
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Neurol. , 26 November 2021
Sec. Neuro-Oncology and Neurosurgical Oncology
Volume 12 - 2021 | https://doi.org/10.3389/fneur.2021.778590
Background and Purpose: The risk factors for progression in vestibular schwannomas (VSs) after incomplete resection (IR) remain to be elucidated. The purpose of this study was to investigate the risk factors for progression in remnant VSs after surgery.
Methods: From January 2009 to January 2018, 140 consecutive patients who underwent IR of VSs via suboccipital retrosigmoid approach in our institution were retrospectively analyzed. During follow-up, if progression was detected, the patient was classified into Progressive Group (PG); if the residual tumor was stable or shrank, the patient was classified into Stable Group (SG). Univariate analysis and multivariate analysis were used to evaluate the risk factors for progression after IR of VSs.
Results: After a mean follow-up of 80.4 months (range, 24–134 months), 35 (25.0%) patients (PG) had a progression, and no progression was detected in 105 (75.0%) patients (SG). The average tumor size was 36.5 ± 8.9 mm in PG and 31.0 ± 9.8 mm in SG, respectively. The residual tumor volume was 304.6 ± 443.3 mm3 in PG and 75.9 ± 60.0 mm3 in SG, respectively. Univariate analysis showed that preoperative tumor size, residual tumor volume, and irregular internal auditory canal (IAC) expansion were significantly different between the two groups, whereas gender, age, cystic component, or Ki-67 labeling index (LI) did not differ significantly between the two groups. Multivariate analysis showed residual tumor volume was the independent risk factor for progression.
Conclusions: VSs that underwent IR with larger preoperative size, greater residual tumor volume, or irregular IAC expansion may have a higher progression rate. Strict follow-up with shorter interval in these patients to detect early progression is necessary.
Vestibular schwannoma (VS) is a common benign neoplasm originating from the VIII cranial nerve sheath, accounting for around 8% of all intracranial tumors and 80% of cerebellopontine angle tumors (1–3). Surgical resection remains to be an optimal treatment regarding VSs, especially in large (≥ 3 cm) VSs (4). Incomplete resection (IR) may be encountered even for an experienced neurosurgeon due to many factors such as severe adhesion between tumor and peripheral neurovascular structures (5). Furthermore, to preserve favorable neurological function, especially facial nerve (FN) function, strategies including IR with adjuvant stereotactic radiosurgery (SRS) for regrowing tumor and planned partial resection (PR) followed by SRS have been proposed (6, 7). Overall, the IR rate of VSs ranges from 2.1 to 49.2% in the literature (8–12). Although VS is a kind of benign neoplasm with slow growth rate, it has the potential for recurrence/regrowth regardless of any extent of resection (EOR), especially in patients with VS receiving IR. According to the literature, VSs have a different recurrence/regrowth rate when they receive different EOR, with 2.4–3.4%, 3–29%, 23.8–52%, and 25–62.5% progression rate after total resection (TR), near total resection (NTR), subtotal resection (STR), and partial resection (PR), respectively (12–17). Thus, patients with VSs who underwent IR had a higher progression (regrowth) rate compared with those who received TR. However, only part of patients with VSs who underwent IR have progression (12).
The literatures on predicting the progression of VSs after surgical resection are limited, mostly focusing on clinical and histopathological parameters, such as age, sex, preoperative tumor size, tumor cystic formation, and Ki-67 labeling index (LI) (8, 12, 17). Risk factors predicting the regrowth of remnant VSs after IR remain to be elucidated.
Therefore, we retrospectively analyzed the patients with VSs who underwent IR in our hospital from January 2009 to January 2018. According to the follow-up results, they were divided into Progressive Group (PG) and Stable Group (SG). We originally investigated the prognostic role of internal auditory canal (IAC) in remnant progression of VSs. To the best of our knowledge, this is one of the largest retrospective cohort work of VSs that underwent IR, and there is no literature exploring the correlation between the type of IAC and tumor progression after surgery (18).
Patients with pathological confirmation of VS who underwent IR via suboccipital retrosigmoid approach from January 2009 to January 2018 were retrospectively enrolled in our study. The protocol was approved by the ethics committee of our institution. Written informed consent was obtained from all the patients or their authorized trustees and it was in accordance with the ethical guidelines of the Declaration of Helsinki.
(1) Patients were pathologically confirmed with VS.
(2) The medical record of the EOR was IR.
(3) Post-operative brain-enhanced magnetic resonance imaging (MRI) performing 3 months after surgery confirmed the presence of residual tumor.
(4) Patient had at least one subsequent brain-enhanced MRI after initial radiological confirmation of tumor remanence.
(1) Patients diagnosed as having neurofibromatosis II.
(2) Patients had a history of surgery for VS.
(3) Patients received radiotherapy of remnant VS before tumor progression was detected.
(4) No brain-enhanced MRI was conducted within 6 months postoperatively.
(5) Follow-up time was <2 years.
The tumor progression was defined as tumor enlargement of ≥2 mm compared with the residual tumor. During follow-up, if progression was detected, the patient was classified into the Progressive Group (PG); if the residual tumor was stable or shrank, it was classified into Stable Group (SG).
Data including age, gender, size, cystic formation, IAC type, residual tumor volume, and Ki-67 LI performed on first operation were retrospectively collected.
(1) The tumor size was calculated as the maximal diameter on preoperative axial, sagittal, or coronal MRI performed before first operation.
(2) Cystic formation: we termed cystic VSs as where those diameters of cystic portion on enhanced MRI were larger than 50% of the whole tumor and those VSs without cystic formation or diameter of cystic portion ≤ 50% of the whole tumor were termed as solid VSs.
(3) Internal auditory canal type: we divided the IAC type into the following two types according to the preoperative thin-slice CT bone window image of the temporal bone of the lesion side: horn-shaped damaged or no expansion of IAC were termed as regular IAC type (Figures 1A–C); curved or angled damage of the IAC wall was termed as irregular IAC type (Figures 1D–F).
(4) Residual tumor volume: we calculated residual tumor volume by segmentation (multiplying the area of each part by the interval thickness and by adding all the interval volumes of each part.) according to enhanced MRI within 3–6 months after surgery.
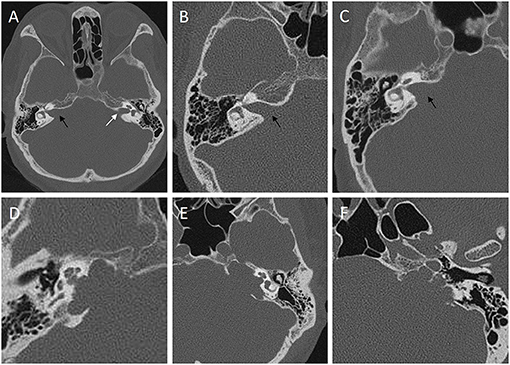
Figure 1. Classification of internal auditory canal (IAC) into regular type (A–C) and irregular type (D–F) according to the preoperative thin-slice CT bone window image of the temporal bone of the lesion side. (A) the right IAC is horn-shaped damaged type (black arrow) caused by a VS compared with the normal side (white arrow). (B) no expansion of the IAC (black arrow). (C) horn-shaped damaged type (black arrow). Curved damaged groove (D,E) or angled damaged groove (F) of the IAC wall could be observed in irregular IAC type.
Follow-up was performed at 3 and 6 months, and then once a year after surgery. During the follow-up period, tumor progression was monitored by enhanced MRI. For patients who underwent IR, we did not perform immediate treatment. Only when progression was detected, we should perform one of the following treatments based on the tumor size, location, and the willingness of the patient: (1) close wait-and-see strategy, (2) gamma-knife radiosurgery, and (3) second operation. The indication for prescribing radiotherapy of remnant VSs was tumor enlargement on radiological examination while the tumor is relatively small ( ≤ 3 cm); the indication for reoperation of regrowing VSs was progressive tumor enlargement radiologically, with or without new symptoms related to the tumor. The whole screening process was performed by two authors (JHL and XYD) independently according to the inclusion and exclusion criteria. In case of any disagreement, a third author (DBK) was consulted.
All statistical analysis was performed with SPSS version 23 software (SPSS, Chicago, IL, USA). The Student's t-test was used to compare continuous variables. Pearson's chi-square test or Fisher's exact probability test was used to compare categorical variables. Univariate and multivariate logistic regression analyses were used to analyze gender, age, preoperative tumor size, cystic formation, ICA type, residual tumor volume, and Ki-67 LI between the two groups so as to seek the risk factors for the progression of residual VSs after IR. p < 0.05 were considered statistically significant. The cutoff point was determined using the receiver operating characteristic (ROC) curve.
From January 2009 to January 2018, a total of 836 patients with sporadic VS received surgical resection in our institution by two independent neurosurgeons, among which 140 (16.7%) cases received IR via suboccipital retrosigmoid approach and met all the defined inclusion and exclusion criteria and were enrolled in our work. During an average follow-up time of 80.4 months (range, 24–134 months), a total of 35 cases (25.0%) had tumor progression (PG) and 105 cases (75.0%) had no sign of progression (SG). In PG, the mean regrowth interval was 54.2 ± 34.5 months (range, 10–120 months). In PG, 15 cases (42.9%) underwent reoperation, among which 10 cases were totally removed after reoperation, three cases still had remnant tumor and two cases had third operation; six patients (17.1%) had gamma-knife radiosurgery and reoperation, among which five cases were totally resected after reoperation, and one case still had remnant tumor; another 14 cases (40%) had gamma-knife radiosurgery alone, among which eight cases were stable, and six were shrank.
Of the 140 cases, 85 (60.7%) were women and 55 (39.3%) were men. There were 25 (71.4%) women and 10 (28.6%) men in PG and 60 (57.1%) women and 45 (42.9%) men in SG. The average age was 42.6 ± 13.0 years (range, 20–65 years) and 47.0 ± 14.5 years (range, 16–74 years) in PG and SG, respectively. Enhanced MRI data were obtained in 27 cases in the PG before first operation, with an average preoperative tumor size of 36.5 ± 8.9 mm (range, 22–58 mm); enhanced MRI data were obtained in 82 cases in the SG before operation, with an average tumor size of 31.0 ± 9.8 mm (range, 11–53 mm). Ten cases (37.0%) were cystic and 17 cases (63.0%) were solid in PG, and 29 cases (35.4%) were cystic and 53 cases (64.6%) were solid in the SG. Preoperative thin-slice CT of the IAC was obtained in 17 cases in PG, of which 10 (58.8%) cases were regular type and seven cases (41.2%) were irregular type; preoperative thin-slice CT of the IAC was obtained in 46 cases in the SG, of which 44 cases (95.7%) were regular type and only two cases (4.3%) were irregular type. The residual tumor volume was 304.6 ± 443.3 mm3 (range, 30–2,160 mm3) in PG and 75.9 ± 60.0 mm3 (range, 10–340 mm3) in SG. The Ki-67 LI was tested in 13 patients in PG, with an average value of 3.2 ± 2.8% (range, 1.0–10.0%); the Ki-67 LI was tested in 32 patients in SG, with an average value of 2.5 ± 2.0% (range, 0.5–8.0%). Ki-67 LI was tested in 10 patients receiving reoperation, with an average value of 5.0 ± 2.9% (range, 2.0–11.0%).
Independent sample t-test showed that preoperative tumor size was statistically larger in PG compared with SG (P = 0.014) and residual tumor volume was statistically greater in PG compared with SG (P < 0.001). Fisher's exact probability test showed the incidence of IAC irregular type in PG was significantly higher compared with SG (P < 0.001). Univariate analysis showed that there was no statistical difference between the two groups regarding age, gender, cystic formation, and Ki-67 LI. The detailed statistical results are shown in Supplementary Table 1.
The ROC curve showed that the optimal cutoff point of the preoperative tumor size was 26.5 mm, with 0.923 sensitivity and 0.354 specificity (Figure 2), and the cutoff point of residual tumor volume was 95 mm3, with 0.615 sensitivity and 0.866 specificity (Figure 2). The area under the curve (AUC) was 0.651 and 0.805, respectively (Figure 2). Multivariate regression analysis showed that residual tumor volume is an independent risk factor contributing the progression of remnant VSs (P = 0.012, OR = 1.017) (Supplementary Table 2).
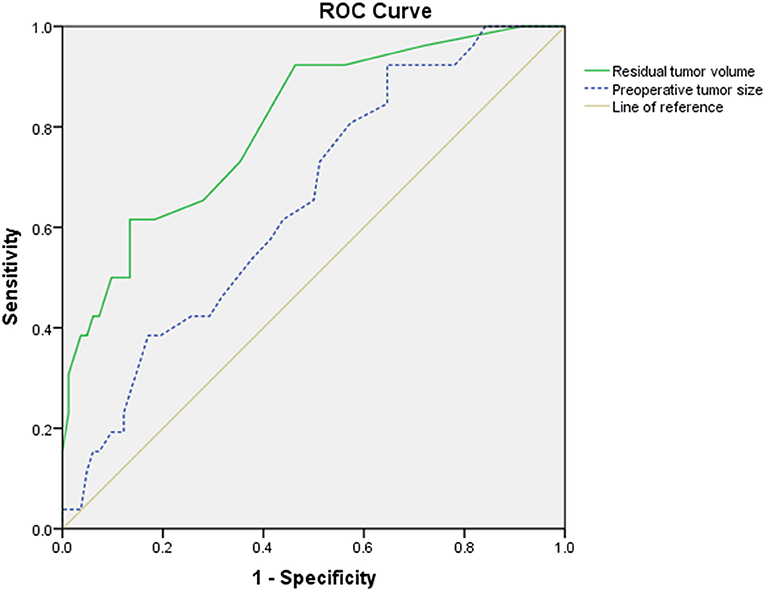
Figure 2. The receiver operating characteristic (ROC) curve of preoperative tumor size (blue dotted line) and residual tumor volume (green solid line). The area under the curve (AUC) was 0.651 and 0.805, respectively. The cutoff point of preoperative tumor size was 26.5 mm, with 0.923 sensitivity and 0.354 specificity, whereas the cutoff point of residual tumor volume was 95 mm3, with 0.615 sensitivity and 0.866 specificity.
The total removal rate of VSs is growing in many neurosurgery departments around the world. However, neurosurgeons sometimes have to compromise to IR for the sake of FN function preservation or because of the obstruction of bony structures, such as IAC wall. In surgical treatment of giant VS, we advocate that every effort should be made to achieve maximal resection. The essence of our institutional experience was that only if the neurophysiologic monitoring indicated or surgeons predicted a risk of facial nerve integrity and vulnerability, FN preservation trumped TR as a surgical goal (14). The fate of VSs that underwent IR includes regrowing, stable, or shrank (12, 19). According to the literature, VSs that received IR had approximately 10 times higher progression rate compared with those received TR (12–17). Besides, only part of remnant VSs would progress during follow-up. Therefore, it would be beneficial if we could find out the risk factors for regrowth in VSs receiving IR. Our study found that preoperative tumor size, residual tumor volume, and IAC type were significantly associated with regrowth in remnant VSs, although other clinical/radiological features, such as age, gender, cystic formation, and Ki-67 LI, did not have significant difference between the PG and SG. Multivariate analysis showed residual tumor volume was the only independent risk factor for progression.
Some investigators found that the growth rate of VSs in elderly patients is slower than that in younger patients (20–22). Our study did not find the predictive role of age and sex in remnant VSs, which is similar to the literature (8, 17, 23, 24). Overall, there is no strong evidence to prove that age and gender could be used as risk factors to predict regrowth of remnant VSs.
Our results revealed that patients in the PG had significantly larger preoperative tumor size than those in the SG (p = 0.014), with AUC value of 0.651 (Figure 2), which means a low prognostic value. Our finding was compatible with the work of Bloch et al., who found that patients with greater remnant tumor had significantly higher rate of tumor progression and had significantly larger preoperative tumor size (12). The reasons may contribute to intrinsic faster growth rate in larger VSs (25, 26), or the larger VSs may have greater residual tumor, resulting in progression (24), although other researchers found that preoperative tumor size was not a significant contributory factor (8, 17, 23, 24). The discrepancy may contribute to the giant VSs (≥ 4 cm) which is more common in developing countries and different from developed countries (14), and larger mean tumor size may have a lower rate of TR. According to univariate analysis and ROC curve, we found that for remnant VSs, the preoperative tumor size >26.5 mm is a risk factor for higher rate of progression. Meanwhile, our multivariate analysis indicated that the residual tumor volume is the only significant risk factor for progression.
Our study explored the correlation between the type of IAC and the progression of residual VSs for the first time. Univariate analysis showed that irregular destruction of the IAC is a risk factor for progression. We considered that the reason may be that it is more difficult to remove the tumor within the IAC because the tumor infiltrates in irregular corners of the IAC, which is hard to achieve adequate exposure of tumor, and thus gross TR is challenging (Figure 3). Also, the FN and bony structure of IAC may obstruct exposing and resecting tumors located in the bottom of IAC, resulting in greater amount of residuals, which leads to progression. However, multivariate analysis showed that IAC was not an independent risk factor for progression. We considered that if we further increase the sample size, the IAC type may be an independent risk factor for progression in remnant VSs. In summary, we recommended that patients with preoperative irregular damaged IAC should be informed that they may have a higher risk of progression if TR could not be achieved. The mechanism is unclear and requires more and larger samples of prospective, multicenter studies.
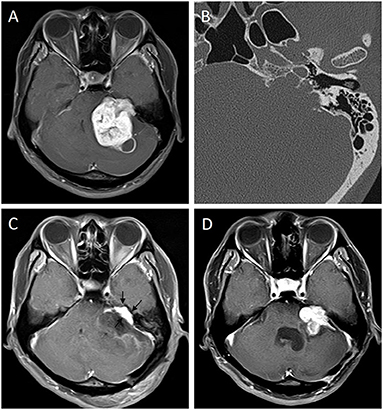
Figure 3. Progression of a remnant vestibular schwannoma with irregular IAC. Preoperative enhanced MRI (A) of a 29-year-old man showed a giant VS of left side. Thin-slice CT bone window image of temporal bone (B) showed irregular IAC type. Three-month postoperative enhanced MRI (C) showed remnant tumor located in the IAC (arrows). The tumor had progression 3 months later (D).
Some investigators pointed out that cystic VSs have higher proliferative activity than solid VSs (27), whereas our study found that there was no significant correlation between cystic formation and the rate of residual tumor progression. Chen et al. (18) found that for those VSs that underwent STR, six of 20 (30%) solid VSs had regrowth whereas only one of 18 (5.56%) cystic VSs had regrowth, but it did not reach statistical significance. Similarly, Fukuda et al. (17) did not find statistically significant correlation either. This was also consistent with the results of Hwang et al., who compared 15 cases of VSs with extremely rapid progression (>15 mm/year) with another 15 cases of non-progressive VSs and found no significant difference regarding cystic formation in the two groups (28). However, the sample size of the above studies is relatively small; thus, a prospective, multicenter study with a larger sample is necessary to explore whether cystic VSs have a significant impact on the progression of residual VSs compared with their solid counterparts.
The best method to evaluate the amount of residual tumor is still controversial. Some scholars believed that there is no significant difference using residual tumor thickness or residual tumor volume (29), whereas others suggested using the latter because measurement errors may occur because of partial volume effect (30). Assessing the thickness of residual tumors is more intuitional and quicker; however, it is more dependent on interobserver variability. Thus, we used the residual tumor volume to evaluate the amount of residual tumors, which is more objective and easily calculated on tumor segmentation.
The mechanism of progression in remnant VSs remains to be clarified. The progression of the residual tumor may be determined by the characteristics of the tumor cells and the blood supply of the tumor. Breshears et al. retrospectively analyzed 66 patients who underwent STR, and greater residual tumor volume and residual tumor located at IAC predicted progression (8). Kasantikul et al. (31) believed that if the diameter of VS is >20 mm, the angiogenesis around the tumor would be significantly accelerated, and so the smaller residual tumor would benefit better tumor control. Fukuda et al. (17) found that the thickness of the residual tumor >7.4 mm was a risk factor for progression and residual tumor thickness was the only independent risk factor. According to univariate analysis and ROC curve, we found that residual tumor volume >95 mm3 was a risk factor for higher rate of progression, with a moderate predictive value of AUC 0.805 (Figure 2). Nevertheless, we hold that every effort should be made to achieve TR, which could achieve lower progression rate compared with IR.
Ki-67 (MIB-1) LI, as a cell proliferation indicator, could be used to predict the progression of some intracranial benign tumors, such as meningiomas or pituitary tumors (32). Panigrahi et al. retrospectively analyzed 144 consecutive patients with sporadic VS, showing that the average Ki-67 LI was significantly higher in patients with recurrence at follow-up, and concluded that Ki-67 LI ≥ 3.5% at initial presentation was associated with recurrence regardless of the EOR (33). Another study found that tumor doubling time of VSs decreased logarithmically with increasing level of Ki-67 LI (34). Fukuda et al. (17) concluded that Ki-67 LI > 1.6% in patients with residual VSs is a risk factor for tumor progression. Hwang et al. (28) compared 15 cases of VSs with rapid progression (> 15 mm/year) after operation with 15 cases of stable VSs and found that Ki-67 LI in the rapid progression group was significantly higher than that in the stable group. According to our unpublished institutional data, the Ki-67 LI of patients with recurrent/regrowing VSs tested at reoperation was significantly higher than that of patients with stable VSs tested at the first surgery. Therefore, we hold that when VS progresses, Ki-67 LI, which reflects tumor proliferation characteristics, may subsequently rise. However, our findings showed that Ki-67 LI could not serve as a predictive factor of progression in remnant VSs. Overall, to determine whether Ki-67 LI could serve as a risk factor to predict progression of residual VSs, further study is warranted.
Our study has limitations. Firstly, this study is a retrospective non-randomized study with its intrinsic data selection bias. Secondly, this study is a single-center analysis of regrowth of VSs cases, which may be limited by the small sample size and may make the conclusion less convincing. Thirdly, our average follow-up time is 80.4 months (range, 24–134 months), which is insufficient to investigate whether VS has progressed. Fourthly, preoperative maximal diameter data could not be obtained in eight cases in the PG and 23 cases in the SG because the patients performed preoperative enhanced MRI in another hospital and the data could not be found, which could bring bias. Finally, Ki-67 LI was available in limited cases because it was not routinely performed before 2013 in our institution. Prospective, multicenter, and large sample size study together with clinicopathological and genetic analysis of VSs with irregular IAC is required.
For patients with IR of VSs, a larger preoperative tumor size, a greater residual tumor volume, and irregular IAC type are the risk factors for progression. These patients require imaging scan with a shorter interval to early detect signs of tumor progression during follow-up.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by Ethics Committee of West China Hospital of Sichuan University. Written informed consent to participate in this study was provided by all the patients or their authorized trustees. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
SZ and XH: conception and design and study supervision. JL, XD, DK, and JC: development of methodology, writing, reviewing, and revisioning of the manuscript. JL, XD, DK, JC, and SZ: acquisition and analysis of data. DK and SZ: technical and material support. All authors have read and approved the manuscript. All authors contributed to the article and approved the submitted version.
This work was supported by Youth Program of National Natural Science Foundation of China (No. 81801178), Program of Science & Technology Department of Sichuan Province (No. 2020YFS0222), and Program of Health Commission of Sichuan Province (No. 20PJ051).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The authors appreciate the patients' and their legal representatives' collaboration to this work.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2021.778590/full#supplementary-material
1. Carlson ML, Link MJ. Vestibular Schwannomas. N Engl J Med. (2021) 384:1335–48. doi: 10.1056/NEJMra2020394
2. Sughrue ME, Kaur R, Rutkowski MJ, Kane AJ, Kaur G, Yang I, et al. Extent of resection and the long-term durability of vestibular schwannoma surgery. J Neurosurg. (2011) 114:1218–23. doi: 10.3171/2010.11.JNS10257
3. Rinaldi V, Casale M, Bressi F, Potena M, Vesperini E, De Franco A, et al. Facial nerve outcome after vestibular schwannoma surgery: our experience. J Neurol Surg B Skull Base. (2012) 73:21–7. doi: 10.1055/s-0032-1304559
4. Matthies C, Samii M. Management of 1000 vestibular schwannomas (acoustic neuromas): clinical presentation. Neurosurgery. (1997) 40:1–9. doi: 10.1227/00006123-199701000-00001
5. Roche P-H, Khalil M, Thomassin J-M. Microsurgical removal of vestibular schwannomas after failed previous microsurgery. Prog Neurol Surg. (2008) 21:158–62. doi: 10.1159/000156903
6. Pan HC, Sheehan J, Sheu ML, Chiu WT, Yang DY. Intracapsular decompression or radical resection followed by Gamma Knife surgery for patients harboring a large vestibular schwannoma. J Neurosurg. (2012) 117:69–77. doi: 10.3171/2012.6.GKS12697
7. Iwai Y, Ishibashi K, Watanabe Y, Uemura G, Yamanaka K. Functional preservation after planned partial resection followed by gamma knife radiosurgery for large vestibular schwannomas. World Neurosurg. (2015) 84:292–300. doi: 10.1016/j.wneu.2015.03.012
8. Breshears JD, Morshed RA, Molinaro AM, McDermott MW, Cheung SW, Theodosopoulos PV. Residual tumor volume and location predict progression after primary subtotal resection of sporadic vestibular schwannomas: a retrospective volumetric study. Neurosurgery. (2020) 86:410–6. doi: 10.1093/neuros/nyz200
9. Samii M, Matthies C. Management of 1000 vestibular schwannomas (Acoustic Neuromas): the facial nerve-preservation and restitution of function. Neurosurgery. (1997) 40:684–95. doi: 10.1097/00006123-199704000-00006
10. Freeman SRM, Ramsden RT, Saeed SR, Alzoubi FQ, Simo R, Rutherford SA, et al. Revision surgery for residual or recurrent vestibular schwannoma. Otol Neurotol. (2007) 28:1076–82. doi: 10.1097/MAO.0b013e318159e76a
11. Pollock BE, Lunsford LD, Flickinger JC, Clyde BL, Kondziolka D. Vestibular schwannoma management. Part I Failed microsurgery and the role of delayed stereotactic radiosurgery. J Neurosurg. (1998) 89:944–8. doi: 10.3171/jns.1998.89.6.0944
12. Bloch DC, Oghalai JS, Jackler RK, Osofsky M, Pitts LH. The fate of the tumor remnant after less-than-complete acoustic neuroma resection. Otolaryngol Head Neck Surg. (2016) 130:104–12. doi: 10.1016/S0194-5998(03)01598-5
13. Ramina R, Coelho Neto M, Bordignon KC, Mattei T, Clemente R, Pires Aguiar PH. Treatment of large and giant residual and recurrent vestibular schwannomas. Skull Base. (2007) 17:109–17. doi: 10.1055/s-2006-953510
14. Zhang S, Liu W, Hui X, You C. Surgical treatment of giant vestibular schwannomas: facial nerve outcome and tumor control. World Neurosurg. (2016) 94:137–44. doi: 10.1016/j.wneu.2016.06.119
15. Roche P-H, Noudel R, Regis J. Management of radiation/radiosurgical complications and failures. Otolaryngol Clin North Am. (2012) 45:367–74. doi: 10.1016/j.otc.2011.12.007
16. Gerganov VM, Giordano M, Samii A, Samii M. Surgical treatment of patients with vestibular schwannomas after failed previous radiosurgery. J Neurosurg. (2012) 116:713–20. doi: 10.3171/2011.12.JNS111682
17. Fukuda M, Oishi M, Hiraishi T, Natsumeda M, Fujii Y. Clinicopathological factors related to regrowth of vestibular schwannoma after incomplete resection. J Neurosurg. (2011) 114:1224–31. doi: 10.3171/2010.11.JNS101041
18. Chen Z, Prasad SC, Di Lella F, Medina M, Piccirillo E, Taibah A, et al. The behavior of residual tumors and facial nerve outcomes after incomplete excision of vestibular schwannomas. J Neurosurg. (2014) 120:1278–87. doi: 10.3171/2014.2.JNS131497
19. Kameyama S, Tanaka R, Honda Y, Hasegawa A, Yamazaki H, Kawaguchi T. The long-term growth rate of residual acoustic neurinomas. Acta Neurochir. (1994) 129:127–30. doi: 10.1007/BF01406491
20. Silverstein H, McDaniel A, Norrell H, Wazen J. Conservative management of acoustic neuroma in the elderly patient. Laryngoscope. (1985) 95:766–70. doi: 10.1288/00005537-198507000-00002
21. El-Kashlan HK, Zeitoun H, Arts HA, Hoff JT, Telian SA. Recurrence of acoustic neuroma after incomplete resection. Am J Otol. (2000) 21:389–92. doi: 10.1016/S0196-0709(00)80049-6
22. Nedzelski JM, Canter RJ, Kassel EE, Rowed DW, Tator CH. Is no treatment good treatment in the management of acoustic neuromas in the elderly? Laryngoscope. (1986) 96:825–9. doi: 10.1288/00005537-198608000-00001
23. Vakilian S, Souhami L, Melancon D, Zeitouni A. Volumetric measurement of vestibular schwannoma tumour growth following partial resection: predictors for recurrence. J Neurol Surg B Skull Base. (2012) 73:117–20. doi: 10.1055/s-0032-1301395
24. Carlson ML, Van Abel KM, Driscoll CL, Neff BA, Beatty CW, Lane JI, et al. Magnetic resonance imaging surveillance following vestibular schwannoma resection. Laryngoscope. (2012) 122:378–88. doi: 10.1002/lary.22411
25. Ogawa K, Kanzaki J, Ogawa S, Yamamoto M, Ikeda S, Shiobara R. The growth rate of acoustic neuromas. Acta oto-laryngologica Supplementum. (1991) 487:157–63. doi: 10.3109/00016489109130462
26. Bederson JB, von Ammon K, Wichmann WW, Yasargil MG. Conservative treatment of patients with acoustic tumors. Neurosurgery. (1991) 28:646–50. doi: 10.1227/00006123-199105000-00002
27. Sinha S, Sharma BS. Cystic acoustic neuromas: surgical outcome in a series of 58 patients. J Clin Neurosci. (2008) 15:511–5. doi: 10.1016/j.jocn.2007.01.007
28. Hwang S-K, Kim DG, Paek SH, Kim C-Y, Kim MK, Chi JG, et al. Aggressive vestibular schwannomas with postoperative rapid growth: clinicopathological analysis of 15 cases. Neurosurgery. (2002) 51:1381–90. doi: 10.1097/00006123-200212000-00008
29. Charabi S, Tos M, Thomsen J, Charabi B, Mantoni M. Vestibular schwannoma growth: the continuing controversy. Laryngoscope. (2000) 110:1720–5. doi: 10.1097/00005537-200010000-00030
30. Herwadker A, Vokurka EA, Evans DGR, Ramsden RT, Jackson A. Size and growth rate of sporadic vestibular schwannoma: predictive value of information available at presentation. Otol Neurotol. (2005) 26:86–92. doi: 10.1097/00129492-200501000-00015
31. Kasantikul V, Netsky MG, Glasscock ME. 3rd, Hays JW. Acoustic neurilemmoma Clinicoanatomical study of 103 patients. J Neurosurg. (1980) 52:28–35. doi: 10.3171/jns.1980.52.1.0028
32. Li J, Deng X, Zhang S, Wang Q, Cheng J, Li X, et al. Intracranial clear cell meningioma: Clinical study with long-term follow-up in 24 patients. Clin Neurol Neurosurg. (2018) 175:74–83. doi: 10.1016/j.clineuro.2018.10.014
33. Panigrahi M, Kumar D, Vooturi S, Madigubba S, MIB. Index as predictor of recurrence in sporadic vestibular schwannomas. World Neurosurg. (2018) 120:e1203–e7. doi: 10.1016/j.wneu.2018.09.039
Keywords: vestibular schwannoma, incomplete resection, progression, risk factor, internal auditory canal, tumor size, residual tumor volume
Citation: Li J, Deng X, Ke D, Cheng J, Zhang S and Hui X (2021) Risk Factors for Progression in Vestibular Schwannomas After Incomplete Resection: A Single Center Retrospective Study. Front. Neurol. 12:778590. doi: 10.3389/fneur.2021.778590
Received: 17 September 2021; Accepted: 27 October 2021;
Published: 26 November 2021.
Edited by:
Sandro M. Krieg, Technical University of Munich, GermanyReviewed by:
Bowen Chang, Anhui Provincial Hospital, ChinaCopyright © 2021 Li, Deng, Ke, Cheng, Zhang and Hui. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Si Zhang, emhhbmdzaTE5ODcxMkAxMjYuY29t; Xuhui Hui, aHVpeHVodWl3Y0AxMjYuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.