1,244 articles were identified through the literature search, of which 99 in CAB ABSTRACTS, 914 in Embase, and 231 in MEDLINE. A total of 233 full-text articles were assessed for eligibility, of which 75 were included in the analysis. In three studies, the nutritional status was evaluated in independent study groups, and this resulted in a total of 78 study groups (Figure 1).
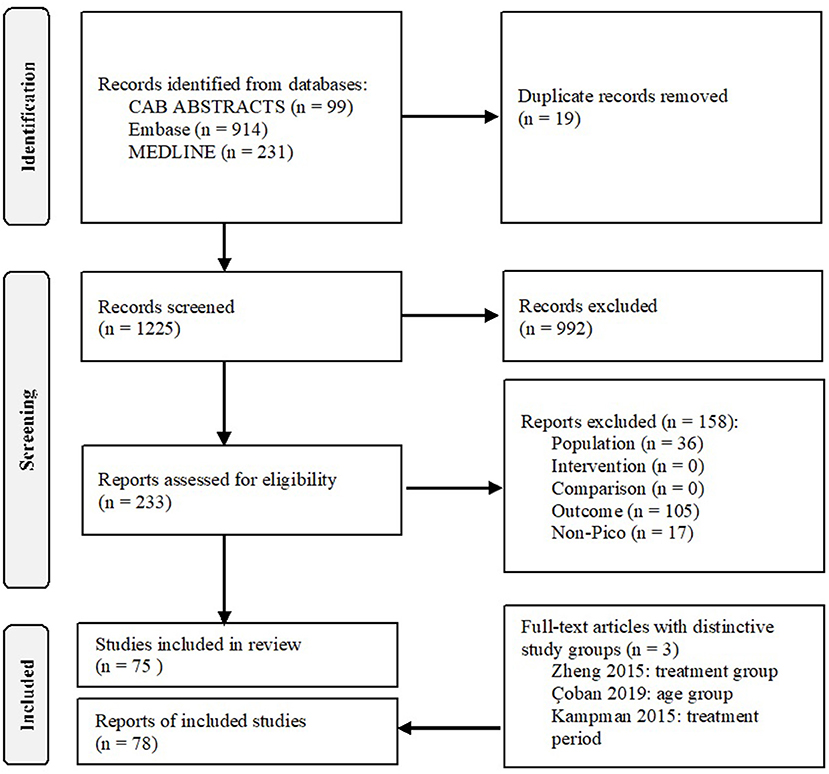
Figure 1. Flowchart. Used under the Creative Commons Attribution License terms, adapted from (22).
Study designs were observational and experimental in 68 (87.2%) and 10 (12.8%) study groups, respectively. Fifty-six (71.8%) study groups were performed in hospitals, 17 (21.8%) in rehabilitation facilities, three (3.8%) in nursing homes/care homes/home, and two (2.6%) in a combination of settings. The type of diagnosis reported among the study groups was mainly ischemic and haemorrhagic stroke. TNE-S-E was available and therefore not estimated in 14 (17.9%) out of 78 study groups. The total number of stroke patients who received the nutritional examination was 25,090 ranging from 12 to 4,023 patients per study group. An overview of the general characteristics of the studies is provided in the Supplemental Table 3.
Out of 78 study groups with data on INC, four (5.1%) were conducted in the hyperacute, 34 (43.6%) in the acute, 34 (43.6%) in the early subacute, three (3.8%) in the late subacute, and three (3.8%) in the chronic phase. Overall, the prevalence of INC across phases ranged from 3.8 to 100.0%. Prevalence of INC ranged from 11.1 to 36.3% in the hyperacute phase, 5.0 to 100% in the acute phase, 3.8 to 100% in the early subacute phase, 12.1 to 27.8% in the late subacute phase and 41 to 91.4% in the chronic phase (Figure 2A). Combining the individual prevalence numbers per phase yielded a pooled prevalence of 19% (95%CI: 7–31) based on four study groups in the hyperacute phase, 34% (95%CI: 25–43) based on 34 study groups in the acute phase, 52% (95%CI: 43–61) based on 34 study groups in the early subacute phase, 21% (95%CI: 12–31) based on three study groups in the late subacute phase, and 72% (95%CI: 41–100) based on three study groups in the chronic phase (Figure 3). In the phases where the pooled prevalence was based on a number of study groups ≤ 5, the results generated with the RE and FE meta-analysis models were overall similar.
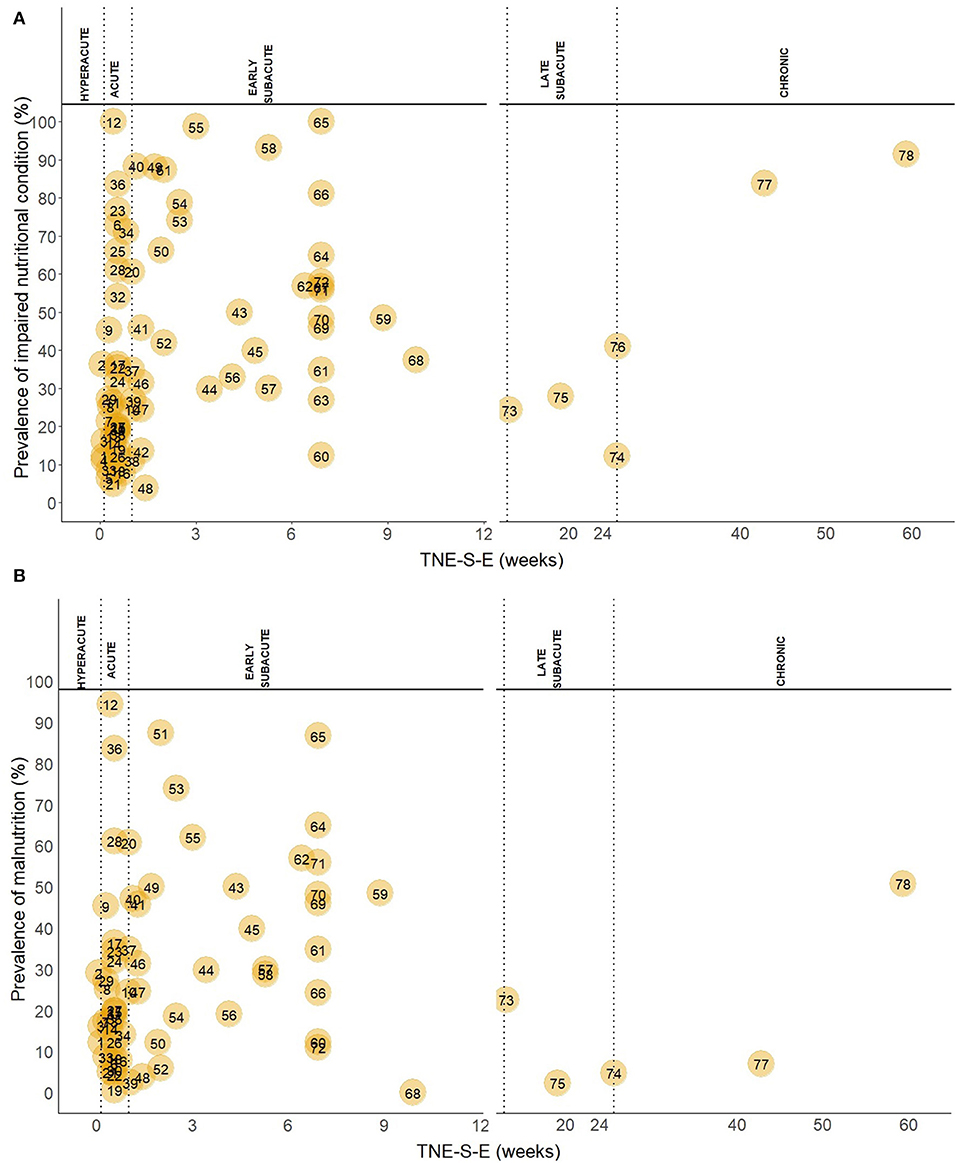
Figure 2. (A) Prevalence of INC in the hyperacute, acute, early subacute, late subacute and chronic phase after stroke. TNE-S-E is shown in a different scale in the hyperacute, acute, and early subacute phase compared to the late subacute and chronic phase. Numbers in the plot indicate the references to the study groups and are listed below. (B) Prevalence of malnutrition in the hyperacute, acute, early subacute, late subacute and chronic phase after stroke. TNE-S-E is shown in a different scale in the hyperacute, acute, and early subacute phase compared to the late subacute and chronic phase. Numbers in the plot indicate the references to the study groups and are listed: 1, Yoo (2); 2, Gomes (3); 3, Davis (32); 4, Kokura (33); 5, Nozoe (34); 6, Nip (8); 7, Sremanakova (35); 8, Diendéré (36); 9, Vajpayee (37); 10, Gandolfo (38); 11, Crary (39); 12, NanZhu (40); 13, Zheng I (41); 14, Zheng II (41); 15, Shen (42); 16, Food Trial 2005(b) (43); 17, Xiang (44); 18, Kokura (45); 19, Barrio (46); 20, Otsuki (47); 21, Robertson (48); 22, López Espuela (49); 23, Aliasghari (50); 24, Crary (51); 25, Çoban I (52); 26, Çoban II (52); 27, Schwarz (53); 28, Porter (54); 29, Pandian (55); 30, Mosselman (56); 31, Martineau (57); 32, Ha (58); 33, Food Trial 2005(a) (59); 34, Medin (60); 35, Isono (61); 36, Far (62); 37, Brynningsen (63); 38, Kokura (64); 39, Kang (65); 40, Drozdz (66); 41, Cai (67); 42, Naito (68); 43, Hirano (69); 44, Nishioka 2020(b) (70); 45, Nishioka 2020(a) (71); 46, Kampman I (72); 47, Kampman II (72); 48, Zhang (73); 49, Shiraishi (74); 50, Hsieh (75); 51, Falsetti (76); 52, Sato (77); 53, Lim (78); 54, James (79); 55, Nishioka (80); 56, Aadal (81); 57, Aquilani (10); 58, Nishioka (82); 59, Garbagnati (83); 60, Westergren (84); 61, Poels (85); 62, Hama (86); 63, Maruyama (87); 64, Shimizu (88); 65, Carlsson (89); 66, Tsai (90); 67, Kaur (91); 68, Jung (92); 69, van Zwienen-Pot (93); 70, Campillo (94); 71, Da Silva (95); 72, Lelli (96); 73, Scrutinio (97); 74, Perry (11); 75, Vilardell (98); 76, Westergren (99); 77, Choi (100); 78, Kim (101).
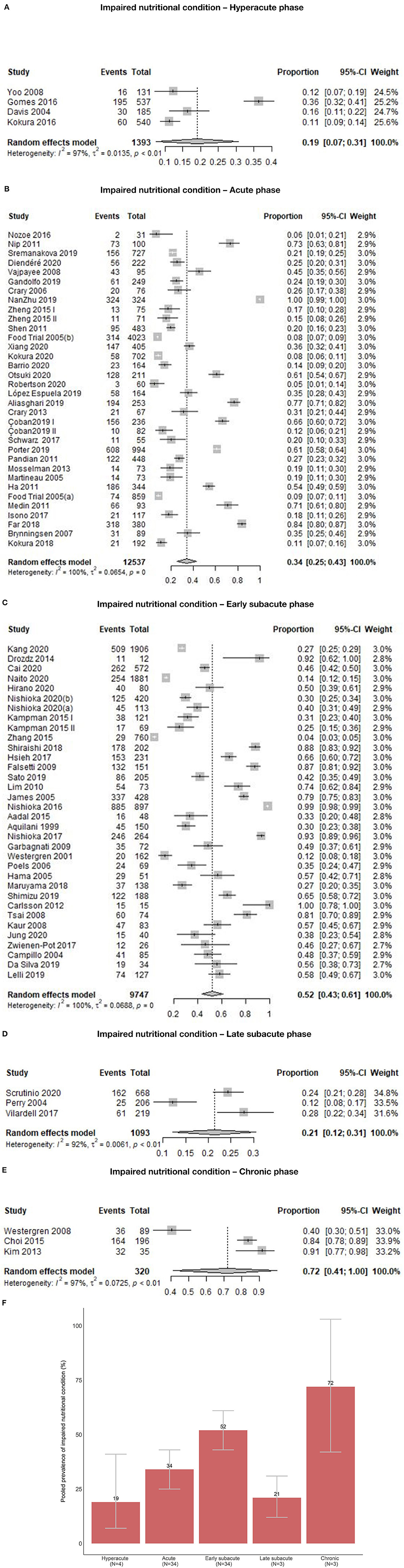
Figure 3. (A) Pooled prevalence of INC in the hyperacute phase. (B) Pooled prevalence of INC in the acute phase. (C) Pooled prevalence of INC in the early subacute phase. (D) Pooled prevalence of INC in the late subacute phase. (E) Pooled prevalence of INC in the chronic phase. (F) Pooled prevalence of INC per phase.
Of 68 study groups with data on malnutrition, three (4.4%) were conducted in the hyperacute, 29 (42.6%) in the acute, 31 (45.6%) in the early subacute, three (4.4%) in the late subacute, and two (2.9%) in the chronic phase after stroke. Overall, the prevalence of malnutrition across phases ranged from 0.0 to 94.4%. Prevalence of malnutrition ranged from 12.2 to 29.1% in the hyperacute phase, 0.6 to 94.4% in the acute phase, 0.0 to 87.4% in the early subacute phase, 2.7 to 24.3% in the late subacute phase, and 7.7 to 54.3% in the chronic phase (Figure 2B). Combining the individual prevalence numbers per phase yielded a pooled prevalence of 19% (95%CI:9–29) based on three study groups in the hyperacute phase, 26% (95%CI:18–35) based on 29 study groups in the acute phase, 37% (95%CI:28–45) based on 31 study groups in the early subacute phase, 11% (95%CI:0–24) based on three study groups in the late subacute phase, and 30% (95%CI:0–76) based on two study groups in the chronic phase (Figure 4). In the phases where the pooled prevalence was based on a number of study groups ≤ 5, the results generated with the RE and FE meta-analysis models were overall similar, except for the chronic phase where the FE meta-analysis model showed a pooled prevalence of 10%.
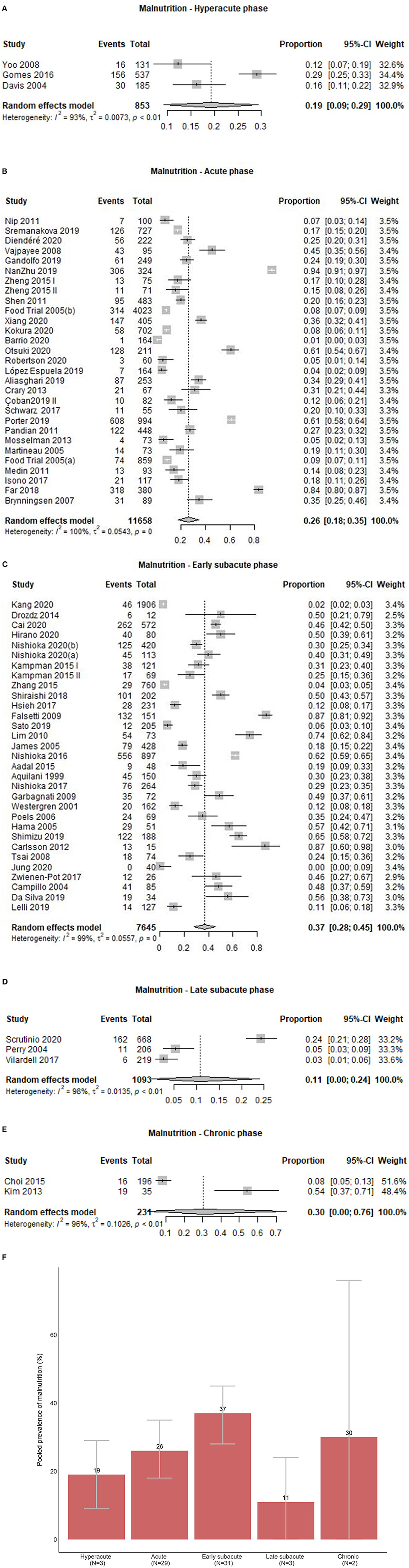
Figure 4. (A) Pooled prevalence of malnutrition in the hyperacute phase. (B) Pooled prevalence of malnutrition in the acute phase. (C) Pooled prevalence of malnutrition in the early subacute phase. (D) Pooled prevalence of malnutrition in the late subacute phase. (E) Pooled prevalence of malnutrition in the chronic phase. (F) Pooled prevalence estimates of malnutrition per phase.
Follow-up data on INC at different time points were available in 13 (16.7%) out of the 78 study groups. An increased prevalence of INC occurred in most of these 13 study groups and within three months after stroke (Figure 5).
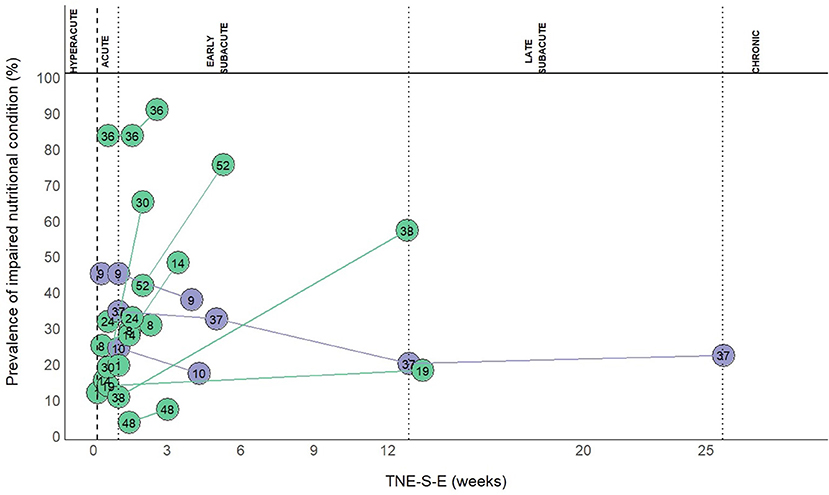
Figure 5. Prevalence of INC at follow-up. Numbers in the plot indicate the references to the study groups: 1, Yoo 2008 (2); 8, Diendéré 2020 (36); 9, Vajpayee 2008 (37); 10, Gandolfo 2019 (38); 14, Zheng 2015 II (41); 19, Barrio 2020 (46); 24, Crary 2013 (51); 30, Mosselman 2013 (56); 36, Far 2018 (62); 37, Brynningsen 2007 (63); 38, Kokura 2018 (64); 48, Zhang 2015 (73); 52, Sato 2019 (77).
Screening/assessment tools and anthropometrical/biochemical measurements were used for the nutritional examination in 56 (71.8%), and 19 (24.4%) out of the 78 study groups, respectively, and three (3.8%) reported various methods. Twenty (35.7%) of the 56 study groups used the MNA (102) or the MNA short-form (MNA-sf) (103), eight (14.3%) used the Geriatric Nutritional Risk Index (GNRI) (104), seven (12.5%) used the Subjective Global Assessment (SGA) (105), seven (12.5%) used the Nutrition Risk Score (NRS) (106), four (7.1%) used the “MUST” (107), three (5.4%) used the Patient-generated Subjective Global Assessment (PG-SGA) (108), two (3.6%) used the Prognostic Nutritional Index (PNI) (109, 110), two (3.6%) used the ESPEN diagnostic criteria for malnutrition (111), one (1.8%) used the Malnutrition Screening Tool (MST) (112), one (1.8%) used the Controlling Nutritional Status score (CONUT) (113), and one (1.8%) used the GLIM criteria (114). Out of the 19 study groups evaluating the nutritional status with anthropometrical/biochemical measurements, nine (47.4%) used a combination of anthropometrical and biochemical measurements, four (21.1%) used anthropometrical measurements, and six (31.6%) used biochemical measurements only. Anthropometrical measurements included BMI, bodyweight (loss), weight index based on actual bodyweight and reference weight (2, 115), arm muscle circumference, triceps skinfold, and the brachial perimeter. Biochemical measurements included albumin, pre-albumin, transferrin, hemoglobin, total cholesterol, lymphocyte count, ferritin, transthyretin, iron, and urea. In three study groups, a combination of screening/assessment tools and anthropometrical/biochemical measurements was used, and it included either a combination of bedside assessment, bodyweight, height, dietary history, blood test, or “MUST” and albumin. Figure 6 shows the prevalence of INC examined with different methods and plotted against TNE-S-E.
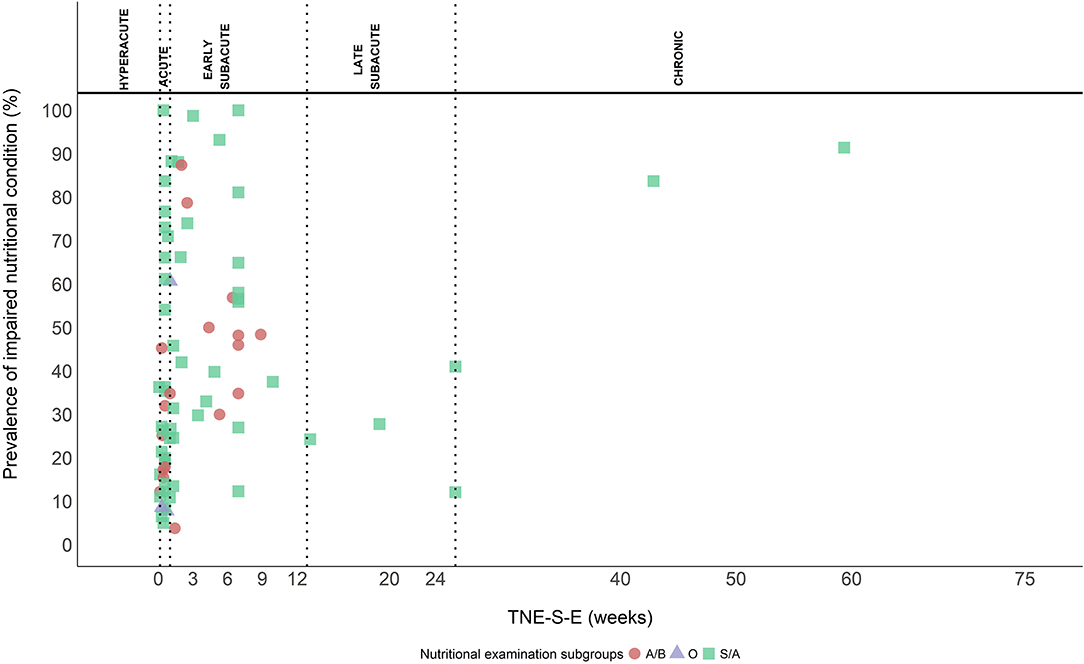
Figure 6. Methods used to examine the prevalence of INC. Screening/assessment tools (S/A) (squares), anthropometrical/biochemical measurements (A/B) (circles), and other (O) (triangles) (combination of S/A tools and A/B measurements).
Thirty-two (41.0%) of the 78 study groups reported on the prevalence of dysphagia at baseline in the stroke patients in whom nutritional status was examined. After excluding study groups that used the presence or absence of dysphagia as an eligibility criterion, the prevalence of dysphagia ranged between 6.0 and 87.5%. A wide variety of screening and diagnostic methods were used to assess dysphagia. Out of the 78 study groups, 20 (25.6%) reported NIHSS scores at baseline in the stroke patients who were examined for the nutritional status. Mean/median NIHSS scores ranged from 1.5 to 14.2. Minor (NIHSS scores 1–4) and moderate (NIHSS scores 5–15) strokes were reported in three (15.0%) and 17 (85.0%) of the 20 study groups, respectively. Poor nutritional status was often linked to adverse events such as post-stroke complications and poor outcomes. Studies reported pressure ulcer development, impaired functional independence, a longer length of hospital stay, hospitalization costs, unfavorable recovery from stroke, and increased mortality. The continent-specific pooled prevalence of INC was 46% (95%CI: 36–56) based on 36 (46.2%) study groups in Asia, 37% (95%CI: 28–45) based on 29 (37.2%) study groups in Europe, 36% (95%CI: 16–56) based on 7 (9.0%) study groups in Australia, 46% (95%CI: 13–79) based on 3 (3.8%) study groups in North-America, 74% (95%CI: 39–100) based on two (2.6%) study groups in South-America, 25% (95%CI: 30–31) based on one (1.3%) study group in Africa, and 42% (95%CI: 36–48) based on the total number of 78 study groups (Supplemental Figure 2). In the continents where the pooled prevalence was based on a number of study groups ≤ 5, the results generated with the RE and FE meta-analysis models were overall similar.
A risk of selection bias was found in 38 out of the 78 study groups (48.7%) as the study population was considered not representative for the stroke population in the assigned phase after stroke. A risk of performance bias was found in 53 out of 78 (67.9%) study groups based on methods used for the confirmation of stroke (performance bias I). In these study groups this information was unknown, not reported, or the diagnosis was confirmed differently, e.g., screening by a board certificated neurologists or extraction of data from the patients' medical dossiers. A risk of performance bias based on validity of the screening/assessment tools for nutritional examination (performance bias II) was found in 10 out of the 56 (17.9%) study groups that used screening/assessment tools for the examination of nutritional status. These study groups used e.g., the GNRI or PNI, that have not been validated in specific patient populations. A risk of detection bias was found in two out of 78 (2.6%) study groups as these did not clearly define the methods used to examine the nutritional status (detection bias I). A risk of detection bias was also found in 39 out of 78 (50.0%) study groups as there was no clear indication of consistent performance of methods (detection bias II). In these study groups, it was unclear who performed the evaluation or who collected the data from medical files or a wide variety of assessors was involved. A risk of reporting bias was found in two of the 78 (2.6%) study groups as these study groups included about 99% of confirmed stroke and remaining subjects were diagnosed with “brain tumor” or as “non-stroke” (reporting bias I). In 16 out of 53 (30.2%) study groups that used screening/assessment tools other than GLIM or ESPEN criteria for the examination of nutritional status, reported incomplete prevalence data on INC according to the criteria (Supplemental Table 2) used in this systematic review (reporting bias II). In these cases, data were missing in one or more categories. A summary of the risk of bias is provided in Figure 7. The risk of bias and relative risk for each individual study group is provided in the Supplemental Table 4.
This systematic review shows the prevalence of INC and malnutrition ranging from 3.8 to 100.0% and from 0.0 to 94.4%, respectively. A high prevalence of INC was reported within three months after stroke. The pooled prevalence of INC was 34% in the acute and 52% in the early subacute phase, respectively. For malnutrition, these numbers were 26 and 37%, respectively. A deterioration of nutritional condition within the first three months was seen from the follow-up data. A poor nutritional condition occurring within three months after stroke parallels the time period associated with the peak of recovery (17, 18). As poor nutritional status negatively impacts the recovery processes, it is advised to intervene within this time window and to address nutrition as an integral component of rehabilitation therapy.
The importance of nutrition in stroke recovery is supported by several studies that demonstrate an association between poor nutritional status and worse stroke outcomes such as disability, complications, extended length of hospital stay, mortality and costs for hospitalization (2, 3). Poor nutritional status, inactivity and immobilization, can lead to muscle loss and sarcopenia and can negatively impact the recovery after stroke (116). A recent meta-analysis by Su (117) reports that sarcopenia is common after stroke. Furthermore, several studies show that improving the nutritional status of stroke patients using specialized nutritional interventions can significantly improve clinical outcomes. In a randomized controlled trial (RCT) with 102 undernourished stroke patients, intensive nutritional supplementation, including oral nutritional supplements (ONS), improved motor function (p < 0.002) (118). In a rehabilitation center the total Functional Independence Measure (FIM) gain (p = 0.036) and efficiency (p = 0.020) were improved in cerebrovascular patients (mainly due to stroke) with poor nutritional status and in whom an improvement of the GNRI and energy intake was achieved (119). A different RCT showed that supplementation of subacute ischemic stroke patients with high protein ONS enhanced the cognitive function evaluated with the Mini-Mental State Examination (p = 0.01) (120). Oral energy and protein-rich (enteral) feeding of acute stroke patients at nutritional risk increased quality of life (p = 0.009) and handgrip strength (p = 0.002) (121). A positive effect on energy (p < 0.0001) and protein (p < 0.001) intake and on albumin (p = 0.025) and iron (p = 0.030) levels were observed in acute ischemic stroke patients using ONS providing 600 Kcal and 20 g protein per day in addition to the hospital diet compared to stroke patients randomized to receive only the hospital diet (122). A recent study investigated the effect of tailored dietary prescription in 454 stroke patients in rehabilitation and reported an inverse correlation between dysphagia and frequency of dietary adjustments in prescriptions (p = 0.032) (123). In addition, more frequent dietary adjustments positively affected FIM motor scores (p = 0.045), muscle mass change (p = 0.028), and length of hospitalization (p = 0.019) (123). The Feed Or Ordinary Diet (FOOD) trial randomized acute non-dysphagic stroke patients to a control group that received a regular hospital diet or a treatment group that received a regular hospital diet with additional ONS that did not measurably affect mortality or outcome (43). However, 77.0% of the population in the FOOD Trial was well-nourished at baseline, and this may have influenced the effectiveness of ONS. Finally, the importance of examination of nutritional status and dysphagia and adequate nutritional status in stroke patients is reflected in several (international) stroke guidelines. These guidelines recommend dysphagia screening prior to first oral intake in all stroke patients, screening for malnutrition and the provision of nutritional support, including the use of ONS, in stroke patients with an impaired nutritional status and/or dysphagia (1, 19, 20, 124–127). These guidelines (1, 19, 126, 127) do not recommend routine administration of ONS in well-nourished stroke patients, in line with the results of the FOOD trial. In these guidelines, also recommendations are given on the use of enteral tube feeding in specific conditions and/or on the route of administration (nasogastric or PEG) (19, 20, 127).
This review shows variation in the prevalence of INC. This may be attributed to the various methods used to screen or assess the nutritional status. A gold standard method and a recognized definition of malnutrition are lacking (128). Only recently, the GLIM reached a global consensus on the diagnostic criteria for malnutrition in adults. Nutritional screening and assessment are both included, and five key health phenotypic and etiologic health criteria such as involuntary weight loss, BMI, decreased muscle mass, reduced nutritional intake or absorption, and disease-induced burden or inflammation are covered (114). Foley (21) suggested that a great part of the variation in the estimates of malnutrition in stroke may be attributed to differences in the nutritional examinations. In this systematic review, 71.8% of the study groups used screening/assessment tools and 24.4% of the study groups used anthropometrical/biochemical measurements. The results showed a higher prevalence of INC when the screening/assessment tools were used, indicating that the prevalence may vary in relation to the methods used for the examination. Additionally, in some cases, the original tools were modified, and the adapted versions were used for the examination. The use of one single method might result in significant prevalence variations as well. Geriatric patients showed a prevalence of malnutrition and risk of malnutrition between 3.0 and 58.0% when the nutritional examination was performed with MNA solely (129). Considerable variation of the prevalence of malnutrition was also observed within patient groups. In cancer patients, the type of cancer was an important determinant (130, 131). In addition, the setting in which patients are residing could also play a role. Cereda (132) reported on the nutritional status in older people examined with MNA in various settings. They found high heterogeneity in the studies, with a prevalence of malnutrition ranging between 3.1% in the community and 29.4% in rehabilitation/post-acute care. Studies in the current review have also been performed in a variety of health care settings. The time of nutritional examination has been suggested to be a reason for the variation of prevalence in stroke patients as well (21). Although in this systematic review, the timing was taken into account by studying each phase after a stroke, a considerable variation of the prevalence remained. The Stroke Recovery and Rehabilitation Roundtable Taskforce (17) encourages research in the field to provide clear guidance on timing. TNE-S-E was only available in 17.9% of the study groups; the allocation of the remaining studies within a pre-defined phase provides a general indication of the time of nutritional examination after a stroke. The studies included in the analysis were not all explicitly performed to examine the nutritional status in stroke, and this might have contributed to the missing data on timing.
When interpreting the data on the prevalence of INC in the hyperacute phase, it is crucial to consider the limited time passed since stroke onset. Data on nutritional status in this phase most likely indicate the state of nutrition before the stroke event rather than an actual stroke-related change in nutritional status. However, some screening/assessment tools determine nutritional risk by allocating a score to reduced or interrupted nutritional intake which would reflect that moment in time. The small number of studies reporting on the nutritional status in the hyperacute phase is likely a result of the significant focus on specific treatments and patient needs within 24 h after stroke. Lack of data in the late subacute and chronic phase might reflect a reduced number of studies performed at later stages or a lack of attention to the nutritional status over time. Considering the high prevalence of INC occurring within three months after stroke, continuous monitoring of the nutritional status during and beyond this stage of rehabilitation is desirable. The current review shows the prevalence of dysphagia up to 87.5%, and Foley (133) reports that dysphagia increases the risk of malnutrition 2.4 fold (p < 0.008). This systematic review highlights the need for future research to increase the knowledge on nutritional status after stroke.
To our knowledge, this systematic review has been performed in the most appropriate way to provide a transparent and comprehensive overview of the existing evidence. Nevertheless, this study has some limitations. The screening of titles and abstracts and data extraction of the exploratory parameters was performed by one reviewer, and data on prevalence and timing were harmonized with specific self-developed criteria. One other limitation is that screening and assessment tools were both included, and a differentiation (134) was not performed. Despite this limitation, all eligible literature on nutritional status in stroke was considered valuable and included in the analysis of the review. The risk of bias was evaluated using a self-developed checklist. This checklist included critical questions regarding selection, performance, detection, and reporting bias and provided a comprehensive risk of bias summary. Due to the high heterogeneity of the data one may not conclude on the exact prevalence of INC; however these results shed light on a problem that is often underestimated.
In summary, results of the current review indicate that INC and malnutrition occur across the continuum of stroke care, from the hyperacute to the chronic phase. The large prevalence range of INC and malnutrition in the different phases underlines the importance of continuously reviewing the nutritional status in stroke patients to identify and take action to prevent nutritional deterioration. The large prevalence range also shows that there is a large heterogeneity in prevalence data amongst different studies. Malnutrition after stroke has been shown to negatively affect clinical outcomes, mortality and overall healthcare expenditure. This suggests that continuous monitoring of the nutritional status and improved nutritional management within the multidisciplinary context of rehabilitation is warranted, to ensure malnutrition does not go unnoticed, untreated, and impede rehabilitation and recovery after stroke.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
SG and ML initiated the study. SG and VH performed the screening of the literature and the data extraction. All authors contributed to the data analysis, review, interpretation of the results, were involved in the writing of the manuscript, and provided their consent on the manuscript.
Support for this work has been received from Maastricht University, Maastricht, Netherlands and from Danone Nutricia Research, Utrecht, Netherlands.
SG, ML, and AHe are employees of Danone Nutricia Research. JS and AHo have been consultants for Danone Nutricia Research. VH and JS received financial support for their research. LB is a consultant for Phagenesis Limited, Manchester, United Kingdom.
The authors declare that this study received funding from Danone Nutricia Research. The authors employed by the funder had co-involvement in the following parts of the study: design, data collection, analysis, interpretation of data, the writing of this article and the decision to submit it for publication.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The authors would like to thank the Research and Innovation Data Science department of Danone Nutricia Research for the contribution to the statistical methods and analysis, Claudia van den Berg for her support as a third reviewer, and Daan Snoeks for the literature search.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2021.780080/full#supplementary-material
1. IntercollegiateStroke, ICSWP, Bowen A, James M, Young G. National clinical guideline for stroke. In: Royal College of Physicians (2016).
2. Yoo S-H, Kim JS, Kwon SU, Yun S-C, Koh J-Y, Kang D-W. Undernutrition as a predictor of poor clinical outcomes in acute ischemic stroke patients. Arch Neurol. (2008) 65:39–43. doi: 10.1001/archneurol.2007.12
3. Gomes F, Emery PW, Weekes CE. Risk of malnutrition is an independent predictor of mortality, length of hospital stay, and hospitalization costs in stroke patients. J Stroke Cerebrovasc Dis. (2016) 25:799–806. doi: 10.1016/j.jstrokecerebrovasdis.2015.12.017
4. Clavé P, Shaker R. Dysphagia: current reality and scope of the problem. Nat Rev Gastroenterol Hepatol. (2015) 12:259. doi: 10.1038/nrgastro.2015.49
5. Winstein CJ, Stein J, Arena R, Bates B, Cherney LR, Cramer SC, et al. Guidelines for adult stroke rehabilitation and recovery: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. (2016) 47:e98–e169. doi: 10.1161/STR.0000000000000098
6. Mijajlović MD, Pavlović A, Brainin M, Heiss W-D, Quinn TJ, Ihle-Hansen HB, et al. (2017). Post-stroke dementia–a comprehensive review. BMC medicine 15, 1–12. doi: 10.1186/s12916-017-0779-7
7. Gariballa S. Malnutrition in hospitalized elderly patients: when does it matter? Clin Nutr. (2001) 20:487–91. doi: 10.1054/clnu.2001.0477
8. Nip W, Perry L, McLaren S, Mackenzie A. Dietary intake, nutritional status and rehabilitation outcomes of stroke patients in hospital. J Hum Nutr Diet. (2011) 24:460–9. doi: 10.1111/j.1365-277X.2011.01173.x
9. Foley N, Finestone H, Woodbury M, Teasell R, Greene-Finestone L. Energy and protein intakes of acute stroke patients. J Nutr Health Aging. (2006) 10:171.
10. Aquilani R, Galli M, Guarnaschelli C, Fugazza G. Prevalence of malnutrition and inadequate food intake in self-feeding rehabilitation patients with stroke. Eur J Phys Rehabil Med. (1999) 35:75.
11. Perry L, McLaren S. An exploration of nutrition and eating disabilities in relation to quality of life at 6 months post-stroke. Health Soc Care Community. (2004) 12:288–297. doi: 10.1111/j.1365-2524.2004.00494.x
12. Muresanu DF, Strilciuc S, Stan A. Current drug treatment of acute ischemic stroke: challenges and opportunities. CNS Drugs. (2019) 33:841–7. doi: 10.1007/s40263-019-00663-x
13. Luengo-Fernandez R, Violato M, Candio P, Leal J. Economic burden of stroke across Europe: A population-based cost analysis. Eur Stroke J. (2020) 5:17–25. doi: 10.1177/2396987319883160
14. Platz T. Evidence-based guidelines and clinical pathways in stroke rehabilitation—an international perspective. Front Neurol. (2019) 10:200. doi: 10.3389/fneur.2019.00200
15. Stinear CM, Lang CE, Zeiler S, Byblow WD. Advances and challenges in stroke rehabilitation. Lancet Neurol. (2020) 19:348–60. doi: 10.1016/S1474-4422(19)30415-6
16. Brainin M. Cerebrolysin: a multi-target drug for recovery after stroke. Expert Rev Neurother. (2018) 18:681–7. doi: 10.1080/14737175.2018.1500459
17. Bernhardt J, Hayward KS, Kwakkel G, Ward NS, Wolf SL, Borschmann K, et al. Agreed definitions and a shared vision for new standards in stroke recovery research: the stroke recovery and rehabilitation roundtable taskforce. Int J Stroke. (2017) 12:444–50. doi: 10.1177/1747493017711816
18. Dobkin BH, Carmichael ST. The specific requirements of neural repair trials for stroke. Neurorehabil Neural Repair. (2016) 30:470–8. doi: 10.1177/1545968315604400
19. Burgos R, Bretón I, Cereda E, Desport JC, Dziewas R, Genton L, et al. ESPEN guideline clinical nutrition in neurology. Clin Nutr. (2018) 37:354–96. doi: 10.1016/j.clnu.2017.09.003
20. Wirth R, Smoliner C, Jäger M, Warnecke T, Leischker AH, Dziewas R. Guideline clinical nutrition in patients with stroke. Exp Transl Stroke Med. (2013) 5:1–11. doi: 10.1186/2040-7378-5-14
21. Foley NC, Salter KL, Robertson J, Teasell RW, Woodbury MG. Which reported estimate of the prevalence of malnutrition after stroke is valid? Stroke. (2009) 40:e66–74. doi: 10.1161/STROKEAHA.108.518910
22. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. (2021) 372. doi: 10.1136/bmj.n71
23. Huppertz V, Guida S. Nutritional status in stroke patients from hyperacute to chronic phase : a systematic review. In: PROSPERO: International prospective register of systematic reviews (2020).
25. Huang X, Lin J, Demner-Fushman D. Evaluation of PICO as a knowledge representation for clinical questions. In: AMIA annual symposium proceedings American Medical Informatics Association (2006). p.359–63.
26. Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol. (2005) 5:1–10. doi: 10.1186/1471-2288-5-13
27. Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. (2002) 21:1539–58. doi: 10.1002/sim.1186
28. Borenstein M, Hedges LV, Higgins JP, Rothstein HR. A basic introduction to fixed-effect and random-effects models for meta-analysis. Res Synth Methods. (2010) 1:97–111. doi: 10.1002/jrsm.12
29. Riley RD, Higgins JP, Deeks JJ. Interpretation of random effects meta-analyses. BMJ. (2011) 342. doi: 10.1136/bmj.d549
30. Higgins JP, Thompson SG, Spiegelhalter DJ. A re-evaluation of random-effects meta-analysis. J R Stat Soc. (2009) 172:137–59. doi: 10.1111/j.1467-985X.2008.00552.x
31. Raudenbush SW. Analyzing effect sizes: Random-effects models. The handbook of research synthesis and meta-analysis. (2009) 2:295–316.
32. Davis JP, Wong AA, Schluter PJ, Henderson RD, O'Sullivan JD, Read SJ. Impact of premorbid undernutrition on outcome in stroke patients. Stroke. (2004) 35:1930–4. doi: 10.1161/01.STR.0000135227.10451.c9
33. Kokura Y, Maeda K, Wakabayashi H, Nishioka S, Higashi S. High nutritional-related risk on admission predicts less improvement of functional independence measure in geriatric stroke patients: a retrospective cohort study. J Stroke Cerebrovasc Dis. (2016) 25:1335–41. doi: 10.1016/j.jstrokecerebrovasdis.2016.01.048
34. Nozoe M, Kanai M, Kubo H, Kitamura Y, Yamamoto M, Furuichi A, et al. Changes in quadriceps muscle thickness, disease severity, nutritional status, and C-reactive protein after acute stroke. J Stroke Cerebrovasc Dis. (2016) 25:2470–4. doi: 10.1016/j.jstrokecerebrovasdis.2016.06.020
35. Sremanakova J, Burden S, Kama Y, Gittins M, Lal S, Smith CJ, et al. An observational cohort study investigating risk of malnutrition using the malnutrition universal screening tool in patients with stroke. J Stroke Cerebrovasc Dis. (2019) 28:104405. doi: 10.1016/j.jstrokecerebrovasdis.2019.104405
36. Diendéré J, Millogo A, Philippe F, Kaboré J, Napon C, Dabilgou A, et al. Post-stroke complications and mortality in burkinabè hospitals: relationships with deglutition disorders and nutritional status. Dysphagia. (2020) 36:85–95. doi: 10.1007/s00455-020-10111-4
37. Vajpayee A, Kalita J, Misra UK. Nutritional deficiency in stroke patients results in poor outcome. E Spen Eur E J Clin Nutr Metab. (2008) 3:e142–6. doi: 10.1016/j.eclnm.2008.04.006
38. Gandolfo C, Sukkar S, Ceravolo MG, Cortinovis F, Finocchi C, Gradaschi R, et al. The predictive dysphagia score (PreDyScore) in the short-and medium-term post-stroke: a putative tool in PEG indication. Neurol Sci. (2019) 40:1619–26. doi: 10.1007/s10072-019-03896-2
39. Crary MA, Carnaby-Mann GD, Miller L, Antonios N, Silliman S. Dysphagia and nutritional status at the time of hospital admission for ischemic stroke. J Stroke Cerebrovasc Dis. (2006) 15:164–71. doi: 10.1016/j.jstrokecerebrovasdis.2006.05.006
40. NanZhu Y, Xin L, Xianghua Y, Jun C, Min L. Risk factors analysis of nosocomial pneumonia in elderly patients with acute cerebral infraction. Medicine. (2019) 98:e15045. doi: 10.1097/MD.0000000000015045
41. Zheng T, Zhu X, Liang H, Huang H, Yang J, Wang S. Impact of early enteral nutrition on short term prognosis after acute stroke. J Clin Neurosci. (2015) 22:1473–6. doi: 10.1016/j.jocn.2015.03.028
42. Shen H-C, Chen H-F, Peng L-N, Lin M-H, Chen L-K, Liang C-K, et al. Impact of nutritional status on long-term functional outcomes of post-acute stroke patients in Taiwan. Arch Gerontol Geriatr. (2011) 53:e149–e152. doi: 10.1016/j.archger.2010.08.001
43. Collaboration FT. Routine oral nutritional supplementation for stroke patients in hospital (FOOD): a multicentre randomised controlled trial. Lancet. (2005) 365:755–63. doi: 10.1016/S0140-6736(05)17982-3
44. Xiang W, Chen X, Ye W, Zhang Li J, Xie XD. Prognostic nutritional index for predicting 3-month outcomes in ischemic stroke patients undergoing thrombolysis. Front Neurol. (2020) 11:599. doi: 10.3389/fneur.2020.00599
45. Kokura Y, Kimoto K, Okada Y, Kawakita S. The Controlling Nutritional Status score as a functional prognostic marker in patients with acute stroke: a multicenter retrospective cohort study. Nutrition. (2020) 79:110889. doi: 10.1016/j.nut.2020.110889
46. Barrio MÁO, Sieiro FV, Fernández MTA, Santamaría SB, Maestro RM. Effect of stroke on nutritional status and its relationship with dysphagia. Revista Científica de la Sociedad de Enfermería Neurológica. (2020) 51:13–21. doi: 10.1016/j.sedeng.2019.04.003
47. Otsuki I, Himuro N, Tatsumi H, Mori M, Niiya Y, Kumeta Y, et al. Individualized nutritional treatment for acute stroke patients with malnutrition risk improves functional independence measurement: a randomized controlled trial. Geriatr Gerontol Int. (2020) 20:176–82. doi: 10.1111/ggi.13854
48. Robertson ST, Grimley RS, Anstey C, Rosbergen IC. Acute stroke patients not meeting their nutrition requirements: investigating nutrition within the enriched environment. Clin Nutr. (2020) 39:1470–7. doi: 10.1016/j.clnu.2019.06.009
49. Lopez Espuela F, Roncero-Martín R, Zamorano JDP, Rey-Sanchez P, Aliaga-Vera I, Portilla Cuenca JC, et al. Controlling Nutritional Status (CONUT) score as a predictor of all-cause mortality at 3 months in stroke patients. Biol Res Nurs. (2019) 21:564–70. doi: 10.1177/1099800419860253
50. Aliasghari F, Izadi A, Khalili M, Farhoudi M, Ahmadiyan S, Deljavan R. Impact of premorbid malnutrition and dysphagia on ischemic stroke outcome in elderly patients: a community-based study. J Am Coll Nutr. (2019) 38:318–26. doi: 10.1080/07315724.2018.1510348
51. Crary MA, Humphrey JL, Carnaby-Mann G, Sambandam R, Miller L, Silliman S. Dysphagia, nutrition, and hydration in ischemic stroke patients at admission and discharge from acute care. Dysphagia. (2013) 28:69–76. doi: 10.1007/s00455-012-9414-0
52. Çoban E. Malnutrition Rate in Stroke Patients on Admission. Sişli Etfal Hastanesi tip Bülteni. (2019) 53:272. doi: 10.14744/SEMB.2018.81994
53. Schwarz M, Coccetti A, Cardell E, Murdoch A, Davis J. Management of swallowing in thrombolysed stroke patients: implementation of a new protocol. Int J Speech Lang Pathol. (2017) 19:551–61. doi: 10.1080/17549507.2016.1221457
54. Porter C, Coleman E, Ross L, Palmer M. Do stroke patients screened as lower-nutritional-risk still receive dietitian assessment if indicated? A retrospective evaluation of two dietetic models of care for adult stroke patients. J Hum Nutr Diet. (2019) 32:267–75. doi: 10.1111/jhn.12619
55. Pandian JD, Jyotsna R, Singh R, Sylaja PN, Vijaya P, Padma MV, et al. Premorbid nutrition and short term outcome of stroke: a multicentre study from India. J Neurol Neurosurg Psychiatry. (2011) 82:1087–92. doi: 10.1136/jnnp.2010.233429
56. Mosselman MJ, Kruitwagen CL, Schuurmans MJ, Hafsteinsdóttir TB. Malnutrition and risk of malnutrition in patients with stroke: prevalence during hospital stay. J Neurosci Nurs. (2013) 45:194–204. doi: 10.1097/JNN.0b013e31829863cb
57. Martineau J, Bauer JD, Isenring E, Cohen S. Malnutrition determined by the patient-generated subjective global assessment is associated with poor outcomes in acute stroke patients. Clin Nutr. (2005) 24:1073–7. doi: 10.1016/j.clnu.2005.08.010
58. Ha L, Sakhi AK, Bøhn SK, Flekkøy K, Blomhoff R, Iversen PO, et al. Antioxidant status after an acute stroke and the association with survival in elderly at nutritional risk. E Spen Eur E J Clin Nutr Metab. (2011) 6:e135–41. doi: 10.1016/j.eclnm.2011.02.004
59. Collaboration FT. Effect of timing and method of enteral tube feeding for dysphagic stroke patients (FOOD): a multicentre randomised controlled trial. Lancet. (2005) 365:764–72. doi: 10.1016/S0140-6736(05)17983-5
60. Medin J, Windahl J, von Arbin M, Tham K, Wredling R. Eating difficulties among stroke patients in the acute state: a descriptive, cross-sectional, comparative study. J Clin Nurs. (2011) 20:2563–72. doi: 10.1111/j.1365-2702.2011.03812.x
61. Isono N, Imamura Y, Ohmura K, Ueda N, Kawabata S, Furuse M, et al. Transthyretin concentrations in acute stroke patients predict convalescent rehabilitation. J Stroke Cerebrovasc Dis. (2017) 26:1375–82. doi: 10.1016/j.jstrokecerebrovasdis.2017.02.020
62. Far AH, Alipour B, Khalili M. Assessment ofthe relationship between nutritional status and serum lipid profile in stroke hospitalized patients. J Forensic Med Toxicol. (2018) 35:10–6. doi: 10.5958/0974-4568.2018.00003.0
63. Brynningsen P, Damsgaard E, Husted S. Improved nutritional status in elderly patients 6 months after stroke. J Nutr Health Aging. (2007) 11:75.
64. Kokura Y, Wakabayashi H, Nishioka S, Maeda K. Nutritional intake is associated with activities of daily living and complications in older inpatients with stroke. Geriatr Gerontol Int. (2018) 18:1334–9. doi: 10.1111/ggi.13467
65. Kang MK, Kim TJ, Kim Y, Nam K-W, Jeong H-Y, Kim SK, et al. Geriatric nutritional risk index predicts poor outcomes in patients with acute ischemic stroke-Automated undernutrition screen tool. Plos ONE. (2020) 15:e0228738. doi: 10.1371/journal.pone.0228738
66. Drozdz D, Mancopes R, Silva AMT, Reppold C. Analysis of the level of dysphagia, anxiety, and nutritional status before and after speech therapy in patients with stroke. Int Arch Otorhinolaryngol. (2014) 18:172–7. doi: 10.1055/s-0033-1364169
67. Cai Z-M, Wu Y-Z, Chen H-M, Feng R-Q, Liao C-W, Ye S-L, et al. Being at risk of malnutrition predicts poor outcomes at 3 months in acute ischemic stroke patients. Eur J Clin Nutr. (2020) 74:796–805. doi: 10.1038/s41430-020-0605-8
68. Naito H, Hosomi N, Nezu T, Kuzume D, Aoki S, Morimoto Y, et al. Prognostic role of the controlling nutritional status score in acute ischemic stroke among stroke subtypes. J Neurol Sci. (2020) 416:116984. doi: 10.1016/j.jns.2020.116984
69. Hirano Y, Nitta O. Effects of nutritional status on prognosis in patients with severe hemiplegia who were recently admitted to a rehabilitation hospital. J Phys Ther Sci. (2020) 32:319–22. doi: 10.1589/jpts.32.319
70. Nishioka S, Omagari K, Nishioka E, Mori N, Taketani Y, Kayashita J. Concurrent and predictive validity of the Mini Nutritional Assessment Short-Form and the Geriatric Nutritional Risk Index in older stroke rehabilitation patients. J Hum Nutr Diet. (2020) 33:12–22. doi: 10.1111/jhn.12699
71. Nishioka S, Yamasaki K, Ogawa K, Oishi K, Yano Y, Okazaki Y, et al. Impact of nutritional status, muscle mass and oral status on recovery of full oral intake among stroke patients receiving enteral nutrition: a retrospective cohort study. Nutr Diet. (2020) 77:456–66. doi: 10.1111/1747-0080.12579
72. Kampman MT, Eltoft A, Karaliute M, Børvik MT, Nilssen H, Rasmussen I, et al. Full implementation of screening for nutritional risk and dysphagia in an acute stroke unit: a clinical audit. Neurohospitalist. (2015) 5:205–11. doi: 10.1177/1941874415588749
73. Zhang J, Zhao X, Wang A, Zhou Y, Yang B, Wei N, et al. Emerging malnutrition during hospitalisation independently predicts poor 3-month outcomes after acute stroke: data from a Chinese cohort. Asia Pac J Clin Nutr. (2015) 24:379.
74. Shiraishi A, Yoshimura Y, Wakabayashi H, Tsuji Y. Prevalence of stroke-related sarcopenia and its association with poor oral status in post-acute stroke patients: Implications for oral sarcopenia. Clin Nutr. (2018) 37:204–7. doi: 10.1016/j.clnu.2016.12.002
75. Hsieh D-Y, Hung J-W, Chang K-C, Huang Y-C, Lee T-H, Chen H-M. Malnutrition in acute stroke patients stratified by stroke severity-a hospital based study. Acta Neurol Taiwan. (2017) 26:120–127.
76. Falsetti P, Acciai C, Palilla R, Bosi M, Carpinteri F, Zingarelli A, et al. Oropharyngeal dysphagia after stroke: incidence, diagnosis, and clinical predictors in patients admitted to a neurorehabilitation unit. J Stroke Cerebrovasc Dis. (2009) 18:329–35. doi: 10.1016/j.jstrokecerebrovasdis.2009.01.009
77. Sato M, Ido Y, Yoshimura Y, Mutai H. Relationship of malnutrition during hospitalization with functional recovery and postdischarge destination in elderly stroke patients. J Stroke Cerebrovasc Dis. (2019) 28:1866–72. doi: 10.1016/j.jstrokecerebrovasdis.2019.04.012
78. Lim HJ, Choue R. Nutritional status assessed by the Patient-Generated Subjective Global Assessment (PG-SGA) is associated with qualities of diet and life in Korean cerebral infarction patients. Nutrition. (2010) 26:766–71. doi: 10.1016/j.nut.2009.10.003
79. James R, Gines D, Menlove A, Horn SD, Gassaway J, Smout RJ. Nutrition support (tube feeding) as a rehabilitation intervention. Arch Phys Med Rehabil. (2005) 86:82–92. doi: 10.1016/j.apmr.2005.07.314
80. Nishioka S, Wakabayashi H, Yoshida T, Mori N, Watanabe R, Nishioka E. Obese Japanese patients with stroke have higher functional recovery in convalescent rehabilitation wards: a retrospective cohort study. J Stroke Cerebrovasc Dis. (2016) 25:26–33. doi: 10.1016/j.jstrokecerebrovasdis.2015.08.029
81. Aadal L, Mortensen J, Nielsen JF. Weight reduction after severe brain injury: a challenge during the rehabilitation course. J Neurosci Nurs. (2015) 47:85–90. doi: 10.1097/JNN.0000000000000121
82. Nishioka S, Okamoto T, Takayama M, Urushihara M, Watanabe M, Kiriya Y, et al. Malnutrition risk predicts recovery of full oral intake among older adult stroke patients undergoing enteral nutrition: secondary analysis of a multicentre survey (the APPLE study). Clin Nutr. (2017) 36:1089–96. doi: 10.1016/j.clnu.2016.06.028
83. Garbagnati F, Cairella G, De Multari A, Multari M, Scognamiglio U, Venturiero U, et al. Is antioxidant and n−3 supplementation able to improve functional status in poststroke patients? Results from the Nutristroke Trial. Cerebrovas Dis. (2009) 27:375–83. doi: 10.1159/000207441
84. Westergren A, Karlsson S, Andersson P, Ohlsson O, Hallberg IR. Eating difficulties, need for assisted eating, nutritional status and pressure ulcers in patients admitted for stroke rehabilitation. J Clin Nurs. (2001) 10:257–69. doi: 10.1046/j.1365-2702.2001.00479.x
85. Poels B, Brinkman-Zijlker H, Dijkstra P, Postema K. Malnutrition, eating difficulties and feeding dependence in a stroke rehabilitation centre. Disabil Rehabil. (2006) 28:637–43. doi: 10.1080/09638280500276612
86. Hama S, Kitaoka T, Shigenobu M, Watanabe A, Imura I, Seno H, et al. Malnutrition and nonthyroidal illness syndrome after stroke. Metabolism. (2005) 54:699–704. doi: 10.1016/j.metabol.2004.11.016
87. Maruyama K, Nakagawa N, Koyama S, Maruyama JI, Hasebe N. Malnutrition increases the incidence of death, cardiovascular events, and infections in patients with stroke after rehabilitation. J Stroke Cerebrovasc Dis. (2018) 27:716–23. doi: 10.1016/j.jstrokecerebrovasdis.2017.10.002
88. Shimizu A, Maeda K, Koyanagi Y, Kayashita J, Fujishima I, Mori N. The global leadership initiative on malnutrition–defined malnutrition predicts prognosis in persons with stroke-related dysphagia. J Am Med Dir Assoc. (2019) 20:1628–33. doi: 10.1016/j.jamda.2019.07.008
89. Carlsson E, Ehnfors M, Eldh AC, Ehrenberg A. Accuracy and continuity in discharge information for patients with eating difficulties after stroke. J Clin Nurs. (2012) 21:21–31. doi: 10.1111/j.1365-2702.2010.03648.x
90. Tsai AC, Shih CL. A population-specific Mini-Nutritional Assessment can effectively grade the nutritional status of stroke rehabilitation patients in Taiwan. J Clin Nurs. (2008) 18:82–8. doi: 10.1111/j.1365-2702.2008.02319.x
91. Kaur S, Miller MD, Halbert JA, Giles LC, Crotty M. Nutritional status of adults participating in ambulatory rehabilitation. Asia Pac J Clin Nutr. (2008) 17:199–207.
92. Jung HJ, Lee YM, Kim M, Uhm KE, Lee J. Suggested assessments for sarcopenia in patients with stroke who can walk independently. Ann Rehabil Med. (2020) 44:20. doi: 10.5535/arm.2020.44.1.20
93. van Zwienen-Pot J, Visser M, Kuijpers M, Grimmerink M, Kruizenga H. Undernutrition in nursing home rehabilitation patients. Clin Nutr. (2017) 36:755–9. doi: 10.1016/j.clnu.2016.06.003
94. Campillo B, Paillaud E, Uzan I, Merlier I, Abdellaoui M, Perennec J, et al. Value of body mass index in the detection of severe malnutrition: influence of the pathology and changes in anthropometric parameters. Clin Nutr. (2004) 23:551–9. doi: 10.1016/j.clnu.2003.10.003
95. Appel-da-Silva MC, Zuchinali P, de Oliveira RF, Boligon CS, Riella C, Salazar GS. Nutritional profile and mortality in patients undergoing percutaneous endoscopic gastrostomy. Nutr Hosp. (2019) 36:499–503. doi: 10.20960/nh.2348
96. Lelli D, Calle A, Pérez LM, Onder G, Morandi A, Ortolani E, et al. Nutritional status and functional outcomes in older adults admitted to geriatric rehabilitations: the SAFARI study. J Am Coll Nutr. (2019) 38:441–6. doi: 10.1080/07315724.2018.1541427
97. Scrutinio D, Lanzillo B, Guida P, Passantino A, Spaccavento S, Battista P. Association between malnutrition and outcomes in patients with severe ischemic stroke undergoing rehabilitation. Arch Phys Med Rehabil. (2020) 101:852–60. doi: 10.1016/j.apmr.2019.11.012
98. Vilardell N, Rofes L, Nascimento W, Muriana D, Palomeras E, Clave P. Cough reflex attenuation and swallowing dysfunction in sub-acute post-stroke patients: prevalence, risk factors, and clinical outcome. Neurogastroenterol Motil. (2017) 29:e12910. doi: 10.1111/nmo.12910
99. Westergren A. Nutrition and its relation to mealtime preparation, eating, fatigue and mood among stroke survivors after discharge from hospital-a pilot study. Open Nurs J. (2008) 2:15. doi: 10.2174/1874434600802010015
100. Choi S-H, Choi-Kwon S, Kim M-S, Kim J-S. Poor nutrition and alcohol consumption are related to high serum homocysteine level at post-stroke. Nutr Res Pract. (2015) 9:503. doi: 10.4162/nrp.2015.9.5.503
101. Kim EJ, Yoon YH, Kim WH, Lee KL, Park JM. The clinical significance of the mini-nutritional assessment and the scored patient-generated subjective global assessment in elderly patients with stroke. Ann Rehabil Med. (2013) 37:66. doi: 10.5535/arm.2013.37.1.66
102. Vellas BJ, Guigoz Y, Garry P, Albarede J. The mini nutritional assessment: MNA. Nutrition in the elderly. SpringerandSerdi Publishing Co., Paris (1997).
103. Rubenstein LZ, Harker JO, Salvà A, Guigoz Y, Vellas B. Screening for undernutrition in geriatric practice: developing the short-form mini-nutritional assessment (MNA-SF). J Gerontol A Biol Sci Med Sci. (2001) 56:M366–72. doi: 10.1093/gerona/56.6.M366
104. Bouillanne O, Morineau G, Dupont C, Coulombel I, Vincent J-P, Nicolis I, et al. Geriatric Nutritional Risk Index: a new index for evaluating at-risk elderly medical patients. Am J Clin Nutr. (2005) 82:777–83. doi: 10.1093/ajcn/82.4.777
105. Detsky AS, Baker J, Johnston N, Whittaker S, Mendelson R, Jeejeebhoy K. What is subjective global assessment of nutritional status? J Parenter Enteral Nutr. (1987) 11:8–13. doi: 10.1177/014860718701100108
106. Kondrup J, Rasmussen HH, Hamberg O, Stanga Z. Group AahEW. Nutritional risk screening (NRS 2002): a new method based on an analysis of controlled clinical trials. Clin Nutr. (2003) 22:321–36. doi: 10.1016/S0261-5614(02)00214-5
107. Elia M. The ‘MUST’report. Nutritional screening for adults: a multidisciplinary responsibility Development and use of the ‘Malnutrition Universal Screening Tool’(‘MUST’) for adults A report by the Malnutrition Advisory Group of the British Association for Parenteral and Enteral Nutrition. 127 (2003).
108. Ottery FD. Definition of standardized nutritional assessment and interventional pathways in oncology. Nutrition. (1996) 12:S15–9. doi: 10.1016/0899-9007(96)90011-8
109. Buzby GP, Mullen JL, Matthews DC, Hobbs CL, Rosato EF. Prognostic nutritional index in gastrointestinal surgery. Am J Surg. (1980) 139:160–7. doi: 10.1016/0002-9610(80)90246-9
110. Onodera T, Goseki N, Kosaki G. Prognostic nutritional index in gastrointestinal surgery of malnourished cancer patients. Nihon Geka Gakkai Zasshi. (1984) 85:1001–5.
111. Cederholm T, Bosaeus I, Barazzoni R, Bauer J, Van Gossum A, Klek S, et al. Diagnostic criteria for malnutrition–an ESPEN consensus statement. Clin Nutr. (2015) 34:335–40. doi: 10.1016/j.clnu.2015.03.001
112. Ferguson M, Capra S, Bauer J, Banks M. Development of a valid and reliable malnutrition screening tool for adult acute hospital patients. Nutrition. (1999) 15:458–64. doi: 10.1016/S0899-9007(99)00084-2
113. Ignacio de Ulíbarri J, González-Madroño A, de Villar NG, González P, González B, Mancha A, et al. CONUT: a tool for controlling nutritional status. First validation in a hospital population. Nutr Hosp. (2005) 20:38–45.
114. Jensen GL, Cederholm T, Correia MIT, Gonzalez MC, Fukushima R, Higashiguchi T, et al. GLIM criteria for the diagnosis of malnutrition: a consensus report from the global clinical nutrition community. JPEN. (2019) 43:32–40. doi: 10.1002/jpen.1440
115. Warnold I, Lundholm K. Clinical significance of preoperative nutritional status in 215 noncancer patients. Ann Surg. (1984) 199:299. doi: 10.1097/00000658-198403000-00009
116. Mas MF, González J, Frontera WR. Stroke and Sarcopenia. Curr Phys Med Rehabil Rep. (2020) 8:452–60. doi: 10.1007/s40141-020-00284-2
117. Su Y, Yuki M, Otsuki M. Prevalence of stroke-related sarcopenia: A systematic review and meta-analysis. J Stroke Cerebrovasc Dis. (2020) 29:105092. doi: 10.1016/j.jstrokecerebrovasdis.2020.105092
118. Rabadi M, Coar P, Lukin M, Lesser M, Blass J. Intensive nutritional supplements can improve outcomes in stroke rehabilitation. Neurology. (2008) 71:1856–61. doi: 10.1212/01.wnl.0000327092.39422.3c
119. Maeda NiiM, Wakabayashi K, Nishioka H, Tanaka S. A Nutritional improvement and energy intake are associated with functional recovery in patients after cerebrovascular disorders. J Stroke Cerebrovasc Dis. (2016) 25:57–62. doi: 10.1016/j.jstrokecerebrovasdis.2015.08.033
120. Aquilani R, Scocchi M, Boschi F, Viglio S, Iadarola P, Pastoris O, et al. Effect of calorie-protein supplementation on the cognitive recovery of patients with subacute stroke. Nutr Neurosci. (2008) 11:235–40. doi: 10.1179/147683008X301586
121. Ha L, Hauge T, Spenning AB, Iversen PO. Individual, nutritional support prevents undernutrition, increases muscle strength and improves QoL among elderly at nutritional risk hospitalized for acute stroke: a randomized, controlled trial. Clin Nutr. (2010) 29:567–73. doi: 10.1016/j.clnu.2010.01.011
122. Gariballa SE, Parker SG, Taub N, Castleden CM. A randomized, controlled, single-blind trial of nutritional supplementation after acute stroke. JPEN. (1998) 22:315–9. doi: 10.1177/0148607198022005315
123. Shimazu S, Yoshimura Y, Kudo M, Nagano F, Bise T, Shiraishi A, et al. Frequent and personalized nutritional support leads to improved nutritional status, activities of daily living, and dysphagia after stroke. Nutrition. (2021) 83:111091. doi: 10.1016/j.nut.2020.111091
124. Powers WJ, Rabinstein AA, Ackerson T, Adeoye OM, Bambakidis NC, Becker K, et al. Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 guidelines for the early management of acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. (2019) 50:e344–418. doi: 10.1161/STR.0000000000000211
125. Smith L. Management of Patients with Stroke: rehabilitation, prevention and Management of Complications, and discharge planning: a National Clinical Guideline. In:SIGN (2010).
126. UK NGC. Stroke and Transient Ischaemic Attack in Over 16s: Diagnosis and Initial Management. London: NICE, National Institute for Health and Care Excellence (UK) (2019).
127. Dziewas R, Michou E, Trapl-Grundschober M, Lal A, Arsava EM, Bath PM, et al. European Stroke Organisation and European Society for Swallowing Disorders guideline for the diagnosis and treatment of post-stroke dysphagia. Eur Stroke J. (2021) 6: LXXXIX–CXV. doi: 10.1177/23969873211039721
128. Sabbouh T, Torbey MT. Malnutrition in stroke patients: risk factors, assessment, and management. Neurocrit Care. (2018) 29:374–84. doi: 10.1007/s12028-017-0436-1
129. Wojzischke J, van Wijngaarden J, van den Berg C, Cetinyurek-Yavuz A, Diekmann R, Luiking Y, et al. Nutritional status and functionality in geriatric rehabilitation patients: a systematic review and meta-analysis. Eur Geriatr Med. (2020) 11:195–207. doi: 10.1007/s41999-020-00294-2
130. Muscaritoli M, Lucia S, Farcomeni A, Lorusso V, Saracino V, Barone C, et al. Prevalence of malnutrition in patients at first medical oncology visit: the PreMiO study. Oncotarget. (2017) 8:79884. doi: 10.18632/oncotarget.20168
131. Marshall KM, Loeliger J, Nolte L, Kelaart A, Kiss NK. Prevalence of malnutrition and impact on clinical outcomes in cancer services: a comparison of two time points. Clinical nutrition. (2019) 38:644–51. doi: 10.1016/j.clnu.2018.04.007
132. Cereda E, Pedrolli C, Klersy C, Bonardi C, Quarleri L, Cappello S, et al. Nutritional status in older persons according to healthcare setting: A systematic review and meta-analysis of prevalence data using MNA®. Clinical nutrition. (2016) 35:1282–90. doi: 10.1016/j.clnu.2016.03.008
133. Foley NC, Martin RE, Salter KL, Teasell RW. A review of the relationship between dysphagia and malnutrition following stroke. J Rehabil Med. (2009) 41:707–13. doi: 10.2340/16501977-0415
Keywords: nutritional status, malnutrition, neurorehabilitation, stroke recovery, stroke rehabilitation, stroke
Citation: Huppertz V, Guida S, Holdoway A, Strilciuc S, Baijens L, Schols JMGA, van Helvoort A, Lansink M and Muresanu DF (2022) Impaired Nutritional Condition After Stroke From the Hyperacute to the Chronic Phase: A Systematic Review and Meta-Analysis. Front. Neurol. 12:780080. doi: 10.3389/fneur.2021.780080
Received: 20 September 2021; Accepted: 09 December 2021;
Published: 01 February 2022.
Edited by:
Steffen Tiedt, LMU Munich University Hospital, GermanyReviewed by:
Pawel Wróbel, University Medical Center Hamburg-Eppendorf, GermanyCopyright © 2022 Huppertz, Guida, Holdoway, Strilciuc, Baijens, Schols, van Helvoort, Lansink and Muresanu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Viviënne Huppertz, di5odXBwZXJ0ekBtYWFzdHJpY2h0dW5pdmVyc2l0eS5ubA==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.