- Department of Neurosurgery, Osaka University Graduate School of Medicine, Suita, Japan
Purpose: Meningiomas are the most common primary intracranial neoplasms and clinical symptom appearance depends on their volume and location. This study aimed to identify factors that influence clinical symptoms and to determine a specific threshold tumor volume for the prediction of symptomatic progression in patients with convexity, parasagittal, and falx meningiomas.
Materials and Methods: We retrospectively studied patients with radiologically suspected convexity, parasagittal, or falx meningiomas at our institution.
Results: The data of three hundred thirty-three patients were analyzed. We further divided patients into two groups based on clinical symptoms: an asymptomatic group (250 cases) and a symptomatic group (83 cases). Univariate analysis revealed significant differences between the groups in terms of sex (p = 0.002), age at the time of volumetric analysis (p < 0.001), hyperintense lesions on T2-weighted images (p = 0.029), peritumoral edema (p < 0.001), maximum tumor diameter (p < 0.001), and tumor volume (p < 0.001). Further multivariate analysis revealed significant differences between the groups in terms of age at the time of volumetric analysis (p = 0.002), peritumoral edema (p < 0.001), and tumor volume (p < 0.001). The receiver operating characteristic curve revealed a threshold tumor volume of 21.1 ml for predicting whether a patient would develop symptoms (sensitivity 0.843, specificity 0.880, an area under the curve 0.919 [95% confidence interval: 0.887–0.951]).
Conclusion: We identified factors predictive of clinical symptoms in patients with convexity, parasagittal, and falx meningiomas and determined the first-ever threshold tumor volume for predicting symptomatic progression in such patients.
Introduction
Meningiomas are the most common primary intracranial tumors, accounting for ~25–38% of all such lesions (1, 2). The number of incidentally discovered meningiomas has increased with the widespread use of neuroimaging modalities such as computed tomography and magnetic resonance imaging (MRI) (3). In fact, radiological studies have revealed that neuroimaging could incidentally reveal suspected meningioma lesions with an incidence ranging from 0.9 to 2.5% in individuals aged in their middle years and older (4, 5). On the other hand, meningiomas are often discovered because of a variety of symptoms, including motor and sensory deficits, cognitive decline, and epilepsy (6–8). However, the factors that determine whether a lesion is symptomatic remain unclear.
Meningiomas are benign neoplasms that can exhibit a variety of growth patterns (9, 10) and, eventually, 67–75% of them enlarge (7, 10, 11). In one study in which the median tumor volume was 35.7 ml (range 1.1–133.1 ml) and 90% (52 patients) were symptomatic, tumor volume was statistically significantly related to the appearance of clinical symptoms (6). In recent meta-analyses, 4.7–8.1% of patients with incidentally discovered intracranial meningiomas developed related symptoms at follow-up visits (11, 12). However, the specific locations of the tumors were not examined in either report. The location of such a tumor is important as it is related to the symptoms a patient will experience (8), as well as the clinical and biological behavior of the tumor (6, 13, 14).
Convexity, parasagittal, and falx meningiomas account for almost 50% of all meningiomas (15). In the report in which the association between tumor volume and clinical symptoms was observed in intracranial meningiomas, nearly half of the cases were skull-base meningiomas (6). Convexity, parasagittal, and falx meningiomas differ from skull-base meningiomas in that they are located in the supratentorial space and do not involve cranial nerves. Thus, the tumor volume that causes clinical symptoms differs between supratentorial and skull-base meningiomas, and it is important to analyze the tumor volume that causes clinical symptoms exclusively for convexity, parasagittal, and falx meningiomas.
The purpose of this retrospective study was to identify factors that influence clinical symptoms and to determine a specific threshold tumor volume for the prediction of symptomatic progression in patients with convexity, parasagittal, and falx meningiomas.
Materials and Methods
Study Design and Patient Selection
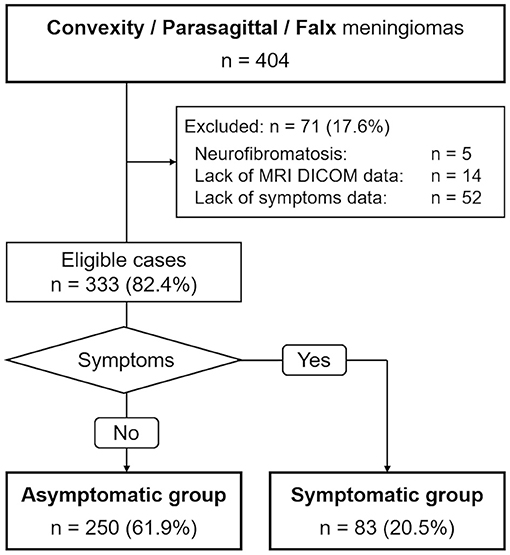
We conducted a retrospective case-control study of patients with primary radiologically suspected convexity, parasagittal, or falx meningiomas. We collected data from patients whose first visit was from 1990 to 2020 at our institution. We excluded patients diagnosed with neurofibromatosis, those for whom MRI Digital Imaging and Communications in Medicine (DICOM) data were not available, and those whose symptoms were unknown due to insufficient medical records of their first visit. When patients had more than one convexity, parasagittal, or falx meningioma, we selected the largest one for analysis. The Osaka University Clinical Research Review Committee approved the study (approval number 14231) and waived the need for written informed consent, as all data were retrospective.
Definition of Symptoms
We determined from their medical records whether patients had clinical findings, which we defined as clinical symptoms. When patients exhibited more than one symptom, we selected the one that mainly interfered with their daily life. We further defined neurological symptoms as excluding epilepsy or non-specific symptoms such as headache.
Volumetric Analysis
We measured the volume of each lesion using the latest MRI DICOM data for asymptomatic patients or the MRI DICOM data at the time of symptom onset for symptomatic patients. We used Horos for macOS to perform the measurements (Horos is a free and open-source code software program that is distributed free of charge under the LGPL license at Horosproject.org and sponsored by Nimble Co LLC d/b/a Purview in Annapolis, MD USA). Using T2-weighed images (T2WIs) or contrast-enhanced T1 weighted images (T1WIs) of ~5 mm slice thickness, we measured the tumor area in each slice by manually tracing the tumor boundary. Thereafter, we multiplied the sum of all the areas by the thickness between slices, including the gaps.
Tumor Diameter
We used the same MRI DICOM data as for volumetric analysis to measure tumor diameter. The maximum tumor diameter was determined using either axial, coronal, or sagittal images.
Tumor Location, Side, and Area
The lead author (SY) carefully determined the locations of the lesions via MRI, which was independently confirmed by the senior author (NKi). We divided tumor location in three ways: convexity, parasagittal angle, and falx cerebri; right, and left; frontal, middle, and occipital area. We used “frontal area” for the anterior one-third, “occipital area” for the posterior one-third, and “middle area” for the rest.
Interpretation of T2-Weighted Images
We classified lesions according to the radiologic characteristics on T2WIs. They were classified as either “T2-hyperintense” or “other” according to the brightness of the lesion. Lesions that were too heterogenous to classify were assigned to the “other” group. One case with a maximum diameter of only 1 mm was excluded from analysis because the lesion was too small to evaluate.
Statistical Analysis
All statistical analyses were performed using R 3.6.3 for Windows (www.R-project.org; R Foundation for Statistical Computing, Vienna, Austria). Statistical differences for categorical variables were examined using a two-tailed Fisher's exact test. Continuous variables were assessed using the Mann-Whitney U test or the Kruskal-Wallis test adjusted by Bonferroni correction. Multivariate logistic regression analysis was performed with variables that were significant in those univariate analyses. The thresholds were calculated by receiver operating characteristic (ROC) analysis using the distance from the upper left-hand corner (16). Probability values <0.05 were considered significant.
Results
Overall Patient Cohort
Figure 1 illustrates the flow of patient selection. The data of 333 patients (84 male and 249 female) were analyzed. The median age at volumetric analysis was 70 years (range 23–90 years). The median tumor volume and the median maximum tumor diameter were 8.2 ml (range 0.1–188.9 ml) and 30 mm (range 5–100 mm), respectively. We further divided patients into two groups based on clinical symptoms: an asymptomatic group (250 cases) and a symptomatic group (83 cases).
Comparison Between the Asymptomatic and Symptomatic Groups
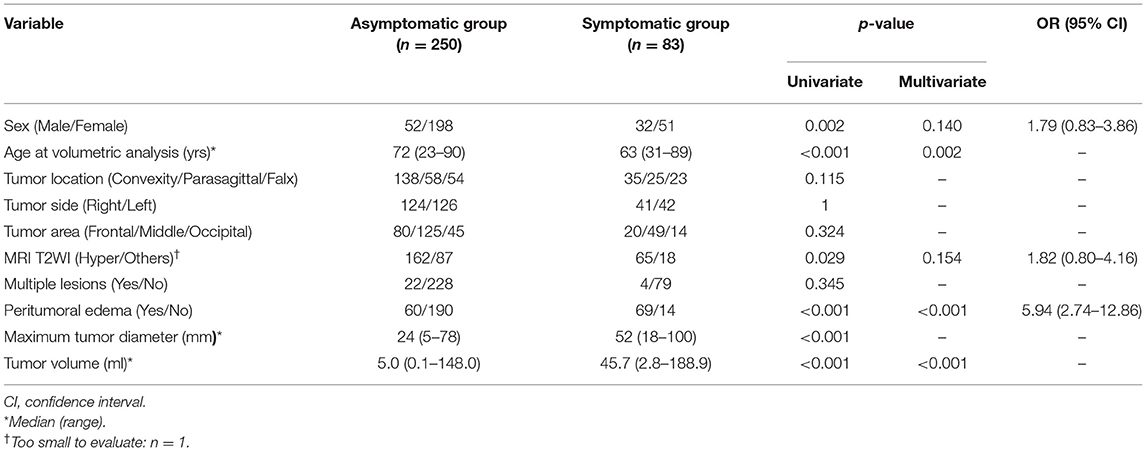
Table 1 summarizes the characteristics of each group. Univariate analysis revealed significant differences between the groups in terms of sex (p = 0.002), age at the time of volumetric analysis (p < 0.001), hyperintense lesions on T2WIs (p = 0.029), peritumoral edema (p < 0.001), maximum tumor diameter (p < 0.001), and tumor volume (p < 0.001, Figure 2A). For multivariate analysis, the maximum tumor diameter was excluded as it is a similar metric to tumor volume (17). Multivariate analysis revealed significant differences between the groups in terms of age at the time of volumetric analysis (p = 0.002), peritumoral edema (p < 0.001), and tumor volume (p < 0.001). The odds ratio (OR) for peritumoral edema was 5.94 (95% confidence interval [CI]: 2.74–12.86).
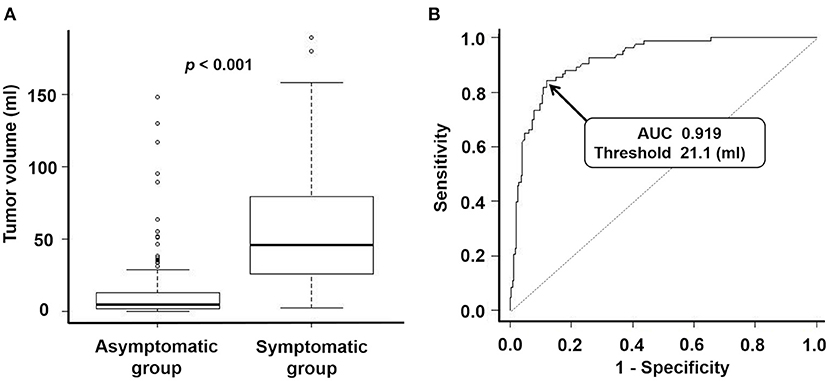
Figure 2. (A) Box-and-whisker plots representing tumor volume of an asymptomatic group and symptomatic group. The median tumor volume of the asymptomatic group and the symptomatic group were 5.0 ml and 45.7 ml, respectively. P-value for the Mann-Whitney U test: <0.001. (B) Receiver operating characteristic curve for predicting which patients will become symptomatic via tumor volume. Area under the curve: 0.919 (95% confidence interval: 0.887–0.951). Threshold for tumor volume: 21.1 ml. Sensitivity: 0.843. Specificity: 0.880.
Thresholds for Predicting Development of Clinical Symptoms
Patients in the symptomatic group experienced motor deficits (37 cases), epilepsy (18 cases), gait disorder (seven cases), visual impairment (seven cases), cognitive decline (six cases), aphasia (four cases), headaches (two cases), sensory deficits (one case), and a subcutaneous mass (one case). The ROC curve revealed a threshold tumor volume of 21.1 ml for predicting whether a patient would develop symptoms, with a sensitivity of 0.843, a specificity of 0.880, and an area under the curve (AUC) of 0.919 (Figure 2B, 95% CI: 0.887–0.951). In addition, a threshold maximum tumor diameter of 40 mm may also be a reliable marker for predicting which patients will become symptomatic, with a sensitivity of 0.819, a specificity of 0.840, and an AUC of 0.893 (95% CI: 0.856–0.930). For 13 patients in the symptomatic group, we obtained MRI DICOM data when they had been asymptomatic at their first visit. Of these, the 21.1-ml and 40-mm threshold correctly predicted the development of symptoms in seven cases (54%).
Threshold for Predicting Development of Neurological Symptoms
When focusing only on neurological symptoms (62 cases), the threshold for tumor volume was also 21.1 ml (sensitivity 0.871, specificity 0.880, AUC 0.937 [95% CI: 0.910–0.965]). The threshold for maximum tumor diameter for prediction of neurological symptoms was almost the same as that for all symptoms: 41 mm (sensitivity 0.839, specificity 0.856, AUC 0.914 [95% CI: 0.880–0.948]).
Comparison by Age at the Time of Volumetric Analysis
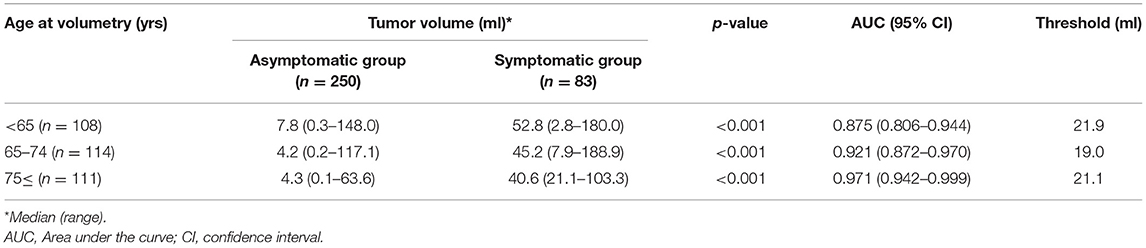
When divided into three age groups; <65, 65–74, 75 ≤, the rates of symptomatic patients were 40.7, 24.6, and 9.9%, and the ORs for presenting clinical symptoms were 1 (Reference), 0.47 (95% CI: 0.27–0.84), and 0.16 (95% CI: 0.08–0.33), respectively. In all age groups, the tumor volume was significantly larger in the symptomatic than in the asymptomatic group (p < 0.001, Table 2). The threshold for predicting patients in which symptoms would develop was similar: 21.9 ml for patients <65 years, 19.0 ml for patients 64–74 years, and 21.1 ml for patients ≥75 years.
Comparison by Tumor Location
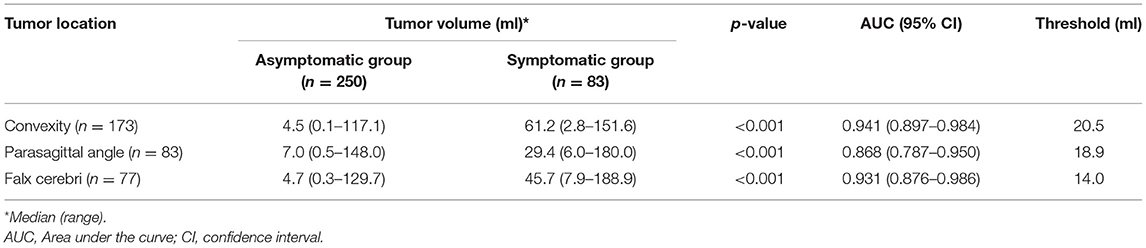
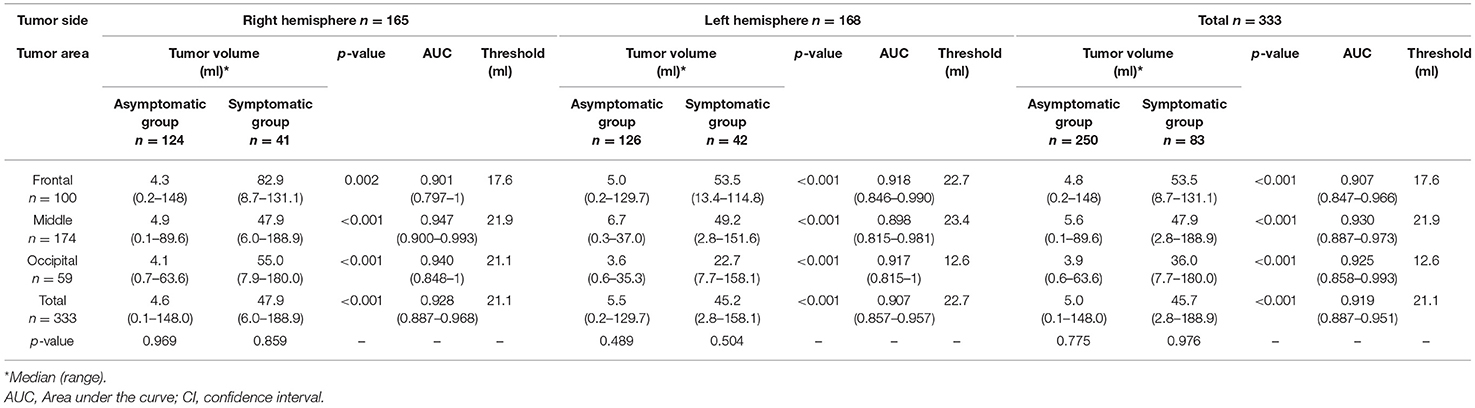
Tumor volume of the asymptomatic and symptomatic group based on tumor location is shown in Table 3. For all locations, tumor volume was significantly larger in the symptomatic than in the asymptomatic group (p < 0.001). Falx meningiomas had a slightly lower threshold for symptomatic progression than other locations.
Correlation Between Tumor Volume and Tumor Side/Area
Table 4 displays the difference in tumor volume between the asymptomatic and symptomatic groups, depending on the side or area of the tumor. For all areas, the symptomatic group had significantly larger tumor volume than the asymptomatic group (p < 0.001). The threshold for predicting patients to develop clinical symptoms was also around 21.1 ml for all areas except the occipital area of the left hemisphere.
Comparison by WHO Grade
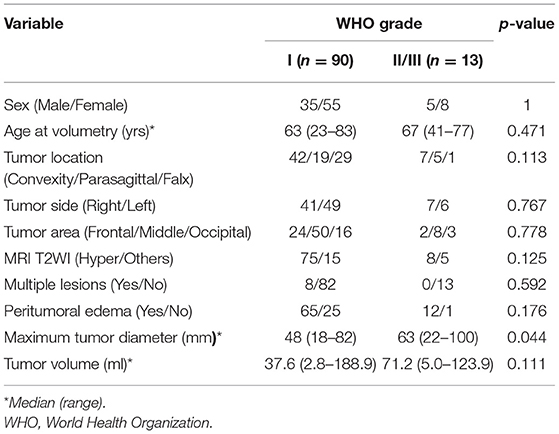
Thirty-three patients in the asymptomatic and 77 in the symptomatic group received surgical treatment. Of the 110 patients, the meningiomas of 90 (81.8%) were World Health Organization (WHO) grade I, 10 (9.1%) were WHO grade II, 3 (2.7%) were WHO grade III, and 7 (6.4%) were not mentioned in WHO grade. There were no significant differences between patients with WHO grade I and those with WHO grade II/III meningiomas, except in the maximum tumor diameter, which was larger in the latter than in the former (Table 5).
Discussion
In the present study, we identified factors that are related to clinical symptoms of patients with convexity, parasagittal, and falx meningiomas, and, to our knowledge, we determined the first-ever threshold of tumor volume for predicting symptomatic progression in such patients. This may allow clinicians to predict when a growing, asymptomatic meningioma will become symptomatic. Currently, observation is the first choice for management of patients with asymptomatic meningiomas (18); these results may be useful to determine the necessity of treatment and its appropriate timing.
In this study, we also determined a threshold maximum tumor diameter of 40 mm for the prediction of symptomatic progression of patients (sensitivity 0.819, specificity 0.840, AUC 0.893). However, a tumor volume of 21.1 ml was a more accurate threshold (sensitivity 0.843, specificity 0.880, AUC 0.919). Maximum tumor diameter and tumor volume are highly correlated (17). However, tumors do not always grow in the direction of their maximum diameter, which may be why we discovered tumor volume to be the more accurate predictive factor. Maximum tumor diameter is one of the most convenient clinical metrics; however, our results indicate that the development and widespread use of a simple method to measure tumor volume is needed.
Meningiomas manifest in a variety of symptoms (8), including non-specific symptoms such as headaches and dizziness (7). Such non-specific symptoms make clinicians wonder if interventions such as surgery or radiotherapy are required for the existing meningioma. In this study, we also calculated thresholds for tumor volume and maximum tumor diameter to predict the development of neurological symptoms. However, the thresholds were similar to those for all clinical symptoms. Therefore, it may be less important for clinicians to examine whether non-specific symptoms are caused by a given meningioma.
The hemisphere in which glioblastomas and strokes occur affects the symptoms that the patient experiences (19, 20); however, in this study of meningiomas, we detected no differences between hemispheres. This may be because few patients presented with cognitive decline (7%) or aphasia (5%). The low threshold of the tumor volume only in the occipital area of the left hemisphere may be due to the small number of symptomatic patients itself. Falx meningiomas also had a lower threshold for symptomatic progression than other locations, but as in previous studies (21–23), the number of cases may not have been sufficient. Therefore, further large-scale studies are needed to validate our location-specific findings.
In multiple meta-analyses (11, 24), a T2-hyperintense sign was correlated with radiological progression, and peritumoral edema was the only imaging metric that correlated with symptomatic progression, which was confirmed in this study. Since T2WIs may be appropriate for follow-up of untreated meningiomas (25), symptom-related radiological indicators that do not require contrast-enhanced T1WIs are needed.
We should note that this study has several limitations. The first is the fact that this was a single-center, retrospective study conducted in Japan. As Japan has the largest number of MRIs per unit population in the world (26), a larger proportion of small, asymptomatic meningiomas may be detected than in other countries. This would have lowered the thresholds of tumor volume and maximum tumor diameter for predicting symptomatic progression of patients in this study. The second limitation is the possibility of errors in volumetric measurements. Volumetric measurement may be inaccurate especially for small tumors (27), and manual segmentation may be inconsistent (28). Finally, this study was conducted on radiologically presumed meningiomas; therefore, 2.9–3.4% of our study population may actually have had other tumors (10, 29).
Conclusion
In the present study, we identified factors predictive of clinical symptoms in patients with convexity, parasagittal, and falx meningiomas and, to our knowledge, determined the first-ever threshold tumor volume for predicting symptomatic progression in such patients. These results may be useful in allowing clinicians to estimate when a growing, asymptomatic meningioma will develop clinical symptoms, thereby improving management of patients with the disease.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Ethics Statement
The studies involving human participants were reviewed and approved by Osaka University Clinical Research Review Committee. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author Contributions
SY and NKi contributed to the concept, drafting, and writing the manuscript. TN contributed to the acquisition of data. RH and NKa contributed to the manuscript editing. MK contributed to the provision of insightful thought and design. HK contributed to the study supervision. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We would like to thank Editage (www.editage.com) for English language editing.
Abbreviations
ROC, receiver operating characteristic; OR, odds ratio; CI, confidence interval; AUC, area under the curve; WHO, World Health Organization.
References
1. Claus EB, Bondy ML, Schildkraut JM, Wiemels JL, Wrensch M, Black PM. Epidemiology of intracranial meningioma. Neurosurgery. (2005) 57:1088–94. doi: 10.1227/01.NEU.0000188281.91351.B9
2. Ostrom QT, Patil N, Cioffi G, Waite K, Kruchko C, Barnholtz-Sloan JS, et al. Statistical report: primary brain and other central nervous system tumors diagnosed in the United States in 2013–2017. Neuro Oncol. (2020) 22:iv1–iv96. doi: 10.1093/neuonc/noaa200
3. Islim AI, Kolamunnage-Dona R, Mohan M, Moon RDC, Crofton A, Haylock BJ, et al. prognostic model to personalize monitoring regimes for patients with incidental asymptomatic meningiomas. Neuro Oncol. (2020) 22:278–89. doi: 10.1093/neuonc/noz160
4. Vernooij MW, Ikram MA, Tanghe HL, Vincent AJPE, Hofman A, Krestin GP, et al. Incidental findings on brain MRI in the general population. N Engl J Med. (2007) 357:1821–8. doi: 10.1056/NEJMoa070972
5. Bos D, Adams HHH, Zonneveld HI, Verlinden VJA, Verbruggen JGJ. Course of incidental findings on brain MR images: the population. Radiology. (2016) 281:507–15. doi: 10.1148/radiol.2016160218
6. Kauke M, Safi AF, Stavrinou P, Krischek B, Goldbrunner R, Timmer M. Does meningioma volume correlate with clinical disease manifestation irrespective of histopathologic tumor grade? J Craniofac Surg. (2019) 30:e799–802. doi: 10.1097/SCS.0000000000005845
7. Kim KH, Kang SJ, Choi J-W, Kong D-S, Seol HJ, Nam D-H, et al. Clinical and radiological outcomes of proactive Gamma Knife surgery for asymptomatic meningiomas compared with the natural course without intervention. J Neurosurg. (2019) 130:1740–9. doi: 10.3171/2017.12.JNS171943
8. Fathi AR, Roelcke U. Meningioma. Curr Neurol Neurosci Rep. (2013) 13:337. doi: 10.1007/s11910-013-0337-4
9. Hashiba T, Moto NH, Izumoto S, Suzuki T, Kagawa N, Maruno M, et al. Serial volumetric assessment of the natural history and growth pattern of incidentally discovered meningiomas: clinical article. J Neurosurg. (2009) 110:675–84. doi: 10.3171/2008.8.JNS08481
10. Behbahani M, Skeie GO, Eide GE, Hausken A, Lund-Johansen M, Skeie BS. A prospective study of the natural history of incidental meningioma-Hold your horses! Neuro Oncol Pract. (2019) 6:438–50. doi: 10.1093/nop/npz011
11. Nakasu S, Nakasu Y. Natural history of meningiomas: review with meta-analyses. Neurol Med Chir (Tokyo). (2020) 60:109–20. doi: 10.2176/nmc.ra.2019-0213
12. Islim AI, Mohan M, Moon RDC, Srikandarajah N, Mills SJ, Brodbelt AR, et al. Incidental intracranial meningiomas: a systematic review and meta-analysis of prognostic factors and outcomes. J Neurooncol. (2019) 142:211–21. doi: 10.1007/s11060-019-03104-3
13. Maiuri F, Mariniello G, Guadagno E, Barbato M, Corvino S, Del Basso De Caro M, et al. grade, proliferation index, and progesterone receptor expression are different according to the location of meningioma. Acta Neurochir (Wien). (2019) 161:2553–61. doi: 10.1007/s00701-019-04084-z
14. Hashimoto N, Rabo CS, Okita Y, Kinoshita M, Kagawa N, Fujimoto Y, et al. Slower growth of skull base meningiomas compared with non–skull base meningiomas based on volumetric and biological studies. J Neurosurg. (2012) 116:574–80. doi: 10.3171/2011.11.JNS11999
15. Sun C, Dou Z, Wu J, Jiang B, Iranmanesh Y, Yu X, et al. The preferred locations of meningioma according to different biological characteristics based on voxel-wise analysis. Front Oncol. (2020) 10:1412. doi: 10.3389/fonc.2020.01412
16. Akobeng AK. Understanding diagnostic tests 3: receiver operating characteristic curves. Acta Paediatr Int J Paediatr. (2007) 96:644–7. doi: 10.1111/j.1651-2227.2006.00178.x
17. Ishi Y, Terasaka S, Yamaguchi S, Yoshida M, Endo S, Kobayashi H, et al. Reliability of the size evaluation method for meningiomas: maximum diameter, ABC/2 formula, and planimetry method. World Neurosurg. (2016) 94:80–8. doi: 10.1016/j.wneu.2016.06.108
18. Goldbrunner R, Minniti G, Preusser M, Jenkinson MD, Sallabanda K, Houdart E, et al. EANO guidelines for the diagnosis and treatment of meningiomas. Lancet Oncol. (2016) 17:e383–91. doi: 10.1016/S1470-2045(16)30321-7
19. Baumann C, Tichy J, Schaefer JH, Steinbach JP, Mittelbronn M, Wagner M, et al. Delay in diagnosing patients with right-sided glioblastoma induced by hemispheric-specific clinical presentation. J Neurooncol. (2020) 146:63–9. doi: 10.1007/s11060-019-03335-4
20. Foerch C, Misselwitz B, Sitzer M, Berger K, Steinmetz H, Neumann-Haefelin T. Difference in recognition of right and left hemispheric stroke. Lancet. (2005) 366:392–3. doi: 10.1016/S0140-6736(05)67024-9
21. Das KK, Gosal JS, Sharma P, Mehrotra A, Bhaisora K, Sardhara J, et al. Falcine meningiomas: analysis of the impact of radiologic tumor extensions and proposal of a modified preoperative radiologic classification scheme. World Neurosurg. (2017) 104:248–58. doi: 10.1016/j.wneu.2017.04.159
22. Zuo FX, Wan JH, Li XJ, Qian HP, Meng XLA. proposed scheme for the classification and surgical planning of falcine meningioma treatment. J Clin Neurosci. (2012) 19:1679–83. doi: 10.1016/j.jocn.2012.01.034
23. Chung S-B, Kim C-Y, Park C-K, Kim DG, Jung H-W. Falx meningiomas: surgical results and lessons learned from 68 cases. J Korean Neurosurg Soc. (2007) 42:276. doi: 10.3340/jkns.2007.42.4.276
24. Sughrue ME, Rutkowski MJ, Aranda D, Barani IJ, McDermott MW, Parsa AT. Treatment decision making based on the published natural history and growth rate of small meningiomas. J Neurosurg. (2010) 113:1036–42. doi: 10.3171/2010.3.JNS091966
25. Boto J, Guatta R, Fitsiori A, Hofmeister J, Meling TR, Vargas MI. Is contrast medium really needed for follow-up MRI of untreated intracranial meningiomas? Am J Neuroradiol. (2021) 42:1421–8. doi: 10.3174/ajnr.A7170
26. Matsumoto M, Koike S, Kashima S, Awai K. Geographic distribution of CT, MRI and PET devices in Japan: a longitudinal analysis based on national census data. PLoS ONE. (2015) 10:e0126036. doi: 10.1371/journal.pone.0126036
27. Pan HC, Cheng FC, Sun MH, Chen CCC, Sheehan J. Prediction of volumetric data errors in patients treated with Gamma Knife radiosurgery. Stereotact Funct Neurosurg. (2007) 85:184–91. doi: 10.1159/000101297
28. Mazzara GP, Velthuizen RP, Pearlman JL, Greenberg HM, Wagner H. Brain tumor target volume determination for radiation treatment planning through automated MRI segmentation. Int J Radiat Oncol Biol Phys. (2004) 59:300–12. doi: 10.1016/j.ijrobp.2004.01.026
Keywords: convexity meningioma, falx meningioma, parasagittal meningioma, symptomatic progression, tumor volume
Citation: Yamada S, Kijima N, Nakagawa T, Hirayama R, Kinoshita M, Kagawa N and Kishima H (2021) How Much Tumor Volume Is Responsible for Development of Clinical Symptoms in Patients With Convexity, Parasagittal, and Falx Meningiomas? Front. Neurol. 12:769656. doi: 10.3389/fneur.2021.769656
Received: 02 September 2021; Accepted: 25 October 2021;
Published: 17 November 2021.
Edited by:
Hailiang Tang, Fudan University, ChinaReviewed by:
Rintaro Hashizume, Northwestern University, United StatesRyota Tamura, Keio University, Japan
Nora F. Dengler, Charité—Universitätsmedizin Berlin, Germany
Copyright © 2021 Yamada, Kijima, Nakagawa, Hirayama, Kinoshita, Kagawa and Kishima. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Noriyuki Kijima, bi1raWppbWFAbnN1cmcubWVkLm9zYWthLXUuYWMuanA=
 Shuhei Yamada
Shuhei Yamada Noriyuki Kijima
Noriyuki Kijima Tomoyoshi Nakagawa
Tomoyoshi Nakagawa Manabu Kinoshita
Manabu Kinoshita Naoki Kagawa
Naoki Kagawa Haruhiko Kishima
Haruhiko Kishima