
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Neurol., 18 November 2021
Sec. Neuromuscular Disorders and Peripheral Neuropathies
Volume 12 - 2021 | https://doi.org/10.3389/fneur.2021.767961
This article is part of the Research TopicPhenotypes of Myasthenia GravisView all 19 articles
Background: Life-threatening myasthenic crisis (MC) occurs in 10–20% of the patients with myasthenia gravis (MG). It is important to identify the predictors of progression to MC and prognosis in the patients with MG with acute exacerbations.
Objective: This study aimed to explore the predictors of progression to MC in the patients with MG with acute onset of dyspnea and their short-term and long-term prognosis.
Methods: This study is a retrospective cohort study. We collected and analyzed data on all the patients with MG with acute dyspnea over a 10-year period in a single center using the univariate and multivariate analysis.
Results: Eighty-six patients with MG were included. In their first acute dyspnea episodes, 36 (41.9%) episodes eventually progressed to MC. A multivariate analysis showed that the early-onset MG (adjusted OR: 3.079, 95% CI 1.052–9.012) and respiratory infection as a trigger (adjusted OR: 3.926, 95% CI 1.141–13.510) were independent risk factors for the progression to MC, while intravenous immunoglobulin (IVIg) treatment prior to the mechanical ventilation (adjusted OR: 0.253, 95% CI 0.087–0.732) was a protective factor. The prognosis did not significantly differ between the patients with and without MC during the MG course, with a total of 45 (52.3%) patients reaching post-intervention status better than minimal manifestations at the last follow-up.
Conclusion: When treating the patients with MG with acute dyspnea, the clinicians should be aware of the risk factors of progression to MC, such as early-onset MG and respiratory infection. IVIg is an effective treatment. With proper immunosuppressive therapy, this group of patients had an overall good long-term prognosis.
Myasthenia gravis (MG) is an autoimmune disease caused by the autoantibodies that affect the structure of the post-synaptic membrane. Its main clinical manifestation is fluctuating muscle weakness (1). Myasthenic crisis (MC) refers to an event that requires mechanical ventilation because of severe involvement of the bulbar muscles or/and respiratory muscles (2). Furthermore, 10–20% of the patients with MG will develop MC (3–6), and most MC occurs within 2 years of the onset of MG (6, 7). IVIg and plasma exchange are recommended as the first-line therapy for impending and manifest myasthenic crisis (2, 8–10). However, the proportion of Chinese patients using IVIg and plasma exchange is significantly lower than that of the United States, and an Indian study on MC mentioned they treat the patients with MC with only corticosteroids and other immunosuppressive agents (11), suggesting that in the developing countries, many patients are not treated adequately due to the high price and unavailability of IVIg and plasma exchange. Therefore, exploring the risk factors of progress to MC in the patients with acute onset of dyspnea will aid clinical decision-making and medical resource allocation, especially in the developing countries. However, the previous studies focused on the clinical characteristics of the patients with MC (4, 12, 13) or the risk factors of MC occurrence after thymectomy (14–16), very few studies aimed to investigate the predictors of progression to MC in the patients with MG with acute onset of dyspnea. This study aimed to explore the predictors of progression to MC in the patients with MG with acute onset of dyspnea and their short-term and long-term prognosis.
This study is a retrospective cohort study. We included all the patients with MG who complained of acute dyspnea and visited the emergency department of the Peking Union Medical College Hospital, Beijing, China from September 2010 to June 2020. The diagnosis of myasthenia gravis was made by satisfying the following three criteria: (1) fluctuating muscle weakness; (2) positive neostigmine test, or decrements of compound motor action potential showed in slow repetitive nerve stimulation, or positive serum anti-acetylcholine receptor (AChR) antibody; (3) other causes of skeletal muscle weakness excluded. The patients with dyspnea occurring within 4 weeks after thymectomy, or positive muscle-specific tyrosine kinase (MuSK) antibodies, or dyspnea because of acute exacerbation of chronic cardiopulmonary disease were excluded from this study. MC (2) was defined as receiving mechanical ventilation (non-invasive or invasive ventilation) or arterial blood gas analyses suggesting that the indications for mechanical ventilation were met (PaCO2 > 45 mmHg and pH < 7.35, or oxygenation index PaO2/FiO2 < 200) (17). Multiple emergency department visits of the same patient were analyzed separately. We recommend IVIg for all the patients complaining of dyspnea. When mechanical ventilation was indicated, non-invasive ventilation was used first if the conditions permitted. All the patients were discharged from the hospital with oral corticosteroids and/or immunosuppressive agents. The final treatment choices were based on the informed consents of the patients and their family members. This study was approved by the Ethics Committee of Clinical Research of Peking Union Medical College Hospital (Beijing, China). The informed consents were obtained from every patient.
We collected data of all acute dyspnea episodes and selected the first episodes of each enrolled patient to explore the predictors of progression to MC. For predictive variables, we retrospectively collected demographic data, clinical features of MG, and comorbidities by reviewing the medical record data. All the patients underwent chest CT or contrast-enhanced chest CT, and the conditions of thymus were documented according to imaging or pathology. We obtained the data on the triggers of dyspnea, AChR antibody status, the Myasthenia Gravis Foundation of America (MGFA) clinical classification at the last follow-up before the episode, the treatments before the dyspnea episode, and the specific treatments (IVIg and glucocorticoid impulse therapy) used before the progression to crisis. For the outcome variables, the primary outcome was the occurrence of MC, the secondary outcomes included short-term prognosis and long-term prognosis, the former including the type and length of mechanical ventilation, total length of hospital stay, and in-hospital complications, and the long-term prognosis included the post-intervention status (PIS) at the last follow-up.
The numerical variables were summarized as mean ± SD or median (inter quartile range, IQR), respectively, depending on whether they followed normal distribution. For univariate analysis, t-test or Wilcoxon's rank sum test was used for group comparisons of numerical variables, and chi-square test or Fisher's exact test was used for the categorical variables. Significance level α was set at 0.05 for both the sides. To find independent risk factors for progression to MC in the patients with acute onset of dyspnea, first dyspnea episodes of each patient were divided into two groups according to whether MC occurred, and the distribution of each predictor variable was compared between the two groups using a univariate analysis. All the variables that achieved p < 0.10 were selected for the bivariate logistic regression model. If multiple selected variables were correlated clinically (e.g., age of onset and early onset MG), only one variable of them was selected for the multivariate analysis. Thereafter, the p-values and adjusted OR values of the selected variables were calculated using the bivariate logistic regression. A principal component analysis was preformed to evaluate and eliminate multicollinearity. The data analysis was conducted using the IBM-SPSS (version 25, IBM, NY, USA).
We collected the clinical data from 86 patients with MG who had 111 visits to the emergency department for acute onset of dyspnea (Table 1). In total, 20 (23.3%) patients experienced multiple episodes of dyspnea (maximum of four episodes). Thirty-six (41.9%) of the patients were male. Of the 86 first dyspnea episodes of each patient, 35 (40.7%) occurred in the patients with thymoma and 23 (26.7%) in the patients who had undergone thymectomy. The mean age of MG onset was 47.2 ± 17.8 years, 45 (52.3%) dyspnea episodes occurred in the patients with early-onset MG (i.e., age of onset not older than 50), and 62 (72.1%) episodes occurred in the patients with MG with ocular onset. Fifty-seven (66.3%) patients had positive AChR antibody, while 22 (25.6%) patients lacked the data. The median time from MG onset to dyspnea episode was 12.0 (4.0–34.5) months, and the median age of dyspnea episode was 51.0 ± 17.1. We divided the skeletal muscles other than respiratory muscles into four groups: ocular muscles, limb muscles, bulbar muscles, and cervical muscles. Thirty (34.9%) dyspnea episodes occurred in the patients with involvement of all the four muscle groups, while 60 (69.8%) episodes in the patients with involvement of three or more groups. We also collected MGFA clinical classification at the last follow-up before the episode, and 53 (61.6%) dyspnea episodes occurred in the patients with MGFA clinical classification ≥3a at the last follow-up. Fifty-eight (67.4%) dyspnea episodes occurred in the patients who were not on corticosteroids or other immunosuppressive agents, while 56 (65.1%) episodes occurred in the patients who had not used corticosteroids before. Twenty-one (24.4%) episodes occurred after a trigger of respiratory infection, while other triggers included long-time fatigue, other infections, and other unspecified predisposing factors. In 55 (64.0%) dyspnea episodes, the patients received IVIg prior to mechanical ventilation. Regarding the comorbidities, the most frequent comorbidity was diabetes mellitus (14 episodes, 16.3%), followed by other autoimmune diseases (10 episodes, 11.6%). Thirty-six (41.9%) dyspnea episodes eventually progressed to MC, and 32 patients received mechanical ventilation, 28 (87.5%) of which ended with invasive mechanical ventilation.
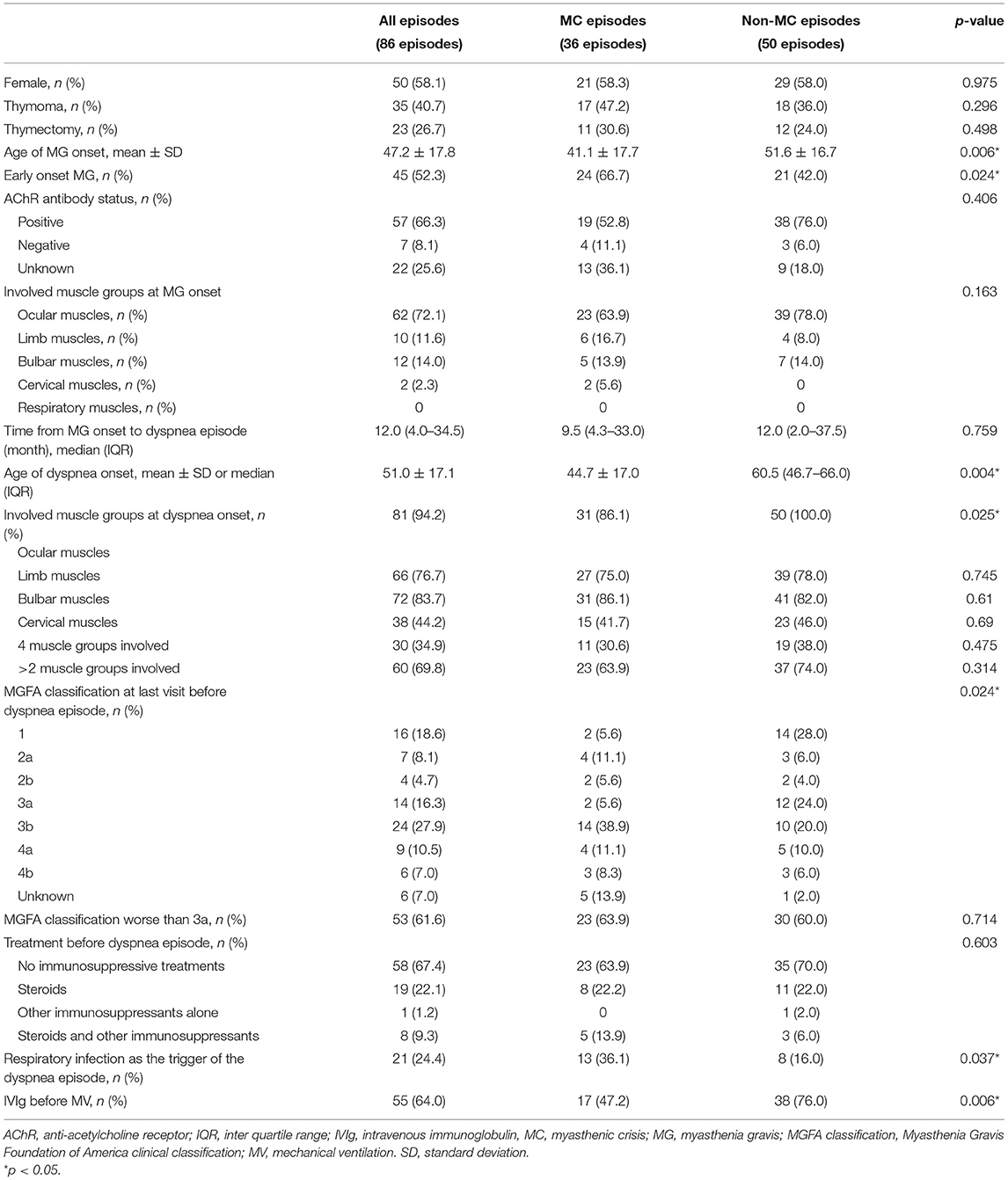
Table 1. The baseline characteristics of first acute dyspnea episodes of the patients with myasthenia gravis (MG) and comparison between myasthenic crisis (MC) and non-MC episodes.
Twenty (23.3%) patients experienced multiple episodes of dyspnea (maximum of four episodes), they had 45 episodes in total. Of the additional 25 episodes after the first episodes, 10 (40.0%) episodes were triggered by respiratory infection, 16 (64.0%) episodes were treated by IVIg. Ten (40.0%) episodes progressed to MC at last, similar to the first episode described above.
We analyzed the usage of IVIg at different time periods. From 2010 to 2013, IVIg was used in 20 out of 34 episodes (58.8%). This proportion was 36/57 (63.2%), 15/20 (75.0%) in different periods of 2014–2017 and 2018–2020, respectively. Although there appears to be an increasing in the use of IVIg, Wilcoxon's rank sum test did not reach statistical significance (p = 0.484).
Of the 86 first dyspnea episodes of each patient, 36 (41.9%) eventually progressed to MC (Table 1). By comparing the baseline characteristics between the MC group and non-MC group using the univariate analysis, we found that the variables with p < 0.10 included age of MG onset (p = 0.006), early-onset MG (p = 0.024), age at the dyspnea episode (p = 0.009), ocular muscle involvement when dyspnea episode occurred (p =0.025), MGFA clinical classification at last follow-up (p = 0.024), respiratory infection as the trigger (p = 0.037), and IVIg therapy before mechanical ventilation (p = 0.006). Before including these variables in the binary logistic regression, we noted that age at onset correlated with early-onset or late-onset MG, and we took only early-onset MG into the regression. Thereafter, MGFA classification at last follow-up was dichotomized (≥3a vs. <3a).
The results of the bivariate logistic regression are shown in Table 2. The final equation suggested early-onset MG (adjusted OR: 3.079, 95% CI 1.052–9.012) and respiratory infection as a trigger (adjusted OR: 3.926, 95% CI 1.141–13.510) were independent risk factors for MC, while IVIg treatment prior to mechanical ventilation (adjusted OR: 0.253, 95% CI 0.087–0.732) was a protective factor for MC. Further the principal component analysis showed no covariance between the selected variables.
Of the 20 patients with multiple dyspnea episodes, seven patients did not experience MC in their first episodes but had MC in subsequent episodes. Thus, in all 86 patients, 49 (57.0%) had no MC during the course of MG, while 37 (43.0%) had MC (Table 3). The patients with early-onset MG were more likely to develop MC (p = 0.025). Thymoma was more frequent in the patients experienced MC, though the statistical difference was not significant (48.6 vs. 34.7%, p = 0.192). Follow-up time was defined as the time from the start of the last episode to the last follow-up visit. There was a significant difference in the follow-up time between the two groups (p = 0.020), with the patients without MC followed for 36.5 ± 22.8 months, while the patients with MC were followed for 60.4 ± 55.2 months. The long-term prognosis was similar in both the groups, with 57.1% of the non-MC group having PIS better than minimal manifestations (MM) at the last follow-up, compared with 45.9% of patients in the MC group.
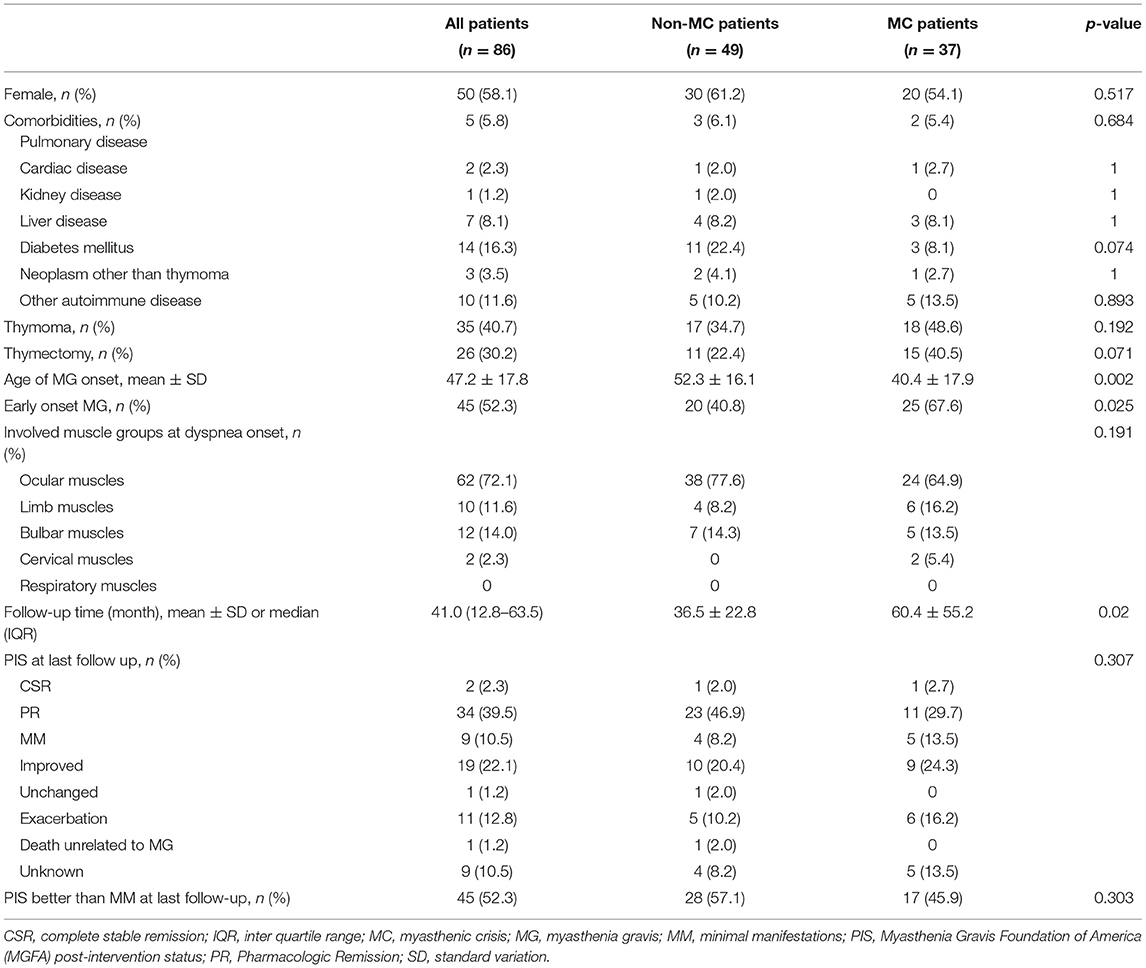
Table 3. The clinical characteristics and prognosis of patients with and without MC during the MG course.
Of the 35 MC episodes requiring invasive ventilation, 12 (34.3%) had ventilation over 15 days (Table 4). One-third of them had comorbid diabetes mellitus, significantly more than the other group (p = 0.038). The patients requiring prolonged mechanical ventilation also had significantly more pneumonia complications (83.3 vs. 39.1%, p = 0.03) and longer total length of hospital stay (46.5 vs. 25.7 days, p = 0.003). In addition, the patients requiring prolonged mechanical ventilation had older age (51.7 ± 13.1 years) than the other group (42.9 ± 20.2 years), but no significant difference was reached (p = 0.13).
In our present study, we demonstrated that in the patients with MG with acute onset of dyspnea, early-onset MG (adjusted OR: 3.079, 95% CI 1.052–9.012) and respiratory infection as a trigger (adjusted OR: 3.926, 95% CI 1.141–13.510) were independent risk factors for progression to MC, while the use of IVIg prior to mechanical ventilation (adjusted OR: 0.253, 95% CI 0.087–0.732) was a protective factor. The occurrence of MC had no significant impact on the long-term prognosis of these patients, and more than half of the patients (52.3%) reached a PIS better than MM at the last follow-up. Of the 35 MC episodes requiring invasive ventilation, 12 (34.3%) needed ventilation for more than 15 days. Comorbid diabetes mellitus (33.3 vs. 14.3%, p = 0.038) and complicated pneumonia (83.3 vs. 39.1%, p = 0.030) was associated with prolonged ventilation.
We found that early-onset MG was an independent risk factor for progression to MC in the patients with MG with acute onset of dyspnea (Table 2). Similar to our findings, A. Ramos-Fransi et al. (7) found that in the patients with MG with life-threatening events (i.e., MGFA class V or class IVB), early-onset MG had a longer time to weaning from ventilation, suggesting that early-onset MG may have more severe life-threatening events and worse response to the treatment. Since most patients in our study had positive AChR antibodies and the positive rate was similar between the MC and non-MC episodes, the reason for early-onset MG was a risk factor may be that in the patients with positive AChR antibodies, early-onset MG and late-onset MG are thought to differ significantly in pathogenesis. The origin of autoimmune antibodies in early-onset MG is mostly in the thymus, while the role of the thymus in the pathogenesis of late-onset MG is unclear, since no detectable inflammation was found in the thymus (1). Besides, the clinical characteristics of early-onset MG differ from of late-onset MG, one of which is that the response of early-onset MG to immunotherapy and the prognosis are worse, which may be associated with an expansion in protective immunomodulatory mechanisms, such as peripheral T-regulatory cells, in the elderly (18).
The previous studies have shown that infection is the most common trigger of MC as well as other life-threatening events in the patients with MG (3, 4, 7, 12, 19, 20). Further, our study also found that among the patients with MG with acute onset of dyspnea, respiratory infection was an independent risk factor for progression to MC. Infection may lead to exacerbation of the symptoms of bulbar palsy and respiratory muscle weakness, while the respiratory infections can aggravate ventilation dysfunction and lead to impaired air exchange, increasing the probability of mechanical ventilation. For the patients with MG with acute dyspnea with respiratory infection, close monitoring of vital signs and preparation for mechanical ventilation is particularly essential.
Coronavirus disease 2019 (COVID-19) is an infectious disease caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) virus and has become a global pandemic (21). Although there were no patients with COVID-19 in our cases, the effect of COVID-19 on the patients with MG is noteworthy in the context of pandemic. Dyspnea caused by SARS-CoV-2 infection may have a greater risk of progression to MC, as we discussed above. The patients with MG may be more vulnerable to COVID-19 due to the immunosuppressive therapies (22). However, the current evidence on the relationship between MG and COVID-19 is highly variable. Some studies suggested that COVID-19 has a limited effect on most of the patients with MG, since most of the patients in these studies do not experience exacerbations of MG, and immunosuppressive therapy is relatively safe (23–25). In contrast, other studies found a high proportion of patients with MG exacerbations requiring rescue therapy or mechanical ventilation (26, 27). High-quality studies on the relationship between COVID-19 and MC are also lacking, which may be explained by the difficulty in diagnosing MC in patients with COVID-19. Besides MC, severe COVID-19 may also lead to respiratory failure. Furthermore, many previous cases used drugs such as hydroxychloroquine and azithromycin that may exacerbate the MG symptoms or cause myopathy, further complicating the relationship between respiratory failure and MC. Further well-designed, and prospective studies are needed.
The efficacy of IVIg in the patients with MG with acute exacerbation has been demonstrated in several randomized controlled trials (8). Likewise, our study found that IVIg was a protective factor for progression to MC in the patients with acute onset of dyspnea. Since IVIg and plasma exchange are equally effective in worsening MG (9), and IVIg is easier to use, our center uses IVIg to treat this group of patients. Although the international consensus guidance (2) and multiple national guidelines (10, 28) recommended IVIg and plasma exchange as first-line therapy for impending and manifest myasthenic crisis, IVIg was not used timely in 31.0% of acute dyspnea episodes in this study. Based on our experience, we speculate that the reason for this is that IVIg is still expensive for the patients with MG in the developing countries, even when health insurance can partially cover the cost, which prevents some patients with MG with acute dyspnea from receiving timely treatment.
All the patients in our study were prescribed with oral corticosteroids and/or immunosuppressant agents after discharge. The overall prognosis was good, with 52.3% of patients having a PIS better than MM at last follow-up, with a median follow-up time of 41.0 months. There was no significant difference in the PIS at the last follow-up between the patients experienced MC and those who did not, which is similar to other studies. Sivadasan, A et al. (29) studied the patients with MC admitted to the intensive care unit. All the patients received oral corticosteroids and immunosuppressant agents and were followed for a median time of 36 months, with 67% of them reaching PIS better than MM at the last follow-up. Spillane, J et al. (20) studied the patients with MG admitted to the intensive care unit due to acute exacerbation. Most of the patients (97%) were on oral steroids and nearly half (45%) were started with other immunosuppressants. At a median follow-up time of 4 years, 19% of patients were asymptomatic at the last follow-up and 48% reached MGFA classification better than type II. Both these studies and our study suggest that the long-term use of immunosuppressive therapy after the acute phase significantly improves the long-term prognosis of patients with MG who experienced acute dyspnea.
Our study found that in the patients requiring invasive ventilation longer than 15 days, a significantly higher proportion of patients had comorbid diabetes mellitus, prolonged length of hospital stay, and complications of pneumonia. The age at onset of dyspnea was also older in this group, although it did not reach significance difference (51.7 ± 13.1 vs. 42.9 ± 20.2, p = 0.13). Similar to other studies, advanced age and more chronic underlying diseases were risk factors for prolonged mechanical ventilation (3, 12), suggesting that this group of patients may require better intensive care unit management and have a higher probability of tracheotomy.
Of the patients included in this study, 67.4% had not used immunosuppressive therapy, such as oral steroids or immunosuppressants before their first dyspnea episodes. Similarly, the proportion of this group of patients in other studies was 40–50% (3, 20). Several studies showed that early immunosuppressive treatment of MG reduces the probability of MC occurrence and recurrence (30). To date, several retrospective studies showed that the use of oral steroids in ocular MG could reduce the probability of progression to generalized MG (31, 32). These results suggest that the use of immunosuppressive therapy in the patients with MG may not only improve symptoms, but also act as a disease modifier to prevent the progression of the disease course in the patients with MG.
The greatest limitation of this study arises from its single-center retrospective nature. The relative rarity of MG and the low incidence of respiratory or bulbar muscle involvement in MG resulted in a small sample size, so the results may be incidental. Selection bias is inevitable in single center studies. The lower incidence of comorbidities and the higher incidence of thymoma in our patients compared with other studies may limit the generalization of the findings to all the patients. However, compared with other studies that focused mostly on the patients with MG who had already developed MC, we expanded the target population to include the patients with MG with acute onset of dyspnea (i.e., the patients may progress to MC), effectively expanded the sample size and increased the credibility of the final conclusions.
The previous studies have focused on the patients with manifest MC, and most studies have been conducted in intensive care unit settings (4, 12, 13). A strength of this study is that we described the clinical characteristics of impending the patients with MC from the emergency department, which makes our study more relevant to the clinical practice of neurologists. Nowadays, many patients with MG with acute dyspnea in the developing countries are unable to receive IVIg because of its high price and unavailability. The significance of this study is that we explored the risk factors for progression to MC in this group of patients, indicating that when treating the patients with MG with acute dyspnea, the clinicians should be more aggressive in advancing the use of IVIg to avoid MC occurrence in early-onset MG or dyspnea triggered by respiratory infection. Initiating social assistance or transferring the patients to a qualified center are effective options.
In conclusion, we demonstrated that early-onset MG and respiratory infection as a trigger were the independent risk factors for progression to MC in the patients with MG with acute onset of dyspnea, while the use of IVIg prior to mechanical ventilation was a protective factor. With proper immunosuppressive therapy, this group of patients had an overall good prognosis.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by Ethics Committee of Clinical Research of Peking Union Medical College Hospital (Beijing, China). Written informed consent to participate in this study was provided by the participants' legal guardian/next of kin.
YH and YG contributed to conception and design of the study. YH, YT, JS, JY, and KL collected the clinical data. YH, YT, and KL contributed to the data analysis and interpretation. YH wrote the manuscript. YT and YG edited the manuscript. All authors read and approved the final manuscript.
This work was supported by the National Clinical Cohort Study of Rare Diseases for key R & D Program (grant number: 2016YFC091501) and the Beijing Municipal Sciences & Technology Commission (Z181100001718145).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Gilhus NE, Tzartos S, Evoli A, Palace J, Burns TM, Verschuuren JJGM. Myasthenia gravis. Nat Rev Dis Prim. (2019) 5:30. doi: 10.1038/s41572-019-0079-y
2. Sanders DB, Wolfe GI, Benatar M, Evoli A, Gilhus NE, Illa I, et al. International consensus guidance for management of myasthenia gravis: executive summary. Neurology. (2016) 87:419–25. doi: 10.1212/WNL.0000000000002790
3. Thomas CE, Mayer SA, Gungor Y, Swarup R, Webster EA, Chang I, et al. Myasthenic crisis: clinical features, mortality, complications, and risk factors for prolonged intubation. Neurology. (1997) 48:1253–60. doi: 10.1212/WNL.48.5.1253
4. Sakaguchi H, Yamashita S, Hirano T, Nakajima M, Kimura E, Maeda Y, et al. Myasthenic crisis patients who require intensive care unit management. Muscle Nerve. (2012) 46:440–2. doi: 10.1002/mus.23445
5. Alshekhlee A, Miles JD, Katirji B, Preston DC, Kaminski HJ. Incidence and mortality rates of myasthenia gravis and myasthenic crisis in US hospitals. Neurology. (2009) 72:1548–54. doi: 10.1212/WNL.0b013e3181a41211
6. Cohen MS, Younger D. Aspects of the natural history of myasthenia gravis: crisis and death. Ann N Y Acad Sci. (1981) 377:670–7. doi: 10.1111/j.1749-6632.1981.tb33765.x
7. Ramos-Fransi A, Rojas-Garcia R, Segovia S, Marquez-Infante C, Pardo J, Coll-Canti J, et al. Myasthenia gravis: descriptive analysis of life-threatening events in a recent nationwide registry. Eur J Neurol. (2015) 22:1056–61. doi: 10.1111/ene.12703
8. Gajdos P, Chevret S, Toyka KV. Intravenous immunoglobulin for myasthenia gravis. Cochrane Database Syst Rev. (2012) 12:CD002277. doi: 10.1002/14651858.CD002277.pub4
9. Dhawan PS, Goodman BP, Harper CM, Bosch PE, Hoffman-Snyder CR, Wellik KE, et al. IVIG versus PLEX in the treatment of worsening myasthenia gravis: what is the evidence?: a critically appraised topic. Neurologist. (2015) 19:145–8. doi: 10.1097/NRL.0000000000000026
10. Evoli A, Antonini G, Antozzi C, DiMuzio A, Habetswallner F, Iani C, et al. Italian recommendations for the diagnosis and treatment of myasthenia gravis. Neurol Sci. (2019) 40:1111–24. doi: 10.1007/s10072-019-03746-1
11. Kalita J, Kohat AK, Misra UK. Predictors of outcome of myasthenic crisis. Neurol Sci. (2014) 35:1109–14. doi: 10.1007/s10072-014-1659-y
12. Neumann B, Angstwurm K, Mergenthaler P, Kohler S, Schonenberger S, Bosel J, et al. Myasthenic crisis demanding mechanical ventilation: a multicenter analysis of 250 cases. Neurology. (2020) 94:e299–313. doi: 10.1212/WNL.0000000000008688
13. Souayah N, Nasar A, Suri MF, Kirmani JF, Ezzeddine MA, Qureshi AI. Trends in outcomes and hospitalization charges among mechanically ventilated patients with myasthenia gravis in the United States. Int J Biomed Sci. (2009) 5:209–14. Available online at: http://www.ijbs.org/User/ContentFullText.aspx?VolumeNO=5&StartPage=209
14. Tian W, Li X, Tong H, Weng W, Yang F, Jiang G, et al. Surgical effect and prognostic factors of myasthenia gravis with thymomas. Thorac Cancer. (2020) 11:1288–96. doi: 10.1111/1759-7714.13396
15. Choi KH, Nam TS, Lee SH, Kim MK. Preoperative pulmonary function is strongly related to myasthenic crisis after thymectomy. Neurol India. (2014) 62:164–8. doi: 10.4103/0028-3886.132361
16. Ando T, Omasa M, Kondo T, Yamada T, Sato M, Menju T, et al. Predictive factors of myasthenic crisis after extended thymectomy for patients with myasthenia gravis. Eur J Cardiothorac Surg. (2015) 48:705–9; discussion 9. doi: 10.1093/ejcts/ezu530
17. Nava S, Hill N. Non-invasive ventilation in acute respiratory failure. Lancet. (2009) 374:250–9. doi: 10.1016/S0140-6736(09)60496-7
18. Cortes-Vicente E, Alvarez-Velasco R, Segovia S, Paradas C, Casasnovas C, Guerrero-Sola A, et al. Clinical and therapeutic features of myasthenia gravis in adults based on age at onset. Neurology. (2020) 94:e1171–80. doi: 10.1212/WNL.0000000000008903
19. Roper J, Fleming ME, Long B, Koyfman A. Myasthenia gravis and crisis: evaluation and management in the emergency department. J Emerg Med. (2017) 53:843–53. doi: 10.1016/j.jemermed.2017.06.009
20. Spillane J, Hirsch NP, Kullmann DM, Taylor C, Howard RS. Myasthenia gravis–treatment of acute severe exacerbations in the intensive care unit results in a favourable long-term prognosis. Eur J Neurol. (2014) 21:171–3. doi: 10.1111/ene.12115
21. Hu B, Guo H, Zhou P, Shi ZL. Characteristics of SARS-CoV-2 and COVID-19. Nat Rev Microbiol. (2021) 19:141–54. doi: 10.1038/s41579-020-00459-7
22. Jacob S, Muppidi S, Guidon A, Guptill J, Hehir M, Howard JF Jr, et al. Guidance for the management of myasthenia gravis (MG) and Lambert-Eaton myasthenic syndrome (LEMS) during the COVID-19 pandemic. J Neurol Sci. (2020) 412:116803. doi: 10.1016/j.jns.2020.116803
23. Jakubíková M, Týblová M, Tesar A, Horáková M, VlaŽná D, Ryšánková I, et al. Predictive factors for a severe course of COVID-19 infection in myasthenia gravis patients with an overall impact on myasthenic outcome status and survival. Eur J Neurol. (2021) 28:3418–25. doi: 10.1111/ene.14951
24. Solé G, Mathis S, Friedman D, Salort-Campana E, Tard C, Bouhour F, et al. Impact of coronavirus disease 2019 in a French cohort of myasthenia gravis. Neurology. (2021) 96:e2109–20. doi: 10.1212/WNL.0000000000011669
25. Businaro P, Vaghi G, Marchioni E, Diamanti L, Arceri S, Bini P, et al. COVID-19 in patients with myasthenia gravis: epidemiology and disease course. Muscle Nerve. (2021) 64:206–11. doi: 10.1002/mus.27324
26. Camelo-Filho AE, Silva AMS, Estephan EP, Zambon AA, Mendonça RH, Souza PVS, et al. Myasthenia gravis and COVID-19: clinical characteristics and outcomes. Front Neurol. (2020) 11:1053. doi: 10.3389/fneur.2020.01053
27. Muppidi S, Guptill JT, Jacob S, Li Y, Farrugia ME, Guidon AC, et al. COVID-19-associated risks and effects in myasthenia gravis (CARE-MG). Lancet Neurol. (2020) 19:970–1. doi: 10.1016/S1474-4422(20)30413-0
28. Sussman J, Farrugia ME, Maddison P, Hill M, Leite MI, Hilton-Jones D. Myasthenia gravis: association of British neurologists' management guidelines. Pract Neurol. (2015) 15:199–206. doi: 10.1136/practneurol-2015-001126
29. Sivadasan A, Alexander M, Aaron S, Mathew V, Nair S, Muthusamy K, et al. Comorbidities and long-term outcomes in a cohort with myasthenic crisis: experiences from a tertiary care center. Ann Indian Acad Neurol. (2019) 22:464–71. doi: 10.4103/aian.AIAN_197_19
30. Juel VC. Myasthenic crisis: smoother sailing ahead. Eur J Neurol. (2009) 16:775–6. doi: 10.1111/j.1468-1331.2009.02629.x
31. Wong SH, Huda S, Vincent A, Plant GT. Ocular myasthenia gravis: controversies and updates. Curr Neurol Neurosci Rep. (2014) 14:421. doi: 10.1007/s11910-013-0421-9
Keywords: myasthenia gravis, myasthenic crisis, impending myasthenic crisis, early-onset, intravenous immunoglobulin, mechanical ventilation, post-intervention status
Citation: Huang Y, Tan Y, Shi J, Li K, Yan J and Guan Y (2021) Patients With Myasthenia Gravis With Acute Onset of Dyspnea: Predictors of Progression to Myasthenic Crisis and Prognosis. Front. Neurol. 12:767961. doi: 10.3389/fneur.2021.767961
Received: 31 August 2021; Accepted: 18 October 2021;
Published: 18 November 2021.
Edited by:
Ghazala Hayat, Saint Louis University, United StatesCopyright © 2021 Huang, Tan, Shi, Li, Yan and Guan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yuzhou Guan, Z3Vhbnl6MDAxQDE2My5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.