
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Neurol. , 16 February 2022
Sec. Dementia and Neurodegenerative Diseases
Volume 12 - 2021 | https://doi.org/10.3389/fneur.2021.768591
This article is part of the Research Topic Frontotemporal Dementia and its Spectrum in Latin America and the Caribbean: a Multidisciplinary Perspective View all 21 articles
 Fernando Henríquez1,2,3,4
Fernando Henríquez1,2,3,4 Victoria Cabello1,3
Victoria Cabello1,3 Sandra Baez5†
Sandra Baez5† Leonardo Cruz de Souza6,7†
Leonardo Cruz de Souza6,7† Patricia Lillo1,8,9†
Patricia Lillo1,8,9† David Martínez-Pernía1,2,10†
David Martínez-Pernía1,2,10† Loreto Olavarría2,3†
Loreto Olavarría2,3† Teresa Torralva11†
Teresa Torralva11† Andrea Slachevsky1,2,3,12*
Andrea Slachevsky1,2,3,12*Frontotemporal dementia (FTD) is the third most common form of dementia across all age groups and is a leading cause of early-onset dementia. The Frontotemporal dementia (FTD) includes a spectrum of diseases that are classified according to their clinical presentation and patterns of neurodegeneration. There are two main types of FTD: behavioral FTD variant (bvFTD), characterized by a deterioration in social function, behavior, and personality; and primary progressive aphasias (PPA), characterized by a deficit in language skills. There are other types of FTD-related disorders that present motor impairment and/or parkinsonism, including FTD with motor neuron disease (FTD-MND), progressive supranuclear palsy (PSP), and corticobasal syndrome (CBS). The FTD and its associated disorders present great clinical heterogeneity. The diagnosis of FTD is based on the identification through clinical assessments of a specific clinical phenotype of impairments in different domains, complemented by an evaluation through instruments, i.e., tests and questionnaires, validated for the population under study, thus, achieving timely detection and treatment. While the prevalence of dementia in Latin America and the Caribbean (LAC) is increasing rapidly, there is still a lack of standardized instruments and consensus for FTD diagnosis. In this context, it is important to review the published tests and questionnaires adapted and/or validated in LAC for the assessment of cognition, behavior, functionality, and gait in FTD and its spectrum. Therefore, our paper has three main goals. First, to present a narrative review of the main tests and questionnaires published in LAC for the assessment of FTD and its spectrum in six dimensions: (i) Cognitive screening; (ii) Neuropsychological assessment divided by cognitive domain; (iii) Gait assessment; (iv) Behavioral and neuropsychiatric symptoms; (v) Functional assessment; and (vi) Global Rating Scale. Second, to propose a multidimensional clinical assessment of FTD in LAC identifying the main gaps. Lastly, it is proposed to create a LAC consortium that will discuss strategies to address the current challenges in the field.
Frontotemporal dementia (FTD) is a clinical neurodegenerative syndrome characterized by alterations in behavior, executive functions, and language (1–3). The FTD constitutes a spectrum of diseases classified according to their clinical presentation and patterns of neurodegeneration (4, 5). There are two main types of FTD: the first is the behavioral FTD variant (bvFTD), characterized by impaired social function, behavior, and personality; and the second are the language variants, namely, semantic dementia (SD), non-fluent or agrammatical aphasia (nfv-PPA), and logopenic aphasia (lv-PPA), which are characterized by progressive deficits in language skills (2, 4, 6). There is a current controversy surrounding lv-PPA, regarding whether to maintain its inclusion as an FTD variant, given that the neuropathological studies show a stronger association with Alzheimer's Disease (AD) pathologies (7, 8). Nevertheless, some current criteria maintain it as an FTD syndrome variant (6). Other types of FTD-related disorders present with motor symptoms and/or parkinsonism. The main disorders associated with motor difficulties are FTD with motor neuron disease (FTD-MND) and FTD with atypical parkinsonism, i.e., progressive supranuclear palsy (PSP) and corticobasal syndrome (CBS) (2, 9, 10).
FTD is one of the most common causes of early-onset dementia (patient age <65 years) and is the third leading cause of dementia after AD (11, 12) and Lewy body dementia (LBD) (1). Its prevalence ranges between 3 and 26% worldwide (1, 13). Precise data regarding its prevalence in Latin America is unknown despite the consequences it causes (14). It is also frequently underdiagnosed, being confused with psychiatric pathologies (15, 16). Studying this syndrome is greatly relevant as it impairs the capacity of the patient to perform activities of daily life (ADL), affecting both basic (feeding, dressing, and bathing) and instrumental (economic management, cooking, housework) activities of daily living (BADLs and IADLs, respectively) (17, 18). This significantly interferes with the capacity of the patient to live independently, their quality of life, along with that of their relatives (19, 20).
Diagnosis is based on identifying the clinical phenotype described above, i.e., behavioral or neuropsychiatric symptoms and/or language impairment, accompanied by impairment in other domains, namely, social cognition, executive functions, functionality, and motor function (2). The clinical interview and examination are complemented by a multidimensional assessment, defined as the evaluation of cognition, behavior, functionality, and motor capacity, with the administration of validated and standardized tests and questionnaires to obtain reliable and accurate information regarding impairment in these domains (21). Broadly speaking, these tests and questionnaires could be administered in the clinical context as a brief screening evaluation, but they can also be a complementary exam when applied as an extensive neuropsychological assessment (22). Cognitive screening tests are brief and straightforward instruments aimed at detecting signs of dementia or cognitive impairment and monitoring the evolution of the disease and response to treatment (22, 23). These instruments are routinely used in a clinical practice. They are crucial for identifying cognitive impairment and for initiating the diagnostic process, which is further supported by blood tests, neuroimaging, and a formal neuropsychological assessment (22, 23), which includes an evaluation to collect information on various dimensions of cognition, behavior, and functioning (24). The validity and reliability of data gathered with brief screening tests and neuropsychological tests depend on their validity in the cultural contexts in which they were applied (25, 26). A test with good psychometric characteristics allows comparing the performance of a subject with groups of the same age, sex, race, and educational level, given that all these factors influence the performance and interpretation of the instruments used. This comparison determines whether a subject performs as expected or with diminished capabilities, which can be quantified and interpreted (24). Although the screening tests are a powerful tool to detect cognitive impairment, there is no specific screening for FTD due to the heterogeneity of the syndrome, which implies a significant difficulty for a timely diagnosis.
Diagnosing FTD is indeed challenging due to its complex clinical phenotype and its insidious presentation, especially in cases with non-specific behavioral features and without brain atrophy (27–31). Usually, an FTD diagnosis is clinically recognized later than AD (15, 16, 32). A significant delay in diagnosis of up to 5 years from the onset of the first symptoms and a high rate of misdiagnosis with psychiatric conditions have been reported (33, 34). Several diagnotic barriers have been reported, such as (i) the heterogeneity of FTD, whose clinical features frequently overlap with other neurological diseases, e.g., the behavioral/dysexecutive variant of AD (35) or psychiatric disorders (36–39); (ii) Lack of knowledge and training of health professionals in Latin America and Caribbean (LAC) on FTD (25, 40, 41); (iii) Limited access to medical care, neuropsychological evaluations, and advanced neuroimaging facilities to support FTD diagnosis in LAC (42, 43); and (iv) Lack of validated instruments for the LAC population that is capable of detecting and differentiating FTD from other pathologies. For these reasons, it is important to review the available evidence on tests and questionnaires for the assessment of FTD in LAC and propose a strategy to address challenges in the field.
Therefore, our paper has three main goals. First, to present a narrative review of the main tests and questionnaires published in LAC to assess FTD and its spectrum. Second, to propose a multidimensional clinical assessment of FTD in LAC, identifying the main gaps. Lastly, it is proposed to create an LAC consortium that will discuss strategies to address current challenges in the field.
First, experts in FTD and its spectrum from Argentina, Brazil, Chile, and Colombia were invited to participate based on two criteria: (i) neurologists, neuropsychologists, and physical therapist working in clinical evaluation and research in FTD and its spectrum, or (ii) clinical researchers in the clinical assessment of FTD and its spectrum. Second, an online literature search for journals indexed by Pubmed Central, Scopus, Lilacs, and Scielo databases was conducted between March 2021 and July 2021 (performed by FH and VC). The Scielo database was incorporated since it indexes many national and Latin American journals from all areas of knowledge. For this review, we searched for articles with the following keywords in English: Frontotemporal Dementia, Primary Progressive Aphasia, Progressive Supranuclear Palsy, Corticobasal Degeneration, Amyotrophic Lateral Sclerosis AND Neuropsychology, Neuropsychiatric, Activities of Daily Living, Functional Assessment, Cognitive Assessment, Screening Test, Gait, Behavior, AND Latin America, South America, Caribbean. Subsequently, the procedure was reproduced with the exact keywords translated into Spanish and Portuguese.
Once the results of the literature review were provided to the experts, they wrote the different sections of the narrative review based on their expertise (LCD, LO, AS, and FH: cognitive screening; SB and TT: neuropsychology assessment; DMP: gait assessment; PL and FH: behavior and neuropsychiatric symptoms; and FH and AS: functional assessment and global rating scale). After the experts wrote the different sections, they met in several online meetings to reach an agreement on the different sections of the narrative review, and to propose a multidimensional clinical assessment and identify the main gaps in the field.
In the following section, we will present the available evidence divided into six dimensions: (i) Cognitive screening; (ii) Neuropsychological assessment divided by cognitive domain; (iii) Gait assessment; (iv) Behavioral and neuropsychiatric symptoms; (v) Functional assessment; and (vi) Global rating scale. We will discuss the relevance of each dimension for the assessment in the FTD diagnosis, describing the instruments generally used along with the available evidence in LAC.
As discussed previously, FTD diagnosis is based on clinical grounds and requires a high level of suspicion from health professionals. When evaluating a patient with suspected dementia, a brief cognitive screening (BCS), defined as an instrument used to detect signs of dementia that does not include caregiver or information interviews, is the first line of cognitive assessment (23). BCSs are crucial for identifying the presence of a cognitive syndrome, initiating the diagnostic process, and contributing to a timely diagnosis (44).
However, there are no specific tools for screening for neurodegenerative syndromes. In line with this, epidemiological surveys on the prevalence of FTD in community-based studies in LAC employed a three-step procedure to establish FTD diagnosis, namely, (1) demographic and clinical questionnaires, including a brief cognitive battery, e.g., Mini-Mental State Examination (MMSE) (45), Montreal Cognitive Assessment (MoCA) (46), third version of Addenbrooke's Cognitive Examination (ACE-III) (47), and a functional assessment such as the Pfeffer Functional Activities Questionnaire (PFAQ) (48); (2) detailed clinical (neurological) and cognitive evaluations, and (3) laboratory and neuroimaging investigation. Thus, FTD diagnosis is established with a consensus diagnosis (14). Moreover, most clinical studies on FTD conducted in LAC included patients selected from the reference centers to diagnose and manage dementia. These studies usually adopt a consensual diagnostic framework. Indeed, cognitive screening tests are recommended for detecting dementia but not for the differential diagnosis of dementia. Thus, it is crucial to use cognitive screening tools sensitive to FTD (49).
BCS is generally used in FTD research, such as the INECO Frontal Screening (IFS) (50), the Frontal Assessment Battery (FAB) (49, 51), or the Mini-social cognition and emotional assessment (mini-SEA) (52, 53). In addition, the behavioral and psychiatric scales answered by an informant, such as the Neuropsychiatric Inventory (54), may also be helpful for FTD diagnosis (55, 56). However, these tools may not be adapted for use in primary care scenarios as they may require specialized training and are time-consuming (23). Moreover, their accuracy for FTD screening in the general population has, so far, not been investigated.
This context, thus, warrants the development or adaptation and validation of screening tools for FTD diagnosis. The ideal FTD screening tool should combine high sensitivity and short application time and should not require specialized training, thus, being beneficial for primary care settings.
In LAC, brief cognitive assessments are available for use in clinical settings. However, evidence on their diagnostic utility in FTD is still limited. Addenbrooke's Cognitive Examination-Revised (ACE-R) was adapted in Argentina (57), Brazil (58), and Chile (59). Another work conducted in Argentina and Chile has validated the third version of Addenbrooke's Cognitive Examination (ACE-III) in a population of patients with bvFTD, AD, and healthy control subjects (60). The ACE-III showed good psychometric properties and allowed differentiating patients with dementia from healthy controls, and demonstrated good discriminative ability between these two groups of patients (60).
Torralva et al. (50) designed the IFS in Argentina, a cognitive instrument that allows a brief assessment of executive functions. The validity and discriminative capacity of the IFS was studied in patients with bvFTD, AD, and healthy controls. The IFS differentiates patients with dementia from healthy controls (50, 61) and patients with bvFTD from AD (50). Two studies, one in Argentina and the other in Peru, suggested that the IFS presented greater clinical utility in differentiating bvFTD from AD in comparison with the FAB (62, 63). In Brazil, Bahia et al. (64) reported that the IFS showed good psychometric properties, but provided a low accuracy, differentiating between bvFTD and AD. In Chile, the psychometric properties and diagnostic accuracy of IFS were studied in a sample of patients with dementia (bvFTD, AD, vascular dementia (VD), LBD, and SD) and healthy controls (65). The Chilean IFS presented adequate indicators of reliability and good diagnostic accuracy in detecting patients with dementia (65).
Although relative sparing of episodic memory has been proposed as one of the distinctive characteristics of FTD (66, 67), recent evidence questions the validity of the preservation of this domain, particularly in bvFTD. For instance, evidence from a recent meta-analysis (68) showed that patients with bvFTD perform intermediately between healthy controls and patients with AD. However, patients with bvFTD showed severe memory impairments in line with previous studies reporting episodic memory impairments in patients with bvFTD (69, 70). In contrast, several studies demonstrate that patients with AD experience even more significant memory problems than patients with bvFTD (71–74), with delayed memory testing being the most discriminative (73, 75). In addition, some patients with bvFTD have shown genuine amnesia affecting storage and consolidation abilities, which are independent of executive dysfunctions (76), and are observed in a similar degree in AD (77, 78).
Concerning PPA, episodic memory seems to be compromised in all variants compared to healthy controls (78, 79). However, patients with SD are impaired to a similar extent as patients with lv-PPA who are in turn more impaired than patients with nfv-PPA. In addition, patients with SD perform better on tests using non-verbal material and show overall better performance on recognition tests (78). Episodic memory deficits in lv-PPA and nfv-PPA, on the other hand, are observed on both verbal and non-verbal measurements, although patients with lv-PPA show more pronounced episodic and working memory deficits when compared to patients with nfv-PPA (79–81). Thus, given that differentiating the language profiles of the PPA variants remains challenging (80), especially for lv-PPA and nfv-PPA, memory testing could be of potential benefit to better differentiate between these variants.
The most frequent tests used to assess memory in FTD (82) are the Rey Auditory-Verbal Learning Test (RAVLT) (83) or similar word list-learning tests, such as the Hopkins Verbal Learning Test (HVLT-R) (84) or the California Verbal Learning Tests (CVLT) (85), the computerized Paired Associate Learning Test (PAL) (86), the Free and Cued Selective Reminding Test (FCSRT) (87), the autobiographical memory interview (88), and the Cambridge Behavioral Prospective Memory Test (CAMPROMPT) (89). These instruments are also commonly used in LAC [e.g., (52, 90)], although most of them are not validated for this population.
LAC validations are available for the RAVLT (91, 92), the HVLT-R (93), and the FCSRT (94). Other validated memory tests for the assessment of patients with dementia include the Rivermead Behavioral Memory Test (RBMT) (95) and the Logical Memory Subtest of the Wechsler Memory Scale (WMS) (96) for the Brazilian population, and the Signoret battery for amnesic efficiency (BEM 144) for the Argentinian population (97). In addition, the Short-term Memory Binding (STMB) test has been used to assess patients with bvFTD in Brazil (98). Results showed that patients with AD performed significantly worse than controls and patients with bvFTD in the STMB test, while both clinical groups showed equivalent performance. Therefore, this test can be used for clinical purposes and may aid in the differential diagnosis of AD (98). Finally, the visual memory test from the Brief Cognitive Screening Battery (99) has also been employed to investigate episodic memory of patients with bvFTD in Brazil (100).
In conclusion, findings suggest that clinicians should carefully use memory performances and interpret them in conjunction with other diagnostic information, namely, medical history, behavioral observations and questionnaires, neuroimaging, and neuropsychological data from other cognitive domains (68, 101).
Visuospatial function is usually conceptualized in three components: visual perception, construction, and visual memory (101). The relative preservation of visuospatial abilities is suggested to be among the critical features that distinguish FTD from other degenerative disorders and, particularly, from AD (67) and LBD (102). However, a recent study (103) showed that the visuospatial measures demonstrate a limited ability to distinguish between AD and bvFTD unless disease severity is considered. Controlling for disease severity reveals a disproportionate visuospatial impairment in AD compared to bvFTD.
One of the most commonly used instruments to assess visual perception is the Visual Object and Space Perception Battery (VOSP) (104). In this battery, patients with the three language FTD variants obtain lower scores than controls, while patients with bvFTD perform normally (105). However, scores deteriorate with the dementia progression in all patient groups (105). Drawing tasks, such as the Clock Drawing Test (CDT) (106) and the Rey-Osterrieth Complex Figure (ROCF) (107) test, are commonly used to assess constructional abilities. Grossi et al. (108) found that patients with bvFTD and patients with AD achieve similar scores on copying tasks, present similar drawing procedures in the ROCF, and make a similar quantitative and qualitative pattern of errors when copying simple geometrical drawings, which suggests that relative preservation of visuospatial abilities in FTD may be found in early stages of the disease. Finally, some tests are widely used to assess visual memory, including the delayed recall component of the ROCF and the Benton Visual Retention Test (BVRT) (109). In this line, a comprehensive systematic review (110) found that ROCF recall and topographical memory tasks show the greatest diagnostic potential in dementia, while the BVRT shows potential as a prognostic marker.
Regarding the PPA variants, patients with lv-PPA have shown significantly lower scores on all visuospatial skills (111). The nfv-PPA variant shows significant difficulty in all visuospatial abilities except the delayed recall. In contrast, SD performs poorly only on delayed recall of visual information. The lower scores of all patients with lv-PPA on visuospatial skills could be explained by the fact that part of the clinical criteria for this disease includes parietal atrophy on structural MRI or hypometabolism on PET/SPECT (111). One possible reason patients with nfv-PPA displayed difficulty on these tasks is that several of the tasks rely on visuomotor abilities, and nfv-PPA has been associated with the degradation of white matter pathways connecting the left inferior frontal gyrus to the premotor and supplementary motor regions (112, 113). Thus, the deficits may relate more to motor planning and sequencing (111). Further, investigation is needed to determine the underlying mechanism.
Some of the most employed measures have been validated for LAC, including the CDT (114–118) and the ROCF test (119, 120). In addition, the VOSP has also been validated for the Brazilian population (121).
Although language in bvFTD is initially spared (101), some patients with this variant may present difficulties in naming action words. Such a deficit has shown an association with executive abilities (122). In addition, due to apathy, patients with bvFTD may not participate in communication, and, thus, may present a reduction in spontaneous speech (101). Social and emotional aspects of speech may also be impaired in bvFTD, with an inability to understand the subtleties and context of conversations (123). Fluency may also be helpful in differentiating bvFTD and AD. While semantic fluency is usually impaired to a greater degree in AD, phonemic fluency is more affected in bvFTD (123).
Regarding PPA, the most prominent early feature of SD is a reduced expressive vocabulary. Word finding is severely impaired, and speech is empty of content (124). Compared to SD, the hallmark feature of nfv-PPA is effortful non-fluent speech. Nfv-PPA is characterized by grammatical errors and omissions, along with the simplification of grammatical forms (125). The third subtype of PPA, lv-PPA, is mainly characterized by problems in lexical retrieval during conversational speech and impaired repetition of sentences and phrases.
Tests of word comprehension, speech production (fluency, naming, and repetition), as well as oral reading (to detect surface dyslexia) and writing (to detect surface agraphia), should be used in the language assessment of FTD variants (82). The main instruments used for language assessment are the Boston Diagnostic Aphasia Examination (BDAE) (126) and the Sydney Language Battery (SYDBAT) (127). The SYDBAT contains four subtests: nomination, repetition, comprehension, and semantic association. The most commonly used instruments for the assessment of memory or semantic knowledge are the Pyramids and Palm Trees (PPT) Test (128), which measures the accessibility of semantic information of words and images, and the Repeat and Point Test (RPT) (129), which assesses the comprehension and repetition of words, differentiating patients with DS and nfv-PPA.
Some of these language measures have been validated for LAC. For example, normative data on the BDAE and verbal fluency tests exist for the LAC Spanish-Speaking Population (130) and for Brazilian Portuguese (131–135).
Apraxia is one of the major sources of disability in patients with brain injury, as it significantly affects Activities of Daily Living (ADLs) (136). Although apraxia is a main sign of other neurodegenerative pathologies, such as CBS, it is also known to present as an additional early cognitive marker in bvFTD (137), and therefore, its assessment is important (138). Some findings also suggest a relationship between praxis and working memory in this type of patients, since frontal involvement, with its corresponding difficulties in executive memory, hinders the performance, for example, of gestures (137, 139). Additionally, there are FTD variants or diseases with overlapping symptoms where this function is particularly affected. For instance, PPA presents speech apraxia (140), and CBS is characterized by the presence of progressive and asymmetric apraxia (141–143).
Scientific evidence in LAC supports apraxia as an early manifestation of bvFTD and as the most significant manifestation in the previous variants described. Several of the findings on the subject have studied a positive relationship between the severity of apraxia and the degree of cognitive impairment (136).
The most commonly used tests to measure this function in FTD are the ROCF Test (83, 107), the CDT (144), the block design Wechsler Adult Intelligence Scale (WAIS) construction subtest (145), the Cognitive Assessment of Apraxias battery (146), and the Mattis Dementia Rating Scale (MDRS) (147). Some of these praxis measures have been validated for LAC. For example, normative data exist on the ROCF (119, 148), on the WAIS IV construction subtest with cubes (149), and on the MDRS (150–152). In addition, the Cognitive Assessment of Apraxias battery (153) was created in Argentina.
Executive functions are defined as an umbrella concept, encompassing multiple functions commanded by the frontal lobe, such as planning, organization, sequencing, inhibitory control, and cognitive flexibility (154, 155). In FTD, their assessment is of vital importance as it implies the involvement of the prefrontal cortex and some of its variants present a dysexecutive profile (82, 156).
The most commonly used tests to measure this function in FTD can be of three types. Executive screening tests, such as the IFS (50, 157) discussed above, provides a general idea of the preservation or impairment of these functions. A group of classic executive functions assessment tests includes the Trail Making Test A and B (TMT) (158), the Wisconsin Card Sorting Test (WCST) (159), the Stroop test (160), the Hayling Test (161), the Tower of London (162, 163), the Tower of Hanoi (164), the Porteus Maze (165), Raven's Progressive Matrices Test (166), WAIS Matrix Reasoning subtests (145), Iowa Gambling Test (IGT) (167), and the classic working memory tests, such as the reverse digits, arithmetic, and WAIS letter ordering (145). Finally, there are ecological evaluation tests, such as the Hotel Test (168) and the Behavioral Assessment of the Dysexecutive Syndrome (BADS) (169), which optimally evaluate the functioning of the patient with tasks designed similarly to their daily life.
Some of these executive functions measures have been validated for LAC. For example, normative data exists on the TMT A and B (118, 170, 171), on the Modified Wisconsin Card Sorting Test (M-WCST) (172, 173), on the Stroop Color-Word Interference Test (173, 174), on the executive subtests WAIS IV (149), on the Hayling Test (175, 176), on the BADS (177), and on the Hotel Test (52, 178). In addition, the IFS was created in Argentina (50).
The existing scientific evidence in Latin America predominates in patients with bvFTD, who, in addition to behavioral symptoms, present a predominant dysexecutive profile in the neuropsychological assessment (179–181).
Social cognition refers to the set of cognitive processes involved in the perception, interpretation, and generation of responses to the intentions, dispositions, and behaviors of others (182). This domain plays a very relevant role in FTD as it is predominantly affected in the behavioral variant, one of the most common variants of FTD, particularly regarding recognition of emotions, theory of mind, empathy, and moral judgment tasks. These failures occur mostly due to the effects on the orbitofrontal cortex and temporal poles (183–187). Various findings highlight difficulties, such as impaired moral judgment, where patients with FTD score are significantly lower on personal moral dilemma tasks and theory of mind tests than the control subjects (183). In addition, other studies suggest that patients with FTD judge intentional damage as more permissible than accidental damage due to a decrease in gray matter in the temporal pole (188). Investigations studying empathy in this group of patients are also especially relevant, finding that patients with FTD present difficulties in the affective, cognitive, and moral aspects of empathy (184).
Therefore, the most commonly used tests for evaluating these difficulties in social cognition are the Facial Expressions Recognition Test (189), the Mind in the Eyes (190), the Faux Pas Test (191), the Social cognition and Emotional Assessment (SEA) (192), and the short version of the Social Cognition and Emotional Assessment (Mini-SEA) (193). Some of these praxis measures have been validated in LAC, or new versions have been created, such as the Facial Expressions Recognition Test for elderly Argentinians (194) and the Facial Emotions Recognition Test in Brazil (195). In addition, normative data exists on the Mind in the Eyes (52, 196), and the Faux-pas tests (52). The Faux-pas test has also been adapted in Brazil (197) and used for bvFTD investigation (198).
Numerous studies on social cognition in patients with FTD have been carried out in LAC, especially the relation to moral judgment, theory of mind, and the recognition of emotions (53, 183–187, 198, 199).
Motor control has long been understood as a mechanical function and reflex, but an extensive body of research shows that motricity depends on different cognitive processes, such as attention, memory, language, and executive function (200, 201). Especially relevant in motor assessment is the study of gait. Gait is a complex task integrating the participation of multiple systems in order to achieve a cyclic pattern of body movements with cognitive function (202, 203), encompassing multiple independent domains [e.g., pace, rhythm, variability, asymmetry, and postural control (204)]. Gait analysis has shown to be a good predictor for health status in older adults and is a global health marker (205, 206). In the dementia population, studies have shown a strong association between gait and cognition (207) where an assessment according to serial quantitative measures of gait velocity prove to be a good predictor of dementia development (208).
Gait speed has been one of the most reported locomotion variables because of its robust properties in clinical settings (209) and its utility in differentiating between healthy older adults and patients with dementia (210). More recently, gait study has incorporated more accurate and sophisticated measurement systems, showing that gait assessment is a more complex multidimensional construct than the gait speed. For instance, Ijmker and Lamoth (211) found that during walking (single task) and walking while performing a letter fluency (dual task) tasks, patients with FTD presented a significantly longer stride time, lower gait speed, and higher stride variability than healthy older adults. In another study, Rucco et al. (212) found that patients with bvFTD performing single and dual tasks (walking while serially subtracting 7s starting from 100) present a significant difference in gait velocity (speed, stride length, cadence) and instability (stance time, swing time) compared to the healthy group.
Despite the scarcity of research regarding gait assessment in FTD (213), it has shown to be critical when differentiating between neurodegenerative diseases. For instance, the study developed by Allali et al. (214) found that patients with bvFTD showed an increase in stride time coefficient variation during a single (walking) and dual tasks (walking and counting backward by one) in comparison to the AD group. A longitudinal study developed by de Cock et al. (215) found multiple significant associations between different components in gait assessment and the future dementia type (AD, FTD, VD, and LBD).
Despite the increasing evidence demonstrating the potential of gait assessment for the diagnostic discrimination between FTD and other dementias, there is no study of these features in LAC.
The core of bvFTD are behavioral features, as stated in the Current Consortium Criteria for bvFTD (67). These symptoms must present within the first 2–3 years from the onset of disease. Onset is insidious and these features are usually reported by family members or caregivers, as the patients often lack insight. Disinhibition is one of the prominent symptoms and is evident in 76% of the cases. It is manifested through impulsivity, inappropriate social behavior, and lack of decorum. Apathy, the other predominant feature, reaches 84% of the cases, presenting inertia and a lack of motivation. Loss of empathy and/or sympathy and stereotyped behaviors are frequent manifestations reaching up to 70% of patients with bvFTD, while almost 60% of cases present eating disturbances (67, 216). Psychotic symptoms, such as delusions and hallucinations, have been described as less commonly (217). One study reported that 14% of patients with FTD presented delusions, mostly of a paranoid or somatic type (218).
Several assessments, mostly caregiver-based questionnaires, have been used to evaluate neuropsychiatric and behavioral symptoms in FTD. One of them is the Frontal Behavioral Inventory (FBI), which can help to distinguish FTD from other types of dementia but cannot differentiate between bvFTD and psychiatric conditions (219). Nevertheless, sub items such as indifference/emotional flatness, inappropriateness, aphasia, verbal apraxia, alien hand, and apraxia are more suggestive of bvFTD (220). The Frontal Systems Behavior Scale (FrSBe) is another test designed to evaluate apathy, executive dysfunction, and disinhibition (221). The Cambridge Behavioral Inventory Revised (CBI-R) is a questionnaire evaluating a wide range of neuropsychiatric features and everyday functionality. This test was able to discriminate the behavioral profiles of the various neurodegenerative diseases, including AD, Parkinson's Disease (PD), and bvFTD (222, 223). The Neuropsychiatric Inventory Questionnaire (NPI-Q) (224), a short version of the Neuropsychiatric Inventory (NPI) (54), is a tool used to evaluate neuropsychiatric symptoms and response to treatment in patients with dementia, and it has also been used for bvFTD. A behavioral inventory based on the current International Consensus Criteria, DAPHNE (225), allows differentiating the bvFTD from the bipolar disorder. Ducharme et al. (226) developed a 17-item tool, the FTD vs Primary Psychiatric Disorder Checklist, which may be useful in clinical settings and showed good diagnostic accuracy.
There are several scales for more specific symptoms, such as: (a) Apathy may be assessed by the Apathy Evaluation Scale (AES) (227) or with the Starkstein Apathy scale (SAS) (228); (b) The Stereotypy Rating Inventory (SRI) (229), which recognizes stereotypies as more frequent features in bvFTD than in other conditions; (c) Lack of empathy can be measured by the Interpersonal Reactivity Index (IRI) (230); and (d) The Appetite and Eating Habits Questionnaire APEHQ used to assess dietary disturbances (231).
Several studies in LAC have investigated neuropsychiatric symptoms in FTD. In Brazil (55), the NPI was used to verify accuracy in the differential diagnosis between FTD and AD. The results showed that all patients with FTD and only half of those with AD presented neuropsychiatric symptoms (55). Similarly, another Brazilian study (232) demonstrated the usefulness of the FBI for the differential diagnosis between FTD and AD. In Colombia, the Columbia University Psychopathological Scale for Alzheimer's Disease (CUSPAD) and the NPI were used to assess how neuropsychiatric symptoms could influence cognitive and functional impairment in patients with FTD and AD (56). Another study that assessed apathy using the Starkstein Apathy Scale (SAS) showed that patients with bvFTD had higher scores than healthy controls. In addition, the severity of apathy was associated with a decreased gray matter volume in the midline prefrontal regions (233). A case study of FTD with late-onset compulsions and cinephilia was described by Slachevsky et al. (234). Pathological gambling was also reported in a case with bvFTD (235).
Impaired ability to carry out ADLs, resulting in a loss of independence, is central to the diagnosis of dementia and establishes the boundary between dementia and pre-dementia (67, 236). Impairment in functional capacity is a common outcome of all dementia syndromes, and their assessment is critical for diagnosing and monitoring disease progression (237). The assessment of functional capacity has focused on the development of objective and sensitive tools (19), which are based on indirect (i.e., informant-based questionnaires) and direct (i.e., performance-based tests) measures (238). These tools assess BADLs, which represent the most basic level of functioning and are necessary for survival, and IADLs, which require more complex skills and enable independent living in the community (19). Recently, Advanced Activities of Daily Living (AADLs) have been incorporated, which are the activities necessary for complex interpersonal and social functioning (239, 240).
This is important considering that the functional decline is present in all types of dementia and that the same functional assessment tools are used for different types of dementia. Research on functional decline assessment in FTD has focused on establishing if there is a specific pattern of functional decline, its progression, associated factors, and its neural basis. Indeed, the rate of functional impairment is marked more significantly in FTD than in AD (17, 237). In this line, one of the research lines has established ADL assessment measures to differentiate between different types of dementia.
In LAC, the study of functionality in FTD is limited. In Argentina (19), the Activities of Daily Living Questionnaire (ADLQ) (241) is available to assess functional impairment in different types of dementia (AD, FTD, and other subtypes). In Chile, the Technology-ADLQ (T-ADLQ) was developed, expanding the ADLQ with an additional subscale to evaluate the use of technology in patients with dementia (AD, FTD, DV, and LBD) (242, 243).
In Brazil, several studies have evaluated the usefulness of different tests: Bahia et al. (232) applied the Disability Assessment for Dementia (DAD) questionnaire (244) for estimating the functional capacity of patients with FTD (bvFTD, SD, and nfv-PPA) and AD, showing promising results. The Direct Assessment of Functional Performance (DAFS) (245) was administered for the study of patients with FTD (238) (unlike the DAD, this is a performance-based test). In addition, Carvalho et al. (246) used the Functional Assessment of Adult Communicative Skills (Asha-Facs) (247) in patients with FTD and AD. The results showed similar performances in both groups of patients (246). Finally, in Chile, the T-ADLQ showed promising results for evaluating functional impairment in FTD (243).
Importantly, all these tools showed good psychometric properties in the applied populations, making them valuable instruments for assessing the functional capacity of patients with FTD in LAC (19, 246). These instruments are sensitive in identifying impaired functional ability and differentiate patients with dementia from control subjects. Although some tools failed to significantly distinguish between FTD and AD, patients with FTD presented a worse performance in some indices of these scales (238, 246).
Two works explored the association of functional impairment with cognitive and behavioral symptoms in bvFTD. A multicentric study in Brazil, Australia, England, and India (20) showed an association between impairment in a global functional capacity and IADLs, evaluated through the DAD, with global cognitive impairment and apathy (20). More recently, a study explored factors associated with domains of functional impairment as assessed with the T-ADLQ [i.e., BADLs, IADLs, and AADLs (243)]. Interestingly, factors associated with the loss of functionality differ according to the functional domain, i.e., impairments in IADLs were associated with apathy and disinhibition, in IADLs with apathy, deficits in executive function, lack of emotion recognition, and in IADLs with apathy. This study suggested that the factors associated with loss of functionality differ according to the functional domain in patients with bvFTD in its early stage, along with a prominent and transverse effect of apathy in the loss of functionality throughout all the ADL domains, and the association of social cognition with functional impairment (243).
Global assessment scales allow clinical characterization and longitudinal assessment of patients with neurodegenerative diseases (248). In addition, these scales allow proper clinical management and personalized care of patients with dementia, monitoring the progression of the disease and the effects of treatments that could modify the course of the illness (249).
The main instrument used for the global classification of dementia is the Clinical Dementia Rating (CDR) (250), which provides information on cognitive and functional aspects of the disease (251). The CDR is a semi-structured interview administered to the patient and to the primary caregiver, which provides information on six specific domains (memory, orientation, judgment and problem solving, community affairs, home, hobbies, and self-care). Each domain and the scale as a whole reports values ranging from low to high severity: 0 (no impairment), 0.5 (very mild), 1 (mild), 2 (moderate), and 3 (severe) (252). However, the CDR was developed based primarily on AD symptoms, making it a less sensitive scale for other types of dementia, such as FTD (30, 249, 253).
To address the low sensitivity of the CDR, Knopman et al. (254) proposed a new version, the Clinical Dementia Rating Scale for Frontotemporal Lobar Degeneration (CDR-FTLD). This scale incorporated language and behavioral domains (249, 252), providing specific information on FTD and its variants (252). On the other hand, Mioshi et al. (30) proposed a specific scale for FTD, the Frontotemporal Dementia Rating Scale (FTD-FRS). The FTD-FRS was designed based on the DAD and the Cambridge Behavioral Inventory (CBI). This scale allows staging the severity of FTD in its different variants, such as bvFTD and PPA (30, 252, 253).
In LAC, the CDR-FTLD was adapted and validated in Argentina (251) and Brazil (249). Lima-Silva et al. (252, 253) translated, adapted, and validated the FTD-FRS into Portuguese and administered it together with the FTD-FRS and the CDR to patients with FTD (bvFTD, PPA), AD, and healthy controls. In these studies, the CDR was observed to underestimate FTD severity, as it classified patients with mild severity (CDR = 1), unlike the FTD-FRS, which indicated moderate levels of severity (253). The same Lima-Silva group evaluated the ability of the FTD-FRS in comparison with the CDR-FTLD and CDR to detect the functional and behavioral changes in patients with bvFTD, PPA, and AD after 12 months of follow-up (249). All three scales detected an increase in symptom severity after the initial assessment. However, the FTD-FRS and CDR-FTDL were more sensitive in establishing the severity level in bvFTD and PPA (249).
In conclusion, global staging scales used to assess FTD in LAC can determine the stage and progression of the disease by identifying changes in behavior and language that the CDR does not consider (253). Therefore, these instruments are appropriate for clinical use in addition to being well-tolerated by patients and their caregivers (253). Finally, global rating scales show excellent psychometric and diagnostic properties for assessing FTD and its spectrum in LAC.
Considering the evidence on the adaptation, validity, diagnostic utility, and standardization in LAC of the reviewed instruments, we propose a multidimensional clinical assessment and the identification of gaps that represent essential barriers for a comprehensive evaluation of FTD. Importantly, cognitive assessment could be limited to cognitive screening in patients with mild symptoms or with a well-established diagnosis in whom further assessment will not contribute to the diagnosis. Otherwise, we recommend a multidimensional evaluation organized in three steps: (1) Tests to be administered to all patients regardless of variant; (2) Specific tests for specific variants, i.e., language or behavior; and (3) Additional tests for the assessment of specific symptoms.
In Table 1, we propose tests for the first level of the multidimensional clinical assessment. The first step, the tests to be administered to all patients, allows assessing the fundamental dimensions for a proper diagnosis of FTD, i.e., cognition, functionality, neuropsychiatry, and motor symptoms. Significantly, clinical symptoms reported by people with dementia and/or a reliable proxy do not necessarily predict the pattern of cognitive impairment or whether they are preserved (255). Therefore, assessing the main cognitive domains in all patients with suspected FTD is necessary to establish the pattern of cognitive impairment correctly.
In Table 2, corresponding to the second level, some of the recommended instruments have been widely used for clinical assessment and investigation of FTD in LAC. However, we must mention that the results of our review suggest that in most LAC countries, there is no information on the adaptation, validation, and standardization of these instruments. Additionally, the diagnostic utility of these tools has been studied mainly for AD but not for other subtypes of dementia. This second step involves specific testing for the different variants of FTD, i.e., behavioral or language variants. Finally, the third step should include evaluating some patients with more atypical or complex presentations who will benefit from additional testing. However, it is challenging to recommend further testing for these atypical presentations. Therefore, more research is needed.
Notably, our review suggests an important variety of practices in the assessment of FTD in LAC. The recommendation of a comprehensive multidimensional assessment of FTD is limited due to the existence of the main knowledge gaps that could be divided into three main areas. First, there is a lack of validated cognitive, functional, behavioral, and motor instruments for diagnosing FTD. Second, there are almost no tools to evaluate the illiterate and indigenous population. Third, there are no guidelines to orient clinicians on which patients would benefit from a multidimensional assessment. Finally, we will propose how to address the future challenges.
To the best of our knowledge, there are no properly adapted and validated tests for assessing semantic memory and social cognition in LAC. The available tools raise doubts about their validity and diagnostic utility. Currently, social cognition is primarily assessed with the Mini-SEA. Although there are promising results on the diagnostic utility of the Mini-SEA for the differential diagnosis of bvFTD of PD and AD (61, 198), social cognition assessment still faces essential limitations.
The investigation of behavioral and neuropsychiatric symptoms is of utmost importance for the correct diagnosis of FTD. While behavioral and psychiatric scales are of value for screening and measuring these symptoms, the cultural context should also be considered in the neuropsychiatric assessment. Indeed, the examiner may perceive some characteristics of interpersonal interaction as “normal” or “abnormal” according to cultural, personal, and social factors. For instance, interpersonal distance and voice volume are features that vary across cultures and may be described as “normal” or “disinhibited” according to the socio-cultural factors. Therefore, it is not enough to have “adapted and validated” tools to measure neuropsychiatric symptoms, but also ways to correctly interpret individual signs in interpersonal interactions in the perspective of a correct clinical diagnosis.
We think it is important to emphasize that gait dysfunction and, more generally, motor dysfunction have a large amount of overlap in genetics and molecular biology with cognitive disorders (256). Nevertheless, they are not part of the routine assessment of patients with dementia (257). This situation must be improved given that, for example, Parkinson's disease dementia (PPD), PSP, CBS, and Huntington's disease (HD), among others, present motor impairments as their main clinical features. Indeed, the apraxia profile or the applause sign could contribute to the differential diagnosis of diseases included in the FTD spectrum (258). Therefore, this manuscript proposes a gait assessment based on quantitative assessment systems (e.g., 3D motion capture, 2D kinematics, and spatiotemporal gait analysis system). However, the main difficulty in incorporating these systems is that they are expensive, making them difficult to access in the hospitals and clinics in LAC. A viable and much more inexpensive alternative is the wearable devices for gait analysis. Recent studies have found that wearable devices can differentiate gait alteration in dementia disease subtypes (259, 260). Nevertheless, we must remain cautious regarding this wearable technology because they have shown limitations in quantifying gait (e.g., the diversity in the sensor placements and the abundance of inertial algorithms) (203).
Unfortunately, as far as we know, there is no validated brief tool for motor assessment in FTD and its spectrum (256, 261). Concerning gait, we still require systematic studies to understand its contributions in FTD diagnosis. Ideally, a motor assessment tool in patients with dementia should include assessment of the gait pattern, parkinsonian gait, cerebellar gait, and higher-order symptoms such as praxis and motor sequencing (261). Such a tool would most likely benefit from the incorporation of wearable devices that could allow a more objective measurement of motor impairment.
Finally, it is important to highlight that the tools reviewed here have been mostly validated in studies with clinical-based FTD diagnoses without a pathological diagnosis confirmation. Considering a huge amount of evidence suggesting that FTD-related clinical syndromes are associated with heterogeneous pathology (262), it is important to emphasize that recommended tests allow prediction of a clinical syndrome, but not of a given specific pathological diagnosis (263). Either way, predicting neuropathology is beyond the scope of neuropsychology, and an etiopathogenic diagnosis of FTD requires a multilevel assessment including clinical, neuroimaging, and molecular biomarkers (264).
In sum, the translation and the validation of neuropsychological tests and their cultural adaptation are warranted to improve cognitive, functional, behavioral, and motor assessment of patients with FTD in LAC.
As suggested in international consensus studies, it is advisable to follow a multi-step approach to define the proper flow for each patient (261, 265). The first step should be applying a brief global screening instrument to all subjects with suspected cognitive impairment. Global tools, such as the ACE-III and the IFS, are recommended in the Spanish and Portuguese-speaking population (see Table 1). If these instruments and the clinical assessment suggest cognitive impairment and a diagnostic doubt persists, a multidimensional assessment should be performed (266).
Nevertheless, there are no clear guidelines on which patients would benefit from a multidimensional assessment. Considering the barriers to access specialist bvFTD evaluation centers (42, 267), the diagnosis process of bvFTD could be improved with the availability of evidence-based guidelines to help identify patients that could benefit from a multidimensional assessment.
Most of the instruments that have been validated in LAC are specifically for a literate population with, in general, a minimum of 4 years of education, which presents a significant drawback for the assessment of the illiterate and low-educated population (268). The absence of validated tests for the low-educated population is a significant limitation in assessment since years of education and age are two of the main variables affecting performance in cognitive assessments (261). Educational level affects instruments with low specificity given the difficulty in classifying subjects who possess diminished academic levels and how these patients obtain low scores despite being healthy. This situation also occurs with low-sensitivity instruments. Classifying subjects with a high academic level and high scores can be difficult despite presenting cognitive impairment (261).
Indeed, almost 4% of the illiterate population or with very low education levels of the world is found in LAC (269). Functional illiteracy is significant in LAC (270). In addition, about 10–17% of the LAC population is indigenous, with an estimated 400 indigenous languages spoken, along with Spanish and Portuguese (271). Finally, there is an increased percentage of non-Spanish or Portuguese-speaking migration (268). For example, in Chile, a large population of Creole-speaking Haitian citizens has recently arrived in the country, which generates a challenge and a limitation regarding the tools currently used in Chile. Economic factors should also be considered when proposing tests for these populations, as their financial vulnerability hampers access to expensive assessments.
In LAC, research and clinical evaluation of FTD and its spectrum have been conducted by a small group of professionals who share common needs and interests (61, 272). Nevertheless, transfer from research to clinical practice is restricted and significant knowledge gaps limit the implementation of multidimensional assessments. Following multicentric and multi-country initiatives in Europe and North America to improve assessment of neurodegenerative diseases, we propose the creation of a LAC consortium as the best strategy to address current challenges in the multidimensional clinical assessment of FTD. In fact, we are not aware of any organized working group to transfer research to a clinical practice. Regarding the clinical practice, there is currently an enormous heterogeneity of tools used in different countries, a lack of standardization of administration and scoring methods, and scarce information on the psychometric properties and diagnostic utility of some instruments. Moreover, the number of reliable instruments to assess the different dementias is limited, and there is no consensual evaluation protocol (261, 273). This problem directly affects the study of FTD and its spectrum, hindering the advancement of clinical and research practice in this type of dementia and not allowing the comparison and sharing of results from different studies conducted in LAC.
As suggested by international initiatives on the dementia assessment (261, 274), the formation of a consortium to share the works of professionals within LAC is probably the best strategy to establish a consensual multidimensional evaluation of FTD and its spectrum, and to overcome the shortcomings and the regional needs. A key point, as widely discussed and demonstrated in international consensus studies for dementia evaluation (261, 274), is the need for evaluation protocols that are consensual and homogenized by different countries and their local study centers. In addition, these evaluation protocols must have a standardized administration and a scoring procedure (274). In this line, the necessity of a standardized evaluation responds to the different backgrounds of the professionals who apply the evaluation instruments, including neuropsychologists, speech therapists, nurses, occupational therapists, and physicians (274). The contexts where the evaluation instruments are applied are also varied, such as primary care facilities, memory clinics, specialized centers, or in a research context (261).
A homogeneous evaluation practice based on a professional consensus for the assessment of cognitive, functional, behavioral, and motor abilities of patients with dementia (261) could guide the framework for different professionals, generating knowledge and shared data repositories of FTD studies and its spectrum in LAC. This effort could be critical for advancing studies on the adaptation, validation, and standardization of assessment tools (which are critical for the correct interpretation of study results) and possible educational processes and training for LAC professionals. Additionally, a homogenous evaluation practice could enable providing guidelines for implementing a multiple step approach in the evaluation. This is particularly relevant in LAC considering the lack of knowledge on FTD and its spectrum in health professionals (40, 61). In this effort, integrating clinical practice and research is relevant for generating new knowledge to evaluate the clinical utility of a multidimensional assessment, identifying patients that could benefit from this assessment, and elaborating the evidence-based guidelines to define the correct flow for each patient.
International evidence regarding consortiums highlights the main steps to succeed in the establishment of a definitive consensus. European experience suggests that the first step is the creation of a working group or a consortium that brings together different researchers and clinicians (neurologists, neuropsychologists, occupational therapists, speech therapists, and among others) from different countries (261). Each country should have one or two representatives from their main centers of dementia care or research who have specific skills in the diagnosis and evaluation of FTD and its spectrum. These representatives should be available to participate in periodic online working sessions. A general organization of the work plan should be established as follows: (i) Review the totality of assessment tools available in the different LAC centers, (ii) Define a global screening assessment for patients with FTD, and (iii) Establish a detailed assessment of the different variants of FTD covering cognitive, functional, behavioral, and motor dimensions.
Researchers or clinicians from different LAC countries, separated in groups, will seek which assessment tools are currently available to study cognitive, behavioral, functional, and motor dimensions. They will search for the psychometric properties (validity and reliability) and diagnostic utility (sensitivity and specificity) of the tools, their main issues, and propose solutions to solve the respective issues. The information obtained from the different working groups will allow for establishing a definitive consensus and develop a standardized evaluation protocol, which will indicate the instruments to be used in each dimension. This standardized protocol will allow the different centers studying patients with FTD in LAC to use a similar method of data collection. It will also allow the development of training and education processes for professionals through websites and free access to manuals and instruments that will have to be adapted and validated in different cultures.
A common methodology should be proposed regarding the adaptation, validation, and standardization of the evaluation instruments. Establishing a strategy is necessary for carrying out these studies among the different LAC countries, allowing the development of multicenter FTD data repositories. Finally, the support and financing of local and international initiatives should be sought out, along with the support and advice of different consensuses carried out in different parts of the world. This will help our local initiative to be carried out successfully. As seen in the international experience, the way to carry out these initiatives starts from formal entities that have sufficient funding to execute consensus regarding the evaluation of patients with dementia (261, 265). This same idea could be replicated in LAC, seeking entities or creating a consortium that can lead this process and establish a multidimensional clinical assessment in FTD and its spectrum. Initiatives, such as the Multi-Partner Consortium to Expand Dementia Research in Latin America (ReDLat), the Latin America and the Caribbean Consortium on Dementia (LAC-CD), or the United Kingdom–Brazil Dementia Workshop, could constitute the first step in this effort.
Our paper is the first joint initiative to establish a multidimensional clinical assessment for FTD and its spectrum in LAC. Our proposal provides valuable input to a future consortium and to the different LAC countries to adopt a uniform assessment method that considers the different local realities of each country.
The multidimensional assessment proposal, which arises from the published evidence and the recent experiences in FTD studies in LAC, allows the establishment of a preliminary standard assessment protocol for this region (see Figure 1). This protocol aims to assess the primary cognitive, functional, behavioral, and motor domains altered in FTD and its spectrum, which can be used to study patients with suspected or established diagnoses. The proposed protocol is broad enough to contribute to the clinical differentiation between FTD and other types of dementia. It could also help differentiate FTD from psychiatric pathologies.
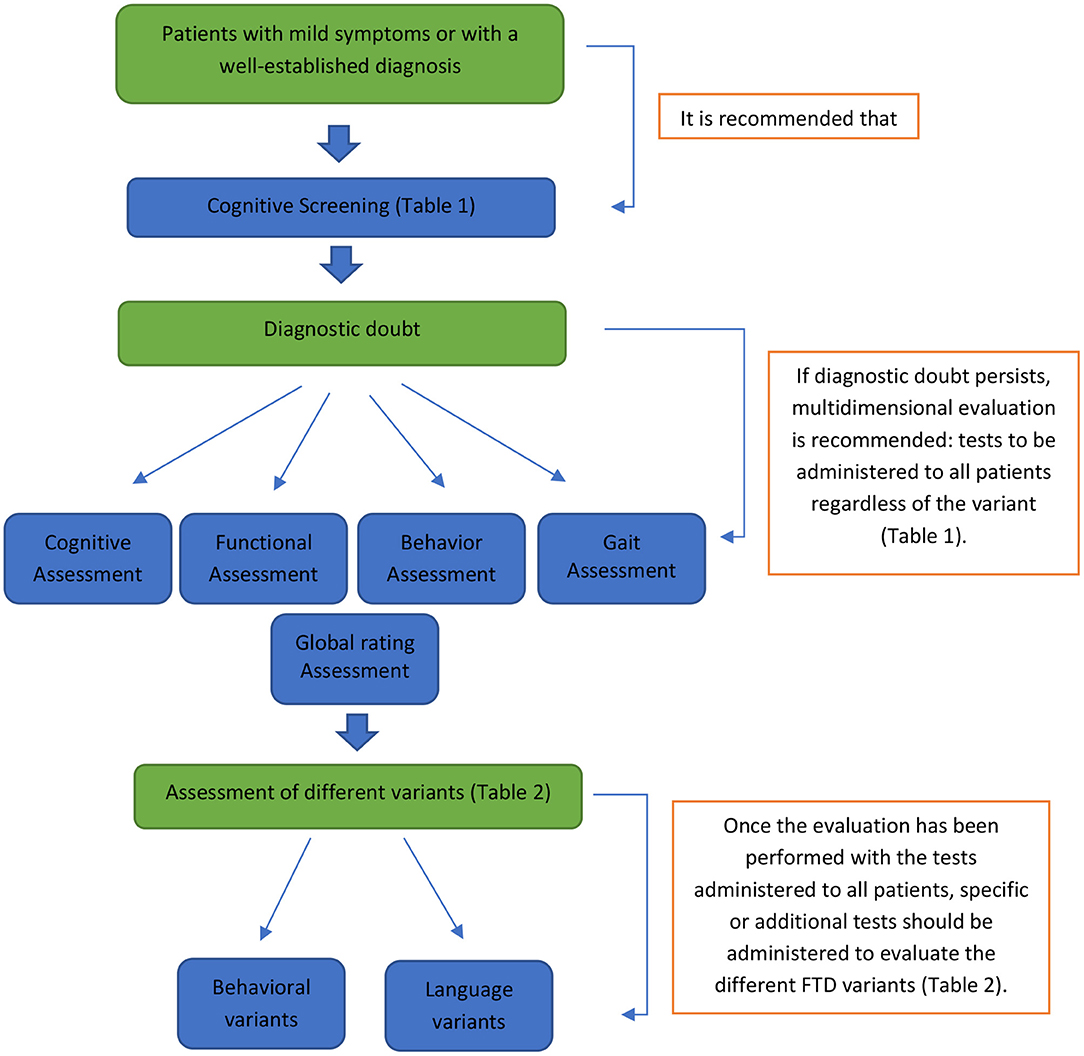
Figure 1. Preliminary standard assessment protocol. This protocol shows the different phases and evaluations to which each patient should be submitted according to the clinical characteristics presented. Suppose we are in the presence of a patient with mild symptoms or with a well-established diagnosis. In that case, it is advisable to evaluate with screening tools (see tools in Table 1). If there is diagnostic doubt, the patient should undergo a multidimensional evaluation (cognitive, functional, behavioral, and motor; see tools in Table 1). After this last step, the administration of additional assessment instruments associated with the specific variant of FTD studied is suggested (see tools in Table 2).
Although this work does not provide information on the normative and psychometric data, or diagnostic utility of all the recommended instruments, it is a first compilation of the minimal and necessary tools for the assessment of FTD. Importantly, valid and reliable tools are recommended in the assessment and follow-up of patients with dementia according to the international evidence.
Patients with FTD and its spectrum face difficulties in access to diagnosis, thereby increasing the burden on patients and their caregivers (267). Therefore, promoting a consensual and multidimensional assessment of FTD and its spectrum through an LAC consortium with validated and reliable tools for the main clinical dimension of FTD, i.e., cognition, functional, behavioral, and motor, could contribute toward addressing diagnosis barriers. The implementation of a multidimensional assessment requires the joint effort of an interdisciplinary team involving physicians, neuropsychologists, occupational therapists, speech therapists, kinesiologists, among others, working to foster both research and sharing of clinical practices. A consortium that brings together an interdisciplinary group represents the best strategy to create the knowledge necessary to facilitate access to diagnosis for patients with FTD in LAC, and to become a more equitable community with better capabilities when facing FTD and its spectrum. Finally, a similar effort is much more needed for dementia in general and its different types, for which we also lack a common approach in LAC.
FH and AS designed the proposal. FH, VC, SB, LS, PL, DM-P, LO, TT, and AS wrote the drafts and discussed contributions from all co-authors. All authors participated in discussing the contents of the paper, contributed to editing, and approved the final version of the article.
AS, PL, and DM-P were supported by grants from ANID/FONDAP/15150012. FH, VC, DM-P, LO, and AS were supported by ANID/FONDEF/ID 18I10113. FH, VC, and AS were supported by ANID/Fondecyt/1191726, 1210176, and 1210195 and MULTI-PARTNER CONSORTIUM TO EXPAND DEMENTIA RESEARCH IN LATIN AMERICA [ReDLat, supported by National Institutes of Health, National Institutes of Aging (R01 AG057234), Alzheimer's Association (SG-20-725707), Tau Consortium, and Global Brain Health Institute], and Alzheimer's Association GBHI ALZ UK-20-639295. FH was supported by grants from ANID-Subdirección de Capital Humano/Doctorado Nacional/2021- 21211349. LS was supported by the Brazilian National Council for Scientific and Technological Development (CNPq—bolsa de produtividade em pesquisa).
The contents of this publication are solely the responsibility of the authors and do not represent the official views of these institutions.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Bang J, Spina S, Miller BL. Frontotemporal dementia. Lancet. (2015) 386:1672–82. doi: 10.1016/S0140-6736(15)00461-4
2. Olney NT, Spina S, Miller BL. Frontotemporal dementia. Neurol Clin. (2017) 35:339–74. doi: 10.1016/j.ncl.2017.01.008
3. Deleon J, Miller BL. Frontotemporal dementia. Handb Clin Neurol. (2018) 148:409–30. doi: 10.1016/B978-0-444-64076-5.00027-2
4. Hodges JR. Frontotemporal dementia (Pick's disease): clinical features and assessment. Neurology. (2001) 56:S6–10. doi: 10.1212/WNL.56.suppl_4.S6
5. Seelaar H, Rohrer JD, Pijnenburg YAL, Fox NC, van Swieten JC. Clinical, genetic and pathological heterogeneity of frontotemporal dementia: a review. J Neurol Neurosurg Psychiatr. (2011) 82:476–86. doi: 10.1136/jnnp.2010.212225
6. Gorno-Tempini ML, Hillis AE, Weintraub S, Kertesz A, Mendez M, Cappa SF, et al. Classification of primary progressive aphasia and its variants. Neurology. (2011) 76:1006–14. doi: 10.1212/WNL.0b013e31821103e6
7. Mesulam M-M, Coventry C, Bigio EH, Geula C, Thompson C, Bonakdarpour B, et al. Nosology of primary progressive aphasia and the neuropathology of language. Adv Exp Med Biol. (2021) 1281:33–49. doi: 10.1007/978-3-030-51140-1_3
8. Warren JD, Rohrer JD, Rossor MN. Frontotemporal dementia. BMJ. (2013) 347:4827. doi: 10.1136/bmj.f4827
9. Baizabal-Carvallo JF, Jankovic J. Parkinsonism, movement disorders and genetics in frontotemporal dementia. Nat Rev Neurol. (2016) 12:175–85. doi: 10.1038/nrneurol.2016.14
10. Burrell JR, Hodges JR, Rowe JB. Cognition in corticobasal syndrome and progressive supranuclear palsy: a review. Mov Disord. (2014) 29:684–93. doi: 10.1002/mds.25872
11. Hodges JR, Davies R, Xuereb J, Kril J, Halliday G. Survival in frontotemporal dementia. Neurology. (2003) 61:349–54. doi: 10.1212/01.WNL.0000078928.20107.52
12. Knopman DS, Roberts RO. Estimating the number of persons with frontotemporal lobar degeneration in the US population. J Mol Neurosci. (2011) 45:330–5. doi: 10.1007/s12031-011-9538-y
13. Vieira RT, Caixeta L, Machado S, Silva AC, Nardi AE, Arias-Carrión O, et al. Epidemiology of early-onset dementia: a review of the literature. Clin Practice Epidemiol Mental Health. (2013) 9:88–95. doi: 10.2174/1745017901309010088
14. Custodio N, Herrera-Perez E, Lira D, Montesinos R, Bendezu L. Prevalence of frontotemporal dementia in community-based studies in Latin America: a systematic review. Dement Neuropsychol. (2013) 7:27–32. doi: 10.1590/S1980-57642013DN70100005
15. Bahia VS. Underdiagnosis of frontotemporal lobar degeneration in Brazil. Dement Neuropsychol. (2007) 1:361–5. doi: 10.1590/S1980-57642008DN10400006
16. Ferrer Soler C, Giatrakou V, Papa S, Scheffler M, Frisoni GB. Démences frontotemporales: mise à jour [Frontotemporal dementia : an update]. Rev Med Suisse. (2017) 13:1917–23.
17. Mioshi E, Kipps CM, Dawson K, Mitchell J, Graham A, Hodges JR. Activities of daily living in frontotemporal dementia and Alzheimer disease. Neurology. (2007) 68:2077–84. doi: 10.1212/01.wnl.0000264897.13722.53
18. Mioshi E, Hodges JR, Hornberger M. Neural correlates of activities of daily living in frontotemporal dementia. J Geriatr Psychiatry Neurol. (2013) 26:51–7. doi: 10.1177/0891988713477474
19. Gleichgerrcht E, Camino J, Roca M, Torralva T, Manes F. Assessment of functional impairment in dementia with the spanish version of the activities of daily living questionnaire. Dement Geriatr Cogn Disord. (2009) 28:380–8. doi: 10.1159/000254495
20. Yassuda MS, Lima da Silva TB, O'Connor CM, Mekala S, Alladi S, Bahia VS, et al. Apathy and functional disability in behavioral variant frontotemporal dementia. Neurol Clin Practice. (2018) 8:120–8. doi: 10.1212/CPJ.0000000000000429
21. Arvanitakis Z, Shah RC, Bennett DA. Diagnosis and management of dementia: review. J Am Med Assoc. (2019) 322:1589–99. doi: 10.1001/jama.2019.4782
22. Carnero-Pardo C. Es hora de jubilar al Mini-Mental? Neurología. (2014) 29:473–81. doi: 10.1016/j.nrl.2013.07.003
23. Custodio N, Duque L, Montesinos R, Alva-Diaz C, Mellado M, Slachevsky A. Systematic review of the diagnostic validity of brief cognitive screenings for early dementia detection in spanish-speaking adults in Latin America. Front Aging Neurosci. (2020) 12:270. doi: 10.3389/fnagi.2020.00270
24. Harvey P. Clinical applications of neuropsychological assessment. Dialogues Clin Neurosci. (2012) 14:91–9. doi: 10.31887/DCNS.2012.14.1/pharvey
25. Custodio N, Becerra-Becerra Y, Cruzado L, Castro-Suárez S, Montesinos R, Bardales Y, et al. Nivel de conocimientos sobre demencia frontotemporal en una muestra de médicos que evalúan regularmente a pacientes con demencia en Lima-Perú. Revista Chilena de Neuro-Psiquiatría. (2018) 56:77–88. doi: 10.4067/s0717-92272018000200077
26. Dua T, Barbui C, Clark N, Fleischmann A, Poznyak V, van Ommeren M, et al. Evidence-based guidelines for mental, neurological, and substance use disorders in low- and middle-income countries: summary of WHO recommendations. PLoS Med. (2011) 8:e1001122. doi: 10.1371/journal.pmed.1001122
27. Neary D, Snowden JS, Gustafson L, Passant U, Stuss D, Black S, et al. Frontotemporal lobar degeneration: a consensus on clinical diagnostic criteria. Neurology. (1998) 51:1546–54. doi: 10.1212/WNL.51.6.1546
28. Hornberger M, Piguet O, Kipps C, Hodges JR. Executive function in progressive and nonprogressive behavioral variant frontotemporal dementia. Neurology. (2008) 71:1481–8. doi: 10.1212/01.wnl.0000334299.72023.c8
29. Kipps CM, Mioshi E, Hodges JR. Emotion, social functioning and activities of daily living in frontotemporal dementia. Neurocase. (2009) 15:182–9. doi: 10.1080/13554790802632892
30. Mioshi E, Hsieh S, Savage S, Hornberger M, Hodges JR. Clinical staging and disease progression in frontotemporal dementia. Neurology. (2010) 74:1591–7. doi: 10.1212/WNL.0b013e3181e04070
31. McKhann GM, Albert M, Grossman M, Miller B, Dickson D, Trojanowski JQ, et al. Clinical and pathological diagnosis of frontotemporal dementia: report of the work group on Frontotemporal Dementia and pick's Disease. Archiv Neurol. (2001) 58:1803–9. doi: 10.1001/archneur.58.11.1803
32. Leroy M., Bertoux M, Skrobala E, Mode E, Adnet-Bonte C, le Ber I, et al. (2021). Characteristics and progression of patients with frontotemporal dementia in a regional memory clinic network. Alzheimer's Research & Therapy 13, 19. doi: 10.1186/s13195-020-00753-9
33. Guimarães HC, Vale TC, Pimentel V, de Sá NC, Beato RG, Caramelli P. Analysis of a case series of behavioral variant frontotemporal dementia: emphasis on diagnostic delay. Dement Neuropsychol. (2013) 7:55–9. doi: 10.1590/S1980-57642013DN70100009
34. Rosness TA, Engedal K, Chemali Z. Frontotemporal dementia. J Geriatr Psychiatry Neurol. (2016) 29:271–80. doi: 10.1177/0891988716654986
35. Ossenkoppele R, Schonhaut DR, Schöll M, Lockhart SN, Ayakta N, Baker SL, et al. Tau PET patterns mirror clinical and neuroanatomical variability in Alzheimer's disease. Brain. (2016) 139:1551–67. doi: 10.1093/brain/aww027
36. Ducharme S, Dols A, Laforce R, Devenney E, Kumfor F, van den Stock J, et al. Recommendations to distinguish behavioural variant frontotemporal dementia from psychiatric disorders. Brain. (2020) 143:1632–50. doi: 10.1093/brain/awaa018
37. Pose M, Cetkovich M, Gleichgerrcht E, Ibáñez A, Torralva T, Manes F. The overlap of symptomatic dimensions between frontotemporal dementia and several psychiatric disorders that appear in late adulthood. Int Rev Psychiatr. (2013) 25:159–67. doi: 10.3109/09540261.2013.769939
38. Rosness TA, Haugen PK, Passant U, Engedal K. Frontotemporal dementia: a clinically complex diagnosis. Int J Geriatr Psychiatry. (2008) 23:837–42. doi: 10.1002/gps.1992
39. Woolley JD, Khan BK, Murthy NK, Miller BL, Rankin KP. The Diagnostic Challenge of Psychiatric Symptoms in Neurodegenerative Disease: rates of and risk factors for prior psychiatric diagnosis in patients with early neurodegenerative disease. J Clin Psychiatry. (2011) 72:126–33. doi: 10.4088/JCP.10m06382oli
40. Gleichgerrcht E, Flichtentrei D, Manes F. How much do physicians in Latin America know about behavioral variant frontotemporal dementia? J Mol Neurosci. (2011) 45:609–17. doi: 10.1007/s12031-011-9556-9
41. Olavarría L, Mardones C, Delgado C, Slachevsky Ch A. Percepción de conocimiento sobre las demencias en profesionales de la salud de Chile. Revista Médica de Chile. (2016) 144:1365–8. doi: 10.4067/S0034-98872016001000019
42. Custodio N, Wheelock A, Thumala D, Slachevsky A. Dementia in Latin America: epidemiological evidence and implications for public policy. Front Aging Neurosci. (2017) 9:221. doi: 10.3389/fnagi.2017.00221
43. Parra MA, Baez S, Allegri R, Nitrini R, Lopera F, Slachevsky A, et al. Dementia in Latin America: assessing the present and envisioning the future. Neurology. (2018) 90:222–31. doi: 10.1212/WNL.0000000000004897
44. Zucchella C, Federico A, Martini A, Tinazzi M, Bartolo M, Tamburin S. Neuropsychological testing. Pract Neurol. (2018) 18:227–37. doi: 10.1136/practneurol-2017-001743
45. Folstein MF, Folstein SE, McHugh PR. Mini-mental state. J Psychiatr Res. (1975) 12:189–98. doi: 10.1016/0022-3956(75)90026-6
46. Nasreddine ZS, Phillips NA, Bãdirian V, Charbonneau S, Whitehead V, Collin I, et al. The montreal cognitive assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soci. (2005) 53:695–9. doi: 10.1111/j.1532-5415.2005.53221.x
47. Hsieh S, Schubert S, Hoon C, Mioshi E, Hodges JR. Validation of the Addenbrooke's cognitive examination III in frontotemporal dementia and Alzheimer's disease. Dement Geriatr Cogn Disord. (2013) 36:242–50. doi: 10.1159/000351671
48. Pfeffer RI, Kurosaki TT, Harrah CH, Chance JM, Filos S. Measurement of functional activities in older adults in the community. J Gerontol. (1982) 37:323–9. doi: 10.1093/geronj/37.3.323
49. Dubois B, Slachevsky A, Litvan I, Pillon B. The FAB: a frontal assessment battery at bedside. Neurology. (2000) 55:1621–6. doi: 10.1212/WNL.55.11.1621
50. Torralva T, Roca M, Gleichgerrcht E, López P, Manes F. INECO Frontal Screening (IFS): a brief, sensitive, and specific tool to assess executive functions in dementia–CORRECTED VERSION. J Int Neuropsychol Soc. (2009) 15:777–86. doi: 10.1017/S1355617709990415
51. Beato RG, Nitrini R, Formigoni AP, Caramelli P. Brazilian version of the Frontal Assessment Battery (FAB): Preliminary data on administration to healthy elderly. Dement Neuropsychol. (2007) 1:59–65. doi: 10.1590/S1980-57642008DN10100010
52. Torralva T, Roca M, Gleichgerrcht E, Bekinschtein T, Manes F. A neuropsychological battery to detect specific executive and social cognitive impairments in early frontotemporal dementia. Brain. (2009) 132:1299–309. doi: 10.1093/brain/awp041
53. Moura MVB, Mariano LI, Teixeira AL, Caramelli P, de Souza LC. Social cognition tests can discriminate behavioral variant frontotemporal dementia from Alzheimer's disease independently of executive functioning. Archiv Clin Neuropsychol. (2021) 36:831–7. doi: 10.1093/arclin/acaa084
54. Cummings JL, Mega M, Gray K, Rosenberg-Thompson S, Carusi DA, Gornbein J. The neuropsychiatric inventory: comprehensive assessment of psychopathology in dementia. Neurology. (1994) 44:2308–14. doi: 10.1212/WNL.44.12.2308
55. Bahia VS, Viana R. Accuracy of neuropsychological tests and the Neuropsychiatric Inventory in differential diagnosis between Frontotemporal dementia and Alzheimer's disease. Dement Neuropsychol. (2009) 3:332–6. doi: 10.1590/S1980-57642009DN30400012
56. Santacruz Escudero JM, Beltrán J, Palacios Á, Chimbí CM, Matallana D, Reyes P, et al. Neuropsychiatric symptoms as predictors of clinical course in neurodegeneration. A longitudinal study. Front Aging Neurosci. (2019) 11:176. doi: 10.3389/fnagi.2019.00176
57. Torralva T, Roca M, Gleichgerrcht E, Bonifacio A, Raimondi C, Manes F. Validación de la versión en español del Addenbrooke's Cognitive Examination-Revisado (ACE-R). Neurología. (2011) 26:351–6. doi: 10.1016/j.nrl.2010.10.013
58. Amaral-Carvalho V, Caramelli P. Normative data for healthy middle-aged and elderly performance on the addenbrooke cognitive examination-revised. Cogn Behav Neurol. (2012) 25:72–6. doi: 10.1097/WNN.0b013e318259594b
59. Muñoz-Neira C, Henríquez ChF, Ihnen JJ, Sánchez CM, Flores MP, Slachevsky A. Propiedades psicométricas y utilidad diagnóstica del Addenbrooke's Cognitive Examination-Revised (ACE-R) en una muestra de ancianos chilenos. Revista Médica de Chile. (2012) 140:1006–13. doi: 10.4067/S0034-98872012000800006
60. Bruno D, Slachevsky A, Fiorentino N, Rueda DS, Bruno G, Tagle AR, et al. Validación argentino-chilena de la versión en español del test Addenbrooke's Cognitive Examination III para el diagnóstico de demencia. Neurología. (2020) 35:82–8. doi: 10.1016/j.nrl.2017.06.004
61. Ibañez A, Fittipaldi S, Trujillo C, Jaramillo T, Torres A, Cardona JF, et al. Predicting and characterizing neurodegenerative subtypes with multimodal neurocognitive signatures of social and cognitive processes. J Alzheimer's Dis. (2021) 83:227–48. doi: 10.3233/JAD-210163
62. Custodio N, Herrera-Perez E, Lira D, Roca M, Manes F, Báez S, et al. Evaluation of the INECO Frontal Screening and the Frontal Assessment Battery in Peruvian patients with Alzheimer's disease and behavioral variant Frontotemporal dementia. ENeurologicalSci. (2016) 5:25–9. doi: 10.1016/j.ensci.2016.11.001
63. Gleichgerrcht E, Roca M, Manes F, Torralva T. Comparing the clinical usefulness of the Institute of Cognitive Neurology (INECO) Frontal Screening (IFS) and the Frontal Assessment Battery (FAB) in frontotemporal dementia. J Clin Exp Neuropsychol. (2011) 33:997–1004. doi: 10.1080/13803395.2011.589375
64. Bahia VS, Cecchini MA, Cassimiro L, Viana R, Lima-Silva TB, de Souza LC, et al. The accuracy of INECO frontal screening in the diagnosis of executive dysfunction in frontotemporal dementia and Alzheimer disease. Alzheimer Dis Assoc Disord. (2018) 32:314–9. doi: 10.1097/WAD.0000000000000255
65. Ihnen J, Antivilo A, Muñoz-Neira C, Slachevsky A. Chilean version of the INECO Frontal Screening (IFS-Ch): psychometric properties and diagnostic accuracy. Dement Neuropsychol. (2013) 7:40–7. doi: 10.1590/S1980-57642013DN70100007
66. Kertesz A, Jesso S, Harciarek M, Blair M, McMonagle P. What Is Semantic Dementia?: a cohort study of diagnostic features and clinical boundaries. Archiv Neurol. (2010) 67:483–9. doi: 10.1001/archneurol.2010.55
67. Rascovsky K, Hodges JR, Knopman D, Mendez MF, Kramer JH, Neuhaus J, et al. Sensitivity of revised diagnostic criteria for the behavioural variant of frontotemporal dementia. Brain. (2011) 134:2456–77. doi: 10.1093/brain/awr179
68. Poos JM, Jiskoot LC, Papma JM, van Swieten JC, van den Berg E. Meta-analytic review of memory impairment in behavioral variant frontotemporal dementia. J Int Neuropsychol Soc. (2018) 24:593–605. doi: 10.1017/S1355617718000115
69. Pennington C, Hodges JR, Hornberger M. Neural correlates of episodic memory in behavioral variant frontotemporal dementia. J Alzheimer's Dis. (2011) 24:261–8. doi: 10.3233/JAD-2011-101668
70. Simons JS, Verfaellie M, Galton CJ, Miller BL, Hodges JR, Graham KS. Recollection-based memory in frontotemporal dementia: implications for theories of long-term memory. Brain. (2002) 125:2523–36. doi: 10.1093/brain/awf247
71. Galton CJ, Gomez-Anson B, Antoun N, Scheltens P, Patterson K, Graves M, et al. Temporal lobe rating scale: application to Alzheimer's disease and frontotemporal dementia. J Neurol Neurosurg Psychiatr. (2001) 70:165–73. doi: 10.1136/jnnp.70.2.165
72. Kramer JH, Jurik J, Sha SJ, Rankin KP, Rosen HJ, Johnson JK, et al. Distinctive neuropsychological patterns in frontotemporal dementia, semantic dementia, and Alzheimer disease. Cogn Behav Neurol. (2003) 16:211–8. doi: 10.1097/00146965-200312000-00002
73. Pasquier F, Grymonprez L, Lebert F, van der Linden M. Memory impairment differs in frontotemporal dementia and Alzhemier's disease. Neurocase. (2001) 7:161–71. doi: 10.1093/neucas/7.2.161
74. Perry RJ, Hodges JR. Differentiating frontal and temporal variant frontotemporal dementia from Alzheimer's disease. Neurology. (2000) 54:2277–84. doi: 10.1212/WNL.54.12.2277
75. Hutchinson AD, Mathias JL. Neuropsychological deficits in frontotemporal dementia and Alzheimer's disease: a meta-analytic review. J Neurol Neurosurg Psychiatry. (2007) 78:917–28. doi: 10.1136/jnnp.2006.100669
76. Bertoux M, Ramanan S, Slachevsky A, Wong S, Henriquez F, Musa G, et al. So close yet so far: executive contribution to memory processing in behavioral variant frontotemporal dementia. J Alzheimer's Dis. (2016) 54:1005–14. doi: 10.3233/JAD-160522
77. Hornberger M, Piguet O, Graham AJ, Nestor PJ, Hodges JR. How preserved is episodic memory in behavioral variant frontotemporal dementia? Neurology. (2010) 74:472–9. doi: 10.1212/WNL.0b013e3181cef85d
78. Hornberger M, Piguet O. Episodic memory in frontotemporal dementia: a critical review. Brain. (2012) 135:678–92. doi: 10.1093/brain/aws011
79. Eikelboom WS, Janssen N, Jiskoot LC, van den Berg E, Roelofs A, Kessels RPC. Episodic and working memory function in Primary Progressive Aphasia: a meta-analysis. Neurosci Biobehav Rev. (2018) 92:243–54. doi: 10.1016/j.neubiorev.2018.06.015
80. Foxe D, Irish M, Roquet D, Scharfenberg A, Bradshaw N, Hodges JR, et al. Visuospatial short-term and working memory disturbance in the primary progressive aphasias: neuroanatomical and clinical implications. Cortex. (2020) 132:223–37. doi: 10.1016/j.cortex.2020.08.018
81. Piguet O, Leyton CE, Gleeson LD, Hoon C, Hodges JR. Memory and emotion processing performance contributes to the diagnosis of non-semantic primary progressive aphasia syndromes. J Alzheimer's Dis. (2015) 44:541–7. doi: 10.3233/JAD-141854
82. Torralva T, Martinez M, Manes F. Neuropsychological assessment of frontotemporal dementia. In Dickerson B, editors, Hodges' Frontotemporal Dementia. 2nd ed. Cambridge: Cambridge University Press (2016). p. 106–124. doi: 10.1017/CBO9781316091586.012
83. Rey A. L'examen psychologique dans les cas d'encéphalopathie traumatique. (Les problems) [The psychological examination in cases of traumatic encepholopathy Problems]. Archives de Psychologie. (1941) 28:215–85.
84. Benedict RHB, Schretlen D, Groninger L, Brandt J. Hopkins verbal learning test – revised: normative data and analysis of inter-form and test-retest reliability. Clin Neuropsychol. (1998) 12:43–55. doi: 10.1076/clin.12.1.43.1726
85. Delis DC, Freeland J, Kramer JH, Kaplan E. Integrating clinical assessment with cognitive neuroscience: construct validation of the California Verbal Learning Test. J Consult Clin Psychol. (1988) 56:123–30. doi: 10.1037/0022-006X.56.1.123
86. Blackwell AD, Sahakian BJ, Vesey R, Semple JM, Robbins TW, Hodges JR. Detecting dementia: novel neuropsychological markers of preclinical Alzheimer's disease. Dement Geriatr Cogn Disord. (2004) 17:42–8. doi: 10.1159/000074081
87. Grober E, Buschke H, Crystal H, Bang S, Dresner R. Screening for dementia by memory testing. Neurology. (1988) 38:900–3. doi: 10.1212/WNL.38.6.900
88. Kopelman MD, Wilson BA, Baddeley AD. The autobiographical memory interview: a new assessment of autobiographical and personal semantic memory in amnesic patients. J Clin Exp Neuropsychol. (1989) 11:724–44. doi: 10.1080/01688638908400928
89. Groot YCT, Wilson BA, Evans J, Watson P. Prospective memory functioning in people with and without brain injury. J Int Neuropsychol Soc. (2002) 8:645–54. doi: 10.1017/S1355617702801321
90. Porto CS, Bahia VS, Brucki SMD, Caramelli P, Nitrini R. Neuropsychological differences between frontotemporal lobar degeneration and Alzheimer's disease. Dement Neuropsychol. (2008) 2:223–7. doi: 10.1590/S1980-57642009DN20300011
91. Fichman HC, Dias LBT, Fernandes CS, Lourenço R, Caramelli P, Nitrini R. Normative data and construct validity of the Rey Auditory Verbal Learning Test in a Brazilian elderly population. Psychol Neurosci. (2010) 3:79–84. doi: 10.3922/j.psns.2010.1.010
92. Malloy-Diniz LF, Lasmar VAP, Gazinelli L, de SR, Fuentes D, Salgado JV. The rey auditory-verbal learning test: applicability for the Brazilian elderly population. Revista Brasileira de Psiquiatria. (2007) 29:324–9. doi: 10.1590/S1516-44462006005000053
93. Arango-Lasprilla JC, Rivera D, Garza MT, Saracho CP, Rodríguez W, Rodríguez-Agudelo Y, et al. Hopkins verbal learning test– revised: normative data for the Latin American Spanish speaking adult population. NeuroRehabilitation. (2015) 37:699–718. doi: 10.3233/NRE-151286
94. Delgado C, Muñoz-Neira C, Soto A, Martínez M, Henríquez F, Flores P, et al. Comparison of the psychometric properties of the “word” and “picture” versions of the free and cued selective reminding test in a Spanish-speaking cohort of patients with mild Alzheimer's disease and cognitively healthy controls. Archiv Clin Neuropsychol. (2016) 31:165–75. doi: 10.1093/arclin/acv107
95. Yassuda MS, Flaks MK, Viola LF, Pereira FS, Memória CM, Nunes PV, et al. Psychometric characteristics of the Rivermead Behavioural Memory Test (RBMT) as an early detection instrument for dementia and mild cognitive impairment in Brazil. Int Psychogeriatr. (2010) 22:1003–11. doi: 10.1017/S1041610210001055
96. Bolognani SAP, Miranda MC, Martins M, Rzezak P, Bueno OFA, Camargo CHP, et al. Development of alternative versions of the Logical Memory subtest of the WMS-R for use in Brazil. Demen Neuropsychol. (2015) 9:136–48. doi: 10.1590/1980-57642015DN92000008
97. Leis A, Allegri R, Roman F, Iturry M, Crotti B, Gatto E, et al. Datos normativos de la versión argentina de la batería de eficacia mnésica Signoret (BEM 144) para ser aplicados en la evaluación neurocognitiva. Neurología Argentina. (2018) 10:127–36. doi: 10.1016/j.neuarg.2018.04.002
98. Cecchini MA, Yassuda MS, Bahia VS, de Souza LC, Guimarães HC, Caramelli P, et al. Recalling feature bindings differentiates Alzheimer's disease from frontotemporal dementia. J Neurol. (2017) 264:2162–9. doi: 10.1007/s00415-017-8614-9
99. Nitrini R, Caramelli P, Herrera E, Porto CS, Charchat-Fichman H, Carthery M, et al. Performance of illiterate and literate nondemented elderly subjects in two tests of long-term memory. J Int Neuropsychol Soc. (2004) 10:634–8. doi: 10.1017/S1355617704104062
100. Resende E, de PF, Hornberger M, Guimarães HC, Gambogi LB, Mariano LI, et al. Different patterns of gray matter atrophy in behavioral variant frontotemporal dementia with and without episodic memory impairment. Int J Geriatr Psychiatr. (2021) 36:1848–57. doi: 10.1002/gps.5503
101. Musa G, Slachevsky A, Muñoz-Neira C, Méndez-Orellana C, Villagra R, González-Billault C, et al. Alzheimer's disease or behavioral variant frontotemporal dementia? review of key points toward an accurate clinical and neuropsychological diagnosis. J Alzheimer's Dis. (2020) 73:833–48. doi: 10.3233/JAD-190924
102. Geser F, Wenning GK, Poewe W, McKeith I. How to diagnose dementia with Lewy bodies: state of the art. Mov Disord. (2005) 20:S11–20. doi: 10.1002/mds.20535
103. Salimi S, Irish M, Foxe D, Hodges JR, Piguet O, Burrell JR. Visuospatial dysfunction in Alzheimer's disease and behavioural variant frontotemporal dementia. J Neurol Sci. (2019) 402:74–80. doi: 10.1016/j.jns.2019.04.019
104. Warrington E, James M. The Visual Object and Space Perception Battery (Bury St. Edmunds, Ed.). London: Thames Valley Test Company (1991).
105. Pal A, Biswas A, Pandit A, Roy A, Guin D, Gangopadhyay G, et al. Study of visuospatial skill in patients with dementia. Ann Indian Acad Neurol. (2016) 19:83–8. doi: 10.4103/0972-2327.168636
106. Shulman KI. Clock-drawing: is it the ideal cognitive screening test? Int J Geriatr Psychiatry. (2000) 15:548–61. doi: 10.1002/1099-1166(200006)15:6<548::aid-gps242>3.0.co;2-u
107. Osterrieth P. Le test de copie d'une figure complexe; contribution à l'étude de la perception et de la mémoire. [Test of copying a complex figure; contribution to the study of perception and memory]. Archives de Psychologie. (1944) 30:206–356.
108. Grossi D, Fragassi NA, Chiacchio L, Valoroso L, Tuccillo R, Perrotta C, et al. Do visuospatial and constructional disturbances differentiate frontal variant of frontotemporal dementia and Alzheimer's disease? An experimental study of a clinical belief. Int J Geriatr Psychiatry. (2002) 17:641–8. doi: 10.1002/gps.654
109. Manna CG, Alterescu K, Borod JC, Bender HA. Benton visual retention test. In: Kreutzer JS, DeLuca J, Caplan B, editors, Encyclopedia of Clinical Neuropsychology. New York, NY: Springer (2011). doi: 10.1007/978-0-387-79948-3_1110
110. Salimi S, Irish M, Foxe D, Hodges JR, Piguet O, Burrell JR. Can visuospatial measures improve the diagnosis of Alzheimer's disease? Alzheimer's Dement Diagn Assess Dis Monitor. (2018) 10:66–74. doi: 10.1016/j.dadm.2017.10.004
111. Watson CL, Possin K, Allen IE, Hubbard HI, Meyer M, Welch AE, et al. Visuospatial functioning in the primary progressive aphasias. J Int Neuropsychol Soc. (2018) 24:259–68. doi: 10.1017/S1355617717000984
112. Budisavljevic S, Dell'Acqua F, Djordjilovic V, Miotto D, Motta R, Castiello U. The role of the frontal aslant tract and premotor connections in visually guided hand movements. NeuroImage. (2017) 146:419–28. doi: 10.1016/j.neuroimage.2016.10.051
113. Mandelli ML, Caverzasi E, Binney RJ, Henry ML, Lobach I, Block N, et al. Frontal white matter tracts sustaining speech production in primary progressive aphasia. J Neurosci. (2014) 34:9754–67. doi: 10.1523/JNEUROSCI.3464-13.2014
114. Fuzikawa C, Lima-Costa MF, Uchoa E, Barreto SM, Shulman K. A population based study on the intra and inter-rater reliability of the clock drawing test in Brazil: the Bambuí Health and Ageing Study. Int J Geriatr Psychiatry. (2003) 18:450–6. doi: 10.1002/gps.863
115. Custodio N, García A, Montesinos R, Lira D, Bendezú L. Validación de la prueba de dibujo del reloj - versión de Manos - como prueba de cribado para detectar demencia en una población adulta mayor de Lima, Perú. Revista Peruana de Medicina Experimental y Salud Pública. (2011) 28:29–34. doi: 10.1590/S1726-46342011000100005
116. Latini MF, Scharovsky D, Glaser A, Brugger R, Zorrilla JP, Sousa L, et al. El test del reloj: reproducibilidad, consistencia interna y variables predictivas de la prueba del reloj utilizando el método de puntuación de Cacho. Análisis de relojes Neurología Argentina. (2011) 3:83–7. doi: 10.1016/S1853-0028(11)70019-2
117. Aguilar-Navarro SG, Mimenza-Alvarado AJ, Samudio-Cruz MA, Hernández-Contreras FJ, Gutiérrez-Gutiérrez LA, Ramírez-González F, et al. Validation of the Clock Drawing Test Scoring Method in older adults with neurocognitive disorder. Salud Mental. (2018) 41:179–86. doi: 10.17711/SM.0185-3325.2018.026
118. Carvalho GA, Caramelli P. Normative data for middle-aged Brazilians in Verbal Fluency (animals and FAS), Trail Making Test (TMT) and Clock Drawing Test (CDT). Dement Neuropsychol. (2020) 14:14–23. doi: 10.1590/1980-57642020dn14-010003
119. Rivera D, Perrin PB, Morlett-Paredes A, Galarza-del-Angel J, Martínez C, Garza MT, et al. Rey–osterrieth complex figure – copy and immediate recall: normative data for the Latin American Spanish speaking adult population. NeuroRehabilitation. (2015) 37:677–98. doi: 10.3233/NRE-151285
120. Vicente SG, Ramos-Usuga D, Barbosa F, Gaspar N, Dores AR, Rivera D, et al. Regression-based norms for the hopkins verbal learning test-revised and the rey–osterrieth complex figure in a portuguese adult population. Archiv Clin Neuropsychol. (2021) 36:587–96. doi: 10.1093/arclin/acaa087
121. Quental NBM, Brucki SMD, Bueno OFA. Visuospatial function in early Alzheimer's disease—the use of the visual object and space perception (VOSP) battery. PLoS ONE. (2013) 8:e68398. doi: 10.1371/journal.pone.0068398
122. Harciarek M, Cosentino S. Language, executive function and social cognition in the diagnosis of frontotemporal dementia syndromes. Int Rev Psychiatr. (2013) 25:178–96. doi: 10.3109/09540261.2013.763340
123. Sawyer RP, Rodriguez-Porcel F, Hagen M, Shatz R, Espay AJ. Diagnosing the frontal variant of Alzheimer's disease: a clinician's yellow brick road. J Clin Mov Disord. (2017) 4:2. doi: 10.1186/s40734-017-0052-4
124. Hodges JR, Patterson K. Semantic dementia: a unique clinicopathological syndrome. Lancet Neurol. (2007) 6:1004–14. doi: 10.1016/S1474-4422(07)70266-1
125. Grossman M. Primary progressive aphasia: clinicopathological correlations. Nat Rev Neurol. (2010) 6:88–97. doi: 10.1038/nrneurol.2009.216
126. Goodglass H, Kaplan E. Evaluación de la Afasia y de Trastornos Similares (The Assessment of Aphasia and Related Disorders). Editorial Medica panamericana, Ed. 2nd ed. (1986).
127. Savage S, Hsieh S, Leslie F, Foxe D, Piguet O, Hodges JR. Distinguishing subtypes in primary progressive aphasia: application of the sydney language battery. Dement Geriatr Cogn Disord. (2013) 35:208–18. doi: 10.1159/000346389
128. Howard D, Patterson K. Pyramids and Palm Trees: A Test of Semantic Access From Pictures and Words (Bury St Edmunds, Ed. London: Thames Valley Test Company (1992).
129. Hodges JR, Martinos M, Woollams AM, Patterson K, Adlam A-LR. Repeat and point: differentiating semantic dementia from progressive non-fluent aphasia. Cortex. (2008) 44:1256–70. doi: 10.1016/j.cortex.2007.08.018
130. Rosselli M, Ardila A, Florez A, Castro C. Normative data on the boston diagnostic aphasia examination in a spanish-speaking population. J Clin Exp Neuropsychol. (1990) 12:313–22. doi: 10.1080/01688639008400977
131. Radanovic M, Mansur LL, Scaff M. Normative data for the Brazilian population in the Boston Diagnostic Aphasia Examination: influence of schooling. Brazil J Med Biol Res. (2004) 37:1731–8. doi: 10.1590/S0100-879X2004001100019
132. Machado TH, Fichman HC, Santos EL, Carvalho VA, Fialho PP, Koenig AM, et al. Normative data for healthy elderly on the phonemic verbal fluency task – FAS. Dement Neuropsychol. (2009) 3:55–60. doi: 10.1590/S1980-57642009DN30100011
133. Olabarrieta-Landa L, Rivera D, Galarza-del-Angel J, Garza M, Saracho C, Rodríguez W, et al. Verbal fluency tests: Normative data for the Latin American Spanish speaking adult population. NeuroRehabilitation. (2015) 37:515–61. doi: 10.3233/NRE-151279
134. Rivera D, Olabarrieta-Landa L, van der Elst W, Gonzalez I, Rodríguez-Agudelo Y, Aguayo Arelis A, et al. Normative data for verbal fluency in healthy Latin American adults: letter M, and fruits and occupations categories. Neuropsychology. (2019) 33:287–300. doi: 10.1037/neu0000518
135. Rodríguez-Lorenzana A, Benito-Sánchez I, Adana-Díaz L, Paz CP, Yacelga Ponce T, Rivera D, et al. Normative data for test of verbal fluency and naming on ecuadorian adult population. Front Psychol. (2020) 11:830. doi: 10.3389/fpsyg.2020.00830
136. Gómez PG, Politis DG. Correlaciones entre praxias y memoria de trabajo visual en pacientes con demencia frontotemporal variante frontal. I Congreso Internacional de Investigación y Práctica Profesional en Psicología XVI Jornadas de Investigación Quinto Encuentro de Investigadores en Psicología del MERCOSUR. Facultad de Psicología – Universidad de Buenos Aires, Buenos Aires. (2009).
137. Gómez PG, Politis DG. Severidad de la demencia y apraxia en demencia frontotemporal variante frontal. Neurología Argentina. (2011) 3:203–9. doi: 10.1016/j.neuarg.2011.06.008
138. Johnen A, Frommeyer J, Modes F, Wiendl H, Duning T, Lohmann H. Dementia Apraxia Test (DATE): a brief tool to differentiate behavioral variant frontotemporal dementia from Alzheimer's dementia based on apraxia profiles. J Alzheimer's Dis. (2015) 49:593–605. doi: 10.3233/JAD-150447
139. Cossini FC, Gomez PG, Rubinstein WY, Politis DG. Reconocimiento facial de emociones básicas y ejecución de gestos en demencia frontotemporal variante conductual. Neurología Argentina. (2016) 8:246–52. doi: 10.1016/j.neuarg.2016.04.005
140. Pérez Lancho MC, García Bercianos S. Caracterización del lenguaje en las variantes lingüísticas de la Afasia Progresiva Primaria. Revista Signos. (2020) 53:198–218. doi: 10.4067/S0718-09342020000100198
141. Leiguarda R. Limb apraxia: cortical or subcortical. Neuroimage. (2001) 14:S137–41. doi: 10.1006/nimg.2001.0833
142. Leiguarda R, Merello M, Balei J. Apraxia in corticobasal degeneration. Adv Neurol. (2000) 82:103–21.
143. Leiguarda RC, Merello M, Nouzeilles MI, Balej J, Rivero A, Nogués M. Limb-kinetic apraxia in corticobasal degeneration: clinical and kinematic features. Mov Disord. (2003) 18:49–59. doi: 10.1002/mds.10303
144. Cacho Gutiérrez LJ, García García R, Arcaya Navarro J, Vicente Villardón JL, Lantada Puebla N. Una propuesta de aplicación y puntuación del test del reloj en la enfermedad de Alzheimer. Revista de Neurología. (1999) 28:648–55. doi: 10.33588/rn.2807.98501
145. Wechsler D. The Measurement of Adult Intelligence. Baltimore, MD: William and Wilkins (1939). doi: 10.1037/10020-000
146. Gómez PG, Politis DG, Rubinstein W. Evaluación de apraxia en pacientes con demencia frontotemporal variante frontal y su vinculación con la cognición social. XV Jornadas de Investigación y Cuarto Encuentro de Investigadores en Psicología Del Mercosur. Buenos Aires: Facultad de Psicología – Universidad de Buenos Aires (2008).
147. Rascovsky K, Salmon DP, Hansen LA, Galasko D. Distinct cognitive profiles and rates of decline on the Mattis Dementia Rating Scale in autopsy-confirmed frontotemporal dementia and Alzheimer's disease. J Int Neuropsychol Soc. (2008) 14:373–83. doi: 10.1017/S135561770808051X
148. Burin D, Ramenzoni V, Arizaga R. Evaluación Neuropsicológica del Envejecimiento: Normas según Edad y Nivel Educacional. Revista Neurológica Argentina. (2003) 28:149–52.
149. Rosas R, Tenorio M, Pizarro M, Cumsille P, Bosch A, Arancibia S, et al. Estandarización de la Escala Wechsler de Inteligencia Para Adultos-Cuarta Edición en Chile. Psykhe. (2014) 23:1–18. doi: 10.7764/psykhe.23.1.529
150. Belaus A, Fernández L, Farias-Sarquis Y, Bueno A. Is the Mattis Dementia Rating Scale appropriate to detect mild cognitive impairment? Revista Chilena de Neuropsicología. (2015) 10:8–13. doi: 10.5839/rcnp.2015.10.01.03
151. Carvalho GA, Caramelli P. Normative data for middle-aged Brazilians in the Mattis Dementia Rating Scale. Dement Neuropsychol. (2020) 14:350–7. doi: 10.1590/1980-57642020dn14-040004
152. Foss MP, Carvalho VA, de, Machado TH, Reis GC, dos, Tumas V, Caramelli P, et al. Mattis Dementia Rating Scale (DRS): normative data for the Brazilian middle-age and elderly populations. Dement Neuropsychol. (2013) 7:374–9. doi: 10.1590/S1980-57642013DN74000004
153. Politis D, Margulis L. Evaluación de las praxias a partir de un modelo cognitivo Neuropsychologia Latina; 3: pp 92 Resumen presentado en V Congreso Latinoamericano de Neuropsicología, Guadalajara, Jalisco Mexico, 4 al 7 de octubre de 1997. (1997).
154. Gilbert SJ, Burgess PW. Executive function. Curr Biol. (2008) 18:R110–4. doi: 10.1016/j.cub.2007.12.014
155. Lezak MD. The problem of assessing executive functions. Int J Psychol. (1982) 17:281–97. doi: 10.1080/00207598208247445
156. Roca M, Manes F, Gleichgerrcht E, Watson P, Ibáñez A, Thompson R, et al. Intelligence and executive functions in frontotemporal dementia. Neuropsychologia. (2013) 51:725–30. doi: 10.1016/j.neuropsychologia.2013.01.008
157. Fiorentino N, Gleichgerrcht E, Roca M, Cetkovich M, Manes F, Torralva T. The INECO Frontal Screening tool differentiates behavioral variant - frontotemporal dementia (bv-FTD) from major depression. Dement Neuropsychol. (2013) 7:33–9. doi: 10.1590/S1980-57642013DN70100006
158. Reitan RM. Validity of the trail making test as an indicator of organic brain damage. Percept Mot Skills. (1958) 8:271–6. doi: 10.2466/pms.1958.8.3.271
159. Axelrod BN, Goldman RS, Heaton RK, Curtiss G, Thompson LL, Chelune GJ, et al. Discriminability of the Wisconsin card sorting test using the standardization sample. J Clin Exp Neuropsychol. (1996) 18:338–42. doi: 10.1080/01688639608408991
160. Golden CJ. The measurement of creativity by the stroop color and word test. J Pers Assess. (1975) 39:502–6. doi: 10.1207/s15327752jpa3905_9
161. Shallice T, Burgess P. The domain of supervisory processes and temporal organization of behaviour. Philos Trans Royal Soc Lond Ser B Biol Sci. (1996) 351:1405–12. doi: 10.1098/rstb.1996.0124
162. Shallice T. Specific impairments of planning. Philos Trans Royal Soc Lond B Biol Sci. (1982) 298:199–209. doi: 10.1098/rstb.1982.0082
163. Shallice T. From Neuropsychology to Mental Structure. Cambridge: Cambridge University Press (1988). doi: 10.1017/CBO9780511526817
164. Cohen NJ, Eichenbaum H, Deacedo BS, Corkin S. Different memory systems underlying acquisition of procedural and declarative knowledge. Ann N Y Acad Sci. (1985) 444:54–71. doi: 10.1111/j.1749-6632.1985.tb37579.x
167. Bechara A. Iowa Gambling Task Professional Manual. Lutz, FL: Psychological Assessment Resources, Inc. (2007).
168. Manly T, Hawkins K, Evans J, Woldt K, Robertson IH. Rehabilitation of executive function: facilitation of effective goal management on complex tasks using periodic auditory alerts. Neuropsychologia. (2002) 40:271–81. doi: 10.1016/S0028-3932(01)00094-X
169. Wilson BA, Evans JJ, Emslie H, Alderman N, Burgess P. The development of an ecologically valid test for assessing patients with a dysexecutive syndrome. Neuropsychol Rehabil. (1998) 8:213–28. doi: 10.1080/713755570
170. Fernández AL, Marino JC, Alderete AM. Estandarización y validez conceptual del test del trazo en una muestra de adultos argentinos. Revista Neurológica Argentina. (2002) 27:83–8.
171. Arango-Lasprilla JC, Rivera D, Aguayo A, Rodríguez W, Garza MT, Saracho CP, et al. Trail making test: normative data for the Latin American Spanish speaking adult population. NeuroRehabilitation. (2015) 37:639–61. doi: 10.3233/NRE-151284
172. Arango-Lasprilla JC, Rivera D, Longoni M, Saracho CP, Garza MT, Aliaga A, et al. Modified Wisconsin Card Sorting Test (M-WCST): normative data for the Latin American Spanish speaking adult population. NeuroRehabilitation. (2015) 37:563–90. doi: 10.3233/NRE-151280
173. Zimmermann N, Cardoso C, de O, Trentini CM, Grassi-Oliveira R, Fonseca RP. Brazilian preliminary norms and investigation of age and education effects on the Modified Wisconsin Card Sorting Test, Stroop Color and Word test and Digit Span test in adults. Dement Neuropsychol. (2015) 9:120–7. doi: 10.1590/1980-57642015DN92000006
174. Rivera D, Perrin PB, Stevens LF, Garza MT, Weil C, Saracho CP, et al. Stroop Color-Word Interference Test: normative data for the Latin American Spanish speaking adult population. NeuroRehabilitation. (2015) 37:591–624. doi: 10.3233/NRE-151281
175. Abusamra V, Miranda MA, Ferreres A. Evaluación de la iniciación e inhibición verbal en español. Adaptación y normas del test de Hayling. Revista Argentina de Neuropsicología. (2007) 27:463–6.
176. Zimmermann N, Cardoso C, de O, Kristensen CH, Fonseca RP. Brazilian norms and effects of age and education on the Hayling and Trail Making Tests. Trends Psychiatr Psychother. (2017) 39:188–95. doi: 10.1590/2237-6089-2016-0082
177. Querejeta AN, Crostelli A, Stecco J, Moreno M, Farias Y, Sabena C, et al. Adaptación Argentina de la Behavioural Assessment of Dysexecutive Syndrome (BADS). Revista Neuropsicologia Latinoamericana. (2015) 7:45–56. doi: 10.5579/rnl.2015.0241
178. Cardoso C, de O, Zimmermann N, Paraná CB, Gindri G, Pereira APA de, Fonseca RP. Brazilian adaptation of the Hotel Task: a tool for the ecological assessment of executive functions. Dement Neuropsychol. (2015) 9:156–64. doi: 10.1590/1980-57642015DN92000010
179. Allegri RF, Harris P, Feldman M, Taragano F, Paz J. Perfiles cognitivos diferenciales entre la demencia frontotemporal y la demencia tipo Alzheimer. Rev Neurol. (1998) 27:463–6. doi: 10.33588/rn.27157.98206
180. Barutta J, Hodges J, Ibáñez A, Gleichgerrcht E, Manes F. Argentina's early contributions to the understanding of frontotemporal lobar degeneration. Cortex. (2011) 47:621–7. doi: 10.1016/j.cortex.2010.05.006
181. Ibáñez A. Brain oscillations, inhibition and social inappropriateness in frontotemporal degeneration. Brain. (2018) 141:e73. doi: 10.1093/brain/awy233
182. Baron-Cohen S, Golan O, Chakrabarti B, Belmonte MK. Social cognition and autism spectrum conditions. In: Sharp C, Fonagy P, Goodyer I, editors, Social Cognition and Developmental Psychopathology. Oxford: Oxford University Press. (2008). p. 29–56. doi: 10.1093/med/9780198569183.003.0002
183. Gleichgerrcht E, Torralva T, Roca M, Pose M, Manes F. The role of social cognition in moral judgment in frontotemporal dementia. Soc Neurosci. (2011) 6:113–22. doi: 10.1080/17470919.2010.506751
184. Baez S, Manes F, Huepe D, Torralva T, Fiorentino N, Richter F, et al. Primary empathy deficits in frontotemporal dementia. Front Aging Neurosci. (2014) 6:262. doi: 10.3389/fnagi.2014.00262
185. Baez S, Morales JP, Slachevsky A, Torralva T, Matus C, Manes F, et al. Orbitofrontal and limbic signatures of empathic concern and intentional harm in the behavioral variant frontotemporal dementia. Cortex. (2016) 75:20–32. doi: 10.1016/j.cortex.2015.11.007
186. Melloni M, Lopez V, Ibanez A. Empathy and contextual social cognition. Cogn Affect Behav Neurosci. (2014) 14:407–25. doi: 10.3758/s13415-013-0205-3
187. Baez S, Pinasco C, Roca M, Ferrari J, Couto B, García-Cordero I, et al. Brain structural correlates of executive and social cognition profiles in behavioral variant frontotemporal dementia and elderly bipolar disorder. Neuropsychologia. (2019) 126:159–69. doi: 10.1016/j.neuropsychologia.2017.02.012
188. Baez S, Kanske P, Matallana D, Montañes P, Reyes P, Slachevsky A, et al. Integration of intention and outcome for moral judgment in frontotemporal dementia: brain structural signatures. Neurodegener Dis. (2016) 16:206–17. doi: 10.1159/000441918
189. Ekman P, Oster H. Facial expressions of emotion. Annu Rev Psychol. (1979) 30:527–54. doi: 10.1146/annurev.ps.30.020179.002523
190. Baron-Cohen S, Wheelwright S, Hill J, Raste Y, Plumb I. The “Reading the mind in the eyes” Test revised version: a study with normal adults, and adults with Asperger syndrome or high-functioning autism. J Child Psychol Psychiatr. (2001) 42:241–51. doi: 10.1111/1469-7610.00715
191. Baron-Cohen S, O'Riordan M, Stone V, Jones R, Plaisted K. Recognition of faux pas by normally developing children and children with Asperger syndrome or high-functioning autism. J Autism Dev Disord. (1999) 29:407–18. doi: 10.1023/A:1023035012436
192. Funkiewiez A, Bertoux M, de Souza LC, Lévy R, Dubois B. The SEA (Social Cognition and Emotional Assessment): a clinical neuropsychological tool for early diagnosis of frontal variant of frontotemporal lobar degeneration. Neuropsychology. (2012) 26:81–90. doi: 10.1037/a0025318
193. Bertoux M, Delavest M, de Souza LC, Funkiewiez A, Lépine J-P, Fossati P, et al. Social cognition and emotional assessment differentiates frontotemporal dementia from depression. J Neurol Neurosurg Psychiatr. (2012) 83:411–6. doi: 10.1136/jnnp-2011-301849
194. Narambuena L, Vaiman M, Pereno GL. Reconocimiento de Emociones Faciales en Adultos Mayores de la Ciudad de Córdoba. Psykhe. (2016) 25:1–13. doi: 10.7764/psykhe.25.1.791
195. de Souza LC, Bertoux M, de Faria ÂRV, Corgosinho LTS, Prado AC de A, Barbosa IG, et al. The effects of gender, age, schooling, and cultural background on the identification of facial emotions: a transcultural study. Int Psychogeriatr. (2018). 30:1861–70. doi: 10.1017/S1041610218000443
196. Román F, Rojas G, Román N, Iturry M, Blanco R, Leis A, et al. Baremos del Test de la Mirada en español en adultos normales de Buenos Aires. Revista Neuropsicología Latinoamericana. (2012) 4:1–5. doi: 10.5579/rnl.2012.0108
197. Watanabe RGS, Knochenhauer AE, Fabrin MA, Siqueira HH, Martins HF, Oliveira Mello CD, et al. Faux Pas Recognition Test: transcultural adaptation and evaluation of its psychometric properties in Brazil. Cogn Neuropsychiatry. (2021) 26:321–34. doi: 10.1080/13546805.2021.1941830
198. Mariano LI, Caramelli P, Guimarães HC, Gambogi LB, Moura MVB, Yassuda MS, et al. Can social cognition measurements differentiate behavioral variant frontotemporal dementia from Alzheimer's disease regardless of apathy? J Alzheimer's Dis. (2020) 74:817–27. doi: 10.3233/JAD-190861
199. Lillo P, Caramelli P, Musa G, Parrao T, Hughes R, Aragon A, et al. Inside minds, beneath diseases: social cognition in amyotrophic lateral sclerosis-frontotemporal spectrum disorder. J Neurol Neurosurg Psychiatr. (2020) 91:1279–82. doi: 10.1136/jnnp-2020-324302
200. Martínez-Pernía D, González-Castán Ó, Huepe D. From ancient Greece to the cognitive revolution: a comprehensive view of physical rehabilitation sciences. Physiother Theory Pract. (2017) 33:89–102. doi: 10.1080/09593985.2016.1266720
201. Toots ATM, Taylor ME, Lord SR. Close JCT associations between gait speed and cognitive domains in older people with cognitive impairment. J Alzheimer's Dis. (2019) 71:S15–21. doi: 10.3233/JAD-181173
202. Montero-Odasso M, Bergman H, Phillips NA, Wong CH, Sourial N, Chertkow H. Dual-tasking and gait in people with Mild Cognitive Impairment. The effect of working memory. BMC Geriatr. (2009) 9:41. doi: 10.1186/1471-2318-9-41
203. Celik Y, Stuart S, Woo WL, Godfrey A. Gait analysis in neurological populations: progression in the use of wearables. Med Eng Phys. (2021) 87:9–29. doi: 10.1016/j.medengphy.2020.11.005
204. Lord S, Galna B, Verghese J, Coleman S, Burn D, Rochester L. Independent domains of gait in older adults and associated motor and nonmotor attributes: validation of a factor analysis approach. J Gerontol Ser A Biol Sci Med Sci. (2013) 68:820–7. doi: 10.1093/gerona/gls255
205. Hausdorff JM, Rios DA, Edelberg HK. Gait variability and fall risk in community-living older adults: a 1-year prospective study. Arch Phys Med Rehabil. (2001) 82:1050–6. doi: 10.1053/apmr.2001.24893
206. Studenski S, Perera S, Patel K, Rosano C, Faulkner K, Inzitari M, et al. Gait speed and survival in older adults. J Am Med Assoc. (2011) 305:50–8. doi: 10.1001/jama.2010.1923
207. Valkanova V, Ebmeier KP. What can gait tell us about dementia? Review of epidemiological and neuropsychological evidence. Gait Posture. (2017) 53:215–23. doi: 10.1016/j.gaitpost.2017.01.024
208. Montero-Odasso M, Speechley M, Muir-Hunter SW, Sarquis-Adamson Y, Sposato LA, Hachinski V, et al. Motor and cognitive trajectories before dementia: results from gait and brain study. J Am Geriatr Soc. (2018) 66:1676–83. doi: 10.1111/jgs.15341
209. Wade DT. Measurement in neurological rehabilitation. Curr Opin Neurol Neurosurg. (1992) 5:682–6.
210. Morris R, Lord S, Bunce J, Burn D, Rochester L. Gait and cognition: mapping the global and discrete relationships in ageing and neurodegenerative disease. Neurosci Biobehav Rev. (2016) 64:326–45. doi: 10.1016/j.neubiorev.2016.02.012
211. Ijmker T, Lamoth CJC. Gait and cognition: the relationship between gait stability and variability with executive function in persons with and without dementia. Gait Posture. (2012) 35:126–30. doi: 10.1016/j.gaitpost.2011.08.022
212. Rucco R, Agosti V, Jacini F, Sorrentino P, Varriale P, de Stefano M, et al. Spatio-temporal and kinematic gait analysis in patients with Frontotemporal dementia and Alzheimer's disease through 3D motion capture. Gait Posture. (2017) 52:312–7. doi: 10.1016/j.gaitpost.2016.12.021
213. Pieruccini-Faria F, Montero-Odasso M, Hausdorff JM. Gait variability and fall risk in older adults: the role of cognitive function. In: Montero-Odasso M, Camicioli R, editors, Falls and Cognition in Older Persons. Cham: Springer; International Publishing (2020). p. 107–38. doi: 10.1007/978-3-030-24233-6_7
214. Allali G, Dubois B, Assal F, Lallart E, de Souza LC, Bertoux M, et al. Frontotemporal dementia: pathology of gait? Mov Disord. (2010) 25:731–7. doi: 10.1002/mds.22927
215. de Cock A-M, Fransen E, Perkisas S, Verhoeven V, Beauchet O, Vandewoude M, et al. Comprehensive quantitative spatiotemporal gait analysis identifies gait characteristics for early dementia subtyping in community dwelling older adults. Front Neurol. (2019) 10:313. doi: 10.3389/fneur.2019.00313
216. Peet BT, Castro-Suarez S, Miller BL. The neuropsychiatric features of behavioral variant frontotemporal dementia. Adv Exp Med Biol. (2021) 1281:17–31. doi: 10.1007/978-3-030-51140-1_2
217. Mendez MF, Shapira JS, Woods RJ, Licht EA, Saul RE. Psychotic symptoms in frontotemporal dementia: prevalence and review. Dement Geriatr Cogn Disord. (2008) 25:206–11. doi: 10.1159/000113418
218. Omar R, Sampson EL, Loy CT, Mummery CJ, Fox NC, Rossor MN, et al. Delusions in frontotemporal lobar degeneration. J Neurol. (2009) 256:600–7. doi: 10.1007/s00415-009-0128-7
219. Kertesz A, Nadkarni N, Davidson W, Thomas AW. The Frontal Behavioral Inventory in the differential diagnosis of frontotemporal dementia. J Int Neuropsychol Soc. (2000) 6:460–8. doi: 10.1017/S1355617700644041
220. Dols A, van Liempt S, Gossink F, Krudop WA, Sikkes S, Pijnenburg YAL, et al. Identifying specific clinical symptoms of behavioral variant frontotemporal dementia versus differential psychiatric disorders in patients presenting with a late-onset frontal lobe syndrome. J Clin Psychiatry. (2016) 77:1391–5. doi: 10.4088/JCP.15m09844
221. Grace J. Frontal systems behavior scale. In: Kreutzer JS, DeLuca J, Caplan B, editors, Encyclopedia of Clinical Neuropsychology. New York, NY: Springer (2011). p. 3. doi: 10.1007/978-0-387-79948-3_1895
222. Bozeat S, Gregory CA, Ralph MA, Hodges JR. Which neuropsychiatric and behavioural features distinguish frontal and temporal variants of frontotemporal dementia from Alzheimer's disease? J Neurol Neurosurg Psychiatr. (2000) 69:178–86. doi: 10.1136/jnnp.69.2.178
223. Wedderburn C, Wear H, Brown J, Mason SJ, Barker RA, Hodges J, et al. The utility of the Cambridge Behavioural Inventory in neurodegenerative disease. J Neurol Neurosurg Psychiatr. (2008) 79:500–3. doi: 10.1136/jnnp.2007.122028
224. Kaufer DI, Cummings JL, Ketchel P, Smith V, MacMillan A, Shelley T, et al. Validation of the NPI-Q, a brief clinical form of the neuropsychiatric inventory. J Neuropsychiatry Clin Neurosci. (2000) 12:233–9. doi: 10.1176/jnp.12.2.233
225. Boutoleau-Bretonnière C, Evrard C, Hardouin JB, Rocher L, Charriau T, Etcharry-Bouyx F, et al. DAPHNE: a new tool for the assessment of the behavioral variant of frontotemporal dementia. Dement Geriatr Cogn Dis Extra. (2015) 5:503–16. doi: 10.1159/000440859
226. Ducharme S, Pearl-Dowler L, Gossink F, McCarthy J, Lai J, Dickerson BC, et al. The frontotemporal dementia versus primary psychiatric disorder (FTD versus PPD) checklist: a bedside clinical tool to identify behavioral variant FTD in patients with late-onset behavioral changes. J Alzheimer's Dis. (2019) 67:113–24. doi: 10.3233/JAD-180839
227. Marin RS, Biedrzycki RC, Firinciogullari S. Reliability and validity of the apathy evaluation scale. Psychiatry Res. (1991) 38:143–62. doi: 10.1016/0165-1781(91)90040-V
228. Guimarães HC, Fialho PPA, Carvalho VA, Santos EL, dos Caramelli P. Brazilian caregiver version of the Apathy Scale. Dement Neuropsychol. (2009) 3:321–6. doi: 10.1590/S1980-57642009DN30400010
229. Shigenobu K, Ikeda M, Fukuhara R, Maki N, Hokoishi K, Nebu A, et al. The Stereotypy Rating Inventory for frontotemporal lobar degeneration. Psychiatry Res. (2002) 110:175–87. doi: 10.1016/S0165-1781(02)00094-X
230. Davis MH. A multidimensional approach to individual differences in empathy. Catalog Select Document Psychol. (1980) 10:1–17.
231. Ahmed RM, Irish M, Kam J, van Keizerswaard J, Bartley L, Samaras K, et al. Quantifying the eating abnormalities in frontotemporal dementia. J Am Med Assoc Neurol. (2014) 71:1540–6. doi: 10.1001/jamaneurol.2014.1931
232. Bahia VS, Silva MNM, da Viana R, Smid J, Damin AE, Radanovic M, et al. Behavioral and activities of daily living inventories in the diagnosis of frontotemporal lobar degeneration and Alzheimer's disease. Dement Neuropsychol. (2008) 2:108–13. doi: 10.1590/S1980-57642009DN20200006
233. Gonçalves S de AB, Caramelli P, Mariano LI, Guimarães HC, Gambogi LB, Resende EdePF, et al. Apathy in frontotemporal dementia is related to medial prefrontal atrophy and is independent of executive dysfunction. Brain Res. (2020) 1737:146799. doi: 10.1016/j.brainres.2020.146799
234. Slachevsky A, Muñoz-Neira C, Nuñez-Huasaf J, Stern TA, Blesius CR, Atri A. Late-onset cinephilia and compulsive behaviors: harbingers of frontotemporal dementia. Primary Care Companion CNS Disord. (2011). 13:PCC10f01115. doi: 10.4088/PCC.10f01115
235. Manes FF, Torralva T, Roca M, Gleichgerrcht E, Bekinschtein TA, Hodges JR. Frontotemporal dementia presenting as pathological gambling. Nat Rev Neurol. (2010) 6:347–52. doi: 10.1038/nrneurol.2010.34
236. Wicklund AH, Johnson N, Rademaker A, Weitner BB, Weintraub S. Profiles of decline in activities of daily living in non-Alzheimer dementia. Alzheimer Dis Assoc Disord. (2007) 21:8–13. doi: 10.1097/WAD.0b013e3180324549
237. Mioshi E, Kipps CM, Hodges JR. Activities of daily living in behavioral variant frontotemporal dementia. Alzheimer Dis Assoc Disord. (2009) 23:70–6. doi: 10.1097/WAD.0b013e318182d293
238. Lima-Silva TB, Bahia VS, Carvalho VA, Guimarães HC, Caramelli P, Balthazar MLF, et al. Direct and indirect assessments of activities of daily living in behavioral variant frontotemporal dementia and Alzheimer disease. J Geriatr Psychiatry Neurol. (2015) 28:19–26. doi: 10.1177/0891988714541874
239. de Vriendt P, Gorus E, Cornelis E, Bautmans I, Petrovic M, Mets T. The advanced activities of daily living: a tool allowing the evaluation of subtle functional decline in mild cognitive impairment. J Nutr Health Aging. (2013) 17:64–71. doi: 10.1007/s12603-012-0381-9
240. Dias EG, Andrade FB de, Duarte YA de O, Santos JLF, Lebrão ML. Atividades avançadas de vida diária e incidência de declínio cognitivo em idosos: Estudo SABE. Cadernos de Saúde Pública. (2015) 31:1623–35. doi: 10.1590/0102-311X00125014
241. Johnson N, Barion A, Rademaker A, Rehkemper G, Weintraub S. The activities of daily living questionnaire: a validation study in patients with dementia. Alzheimer Dis Assoc Disord. (2004) 18:223–30. doi: 10.1037/t28752-000
242. Muñoz-Neira C, López OL, Riveros R, Núñez-Huasaf J, Flores P, Slachevsky A. The technology – activities of daily living questionnaire: a version with a technology-related subscale. Dement Geriatr Cogn Disord. (2012) 33:361–71. doi: 10.1159/000338606
243. Musa G, Lillo P, van der Hiele K, Mendez-Orellana C, Ibañez A, Slachevsky A. Apathy, executive function, and emotion recognition are the main drivers of functional impairment in behavioral variant of Frontotemporal Dementia. Front Neurol. (2021). doi: 10.3389/fneur.2021.734251
244. Gelinas I, Gauthier L, McIntyre M, Gauthier S. Development of a functional measure for persons with Alzheimer's disease: the disability assessment for dementia. Am J Occup Ther. (1999) 53:471–81. doi: 10.5014/ajot.53.5.471
245. Pereira FS, Oliveira AM, Diniz BS, Forlenza Ov, Yassuda MS. Cross-cultural adaptation, reliability and validity of the DAFS-R in a sample of Brazilian older adults. Archiv Clin Neuropsychol. (2010) 25:335–43. doi: 10.1093/arclin/acq029
246. Carvalho I, Bahia A, Mansur L. Functional communication ability in frontotemporal lobar degeneration and Alzheimer's disease. Dement Neuropsychol. (2008) 2:31–6. doi: 10.1590/S1980-57642009DN20100007
247. Fratalli C, Thompson C, Holland A, Wohl C, Ferketic M. Functional Assessment of Communication Skills for Adults (ASHA FACS). Rockville, MD. American Speech-Language-Hearing Association (1995).
248. Reisberg B. Global measures: utility in defining and measuring treatment response in dementia. Int Psychogeriatr. (2007) 19:421–56. doi: 10.1017/S1041610207005261
249. Lima-Silva TB, Mioshi E, Bahia VS, Cecchini MA, Cassimiro L, Guimarães HC, et al. Disease progression in frontotemporal dementia and Alzheimer disease: the contribution of staging scales. J Geriatr Psychiatry Neurol. (2021) 34:397–404. doi: 10.1177/0891988720944239
250. Morris JC. The clinical dementia rating (CDR). Neurology. (1993) 43:2412–4. doi: 10.1212/WNL.43.11.2412-a
251. Russo G, Russo MJ, Buyatti D, Chrem P, Bagnati P, Suarez MF, et al. Utility of the Spanish version of the FTLD-modified CDR in the diagnosis and staging in frontotemporal lobar degeneration. J Neurol Sci. (2014) 344:63–8. doi: 10.1016/j.jns.2014.06.024
252. Lima-Silva TB, Bahia VS, Cecchini MA, Cassimiro L, Guimarães HC, Gambogi LB, et al. Validity and reliability of the frontotemporal dementia rating scale (FTD-FRS) for the progression and staging of dementia in Brazilian Patients. Alzheimer Dis Assoc Disord. (2018) 32:220–5. doi: 10.1097/WAD.0000000000000246
253. Lima-Silva TB, Bahia VS, Carvalho VA, Guimarães HC, Caramelli P, Balthazar M, et al. Translation, cross-cultural adaptation and applicability of the Brazilian version of the Frontotemporal Dementia Rating Scale (FTD-FRS). Dement Neuropsychol. (2013) 7:387–96. doi: 10.1590/S1980-57642013DN74000006
254. Knopman DS, Kramer JH, Boeve BF, Caselli RJ, Graff-Radford NR, Mendez MF, et al. Development of methodology for conducting clinical trials in frontotemporal lobar degeneration. Brain. (2008) 131:2957–68. doi: 10.1093/brain/awn234
255. Ruchinskas RA, Cullum CM. Neuropsychology in a memory disorder clinic. Archiv Clin Neuropsychol. (2018) 33:301–9. doi: 10.1093/arclin/acx128
256. Bak T. Why patients with dementia need a motor examination. J Neurol Neurosurg Psychiatr. (2016) 87:1157. doi: 10.1136/jnnp-2016-313466
257. Symonds A, Bak T. What is the current practice of cognitive and motor screening in dementia clinics? A worldwide on-line survey. Eur J Neurol. (2015) 22:424.
258. Johnen A, Reul S, Wiendl H, Meuth SG, Duning T. Apraxia profiles—a single cognitive marker to discriminate all variants of frontotemporal lobar degeneration and Alzheimer's disease. Alzheimer's Dement Diagn Assess Dis Monitor. (2018) 10:363–71. doi: 10.1016/j.dadm.2018.04.002
259. Mc Ardle R, del Din S, Donaghy P, Galna B, Thomas AJ, Rochester L. The impact of environment on gait assessment: considerations from real-world gait analysis in dementia subtypes. Sensors. (2021) 21:813. doi: 10.3390/s21030813
260. Mc Ardle R, del Din S, Galna B, Thomas A, Rochester L. Differentiating dementia disease subtypes with gait analysis: feasibility of wearable sensors? Gait Posture. (2020) 76:372–6. doi: 10.1016/j.gaitpost.2019.12.028
261. Costa A, Bak T, Caffarra P, Caltagirone C, Ceccaldi M, Collette F, et al. The need for harmonisation and innovation of neuropsychological assessment in neurodegenerative dementias in Europe: consensus document of the Joint Program for Neurodegenerative Diseases Working Group. Alzheimer's Res Ther. (2017) 9:27. doi: 10.1186/s13195-017-0254-x
262. Perry DC, Brown JA, Possin KL, Datta S, Trujillo A, Radke A, et al. Clinicopathological correlations in behavioural variant frontotemporal dementia. Brain. (2017) 140:3329–45. doi: 10.1093/brain/awx254
263. Ahmed RM, Devenney EM, Irish M, Ittner A, Naismith S, Ittner LM, et al. Neuronal network disintegration: common pathways linking neurodegenerative diseases. J Neurol Neurosurg Psychiatr. (2016) 87:1234–41. doi: 10.1136/jnnp-2014-308350
264. Slachevsky A, Ramos T, Olavarria L. Syndromes and diseases studied by behavioral neurology. In: Encyclopedia of Behavioral Neuroscience. 2nd ed. Amsterdam: Elsevier (2022). p. 1–16. doi: 10.1016/B978-0-12-819641-0.00165-1
265. Paulino Ramirez Diaz S, Gil Gregório P, Manuel Ribera Casado J, Reynish E, Jean Ousset P, Vellas B, et al. The need for a consensus in the use of assessment tools for Alzheimer's disease: the Feasibility Study (assessment tools for dementia in Alzheimer Centres across Europe), a European Alzheimer's Disease Consortium's (EADC) survey. Int J Geriatr Psychiatry. (2005) 20:744–8. doi: 10.1002/gps.1355
266. Rascovsky K. A Primer in Neuropsychological Assessment for Dementia Neuropsychological assessments can be extremely useful in the detection, diagnosis, and management of dementia syndromes. Practical Neurol. (2016) 2016:20–2.
267. Piña-Escudero SD, Aguirre GA, Javandel S, Longoria-Ibarrola EM. Caregiving for patients with frontotemporal dementia in Latin America. Front Neurol. (2021) 12:665694. doi: 10.3389/fneur.2021.665694
268. Calil V, Elliott E, Borelli WV, Barbosa BJAP, Bram J, Silva F, et al. Challenges in the diagnosis of dementia: insights from the United Kingdom-Brazil Dementia Workshop. Dement Neuropsychol. (2020) 14:201–8. doi: 10.1590/1980-57642020dn14-030001
270. Vágvölgyi R, Coldea A, Dresler T, Schrader J, Nuerk H-C. A review about functional illiteracy: definition, cognitive, linguistic, and numerical aspects. Front Psychol. (2016) 7:1617. doi: 10.3389/fpsyg.2016.01617
271. Montenegro RA, Stephens C. Indigenous health in Latin America and the Caribbean. Lancet. (2006) 367:1859–69. doi: 10.1016/S0140-6736(06)68808-9
272. Parra MA, Baez S, Sedeño L, Gonzalez Campo C, Santamaría-García H, Aprahamian I, et al. Dementia in Latin America: paving the way toward a regional action plan. Alzheimer's Dement. (2021) 17:295–313. doi: 10.1002/alz.12202
273. Daffner KR, Gale SA, Barrett AM, Boeve BF, Chatterjee A, Coslett HB, et al. Improving clinical cognitive testing: report of the AAN Behavioral Neurology Section Worgroup. Neurology. (2015) 85:910–8. doi: 10.1212/WNL.0000000000001763
274. Weintraub S, Salmon D, Mercaldo N, Ferris S, Graff-Radford NR, Chui H, et al. The Alzheimer's Disease Centers' Uniform Data Set (UDS): the neuropsychilogic test battery. Alzheimer Dis Assoc Disord. (2009) 23:91–101. doi: 10.1097/WAD.0b013e318191c7dd
AADL, Advanced Activities of Daily Living; ACE-III, Addenbrooke's Cognitive Examination - Third version; ACE-R, Addenbrooke's Cognitive Examination - Revised; AD, Alzheimer's disease; ADLQ, Activities of Daily Living Questionnaire; ADL, Activities of Daily Living; AES, Apathy Evaluation Scale; ALS, Amyotrophic Lateral Sclerosis; APEHQ, Appetite and Eating Habits Questionnaire; Asha-Facs, Functional Assessment of Adult Communication Skills; BADL, Basic Activities of Daily Living; BADS, Behavioral Assessment Dysexecutive Syndrome; BCS, rief Cognitive Screening; BDAE, Boston Diagnostic Aphasia Examination; BEM 144, Signoret battery for mnesic efficiency; bvFTD, behavioral variant of FTD; BVRT, Benton Visual Retention Test; CAMPROMPT, cambridge Behavioral Prospective Memory Test; CBI, Cambridge Behavioral Inventory; CBI-R, Cambridge Behavioral Inventory Revised; CBS, Corticobasal syndrome; CDR, Clinical Dementia Rating;; CDR-FTLD, Clinical Dementia Rating Scale for Frontotemporal Lobar Degeneration;; CDT, Clock Drawing Test;; CUSPAD, Columbia University Psychopathology Scale for Alzheimer's Disease; CVLT, California Verbal Learning Test; DAD, Disability Assessment for Dementia; DAFS, Direct Assessment of Functional Performance; FAB, Frontal Assessment Battery; FBI, Frontal Behavioral Inventory; FCSRT, Free and Cued Selective Reminding Test; FrSBe, Frontal Systems Behavior Scale; FTD, Frontotemporal Dementia; FTD-FRS, Frontotemporal Dementia Rating Scale; FTD-MND, FTD with motor neuron disease; HD, Huntington's disease; HVLT-R, Hopkins Verbal Learning Test; IADL, Instrumental Activities of Daily Living; IFS, INECO Frontal Screening; IGT, Iowa Gaming Test; IRI, Interpersonal Reactivity Index; LAC, Latin America and the Caribbean; LBD, Lewy Body Dementia; lv-PPA, Logopenic variant of PPA; MDRS, Mattis Dementia Rating Scale; Mini-SEA, Social cognition and Emotional Assessment - short version; MMSE, Mini-Mental State Examination; MoCA, Montreal Cognitive Assessment; M-WCST, Modified Wisconsin Card Sorting Test; nfv-PPA, Non-fluent or agrammatical variant of PPA; NPI, Neuropsychiatric Inventory; NPI-Q, Neuropsychiatric Inventory Questionnaire; PAL, Paired Associate Learning Test; PD, Parkinson's Disease; PFAQ, Pfeffer Functional Activities Questionnaire; PPA, Primary progressive aphasia; PPD, Parkinson's disease dementia; PPT, Pyramids and Palm Trees Test; PSP, Progressive supranuclear palsy; RAVLT, Rey Auditory-Verbal Learning Test; RBMT, Rivermead Behavioral Memory Test; ROCF, Rey-Osterrieth Complex Figure; RPT, Repeat and Point Test; SAS, Starkstein Apathy Scale; SD, Semantic Dementia; SEA, Social cognition and Emotional Assessment; SRI, Stereotypy Rating Inventory; STMB, Short-Term Memory Binding; SYDBAT, Sydney Language Battery; T-ADLQ, Technology-ADLQ; TMT, Trail Making Test; VD, Vascular Dementia; WAIS, Wechsler Adult Intelligence Scale; WCST, Wisconsin Card Sorting Test; WMS, Wechsler Memory Scale; VOSP, Visual Object and Space Perception Battery.
Keywords: frontotemporal dementia, neuropsychological assessment, functional assessment, gait assessment, behavior assessment, neuropsychiatric symptoms, multidimensional assessment, consortium
Citation: Henríquez F, Cabello V, Baez S, Souza LCd, Lillo P, Martínez-Pernía D, Olavarría L, Torralva T and Slachevsky A (2022) Multidimensional Clinical Assessment in Frontotemporal Dementia and Its Spectrum in Latin America and the Caribbean: A Narrative Review and a Glance at Future Challenges. Front. Neurol. 12:768591. doi: 10.3389/fneur.2021.768591
Received: 31 August 2021; Accepted: 13 December 2021;
Published: 16 February 2022.
Edited by:
Sudeshna Das, Massachusetts General Hospital and Harvard Medical School, United StatesReviewed by:
Philip Wade Tipton, Mayo Clinic Florida, United StatesCopyright © 2022 Henríquez, Cabello, Baez, Souza, Lillo, Martínez-Pernía, Olavarría, Torralva and Slachevsky. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Andrea Slachevsky, andrea.slachevsky@uchile.cl
†These authors share senior authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.