
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Neurol., 17 December 2021
Sec. Applied Neuroimaging
Volume 12 - 2021 | https://doi.org/10.3389/fneur.2021.769511
This article is part of the Research TopicNovel Imaging Technologies for NeuroinflammationView all 5 articles
Background: Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS) is a multisystem medical condition with heterogeneous symptom expression. Currently, there is no effective cure or treatment for the standard care of patients. A variety of ME/CFS symptoms can be linked to the vital life functions of the brainstem, the lower extension of the brain best known as the hub relaying information back and forth between the cerebral cortex and various parts of the body.
Objective/Methods: Over the past decade, Magnetic Resonance Imaging (MRI) studies have emerged to understand ME/CFS with interesting findings, but there has lacked a synthesized evaluation of what has been found thus far regarding the involvement of the brainstem. We conducted this study to review and evaluate the recent MRI findings via a literature search of the MEDLINE database, from which 11 studies met the eligibility criteria.
Findings: Data showed that MRI studies frequently reported structural changes in the white and gray matter. Abnormalities of the functional connectivity within the brainstem and with other brain regions have also been found. The studies have suggested possible mechanisms including astrocyte dysfunction, cerebral perfusion impairment, impaired nerve conduction, and neuroinflammation involving the brainstem, which may at least partially explain a substantial portion of the ME/CFS symptoms and their heterogeneous presentations in individual patients.
Conclusions: This review draws research attention to the role of the brainstem in ME/CFS, helping enlighten future work to uncover the pathologies and mechanisms of this complex medical condition, for improved management and patient care.
Myalgic Encephalomyelitis, also known as Chronic Fatigue Syndrome (ME/CFS), is a multisystem medical condition affecting the central nervous, cardiovascular, and immune system functions (1). It is estimated that 17 million people worldwide (2, 3) including over 580,000 Canadians (4) are currently living with ME/CFS, with 25% of that population being either housebound or bedbound (5). ME/CFS is characterized by post-exertional malaise, i.e., a worsening of symptoms following minimal mental or physical exertions, and sensory overload with symptoms lasting for up to several months. Other common symptoms can include autonomic dysfunction, e.g., orthostatic intolerance and postural tachycardia problems (6); cognitive deficits including impairments in attention and working memory (7, 8), and substantial reductions in activity engagement (8). ME/CFS is predominant in females and typically has an onset following an infection, environmental toxin exposure, or traumatic accidents. Due to the absence of diagnostic biomarkers and the heterogeneous disease expression among individual patients, diagnosis of ME/CFS involves the careful consideration of other diseases and conditions with similar symptoms (1, 9). The National Institute for Health and Care Excellence (NICE) guidelines on ME/CFS (10) confirm the non-existence of curative treatments for ME/CFS. There are no Food and Drug Administration or Health Canada approved treatments or cures, presumably owing to its unknown pathologies and mechanisms.
The brainstem (or brain stem) is a compact brain structure that lies between the cerebrum and the cervical spinal cord. It consists of three components: the midbrain, pons, and the medulla; consisting of both gray matter (GM) and white matter (WM). The brainstem has connections with numerous other brain structures, including the prefrontal cortex, hypothalamus, cerebellum, hippocampus, and sensorimotor cortex and performs conduit, cranial nerve, and integrative functions that are prerequisite for living (11, 12). The conduit function involves the passage for all WM tracts that connect the brain and the body, e.g., the corticospinal tract (delivering motor output from the brain), spinothalamic tract and dorsal column-medial lemniscus (providing sensory input to the brain), and the autonomic nervous system sympathetic and parasympathetic tracts (13). The brainstem also contains the cranial nuclei for cranial nerves 3-12, bringing in sensory input like facial sensation and delivering both voluntary and involuntary motor output (14). The vasomotor and respiratory centers of the medulla and pons (part of the reticular formation) integrate the regulation of heart rate, blood pressure, and breathing (15, 16). The substantia nigra and ventral tegmental area of the midbrain execute dopaminergic control of motivation, concentration, and learning (17, 18). The reticular activating system (RAS) nuclei of the brainstem are fundamental for regulating the sleep/wakefulness cycles and consciousness (19, 20).
Many symptoms shown in patients with ME/CFS may be associated with brainstem dysfunctions of different extents. For example, sleep disorders have been linked to impaired brainstem reticular formation especially the RAS nuclei (3). Dysregulation of sleep/wakefulness cycles can cause decreased consciousness, leading to impaired cognitive function (11). Also, autonomic dysfunctions such as inappropriate adjustments of the heart rate to exertion have been linked to damaged dorsal motor nucleus of the vagus nerve (cranial nerve ten) that innervates the brainstem vasomotor center (21). Furthermore, widespread neuroinflammation as seen in ME/CFS especially post exertion (22) has been related to the role the vagus nerve has in triggering neuroinflammation (21, 23). In addition, altered dopaminergic function can arise from abnormal dopaminergic brainstem areas such as the substantia nigra and the ventral tegmental nuclei (24). Finally, several cardiac and respiratory symptoms such as orthostatic intolerance and abnormal breathing (8) can involve the brainstem, specifically the vasomotor, cardiac, and respiratory centers of the medulla and pons. Taken together, the associations between the typical ME/CFS symptoms and the brainstem functions indicate that the brainstem potentially plays a significant role in ME/CFS pathomechanism.
Brain magnetic resonance imaging (MRI) has been used in better understanding ME/CFS (7, 22, 25, 26). MRI uses a strong magnetic field to align the protons in the tissues and a radiofrequency pulse to make the selective protons spin out of equilibrium such that when the radiofrequency is removed, the protons realign to the magnetic field. The time it takes for realignment and the energy released result in differing hyperintensities (i.e., brightness) creating imaging contrast between tissue types including WM, GM, and cerebrospinal fluid (CSF). Compared to other common neuroimaging methods, MRI is non-invasive without needing radiation or radioactive/iodinated tracers. These features, together with the relatively high resolution, make MRI preferable for both clinical diagnosis and research.
MRI techniques, such as T1-weighted (T1W) imaging and T2-weighted (T2W) imaging, are commonly used to reveal brain anatomy, parenchymal lesions and other structural changes in WM volume, GM volume, and myelination (27). Diffusion Tensor Imaging (DTI) has been used to capture the rate of water diffusion between cells to create the fiber trajectory map representing WM integrity based on parameters such as the diffusivity and fractional anisotropy (28). In addition, functional MRI (fMRI) can be used to study functional brain response to stimuli or functional connectivity (FC), through detecting regional blood flow changes (e.g., perfusion-weighted Arterial Spin Labeling-ASL) or coupled with neuron activity induced oxygen consumption (e.g., blood oxygen level dependent BOLD echo planner imaging-EPI), either during a resting phase (in absence of implicit brain input/output) or during a task (visual, auditorial, motor, cognitive, etc.). Magnetic Resonance Spectroscopy (MRS) or MRS Imaging (MRSI) can be used to reveal the concentration of the major brain metabolites (e.g., N-acetyl aspartate, Creatinine, Choline), based upon which the brain tissue temperatures may be measured (22, 29).
Despite a lacking consensus on the pathologies and mechanisms of ME/CFS, MRI studies have started to show the involvement of the brainstem. Emerging studies have reported global and regional GM and WM volume reductions in the patients, while inconsistency exists especially regarding brainstem structural changes (30). Similarly, studies have suggested that ME/CFS patients may recruit additional brain regions or increase brain activities in dealing with the symptoms, although inconsistencies remain (30). Changes in brain metabolites (22, 31), BOLD signal complexity (32, 33), and functional connectivity (33) have also been reported.
Considering the vital functions of the brainstem and the associations of these functions with the variety of ME/CFS symptoms, there presents a need of a review and evaluation of the MRI findings concerning the involvement of the brainstem's role in ME/CFS. This has motivated our research aiming to 1) integrate the relevant MRI findings of the research field, and 2) derive a synthesized evaluation of the brainstem's role in ME/CFS based on the findings. The study is intended to provide new insights to advancing the research line, informing future solutions for improved clinical management and patient care of the complex medical condition. The work is also relevant with addressing the challenges of the ongoing pandemic, given that ME/CFS has become one of the long-term consequences of COVID-19 (34).
This scoping review followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses extension for Scoping Reviews (PRISMA-SR) checklist (35).
To be included in the evaluation, publications must investigate the brainstem in relation to ME/CFS, use MRI techniques, be original research, be conducted on human participants and published in English (as English is the preferred language of this review). Journal articles were included in the search if they were published before November 1st, 2021 (Figure 1).
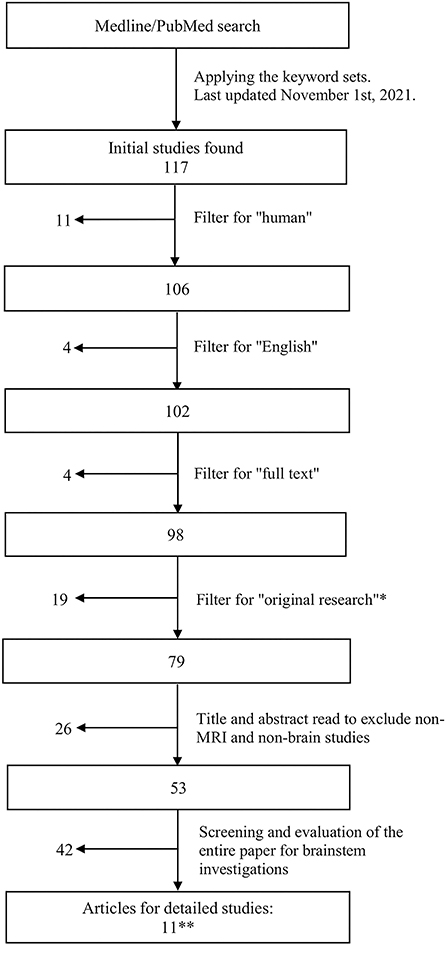
Figure 1. Flowchart showing the literature search and article retrieval process. *Included papers that had original findings, including case reports. **The search revealed a final set of 11 original research studies, none of which were case reports.
Papers that only mentioned the brainstem in ME/CFS but did not involve the investigation of the brainstem's role within the study were excluded. Studies that did not fit into the conceptual framework, i.e., the scope and scale of the present review, were filtered out (Figure 1).
The literature review was conducted by two researchers (TN and LXZ), applying the MEDLINE (the National Library of Medicine's premier bibliographic database that contains 27 million+ references of 5,200 journals in life sciences especially biomedicine) through PubMed search of targeted publications (last updated on November 1st, 2021). Articles were retrieved through subscriptions provided by Simon Fraser University and the University of Toronto.
The initial search was conducted using three sets of keywords: Set 1: “ME*CFS” or “Myalgic Encephalomyelitis” or “Chronic Fatigue Syndrome”; Set 2: “MRI” or “Multimodal MRI” or “Magnetic Resonance Imaging” or “fMRI” or “functional Magnetic Resonance Imaging” or “functional MRI” or “MRSI” or “MRS imaging” or “Magnetic Resonance Spectroscopic Imaging” or “MRS” or “Magnetic Resonance Spectroscopy” or “MR Spectroscopy” or “Proton Density Weighted Imaging” or “DWI” or “Diffusion Weighted Imaging” or “DTI” or “Diffusion Tensor Imaging” or “EPI” or “Echo Planar Imaging” or “PWI” or “Perfusion Weighted Imaging” or “ASL” or “Arterial Spin Labeling” or “MRA” or “Magnetic Resonance Angiography” or “MRV” or “Magnetic Resonance Venography” or “Perfusion MRI” or “Perfusion Weighted Imaging” or “PWI” or “anatomical MRI”; and Set 3: “human” or “brain” or “human brain.” This initial search retrieved 117 articles, as shown in Figure 1.
Filters were used to select the publication from the initial set of articles (Figure 1). These included “Humans” (to include only human subjects), “English” (to include only English text), and “Full text” (to include only full text). To include only original research (including case reports), the database filters: “Systematic Review,” “Review,” and “Meta-Analysis” were employed. Abstract reading was also done to ensure removal of non-original research articles.
The remaining subset of articles after the filtering steps was subjected to further evaluation in detail. First, a title and abstract read of each paper was conducted to remove articles that were not brain MRI studies on ME/CFS. This was followed by a full text screening to exclude any entries that did not include the brainstem or its components. Thereafter, articles that contained “brainstem, brain stem, pons, pontine, midbrain, medulla” were identified and studied further with full text reading.
These processing steps yielded a final set of 11 articles, all of which were original research publications (rather than case studies or reviews), as presented below (Figure 1 and Table 1). Any disagreements or inconsistencies between TN and LXZ during processing and selection were resolved with additional researchers (e.g., HG and XS).
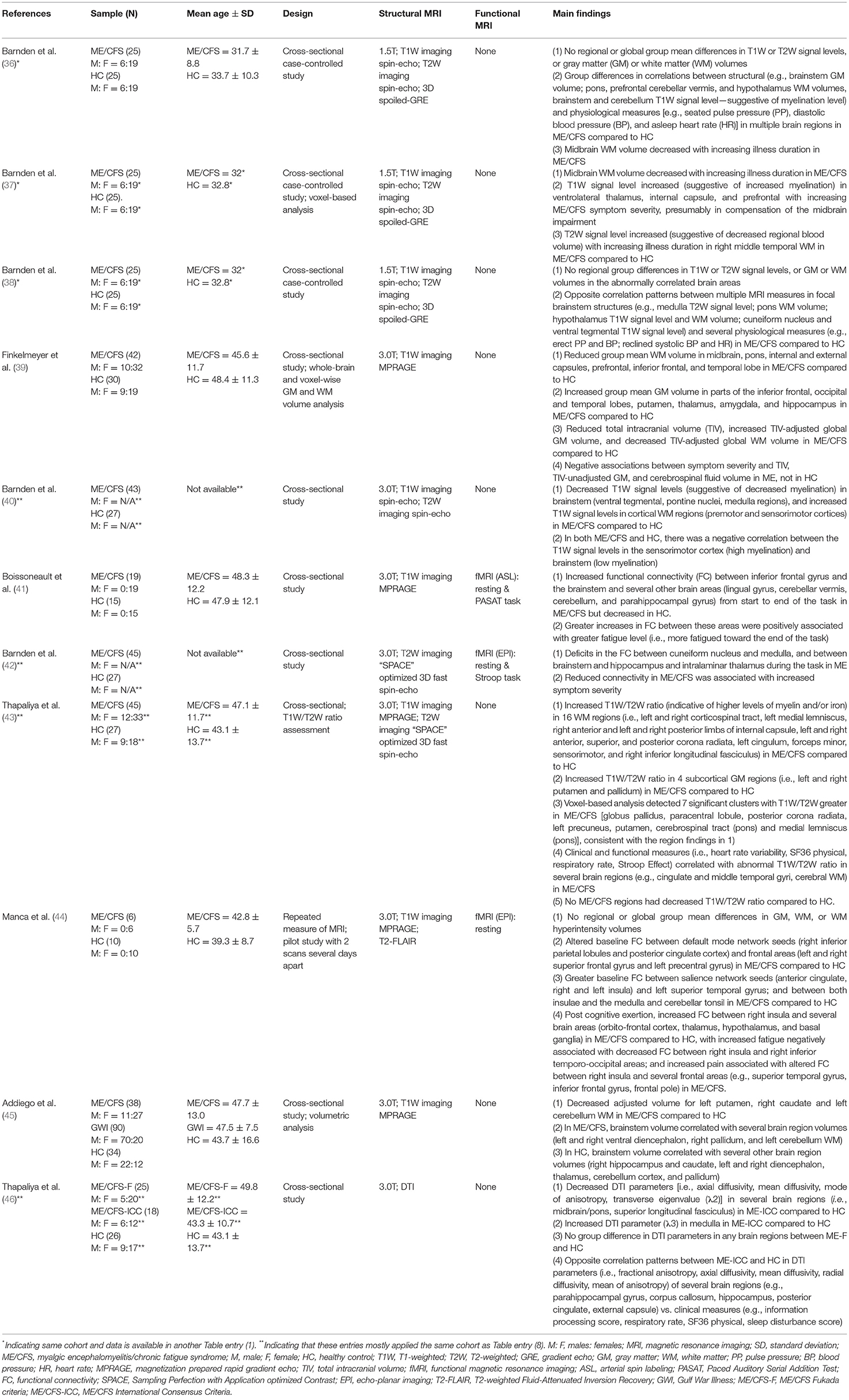
Table 1. MRI studies investigating the role of the brainstem in myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS).
The studies were summarized for main findings. Data was grouped and presented in the context of structural or functional brain changes. For each study, participant characteristics including mean age, MRI techniques including the sequence, study design, publication year, and main findings concerning the brainstem-specific and non-brainstem were provided. Studies included in other reviews and meta-analyses on ME/CFS research that met the criteria for the present review (i.e., MRI research on the brainstem in ME/CFS) were included.
Data showed that ME/CFS has been a subject of MRI studies for decades. Even so, it was not until the past decade when the brainstem emerged in the research line applying MRI, especially in terms of brainstem function (Table 1 and Figure 2). The fMRI studies were published in 2018, 2019, and 2021, using perfusion or BOLD based techniques. Seven of the articles (while some of them used the same dataset) enrolled both adult sexes/genders; two enrolled only adult women; one included Gulf War Illness participants together with ME/CFS and controls; two missed presenting the sex/gender information. The ME/CFS participants in the 11 studies aged from 31.7 ± 8.8 to 49.8 ± 12.2 compared to 32.8–48.4 for healthy controls (HC). The studies all used structural MRI, chiefly T1W imaging for examining the anatomy and the co-registration of the functional images upon structural ones. Six studies also acquired T2W imaging and one acquired T2-weighted Fluid-Attenuated Inversion Recovery (T2-FLAIR) to more effectively investigate pathological changes, and one study pioneered the use of DTI in understanding the brainstem. Most studies performed a one-time MRI scan, except for one that had two scans separated by several days (Table 1). It is noted that the MRI studies investigating the brainstem's role in ME/CFS were conducted by a limited number of research groups (Table 1).
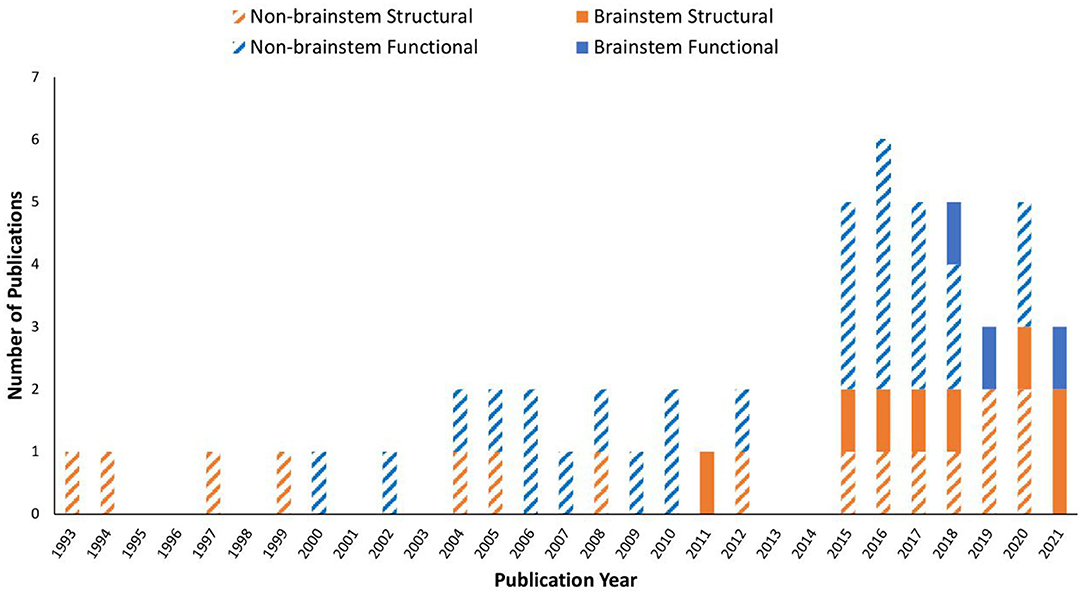
Figure 2. Publication trend in brain MRI research on myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) from 1993 to 2021. A MEDLINE and PubMed search using the keywords described yielded a total of 53 MRI original research papers. Studies investigated the brainstem are shown in filled colors, while those excluded the brainstem are in striped colors: orange: structural MRI only; blue: functional MRI involved.
Barnden et al. (36) reported changes in several brain structures (brainstem, prefrontal WM, and hypothalamus) in ME/CFS using a sample consisting of 25 ME/CFS patients and 25 HC. By regressing volumetric brain MRI data and ME/CFS clinical scores, the authors identified a negative correlation between midbrain WM volume (explained as a loss/shrinkage of glial cells) and the duration of fatigue. By comparing regressions between brain MRI measures and physiological measures across the two groups, the authors reported group differences suggesting abnormalities of the brain areas involved in ME/CFS. For example, the absolute GM volume of the brainstem and the seated pulse pressure (PP) were positively correlated in ME/CFS, but not in HC. The correlation between WM volume of the basal pons and the seated diastolic blood pressure (BP) was positive in ME/CFS, but negative in HC. The seated PP was also correlated with the T1W signal level (suggestive of myelination) in the brainstem and cerebellum, negatively in ME/CFS and positively in HC. Regressions between the asleep heart rate and the WM volume of the prefrontal cortex, hypothalamus, and cerebellar vermis also differed between the two groups (36). The study found no group mean differences in T1W or T2W signal levels, or WM or GM volumes, in the whole brain or in any specific brain regions.
Later, the research group published additional results using the same dataset (37). In addition to assuring that the midbrain WM volume was smaller in ME/CFS patients with longer periods of illness onset, the authors further reported an increasing T1W signal level (suggestive of increased myelination) in the ventrolateral thalamus, internal capsule, and prefrontal WM with increased disease severity. Other findings reported by this study included increased T2W signal level (suggestive of decreased regional blood volume) in the right middle temporal WM in ME/CFS, compared to HC.
Again, analyzing the same dataset, the research group further focused on understanding the involvement of the vasomotor centers in the brainstem and the hypothalamus in autonomic functional changes in ME/CFS (38). They reported abnormal correlations between multiple MRI measures in focal brainstem structures and physiological measures in ME/CFS compared to HC (e.g., medulla T2W signal level; pons WM volume; hypothalamus T1W signal level and WM volume; cuneiform nucleus of the reticular formation and ventral tegmental T1W signal level; erect PP and BP, reclined systolic BP and heart rate). The authors also found no group differences in these structures such as T1W or T2W signal level, or GM or WM volumes. The study found opposite correlation patterns involving additional brain structures (limbic nuclei, and prefrontal WM) with physiological measures for ME/CFS and HC.
Finkelmeyer et al. investigated brainstem abnormalities in ME, focusing on detecting a regional difference in the WM or GM volume (39). The study reported reduced WM volume in the midbrain and pons in ME/CFS compared to HC. A reduced WM volume in the internal capsule, external capsule, prefrontal lobe, inferior frontal lobe, and temporal lobe was also reported for ME/CFS. Surprisingly, the study reported higher adjusted GM volumes in the temporal, frontal, and occipital lobes in ME/CFS patients compared to HC but a possible explanation of the group difference was not found. Other findings included reduced total intracranial volume (TIV), increased TIV-adjusted global GM volume, and decreased TIV-adjusted global WM volume, and negative associations between symptom severity and the TIV, TIV-unadjusted GM, and cerebrospinal fluid volume in ME/CFS.
Barnden et al. (40) employed a larger sample (compared to their earlier publications) of 43 ME/CFS patients and 27 HC. The authors reported decreased T1W signal levels (suggestive of decreased myelination) in the brainstem (ventral tegmental, pontine nuclei, medulla regions) and increased T1W signal levels in cortical WM regions (i.e., premotor and sensorimotor cortices) in ME/CFS compared to HC (as in the internal capsule as they reported in the 2015 paper). Negative correlations in T1W signal levels between the brainstem (low myelination) and sensorimotor cortex (high myelination) were found in both groups.
Thapaliya et al. applied a novel T1W/T2W ratio (a higher ratio is indicative of higher levels of myelin and/or iron), to investigate differences in the levels of myelin and/or iron using 45 ME/CFS and 27 HC participants (43). The study reported increased ratio in ME/CFS compared to HC in 16 WM regions (i.e., brainstem regions-of-interest like the corticospinal and medial lemniscus tracts), projection tracts including the internal capsule, corona radiata, and others (cingulum, forceps minor, sensorimotor, and inferior longitudinal fasciculus). Also reported was the increased ratio in all four subcortical GM regions studied (i.e., the left and right putamen and pallidum) in ME/CFS compared to HC. The abnormality was correlated with clinical and functional measures. A lower ratio was not found in any brain regions in ME/CFS compared to HC, reflecting lack of increased demyelination.
Recently, Addiego et al. (45) investigated the volume of brain regions across three participant groups: 38 ME/CFS, 90 Gulf War Illness, and 34 HC participants. The study showed decreased volume for the putamen, caudate and cerebellum WM in ME/CFS compared to HC, adjusted for intracranial volume and age. The brainstem volume was correlated with the volume of several other brain regions including ventral diencephalon, pallidum, cerebellum WM in ME/CFS. The correlations were in contrast to those observed in HC in different brain regions (e.g., hippocampus, caudate, diencephalon, thalamus, cerebellum cortex).
Most recently, Thapaliya et al. (46) conducted the only DTI study so far, investigating the brainstem in ME/CFS. The authors examined 25 ME/CFS-F (a subgroup meeting the Fukuda criteria), 18 ME/CFS-ICC (a subgroup meeting the International Consensus Criteria) and 26 HC participants. They reported decreased transverse diffusivity and fractional anisotropy in several brain regions including midbrain/pons and superior longitudinal fasciculus in ME/CFS-ICC compared to HC. In addition, this patient subgroup showed correlations between the DTI and clinical measures in several brain regions (e.g., external capsule, corpus callosum). No group differences were seen in DTI parameters in any brain regions between ME/CFS-F and HC.
Boissoneault et al. published the first fMRI study on ME/CFS examining the brainstem in 2018 in 19 ME/CFS and 15 HC participants (Table 1). The authors examined changes in FC during the Paced Auditory Serial Addition Test (PASAT, a fatiguing cognitive task). Participants were presented with numbers sequentially and were asked to determine whether the sum equaled 13, while self-reporting their fatigue level. The study showed progressively increased FC between the inferior frontal gyrus (IFG) and the brainstem and several other brain areas (lingual gyrus, cerebellar vermis, cerebellum, parahippocampal gyrus) during the task in ME/CFS patients, in contrast to a decrease in FC involving the IFG in HC (41). Such increase in FC in ME/CFS was associated with higher fatigue level toward the end of the task.
Another fMRI study of the brainstem in ME/CFS confirmed an impairment in brainstem nerve conduction in ME/CFS (42). The study assessed FC within, and from, the brainstem using a sample of 45 ME/CFS patients and 27 HC. Participants were asked to complete a version of the Stroop task (for word meaning and color matching). The study reported connectivity deficits between the cuneiform nucleus and medulla, and from the brainstem to both the hippocampus and intralaminar thalamus in ME/CFS. The reduced connectivity was associated with an increased level of symptom severity.
Manca et al. (44) investigated FC changes associated with post-exertional malaise in the default mode network and the salience network (key for autonomic function), in six ME/CFS and 10 HC participants (44). Baseline FC data were collected, and group differences were examined, following which a series of cognitively fatiguing tasks were administrated (Stroop task, Trail making test, Category Fluency Task, Letter Fluency task, PASAT, N-back task) to elicit post-exertional malaise. Participants had another MRI scan several days later (at the onset of post-exertional malaise for ME/CFS patients). The study showed differential baseline FC in ME/CFS and HC (e.g., greater between the right inferior parietal lobules and both the posterior cingulate cortex and the right superior frontal gyrus; lower between the posterior cingulate cortex and both the left superior frontal gyrus and left precentral gyrus in ME/CFS). Greater baseline FC in ME/CFS was also found between the salience network (seeds in the anterior cingulate and the right and left insula) and the left superior temporal gyrus; and between both insulae and the medulla and cerebellar tonsil. At the second scan time, increased FC between the right insula (and the orbito-frontal cortex, thalamus, hypothalamus, and basal ganglia) and decreased FC between the right insula and right inferior temporo-occipital were found in ME/CFS, companied by increased level of fatigue and pain.
We conducted a literature review to summarize and evaluate MRI research findings to better understand the role of the brainstem in ME/CFS. The brainstem has only been subject to focused MRI investigations for a decade, with the functional studies seen only since 2018 (Figure 2). Even so, the research published so far has demonstrated the critical involvement of the brainstem in ME/CFS associated deficits (Table 2).
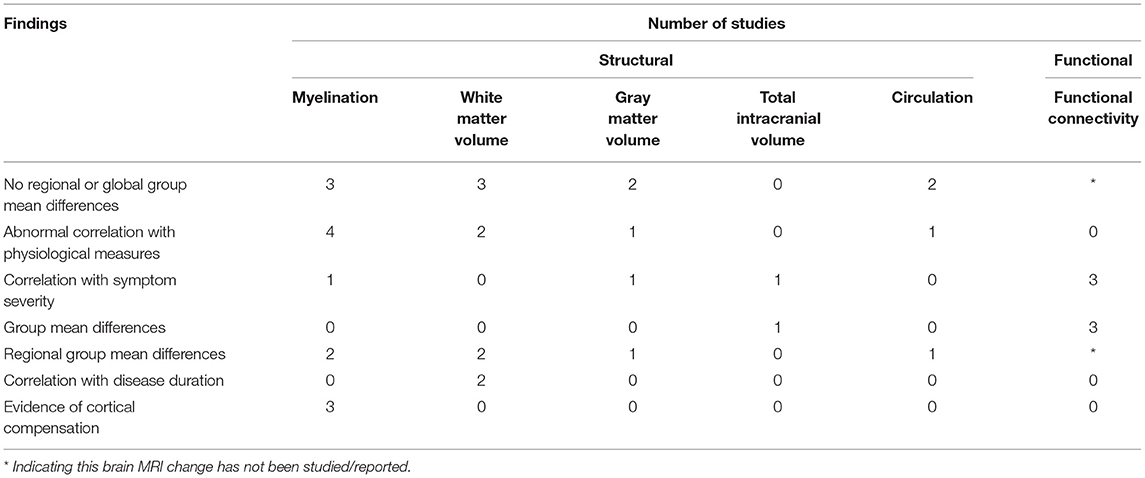
Table 2. Number of studying reporting structural and functional brain MRI changes in relation to myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS).
The studies pointed to a reduced WM volume (36–39), impairments in myelination (36, 38, 40, 43, 46), reduced conduction (37, 38, 40, 46), abnormal FC linking the brainstem and other brain regions (41, 42, 44), and compensatory brain changes (37, 40). The research findings also demonstrated a linkage between brainstem abnormalities and other brainstem structures, highlighting myelination impairment and dysregulation underlying the disease (Table 2 and Figure 2). The reports of midbrain WM volume loss, brainstem myelination impairments, and connectivity deficits within the brainstem also indicated brainstem nerve conduction impairment in ME/CFS, even when the brainstem nuclei themselves remained unaffected. Meanwhile, the increased myelination of multiple brain regions (ventrolateral thalamus, internal capsule, and prefrontal, premotor and sensorimotor cortices) showed a compensatory response in maintaining nerve conduction (e.g., increased myelination of tract segments rostral to the brainstem). Also in support of the regulatory hypothesis, the region based approach in Thapaliya et al. (43) detected increased T1W/T2W ratio in the internal capsule, coronal radiata, cingulum forceps minor, sensory motor, and inferior longitudinal fasciculus, while the voxel based analysis, which does not require a-priori region selection and tests the whole brain, detected increased T1W/T2W ratio in the globus pallidus, paracentral lobule, posterior corona radiata, left precuneus, putamen, cerebrospinal tract (pons) and medial lemniscus (pons).
As summarized in Figure 3, the MRI brain changes in ME/CFS may reflect dysfunctions of the brainstem in several key aspects, including autonomic control and regulation, and afferent and efferent conduction. Such dysfunctions in the brainstem can have consequences on ME/CFS, relating to symptoms such as fatigue, physiological dysregulation, and even, most likely secondary, cognitive decline (7, 8).
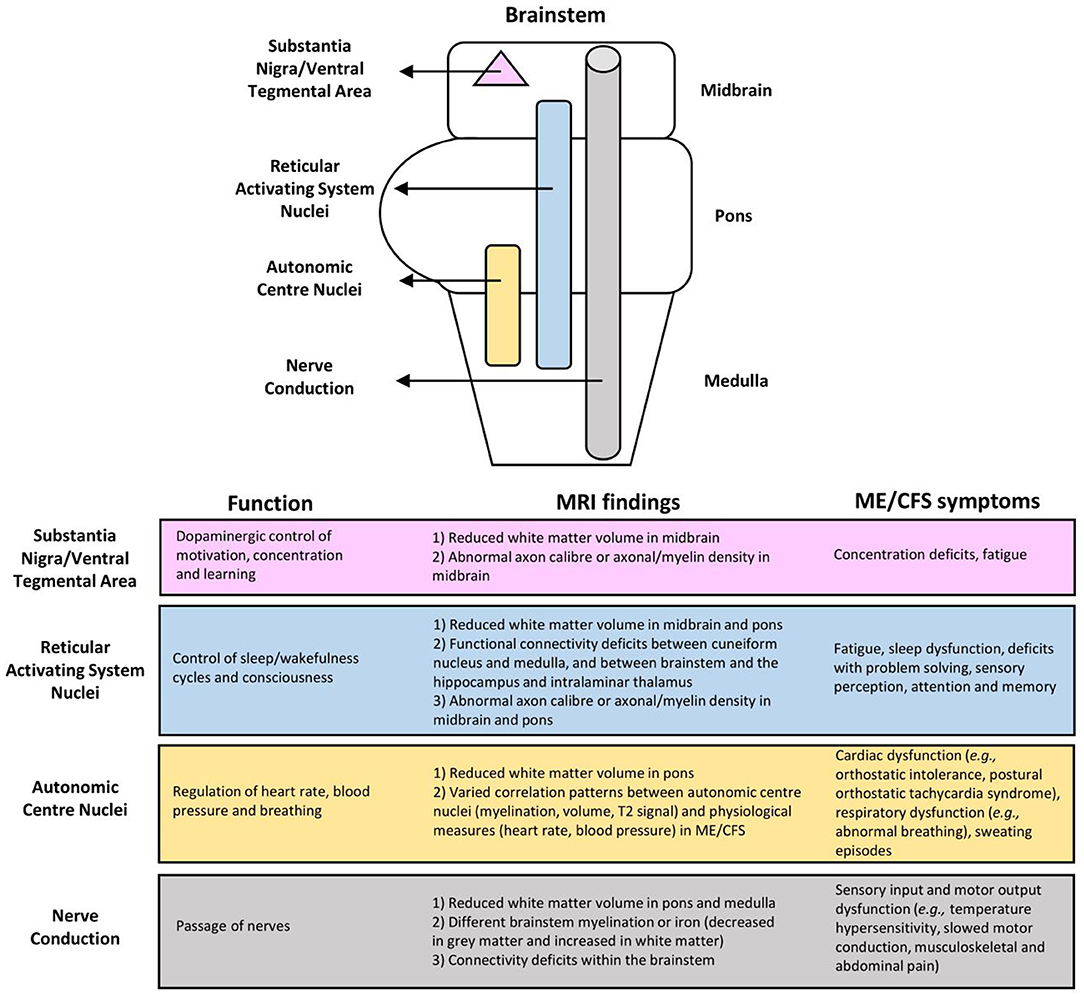
Figure 3. Associations of myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) symptoms with brainstem dysfunction based on MRI findings.
The limited number of existing fMRI studies demonstrated clear abnormalities in the neural network connectivity between several brain areas thought to be involved in ME/CFS symptomology (Figure 3). Abnormalities of the RAS nuclei of the brainstem, which are crucial for the coherence of thalamo-cortical oscillations, are suggested to result in the dysfunction of problem solving, sensory perception, attention, and memory in ME/CFS (42). Abnormalities within the default mode network are suggested to play a role in post-exertional malaise (44), and spatial sensing and coordination difficulties (33), while those within the salience network possibly reflect an increased challenge of salience processing with cognitive performance, interoceptive processing, and visceromotor control related to fatigue and pain perception in ME/CFS (44). Abnormalities from the brainstem to the prefrontal lobe [e.g., the IFG, the known executive control centre; (47)] could lead to executive dysfunctions, while abnormalities between the brainstem and other brain regions (i.e., lingual gyrus, cerebellar vermis, cerebellum, parahippocampal gyrus) are suggestive of a failure of compensatory mechanisms for fatigue (41).
Also importantly, these MRI brain changes were associated with, and supported by, a wide range of clinical assessments, including the vital signs (36, 38, 43, 46), sleep parameters (36, 38, 46), disease duration (36, 37) and severity levels (37, 39, 41, 42, 44).
Our study has identified several challenges of the field. Notably, controversies exist in identifying mean group differences in regional or global T1W and T2W signal levels, or GM and WM volumes in ME/CFS compared to controls. The initial conventional 1.5 Tesla MRI studies failed to detect such a change using relatively small samples (36, 38). More recent studies (37, 39, 40, 43, 45) were able to detect such differences with increased sample sizes, especially at 3.0 T high-field MRI (Table 1). Notably, most MRI on ME/CFS studies thus far applied a relatively small sample size of ME/CFS patient participants, ranging between 6 and 45. Finkelmeyer et al. (39) attributed the lack of regional differences in WM and GM volumes to small sample sizes used, even though the study included 42 patients. The present study informs future research to pay a closer attention to enforcing the effect size to allow adequate statistical power in detecting a targeted difference. Also, several studies under review failed to provide the basic demographics of the study sample. Considering that ME/CFS is a multi-system disease with heterogeneous symptom expression among individuals, a thorough description of the sample will allow appropriate result interpretation and the evaluation of the generalizability of the study finding.
Despite the widely available MRI technologies as applied in studying other clinical conditions (48), they have yet to be sufficiently employed in better understanding ME/CFS. For example, structural scans have only involved T1W and T2W sequences in investigating the brainstem, save for the most recent pioneer DTI work (46). Future research using different MRI techniques, including DTI and other advanced methods may help detect WM changes more sensitively. Furthermore, MRS studies can be invaluable to understanding brainstem metabolites, as widespread brain abnormalities have been reported as suggestive of neuroinflammation in correlation with ME/CFS symptom severity (22). It is anticipated that multimodal MRI studies combining various MRI methods investigating the brainstem can be beneficial in providing confirmatory evidence. Certainly, these undertakings can require vigilant experimental design and implementation, given that the brainstem is deeply located in the brain and relatively difficult to shim and image (21).
Also importantly, to date, the MRI on brainstem publications have largely shown a group-wise comparison of the mean values and the regression patterns, and adopted cross-sectional design with single-time MRI. Studies with longitudinal follow-ups will be needed to understand the progress of the brain deficits and the consequences regarding disease expression. Similarly, clinical trials examining the effect of standard care and novel interventions have yet to become available. Clinical trials are important especially given the consideration that ME/CFS has been recently suggested to be one of the long-term consequences of COVID-19 and has been estimated to double in its prevalence in 1 year in the United States (34), and likely also in other countries.
Over the past decades, MRI on ME/CFS has drawn an increasingly greater attention, as demonstrated by the increase in the number of studies, specifically functional ones (Figure 2). This has reflected, and helped with, the growing body of recognition of ME/CFS as a medical condition, which has become the topic of several recent reviews. While structural and functional abnormalities in ME/CFS are typically found, inconsistencies exist among individual studies (30, 49). Impairments in the autonomic nervous system in ME/CFS are also detected by other imaging methods including positron emission tomography and electroencephalography (50). Unfortunately, most MRI on ME/CFS studies till now have not targeted the role of the brainstem (Figure 1).
Our study has several limitations. First, we applied only the MEDLINE database. Even though it is well-established and provides a comprehensive coverage of peer-reviewed biomedical articles in biomedicine of recognized quality, some abstracts, conference reports, etc., indexed only on less prominent resources might have been excluded. Our findings have also been limited to reports published in English and those targeting the brainstem using structural or functional MRI. Reports with other focuses outside of the scope of the present research may warrant separate reviews (Figure 3). As demonstrated by the present review, MRI studies investigating the brainstem's role in ME/CFS are emerging quickly, calling for future attentive actions of MRI researchers.
Our research has made a helpful contribution to the literature. The study integrated the MRI findings on the role of the brainstem in ME/CFS and revealed brainstem abnormalities in relation to various symptoms that present heterogeneously in patients. The review has also resulted in a model for better understanding the role of the brainstem dysfunction in ME/CFS, showing changes within the brainstem, and linking with other brain regions, as detailed below (Figure 3).
First, the abnormal connectivity between RAS nuclei possibly lessened the cortical oscillatory coherence as they control the thalamocortical state between sleeping and wakefulness, which can consequently affect consciousness and cognitive function (19, 20). Also, given the distribution of the RAS nuclei throughout the brainstem (11), their functions may be affected by the reduced WM volume and functional connectivity, and the suggested abnormal axon caliber and axonal/myelin density in the midbrain and pons (46). RAS dysfunction may lead to fatigue, impaired attention, short term memory, cognitive function, and sleep quality, all of which have been seen in the ME/CFS population (7, 8).
Also vital for autonomic functions, the brainstem plays a role in the regulation of heart rate, blood pressure, and respiration (15, 16). The reduced WM volume in the pons and the varied correlation pattern of the autonomic centre nuclei on physiological measures reflect autonomic centre dysfunction in ME/CFS, such as cardiac (e.g., postural orthostatic tachycardia syndrome, orthostatic intolerance, arrhythmia); respiratory (e.g., abnormal breathing); and other dysfunctions including sweating episodes (7, 8).
Impairments also include the crucial passage of nerves (i.e., conduit function) in both afferent and efferent directions. Reduced WM volume in the pons and medulla, different brainstem myelination or iron (decreased in GM and increased in WM), and connectivity deficits within the brainstem in ME/CFS are indicative of conduit dysfunction. Symptoms may arise directly from the affected nerves and/or indirectly from cortical myelination as a compensatory response. Related symptoms may include sensory input and motor output dysfunctions. Meanwhile, patients with excessive myelination of tracts to the primary somatosensory cortex may have increased sensory awareness, which can manifest into symptoms such as increased touch and pain perception (8, 51, 52), musculoskeletal and abdominal pain (8, 53), and intolerance to extreme temperatures (8). Alternatively, the prolonged motor conduction velocity in ME/CFS (54), which is indicative of motor disturbances (8) may be attributed to insufficient myelination (i.e., under compensation) of tracts from the motor cortex.
Moreover, the midbrain uniquely contains the substantia nigra and ventral tegmental area for dopaminergic control of motivation, concentration, and learning (17, 18). Dopaminergic dysfunction in ME/CFS has been suggested but is yet to be tested by MRI. Specifically, it has been shown that methylphenidate, a dopamine reuptake inhibitor, decreases the levels of fatigue and disrupted concentration in adults with ME/CFS (55). Functional impairment of the putamen, which is innervated by, and is a part of the same functional network as the substantia nigra and ventral tegmental area, has also been reported in ME/CFS (24), suggesting a topic for future investigation.
The review is in favor of the hypothesis that ME/CFS involves brainstem specific astrocyte dysfunctions, contributing to impaired brainstem cerebrovascular autoregulation and reduced blood flow (36, 56). Oxidative stress appears to induce ME/CFS symptoms, associated with reduced blood flow and neuroinflammation. An infection (the most frequently reported trigger for ME/CFS onset) has been linked to peroxynitrite production, a proinflammatory oxygen/nitrogen species (57, 58), triggering neuroinflammation. This can lead to the production of isoprostanes and cause vasoconstriction when the level of antioxidants is insufficient (59). Oxidative stress and increased isoprostanes in ME/CFS have been correlated with clinical symptoms (60). Reduced brain blood flow is common in ME/CFS patients, accompanied by lowered circulation and nutrient/waste exchange (61–65).
Taken together, the present review highlights the brainstem as a potential brain centre that may have a key role in the physiological defects in ME/CFS and recommends future MRI on ME/CFS studies to make the brainstem a specific target. Given the close association between the brainstem function impairments and the complex clinical expression of the disease, MRI, with its relatively high spatial and temporal resolutions for non-invasive in vivo applications, holds promise to uncover the mechanisms of the disease, and in turn enlightens effective strategies for improved patient care. This review contributes to the research line by bringing the existing studies together and integrating them while highlighting the potential for more. As the big questions around ME/CFS remain unanswered (e.g., why does an initial viral event result in a debilitating ongoing life-long disease in some people, why does it not resolve like a typical infectious illness, and why are ME/CFS patients subject to frequent relapses), highlighting of the brainstem as a specific target is meaningful from both research and clinical practice perspectives.
This study targets a better understanding of the involvement of the brainstem in ME/CFS using MRI. Data demonstrated that structural and functional deficits of the brainstem are associated with other brain changes and linked to clinical expression. The study suggested a model summarizing the possible brainstem role in connecting various functional brain centres. The paper draws increased attention to brainstem research in ME/CFS using multi-modality MRI, calling for improved experimental design, and increased sample size and follow-up duration. Targeting the brainstem abnormalities in relation to the heterogeneous symptoms has implications for uncovering ME/CFS mechanisms and thus improving management and patient care.
TN and LXZ conducted the literature search, review and evaluation, summarized and presented the results, and drafted the initial version of the manuscript. HG provided consultations on MRI technologies and evaluations and the diagnostic medical imaging, and reviewed the result and data presentation. LN enabled the funding support, provided medical consultations about the evaluations and treatments of the medical condition, reviewed the result presentation, and edited the manuscript. XS enabled funding support, conceptualized and supervised the study, reviewed the literature evaluation and result presentation, and co-drafted the first version of the manuscript. All authors participated in the result interpretation and the manuscript revisions.
This study received research grant support from the British Columbia Strategy for Patient Oriented Research Unit (FHG2020-014; FHG2020-005) and the Surrey Hospitals Foundation (FHG2017-001). Additional support for training was from the Natural Sciences and Engineering Research Council of Canada (USRA-507466) and the BioTalent Canada (SWPP-FH2020).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The authors sincerely acknowledge Roger Chan, Kati Debelic, and Hilary Robertson for providing valuable patient research partners' insights about ME/CFS and for helping with student supervision. Sincere acknowledgments also go to Drs. Christopher Condin and Richard Sobel for critical discussions and comments, Nicole Prestley and Travis Boulter for administrative assistance, and Margit Glashutter for proofreading the manuscript. The authors acknowledge Surrey Memorial Hospital and the Department of Evaluation and Research Services of Fraser Health for administrative support. We would also like to sincerely acknowledge the three peer reviewers for their valuable comments and suggestions, which helped improve the manuscript remarkably.
ASL, arterial spin labeling; BOLD, blood oxygenation level dependent; DTI, diffusion tensor imaging; EPI, echo-planar imaging; FC, functional connectivity; fMRI, functional magnetic resonance imaging; GM, gray matter; HC, healthy controls; IFG, inferior frontal gyrus; ME/CFS, myalgic encephalomyelitis/chronic fatigue syndrome; MRI, magnetic resonance imaging; MRS, magnetic resonance spectroscopy; PASAT, paced auditory serial addition test; PP, pulse pressure; RAS, reticular activating system; TIV, total intracranial volume; T1W, T1-weighted; T2W, T2-weighted; T2-FLAIR, T2-weighted fluid-attenuated inversion recovery; WM, white matter.
1. Nacul L, O'Boyle S, Palla L, Nacul FE, Mudie K, Kingdon CC, et al. How myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) progresses: the natural history of ME/CFS. Front Neurol. (2020) 11:826. doi: 10.3389/fneur.2020.00826
2. Johnston S, Brenu EW, Staines D, Marshall-Gradisnik S. The prevalence of chronic fatigue syndrome/myalgic encephalomyelitis: a meta-analysis. Clin Epidemiol. (2013) 5:105. doi: 10.2147/CLEP.S39876
3. Watanabe Y. PET/SPECT/MRI/fMRI studies in the myalgic encephalomyelitis/chronic fatigue syndrome. In: PET and SPECT in Psychiatry. Cham: Springer (2021). doi: 10.1007/978-3-030-57231-0_32
4. Canadian Community Health Survey Share File. Statistics Canada. Ontario Ministry of Health and Long-Term Care (2018).
5. Pendergrast T, Brown A, Sunnquist M, Jantke R, Newton JL, Strand EB, et al. Housebound versus nonhousebound patients with myalgic encephalomyelitis and chronic fatigue syndrome. Chronic Illn. (2016) 12:292–307. doi: 10.1177/1742395316644770
6. Frith J, Zalewski P, Klawe JJ, Pairman J, Bitner A, Tafil-Klawe M, et al. Impaired blood pressure variability in chronic fatigue syndrome–a potential biomarker. QJM. (2012) 105:831–8. doi: 10.1093/qjmed/hcs085
7. Carruthers BM, Jain AK, De Meirleir KL, Peterson DL, Klimas NG, Lerner AM, et al. Myalgic encephalomyelitis/chronic fatigue syndrome. J Chronic Fatigue Syndr. (2003) 11:7–115. doi: 10.1300/J092v11n01_02
8. Carruthers BM, van de Sande MI, De Meirleir KL, Klimas NG, Broderick G, Mitchell T, et al. Myalgic encephalomyelitis: international consensus criteria. J Intern Med. (2011) 270:327–38. doi: 10.1111/j.1365-2796.2011.02428.x
9. Clayton EW. Beyond myalgic encephalomyelitis/chronic fatigue syndrome: an IOM report on redefining an illness. JAMA. (2015) 313:1101–2. doi: 10.1001/jama.2015.1346
10. National Institute for Health Care Excellence (NICE). Overview: Myalgic Encephalomyelitis (or Encephalopathy)/Chronic Fatigue Syndrome: Diagnosis and Management: Guidance. (2021). Available online at: https://www.nice.org.uk/guidance/ng206
11. Vanderah TW, Gould DJ. Organization of the brainstem. In: Nolte's The Human Brain: An Introduction to Its Functional Anatomy. Philadelphia, PA: Elsevier (2015).
12. Haines DE, Mihailoff GA. Fundamental Neuroscience for Basic and Clinical Applications. Philadelphia, PA: Elsevier (2018).
13. Angeles Fernández-Gil M, Palacios-Bote R, Leo-Barahona M, Mora-Encinas JP. Anatomy of the brainstem: a gaze into the stem of life. Semi Ultrasound. (2010) 31:196–219. doi: 10.1053/j.sult.2010.03.006
14. Jha RM, Klein JP. Clinical anatomy and imaging of the cranial nerves and skull base. Semin Neurol. (2012) 32:332–46. doi: 10.1055/s-0032-1331807
15. Alexander RS. Tonic and reflex functions of medullary sympathetic cardiovascular centers. J Neurophysiol. (1946) 9:205–17. doi: 10.1152/jn.1946.9.3.205
16. Millhorn DE, Eldridge FL. Role of ventrolateral medulla in regulation of respiratory and cardiovascular systems. J Appl Physiol. (1986) 61:1249–63. doi: 10.1152/jappl.1986.61.4.1249
17. Van Domburg PHMF, ten Donkelaar HJ. The human substantia nigra and ventral tegmental area. Hum Subst Nigra Ventral Tegmental Area. (1991) 1991:32–69. doi: 10.1007/978-3-642-75846-1_4
18. Halliday G, Reyes S, Double K. Substantia nigra, ventral tegmental area and retrorubral fields. Hum Nerv Syst. (2012) 2012:439–55. doi: 10.1016/B978-0-12-374236-0.10013-6
19. Mesulam MM. Cholinergic pathways and the ascending reticular activating system of the human brain. Ann N Y Acad Sci. (1995) 757:169–79. doi: 10.1111/j.1749-6632.1995.tb17472.x
20. Garcia-Rill E, Kezunovic N, Hyde J, Simon C, Beck P, Urbano FJ. Coherence and frequency in the reticular activating system (RAS). Sleep Med Rev. (2013) 17:227–38. doi: 10.1016/j.smrv.2012.06.002
21. VanElzakker MB, Brumfield SA, Lara Mejia PS. Neuroinflammation and cytokines in myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS): a critical review of research methods. Front Neurol. (2019) 9:1033. doi: 10.3389/fneur.2018.01033
22. Mueller C, Lin JC, Sheriff S, Maudsley AA, Younger JW. Evidence of widespread metabolite abnormalities in Myalgic encephalomyelitis/chronic fatigue syndrome: assessment with whole-brain magnetic resonance spectroscopy. Brain Imaging Behav. (2019) 14:562–72. doi: 10.1007/s11682-018-0029-4
23. Rodriguez LS, Pou C, Tadepally L, Zhang J, Mugabo CH, Wang J, et al. Achieving symptom relief in patients with Myalgic encephalomyelitis by targeting the neuro-immune interface and inducing disease tolerance. BioRxiv. (2020). doi: 10.1101/2020.02.20.958249. [Epub ahead of print].
24. Mizuno K, Kawatani J, Tajima K, Sasaki AT, Yoneda T, Komi M, et al. Low putamen activity associated with poor reward sensitivity in childhood chronic fatigue syndrome. NeuroImage: Clinical. (2016) 12:600–6. doi: 10.1016/j.nicl.2016.09.016
25. Josev EK, Malpas CB, Seal ML, Scheinberg A, Lubitz L, Rowe K, et al. Resting-state functional connectivity, cognition, and fatigue in response to cognitive exertion: a novel study in adolescents with chronic fatigue syndrome. Brain Imaging Behav. (2019) 14:1815–30. doi: 10.1007/s11682-019-00119-2
26. Provenzano D, Washington SD, Baraniuk JN. A Machine learning approach to the differentiation of functional magnetic resonance imaging data of chronic fatigue syndrome (CFS) from a sedentary control. Front Comput Neurosci. (2020) 14:2. doi: 10.3389/fncom.2020.00002
27. Widdess-Walsh P, Diehl B, Najm I. Neuroimaging of focal cortical dysplasia. J Neuroimag. (2006) 16:185–96. doi: 10.1111/j.1552-6569.2006.00025.x
28. Filler A. MR neurography and diffusion tensor imaging: origins, history & clinical impact. Nat Prec. (2009) 2009:1–1. doi: 10.1038/npre.2009.2877
29. Maudsley AA, Goryawala MZ, Sheriff S. Effects of tissue susceptibility on brain temperature mapping. Neuroimage. (2017) 146:1093–101. doi: 10.1016/j.neuroimage.2016.09.062
30. Shan ZY, Barnden LR, Kwiatek RA, Bhuta S, Hermens DF, Lagopoulos J. Neuroimaging characteristics of myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS): a systematic review. J Transl Med. (2020) 18:335. doi: 10.1186/s12967-020-02506-6
31. Natelson BH, Vu D, Coplan JD, Mao X, Blate M, Kang G, et al. Elevations of ventricular lactate levels occur in both chronic fatigue syndrome and fibromyalgia. Fatigue. (2017) 5:15–20. doi: 10.1080/21641846.2017.1280114
32. Shan ZY, Finegan K, Bhuta S, Ireland T, Staines DR, Marshall-Gradisnik SM, et al. Brain function characteristics of chronic fatigue syndrome: A task fMRI study. NeuroImage Clin. (2018) 19:279–86. doi: 10.1016/j.nicl.2018.04.025
33. Shan ZY, Finegan K, Bhuta S, Ireland T, Staines DR, Marshall-Gradisnik SM, et al. Decreased connectivity and increased blood oxygenation level dependent complexity in the default mode network in individuals with chronic fatigue syndrome. Brain Connect. (2018) 8:33–9. doi: 10.1089/brain.2017.0549
34. Komaroff AL, Bateman L. Will COVID-19 lead to myalgic encephalomyelitis/chronic fatigue syndrome?. Front Med. (2021) 7:1132. doi: 10.3389/fmed.2020.606824
35. Tricco AC, Lillie E, Zarin W, O'Brien KK, Colquhoun H, Levac D, et al. PRISMA extension for scoping reviews (PRISMA-ScR): checklist and explanation. Ann Intern Med. (2018) 169:467–73. doi: 10.7326/M18-0850
36. Barnden LR, Crouch B, Kwiatek R, Burnet R, Mernone A, Chryssidis S, et al. A brain MRI study of chronic fatigue syndrome: evidence of brainstem dysfunction and altered homeostasis. NMR Biomed. (2011) 24:1302–12. doi: 10.1002/nbm.1692
37. Barnden LR, Crouch B, Kwiatek R, Burnet R, Del Fante P. Evidence in chronic fatigue syndrome for severity dependent upregulation of prefrontal myelination that is independent of anxiety and depression. NMR Biomed. (2015) 28:404–13. doi: 10.1002/nbm.3261
38. Barnden LR, Kwiatek R, Crouch B, Burnet R, Del Fante P. Autonomic correlations with MRI are abnormal in the brainstem vasomotor centre in Chronic Fatigue Syndrome. NeuroImage: Clin. (2016) 11:530–7. doi: 10.1016/j.nicl.2016.03.017
39. Finkelmeyer A, He J, Maclachlan L, Watson S, Gallagher P, Newton JL, et al. Grey and white matter differences in Chronic Fatigue Syndrome - A voxel-based morphometry study. NeuroImage: Clin. (2017) 17:24–30. doi: 10.1016/j.nicl.2017.09.024
40. Barnden LR, Shan ZY, Staines DR, Marshall-Gradisnik S, Finegan K, Ireland T, et al. Hyperintense sensorimotor T1 spin echo MRI is associated with brainstem abnormality in chronic fatigue syndrome. NeuroImage: Clin. (2018) 20:102–9. doi: 10.1016/j.nicl.2018.07.011
41. Boissoneault J, Letzen J, Lai S, Robinson ME, Staud R. Static and dynamic functMancional connectivity in patients with chronic fatigue syndrome: use of arterial spin labelling fMRI. Clin Physiol Funct Imaging. (2018) 38:128–37. doi: 10.1111/cpf.12393
42. Barnden LR, Shan ZY, Staines DR, Marshall-Gradisnik S, Finegan K, Ireland T, et al. Intra brainstem connectivity is impaired in chronic fatigue syndrome. NeuroImage: Clin. (2019) 24:102045. doi: 10.1016/j.nicl.2019.102045
43. Thapaliya K, Marshall-Gradisnik S, Staines D, Barnden L. Mapping of pathological change in chronic fatigue syndrome using the ratio of T1- and T2-weighted MRI scans. NeuroImage Clin. (2020) 28:102366. doi: 10.1016/j.nicl.2020.102366
44. Manca R, Khan K, Mitolo M, De Marco M, Grieveson L, Varley R, et al. Modulatory effects of cognitive exertion on regional functional connectivity of the salience network in women with ME/CFS: A pilot study. J Neurol Sci. (2021) 422:117326. doi: 10.1016/j.jns.2021.117326
45. Addiego FM, Zajur K, Knack S, Jamieson J, Rayhan RU, Baraniuk JN. Subcortical brain segment volumes in Gulf War illness and myalgic encephalomyelitis/chronic fatigue syndrome. Life Sci. (2021) 282:119749. doi: 10.1016/j.lfs.2021.119749
46. Thapaliya K, Marshall-Gradisnik S, Staines D, Barnden L. Diffusion tensor imaging reveals neuronal microstructural changes in myalgic encephalomyelitis/chronic fatigue syndrome. Eur J Neurosci. (2021) 54:6214–28. doi: 10.1111/ejn.15413
47. Otero TM, Barker LA. The frontal lobes and executive functioning. Handb Exec Funct. (2013) 2013:29–44. doi: 10.1007/978-1-4614-8106-5_3
48. Viard A, Eustache F, Segobin S. History of magnetic resonance imaging: a trip down memory lane. Neuroscience. (2021) 474:3–13. doi: 10.1016/j.neuroscience.2021.06.038
49. Almutairi B, Langley C, Crawley E, Thai NJ. Using structural and functional MRI as a neuroimaging technique to investigate chronic fatigue syndrome/myalgic encephalopathy: a systematic review. BMJ Open. (2020) 10:e031672. doi: 10.1136/bmjopen-2019-031672
50. Maksoud R, du Preez S, Eaton-Fitch N, Thapaliya K, Barnden L, Cabanas H, et al. A systematic review of neurological impairments in myalgic encephalomyelitis/chronic fatigue syndrome using neuroimaging techniques. PLoS ONE. (2020) 15:e0232475. doi: 10.1371/journal.pone.0232475
51. Bushnell MC, Duncan GH, Hofbauer RK, Ha B, Chen JI, Carrier B. Pain perception: is there a role for primary somatosensory cortex? Proc Natl Acad Sci USA. (1999) 96:7705–9. doi: 10.1073/pnas.96.14.7705
52. Ullrich PM, Afari N, Jacobsen C, Goldberg J, Buchwald D. Cold pressor pain sensitivity in monozygotic twins discordant for chronic fatigue syndrome. Pain Med. (2007) 8:216–22. doi: 10.1111/j.1526-4637.2006.00277.x
53. Fukuda K, Straus SE, Hickie I, Sharpe MC, Dobbins JG, Komaroff A. The chronic fatigue syndrome: a comprehensive approach to its definition and study. International Chronic Fatigue Syndrome Study Group. Ann Internal Med. (1994) 121:953–9. doi: 10.7326/0003-4819-121-12-199412150-00009
54. Hilgers A, Frank J, Bolte P. Prolongation of central motor conduction time in chronic fatigue syndrome. J Chronic Fatigue Syndr. (1998) 4:23–32. doi: 10.1300/J092v04n02_03
55. Blockmans D, Persoons P, Van Houdenhove B, Bobbaers H. Does methylphenidate reduce the symptoms of chronic fatigue syndrome? Am J Med. (2006) 119:167.e23–167.e1.67E30. doi: 10.1016/j.amjmed.2005.07.047
56. Marina N, Christie IN, Korsak A, Doronin M, Brazhe A, Hosford PS, et al. Astrocytes monitor cerebral perfusion and control systemic circulation to maintain brain blood flow. Nat Commun. (2020) 11:131. doi: 10.1038/s41467-019-13956-y
57. Pall ML. Elevated, sustained peroxynitrite levels as the cause of chronic fatigue syndrome. Med Hypotheses. (2000) 54:115–25. doi: 10.1054/mehy.1998.0825
58. Salvemini D, Doyle TM, Cuzzocrea S. Superoxide, peroxynitrite and oxidative/nitrative stress in inflammation. Biochem Soc Transact. (2006) 34:965–70. doi: 10.1042/BST0340965
59. Shungu DC, Weiduschat N, Murrough JW, Mao X, Pillemer S, Dyke JP, et al. Increased ventricular lactate in chronic fatigue syndrome. III. Relationships to cortical glutathione and clinical symptoms implicate oxidative stress in disorder pathophysiology. NMR Biomed. (2012) 25:1073–87. doi: 10.1002/nbm.2772
60. Kennedy G, Spence VA, McLaren M, Hill A, Underwood C, Belch JJ. Oxidative stress levels are raised in chronic fatigue syndrome and are associated with clinical symptoms. Free Radic Biol Med. (2005) 39:584–9. doi: 10.1016/j.freeradbiomed.2005.04.020
61. Costa DC, Tannock C, Brostoff J. Brainstem perfusion is impaired in chronic fatigue syndrome. QJM. (1995) 88:767–73.
62. Yoshiuchi K, Farkas J, Natelson BH. Patients with chronic fatigue syndrome have reduced absolute cortical blood flow. Clin Physiol Funct Imaging. (2006) 26:83–6. doi: 10.1111/j.1475-097X.2006.00649.x
63. Petcharunpaisan S, Ramalho J, Castillo M. Arterial spin labeling in neuroimaging. World J Radiol. (2010) 2:384–98. doi: 10.4329/wjr.v2.i10.384
64. Biswal B, Kunwar P, Natelson BH. Cerebral blood flow is reduced in chronic fatigue syndrome as assessed by arterial spin labeling. J Neurol Sci. (2011) 301:9–11. doi: 10.1016/j.jns.2010.11.018
Keywords: myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS), brainstem, brain structure and function, brainstem dysfunction, symptoms and expressions, pathologies and mechanisms, magnetic resonance imaging (MRI), neuroinflammation
Citation: Nelson T, Zhang LX, Guo H, Nacul L and Song X (2021) Brainstem Abnormalities in Myalgic Encephalomyelitis/Chronic Fatigue Syndrome: A Scoping Review and Evaluation of Magnetic Resonance Imaging Findings. Front. Neurol. 12:769511. doi: 10.3389/fneur.2021.769511
Received: 02 September 2021; Accepted: 29 November 2021;
Published: 17 December 2021.
Edited by:
Wei Zhang, Peking University First Hospital, ChinaReviewed by:
Rebekah Maksoud, Griffith University, AustraliaCopyright © 2021 Nelson, Zhang, Guo, Nacul and Song. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiaowei Song, eGlhb3dlaS5zb25nQGZyYXNlcmhlYWx0aC5jYQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.