- Department of Neurology, The First Affiliated Hospital of Zhengzhou University, Zhengzhou, China
Background and Purpose: To investigate the association of monocyte to high-density lipoprotein ratio (MHR) with disease severity and prognosis in patients with neuromyelitis optica spectrum disorders (NMOSD).
Methods: This retrospective study included 125 patients with NMOSD. Demographic and clinical parameters, including the MHR, were assessed. The initial Expanded Disability Status Scale (EDSS) score and relapse rate were used to evaluate disease severity and prognosis, respectively. Correlations between MHR and disease severity and relapse rate were analyzed. The predictive value of MHR for prognosis was evaluated using receiver operating characteristic (ROC) curve analysis.
Results: Compared with the low MHR group, the initial EDSS score (median 4.5 vs. 5.5%, P = 0.025) and relapse rate (51.61 vs. 30.16%, P = 0.015) were significantly higher in the high MHR group. MHR was positively correlated with the initial EDSS score (r = 0.306, P = 0.001). Multivariate analysis showed that MHR was significantly associated with severity (odds ratio = 7.90, 95% confidence interval [CI] = 1.08–57.82, P = 0.041), and it was a significant predictor of disease prognosis (hazard ratio = 3.12, 95% CI = 1.02–9.53, P = 0.046). The median relapse interval of the high MHR group was 24.40 months. When the MHR was higher than 0.565, the risk of relapse was high [sensitivity, 33.3%; specificity, 91.9%; area under the ROC curve, 0.642 (95% CI = 0.54–0.74, P = 0.007)].
Conclusion: MHR is a novel predictive marker of disease severity and prognosis in patients with NMOSD. Early monitoring and reduction of MHR may allow earlier intervention and improved prognosis.
Introduction
Neuromyelitis optica is an autoimmune demyelinating disease of the central nervous system that is characterized by acute optic neuritis and transverse myelitis occurring simultaneously or continuously (1–3), with an estimated prevalence of 1–2 per 100,000 people. Approximately 80% of patients have specific antibodies to astrocyte aquaporin 4 (AQP4), and this is one of the key diagnostic criteria (4–6). Patients with neuromyelitis optica spectrum disorders (NMOSD) have severe immune-mediated attacks that usually lead to severe residual disability and a high relapse rate (7). Therefore, accurate prediction of relapse is important to help clinicians initiate early preventive treatment and improve patient prognosis (8, 9).
As an important effector cell of the innate immune response, monocytes play a key role in the pathogenesis of autoimmune-related central nervous system diseases, including NMOSD (10–13). Studies have shown that anti-AQP4 antibodies can stimulate astrocytes to release chemokines, recruit monocytes and promote their activation, enhance the natural immune response, and destroy the blood-brain barrier, which plays a key role in accelerating the formation of NMOSD lesions (14–17). High-density lipoprotein (HDL) is considered an anti-inflammatory factor that has immunomodulatory and antioxidant effects on endothelial cells (18–20) and can prevent the production of pro-inflammatory cytokines. Studies have shown that low HDL is related to disease activity and disability in patients with AQP4-positive NMOSD, which may be associated with the decrease in the levels of apolipoprotein (apo) A-I, the main component of HDL in serum (21, 22).
The monocyte to high-density lipoprotein ratio (MHR) is a novel marker that reflects the degree of inflammation and oxidative stress. Many studies have shown that the MHR is closely related to the occurrence, development, and prognosis of cardiovascular, cerebrovascular (23–25), immune system (26), and rheumatic diseases (27–30). For example, one study found that the MHR was significantly higher in patients with multiple sclerosis than in healthy controls and that it was related to disease severity and disability, suggesting that the MHR may be used as an independent index to predict disability (31). However, the correlation between MHR and prognosis and relapse in patients with NMOSD remains unclear. The purpose of this study was to explore the relationship between MHR and disease severity and prognosis in patients with NMOSD and to determine the best cutoff value of MHR to predict the prognosis of patients with NMOSD.
Materials and Methods
Patients
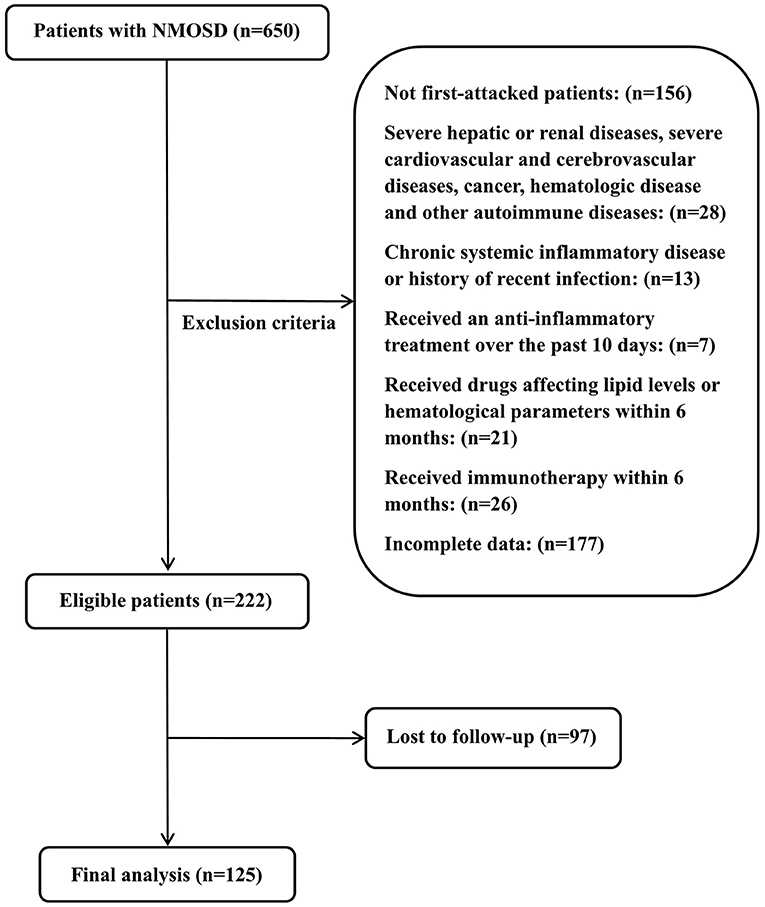
This study was approved by the Ethics Committee of Zhengzhou University (2019-KY-018). All patients provided written informed consent to participate in this study. In this retrospective study, we collected the clinical data of 650 patients newly diagnosed with NMOSD from September 2013 to June 2020 at the First Affiliated Hospital of Zhengzhou University. The inclusion criteria were: (1) diagnosed with NMOSD for the first time according to the 2006 Wingerchuk standard or the 2015 McDonald NMOSD international general diagnostic standard (32, 33); (2) no serious liver or kidney damage, serious cardiovascular or cerebrovascular diseases, malignant tumors, blood system diseases, or other immune diseases; (3) no chronic systemic inflammatory diseases or history of recent infection; (4) did not receive treatment with anti-inflammatory drugs within 10 days before admission, no lipid-lowering treatment within 6 months before admission, and were not taking drugs that affect the number of white blood cells and blood lipid levels; (5) did not receive immunosuppressant treatment within 6 months before admission, and (6) had complete clinical data and follow-up data. A total of 125 patients met these criteria and were included in this cohort study. The specific screening process is shown in Figure 1.
Data Collection
Clinical data were obtained through a retrospective review of the hospital electronic case system. The following clinical information was collected: gender, age, clinical phenotype at onset, comorbidities, blood cell count, triglyceride (TG), total cholesterol (TC), low-density lipoprotein (LDL), HDL, MHR, erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), anti-AQP4 antibody status, spinal magnetic resonance imaging (MRI), and treatment plan.
Blood samples were collected from the antecubital vein at 8:00 am after an overnight fast and stored on ice before testing (within 30 min after collection). Blood samples were analyzed in the biochemistry laboratory of the hospital. Blood cell count and all biochemical parameters were determined by standard methods, and the enzyme kit (Sigma-Aldrich, Saint Louis, MO, USA) was used to evaluate fasting TC, TG, LDL, and HDL levels. The anti-AQP4 antibody status in serum or cerebrospinal fluid samples was analyzed by the cell-based assay method. MRI of the spinal cord was performed using a 3T MAGNETOM Skyra scanner (Siemens Healthcare, Erlangen, Germany) in the Department of Magnetic Resonance of the hospital at admission. Longitudinally extensive transverse myelitis (LETM) was considered as extending 3 or more vertebral segments on T2-weighted imaging (33). When more than one test was performed during hospitalization, the data from the first test were included in the analysis. All tests were conducted according to the manufacturer's instructions, and the inspectors were blinded to the diagnosis or clinical symptoms.
Clinical Assessment
To calculate the extended disability status scale (EDSS) score at the time of admission, at least two professional neurologists carefully reviewed the patient's clinical records. This was recorded as the initial EDSS score, which was used to evaluate disease severity. The baseline EDSS scores of all patients before the first attack were considered normal. Patients in the cohort were divided into those with mild disability (EDSS score 0–3.5) or moderate/severe disability (EDSS score 4–9.5) (34–36).
The main index for evaluating prognosis was the relapse rate, which was defined as new or relapsed neurological symptoms caused by demyelinating diseases of the central nervous system without fever or infection, lasting at least 24 h, and increasing the existing EDSS score of patients by 0.5 points (37). Follow-up data were obtained by a clinical visit or telephone review every 6 months.
Statistical Analysis
Statistical analysis was performed using SPSS version 26.0 (International Business Machines Corporation, Chicago, Illinois, USA). According to the median MHR, the cohort was divided into two groups, and the classification data were expressed as percentages (%). The chi-square test or Fisher's exact test was used to compare the two groups. The Kolmogorov-Smirnov test was used for normality testing, and the measurement data conforming to normal distribution were expressed as mean and standard deviation; the independent sample t-test was used for comparison between the two groups. Data with non-normal distribution were expressed as median and interquartile range, and the Mann-Whitney U test was used for comparison between the two groups. The correlation between MHR and other clinical indices and the initial EDSS score was obtained by Spearman correlation analysis. Patients in the cohort were divided into those with mild disability (EDSS score 0–3.5) or moderate/severe disability (EDSS score 4–9.5) (34–36). We used a binary logistic regression model to analyze the correlation between MHR and other clinical-related indicators and disease severity. Variables with P < 0.2 in univariate analysis and those that were closely related to dependent variables in the clinic were included in the multivariate model, and the results were expressed with the odds ratio (OR) and 95% confidence interval (95% CI). The Kaplan-Meier curve was used to analyze whether different MHRs had an independent influence on the time of the first relapse, and univariate Cox survival analysis was used to screen the variables with p < 0.2. Then the multivariate regression model was used to analyze whether MHR was a predictor of relapse in patients with NMOSD. The results were expressed as the risk ratio with the 95% CI. We used a receiver operating characteristic (ROC) curve to analyze the predictive value of MHR for disease prognosis, determine its optimal critical value, and calculate the area under the curve to evaluate the accuracy of the cutoff value. Differences were considered statistically significant at p < 0.05.
Results
Demographic and Clinical Characteristics of Participants
A total of 125 patients with NMOSD were enrolled in this cohort study (Figure 1). The average age at onset was 42.53 ± 15.06, 106 patients were women (84.80%), 75 were positive for anti-AQP4 antibody (60%), and the average follow-up time was 37.87 (20.27–51.85) months. The most common clinical phenotype at onset in the whole cohort was optic neuritis (72%), and 66.40% of patients had extensive transverse myelitis on MRI of the spinal cord. To better evaluate the relationship between different MHRs and disease severity and prognosis, we divided patients into MHR ≤ 0.40 and MHR > 0.40 groups, according to the median MHR. There were no significant differences in age, gender, clinical phenotype and hypertension between the two groups. Compared to the low MHR group, the high MHR group had significantly lower TC levels (3.93 vs. 4.44 mmol/L, P = 0.005), and there were significantly more patients with LETM on MRI of the spinal cord (47 vs. 36, P = 0.027). Other clinical parameters, such as lymphocyte count, TG, LDL, ESR, and CRP, were not significantly different between the two groups. There was a significant difference in the initial EDSS score between the two groups (5.5 vs. 4.5, P = 0.025). The relapse rate of the high MHR group was higher than that of the low MHR group (51.61 vs. 30.16%, P = 0.015). Other demographic and clinical characteristics are shown in Table 1.
Correlations Between MHR and Disease Severity in Patients With NMOSD
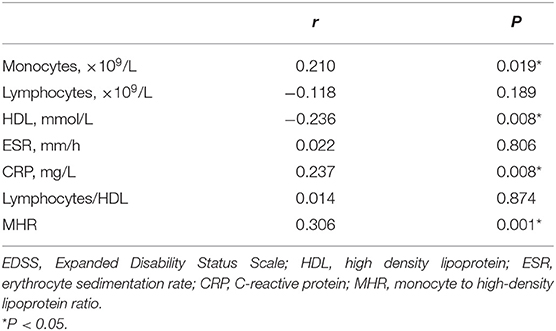
Spearman correlation analysis showed that the levels of monocytes (r = 0.210, P = 0.019), HDL (r = −0.236, P = 0.008), and CRP (r = 0.237, P = 0.008) were significantly correlated with the initial EDSS score of patients with NMOSD, but the correlation was weak (Figures 2A–C). There was no obvious correlation between the blood lymphocyte count, ESR, blood lymphocyte to HDL ratio, and disease severity (P > 0.05) (Table 2). In addition, the MHR was positively correlated with the initial EDSS score (r = 0.306, P = 0.001) (Figure 2D).
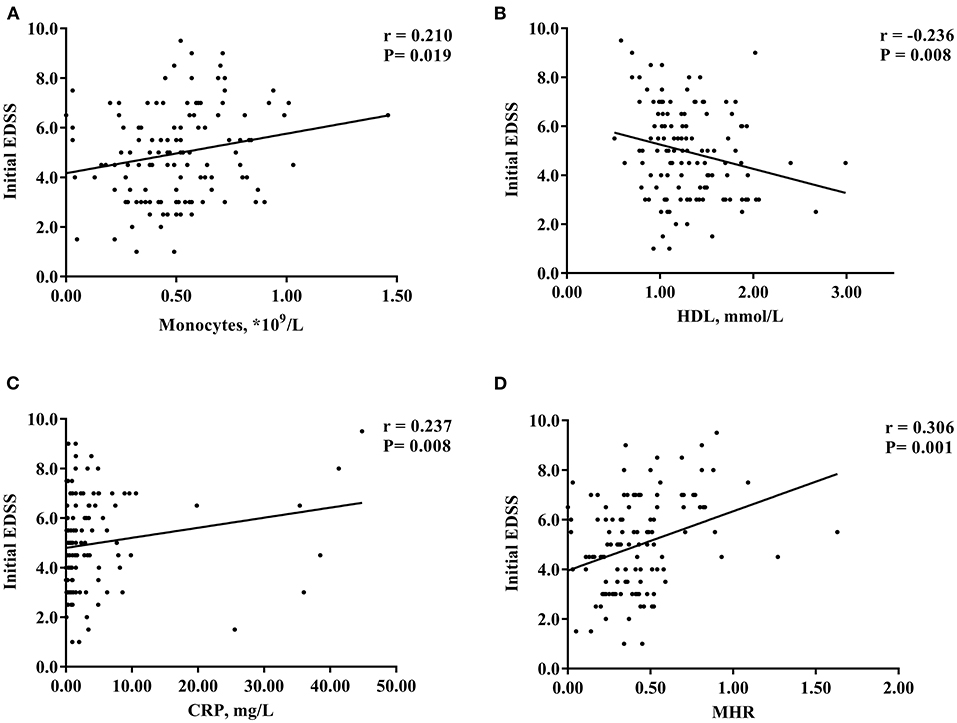
Figure 2. Scatter plot of the correlation between monocytes (A), HDL (B), CRP (C), MHR (D) and initial EDSS scores. HDL, high-density lipoprotein; MHR, monocyte to high-density lipoprotein ratio; CRP, C-reactive protein; EDSS, Expanded Disability Status Scale. *P < 0.05.
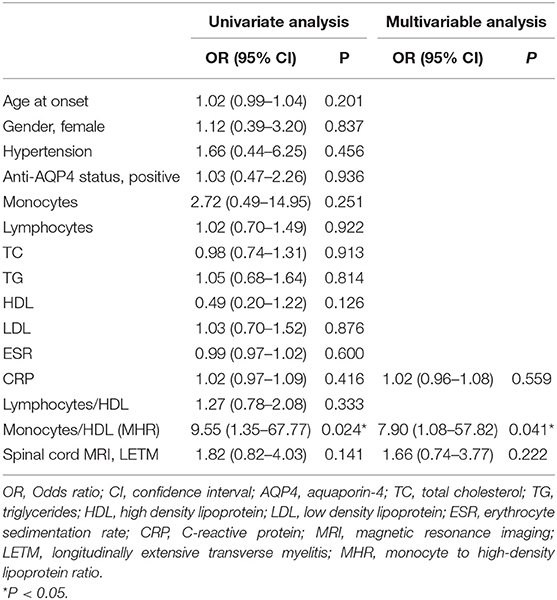
Univariate logistic regression analysis showed that the MHR was significantly correlated with disease severity (OR = 9.55, 95% CI = 1.35–67.77, P = 0.024). HDL levels (OR = 0.49, 95% CI = 0.20–1.22, P = 0.126) and MRI of the spinal cord showing LETM (OR = 1.82, 95% CI = 0.82–4.03, P = 0.141) had a moderate influence on the initial EDSS score. However, age, gender, hypertension, anti-AQP4 antibody status, blood monocytes, lymphocyte count, TC, TG, LDL, ESR, CRP, and lymphocyte to HDL ratio were not significantly correlated with the initial EDSS score. In the multivariate model, MHR was an independent risk factor for disease severity (OR = 7.90, 95% CI = 1.08–57.82, P = 0.041) (Table 3).
Correlations Between MHR and Prognosis in Patients With NMOSD
Kaplan-Meier analysis was used to evaluate the correlation between MHR and the relapse rate of patients with NMOSD. MHR was a significant predictor of disease prognosis (log-rank P = 0.046). The median relapse interval of the high MHR group was 24.40 months, whereas 50% of patients in the high MHR group relapsed 28.60 months after treatment (Figure 3).
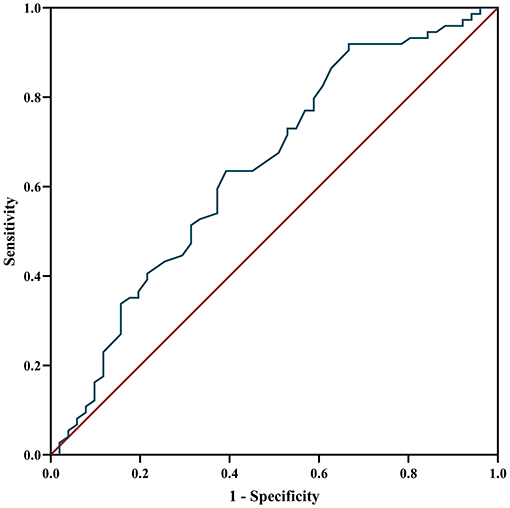
Figure 3. Kaplan-Meier curves categorized according to MHR. MHR, monocyte to high-density lipoprotein ratio. *P < 0.05.
Cox survival analysis was used to test the independent influence of MHRs on the relapse rate of patients with NMOSD. Univariate analysis showed that the MHR (HR = 3.01, 95% CI = 1.19–7.60, p = 0.020) was significantly correlated with relapse risk. However, there was no correlation between age, gender, hypertension, anti-AQP4 antibody, blood lymphocyte count, CRP, lymphocyte-to-HDL ratio, initial EDSS score, treatment plan, and relapse rate (P > 0.05). Blood mononuclear cell count, TC, TG, HDL, LDL, ESR, and MRI of the spinal cord showing LTEM had a moderate influence on disease relapse (Figure 4A). To eliminate these confounding factors, a multivariate Cox analysis model was established to obtain the corrected hazard ratio value. Because MHR is collinear with blood mononuclear cell count and HDL, these parameters were not included in the multivariate analysis. The results showed that the above confounding factors had no significant influence on the experimental results and that MHR was an independent risk factor for disease relapse (HR = 3.12, 95% CI = 1.02–9.53, P = 0.046) (Figure 4B).
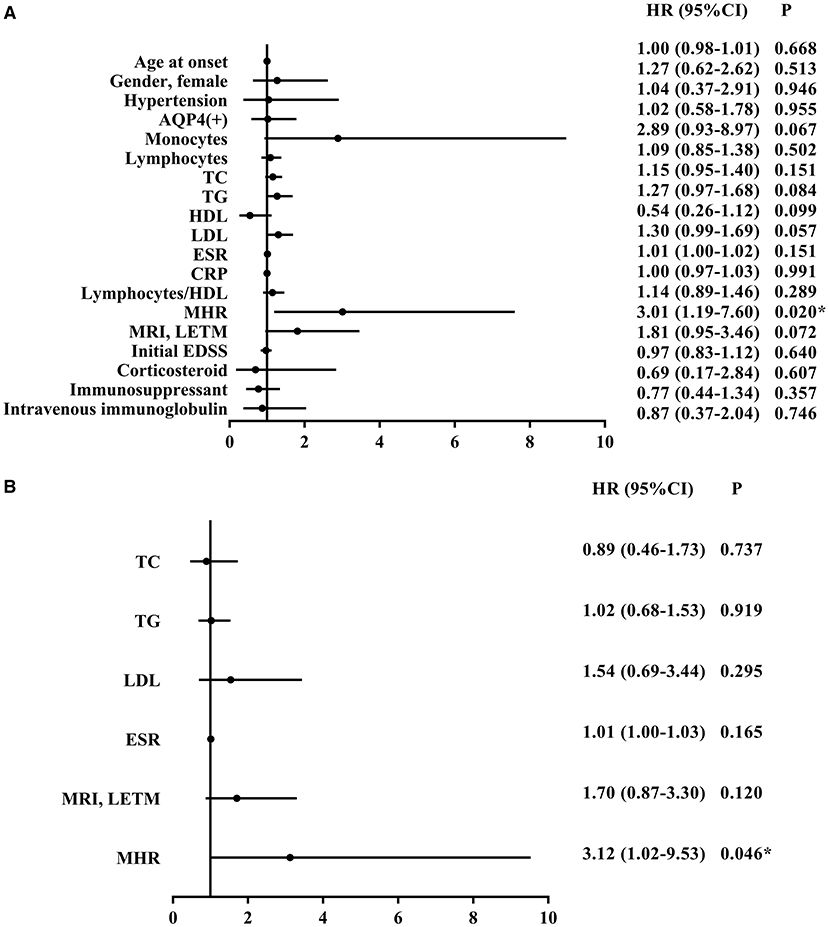
Figure 4. Univariate (A) and multivariate (B) cox survival analysis of potential factors associated with relapse of NMOSD patients. NMOSD, neuromyelitis optica spectrum disorders; AQP4, aquaporin-4; TC, total cholesterol; TG, triglycerides; HDL, high density lipoprotein; LDL, low density lipoprotein; ESR, erythrocyte sedimentation rate; CRP, C-reactive protein; MRI, magnetic resonance imaging; LETM, longitudinally extensive transverse myelitis; STM, short-segment transverse myelitis; EDSS, Expanded Disability Status Scale; MHR, monocyte to high-density lipoprotein ratio; HR, hazard ratio; CI, confidence interval. *P < 0.05.
The ROC curve was used to analyze the predictive value of MHR for disease prognosis. The area under the curve was 0.642 (95% CI = 0.54–0.74, P = 0.007), the best cutoff value was MHR = 0.565, the sensitivity was 0.333, and the specificity was 0.919, showing a good predictive ability for disease relapse. When the MHR is higher than 0.565, the risk of relapse is high (Figure 5).
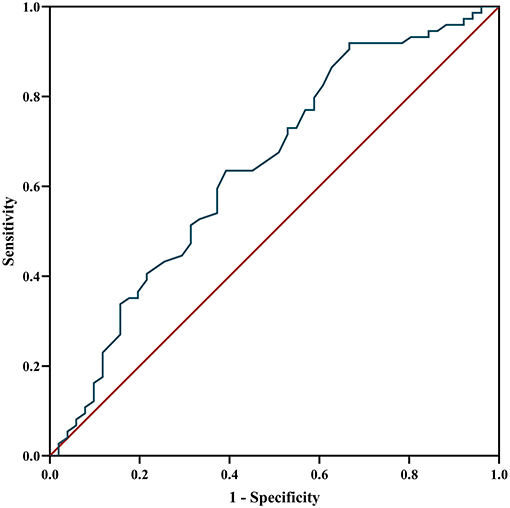
Figure 5. ROC curve analysis of the prediction of relapse based on MHR. ROC curve, receiver operating characteristic curve; MHR, monocyte to high-density lipoprotein ratio.
Discussion
Previous studies have demonstrated the prognostic value of the MHR in immune-mediated diseases, and in this study, we evaluated the effect of the MHR on the disease severity and prognosis of patients with NMOSD. We found that high MHR was associated with more severe disease at onset and a higher relapse rate, and the MHR at the onset of NMOSD was positively correlated with the initial EDSS score. Further, MHR was found to be an independent predictor of the severity and prognosis of NMOSD, and the best cutoff value to predict prognosis was 0.565.
As important effector cells of the innate immune response, monocytes have many functions, including antigen presentation, phagocytosis, and cytokine production, and their role in autoimmune diseases has attracted increasing attention (10–13). The blood mononuclear cell count is closely related to the early clinical severity of MS and can be used as a prognostic index (11). In our study, the blood mononuclear cell count was positively correlated with the NMOSD initial EDSS score. Studies have shown that in NMOSD, anti-AQP4 antibodies can stimulate astrocytes to release chemokines (14) and enhance monocyte recruitment and activation. Activated monocytes promote the production of inflammatory cytokines, such as tumor necrosis factor α, interleukin (IL)-6, IL-1β, IL-12, and IL-23 (38), and increase the expression of costimulatory molecules, such as CD80, ICAM-1, and HLA-DR (39), whereas the level of anti-inflammatory cytokines (IL-10) decreases accordingly (40). Among these cytokines, IL-6 plays a key role in the pathogenesis of NMOSD, and treatment with IL-6 has shown clinical benefits in patients with NMOSD (41). Anti-AQP4 antibody is the main pathogenic antibody of NMOSD (42), but it alone is not enough to cause the disease. AQP4-specific T cells, especially Th17, can help peripheral blood B cells produce autoantibodies, induce tissue inflammation, and promote further injury to the central nervous system. IL-1β (43) induced by monocyte activation not only promotes Th17 differentiation but also causes blood–brain barrier leakage and enhances T cell migration (44); therefore, monocytes may play an important role in the pathological mechanism underlying NMOSD progression (45).
HDL is considered an anti-inflammatory factor that can prevent the production of pro-inflammatory cytokines and affect a series of immune cell reactions, including those involving macrophages and B and T lymphocytes (21). HDL is closely related to monocytes and can regulate the activation, adhesion, and migration of monocytes (46, 47). Apolipoprotein A-1, the main protein component of HDL, has a specific inhibitory effect on the production of inflammatory cytokines by monocytes through prevention of the activation of CD11b (48, 49). The production of anti-AQP4 antibodies in patients with acute NMOSD leads to the loss of astrocytes in specific areas of the central nervous system through complement-mediated cytotoxicity (50). It has been shown that astrocytes can produce apolipoprotein A-I (51) in rats; hence, the extensive loss of astrocytes leads to a significant decrease in the production of apolipoprotein A-I and a decrease in HDL levels. Previous studies have shown that the serum apolipoprotein A-1 levels in patients with NMOSD are significantly lower than those in healthy controls (22), and HDL levels are significantly lower during active disease than during remission. Further, dyslipidemia with low HDL is related to disease activity and disability in patients with AQP4 positive NMOSD (21). Similar results were observed in the present study. The lower the HDL levels, the higher the initial EDSS score of patients with NMOSD. Although TC, TG, and LDL had a moderate influence on disease prognosis in univariate analysis, no significant correlation was observed in multivariate analysis, and abnormal lipid metabolism in NMOSD has been confirmed by many studies. Some studies have found that compared with healthy controls, the levels of TC, TG, and LDL in patients with NMOSD are higher, and the level of TGs is positively correlated with poor recovery of first-time patients with NMOSD (20, 52). This, combined with our research results, suggests that early lipid-lowering treatment may play an important role in improving prognosis.
Compared with single monocytes and HDL levels, combining monocytes and HDL to form a new comprehensive inflammatory index that incorporates both the injury mechanism and protection mechanism, namely the MHR, may have greater clinical value as this biomarker can reflect the degree of inflammation and oxidative stress. Many studies have shown that MHR is closely related to Parkinson's disease (53), ischemic stroke (54), cardiovascular disease (23), metabolic syndrome (55), immune system disease (26), and rheumatic disease (27). Related studies have also reported that the neutrophil-to-lymphocyte ratio (NLR) was related to disease activity at the onset of MS (56). However, another study provided results that did not support the use of NLR as a marker of disease activity and disability in MS patients (57). The association between NLR and disease severity was also confirmed in NMOSD (58). However, the effect of MHR on the pathogenesis and prognosis of patients with NMOSD has not been previously reported. In our study, we found that MHR was positively correlated with the EDSS score of patients with NMOSD (r = 0.306, P = 0.001), and patients with high MHR had a higher relapse rate, which was an independent risk factor for disease severity and poor prognosis. Recently, it has been reported that the MHR of patients with multiple sclerosis with EDSS score ≥4 is significantly higher than that of patients with EDSS score < 4, which proves that MHR is related to the severity and disability of MS and can be used as an independent indicator to predict disability (31). This previous report, combined with our research, suggests the potential role of MHRs in autoimmune demyelinating diseases of the central nervous system, and can also be used for risk assessment in the diagnosis and treatment of NMOSD. In our ROC curve analysis, MHR > 0.565 was associated with a high risk of relapse; therefore, intervention in the early stage of the disease process to reduce the MHR may have therapeutic potential, improve disease prognosis, and reduce the risk of relapse in clinical practice.
In our study, univariate analysis showed that spinal cord MRI showing LETM had a moderate influence on the initial EDSS score and prognosis of NMOSD, but there was no significant correlation in multivariate analysis, and previous studies have found that the length of spinal cord lesions is related to the initial severity of the disease and residual disability (59). The ability of MRI parameters to predict NMOSD prognosis requires further exploration. Consistent with previous studies, we found that the CRP was closely related to the disease, and the CRP level was positively correlated with the initial EDSS score (r = 0.237, P = 0.008) (60). The serum CRP level of patients with NMOSD was significantly higher than that of healthy individuals, which may be related to the oxidative stress and inflammatory reactions that occur during disease pathogenesis (61). CRP level is also related to the destruction of the blood–brain barrier and is a useful index for monitoring NMOSD disease activity (60, 61).
Our study also has several limitations worth noting. First, the retrospective study design has inherent defects. Second, this was a single-center cohort study with a relatively short follow-up time; therefore, a larger multi-center study with a longer follow-up period is warranted to confirm our results. Third, our hospital did not evaluate MOG antibody levels prior to 2019, and the lack of data prior to this date may have affected the research results. Fourth, selection bias should be considered. Because of incomplete test data, loss to follow-up, and other reasons, we only studied a small number of patients (125/650). Finally, some uncontrollable confounding factors may have affected the research results, such as smoking, drinking, eating habits, and drug intake.
In conclusion, our results showed that MHR was correlated with the severity of early NMOSD and could further predict disease relapse. This new biomarker, which is simple, reliable, economical, and easy to obtain, can also be used for NMOSD risk assessment in clinical practice and may facilitate earlier treatment and improvement of prognosis. Further studies are required to elucidate the specific mechanisms underlying the effect of MHR on NMOSD pathogenesis and disease progression.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Ethics Statement
The studies involving human participants were reviewed and approved by the Ethics Committee of Zhengzhou University. Written informed consent to participate in this study was provided by the participants' legal guardian/next of kin.
Author Contributions
JZ and YL contributed to conception and design of the research. YZho, CP, and YZha organized the database. JZ, YL, and HX performed the statistical analysis. JZ and KW conducted regular follow-up of all cases. JZ wrote the first draft of the manuscript. RD, ZG, and YJ undertook the task of revising the manuscript critically. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2021.763793/full#supplementary-material
References
1. Jarius S, Wildemann B. The history of neuromyelitis optica. Part 2: ‘Spinal amaurosis', or how it all began. J Neuroinflammation. (2019) 16:280. doi: 10.1186/s12974-019-1594-1
2. Oh J, Levy M. Neuromyelitis optica: an antibody-mediated disorder of the central nervous system. Neurol Res Int. (2012) 2012:460825. doi: 10.1155/2012/460825
3. Levy M, Fujihara K, Palace J. New therapies for neuromyelitis optica spectrum disorder. Lancet Neurol. (2021) 20:60–7. doi: 10.1016/S1474-4422(20)30392-6
4. Jarius S, Paul F, Weinshenker BG, Levy M, Kim HJ, Wildemann B. Neuromyelitis optica. Nat Rev Dis Primers. (2020) 6:85. doi: 10.1038/s41572-020-0214-9
5. Lennon V, Wingerchuk D, Kryzer T, Pittock S, Lucchinetti C, Fujihara K, et al. A serum autoantibody marker of neuromyelitis optica: distinction from multiple sclerosis. Lancet. (2004) 364:2106–12. doi: 10.1016/S0140-6736(04)17551-X
6. Mealy M, Wingerchuk D, Greenberg B, Levy M. Epidemiology of neuromyelitis optica in the United States: a multicenter analysis. Arch Neurol. (2012) 69:1176–80. doi: 10.1001/archneurol.2012.314
7. Shi Z, Du Q, Chen H, Zhang Y, Qiu Y, Zhao Z, et al. Effects of immunotherapies and prognostic predictors in neuromyelitis optica spectrum disorder: a prospective cohort study. J Neurol. (2020) 267:913–24. doi: 10.1007/s00415-019-09649-7
8. Palace J, Lin DY, Zeng D, Majed M, Elsone L, Hamid S, et al. Outcome prediction models in AQP4-IgG positive neuromyelitis optica spectrum disorders. Brain. (2019) 142:1310–23. doi: 10.1093/brain/awz054
9. He Q, Li L, Li Y, Lu Y, Wu K, Zhang R, et al. Free thyroxine level is associated with both relapse rate and poor neurofunction in first-attack Neuromyelitis Optica Spectrum Disorder (NMOSD) patients. BMC Neurol. (2019) 19:329. doi: 10.1186/s12883-019-1560-7
10. Ziegler-Heitbrock L. The CD14+ CD16+ blood monocytes: their role in infection and inflammation. J Leukoc Biol. (2007) 81:584–92. doi: 10.1189/jlb.0806510
11. Akaishi T, Takahashi T, Nakashima I. Peripheral blood monocyte count at onset may affect the prognosis in multiple sclerosis. J Neuroimmunol. (2018) 319:37–40. doi: 10.1016/j.jneuroim.2018.03.016
12. Asavapanumas N, Ratelade J, Verkman A. Unique neuromyelitis optica pathology produced in naïve rats by intracerebral administration of NMO-IgG. Acta Neuropathol. (2014) 127:539–51. doi: 10.1007/s00401-013-1204-8
13. Kurosawa K, Misu T, Takai Y, Sato D, Takahashi T, Abe Y, et al. Severely exacerbated neuromyelitis optica rat model with extensive astrocytopathy by high affinity anti-aquaporin-4 monoclonal antibody. Acta Neuropathol Commun. (2015) 3:82. doi: 10.1186/s40478-015-0259-2
14. Shimizu M, Okuno T, Kinoshita M, Sumi H, Fujimura H, Yamashita K, et al. Mitochondrial DNA enhance innate immune responses in neuromyelitis optica by monocyte recruitment and activation. Sci Rep. (2020) 10:13274. doi: 10.1038/s41598-020-70203-x
15. Zhao M, Tuo H, Wang S, Zhao L. The roles of monocyte and monocyte-derived macrophages in common brain disorders. Biomed Res Int. (2020) 2020:9396021. doi: 10.1155/2020/9396021
16. Kothur K, Wienholt L, Brilot F, Dale R. CSF cytokines/chemokines as biomarkers in neuroinflammatory CNS disorders: a systematic review. Cytokine. (2016) 77:227–37. doi: 10.1016/j.cyto.2015.10.001
17. Waschbisch A, Schröder S, Schraudner D, Sammet L, Weksler B, Melms A, et al. Pivotal role for CD16+ monocytes in immune surveillance of the central nervous system. J Immunol. (2016) 196:1558–67. doi: 10.4049/jimmunol.1501960
18. Assmann G, Gotto A. HDL cholesterol and protective factors in atherosclerosis. Circulation. (2004) 109:III8–14. doi: 10.1161/01.CIR.0000131512.50667.46
19. Vuilleumier N, Dayer J, von Eckardstein A, Roux-Lombard P. Pro- or anti-inflammatory role of apolipoprotein A-1 in high-density lipoproteins? Swiss Med Wkly. (2013) 143:w13781. doi: 10.4414/smw.2013.13781
20. Wu K, Wen L, Duan R, Li Y, Yao Y, Jing L, et al. Triglyceride level is an independent risk factor in first-attacked neuromyelitis optica spectrum disorders patients. Front Neurol. (2019) 10:1230. doi: 10.3389/fneur.2019.01230
21. Cho E, Cho H, Choi M, Seok J, Shin H, Kim B, et al. Low high-density lipoprotein cholesterol and high triglycerides lipid profile in neuromyelitis optica spectrum disorder: associations with disease activity and disability. Mult Scler Relat Disord. (2020) 40:101981. doi: 10.1016/j.msard.2020.101981
22. Zhong Y, Liu J, Li M, Wang X, Yuan Y, Zhong X, et al. Distinct serum apolipoprotein A-I levels in neuromyelitis optica and acute transverse myelitis. Lipids Health Dis. (2013) 12:150. doi: 10.1186/1476-511X-12-150
23. Ganjali S, Gotto A, Ruscica M, Atkin S, Butler A, Banach M, et al. Monocyte-to-HDL-cholesterol ratio as a prognostic marker in cardiovascular diseases. J Cell Physiol. (2018) 233:9237–46. doi: 10.1002/jcp.27028
24. Ceyhun G, Engin M. The Monocyte/High Density Lipoprotein Cholesterol Ratio (MHR) as an indicator of the need for amputation in patients with peripheral artery disease developing critical limb ischemia. Angiology. (2021) 72:268–73. doi: 10.1177/0003319720965808
25. Villanueva D, Tiongson M, Ramos J, Llanes E. Monocyte to High-Density Lipoprotein Ratio (MHR) as a predictor of mortality and Major Adverse Cardiovascular Events (MACE) among ST Elevation Myocardial Infarction (STEMI) patients undergoing primary percutaneous coronary intervention: a meta-analysis. Lipids Health Dis. (2020) 19:55. doi: 10.1186/s12944-020-01242-6
26. Acikgoz N, Kurtoglu E, Yagmur J, Kapicioglu Y, Cansel M, Ermis N. Elevated monocyte to high-density lipoprotein cholesterol ratio and endothelial dysfunction in Behçet disease. Angiology. (2018) 69:65–70. doi: 10.1177/0003319717704748
27. Wang Q, Meng Y, Cao W, Du W, Liu Y, Yan Y, et al. Association of monocyte to high-density lipoprotein cholesterol ratio with carotid artery intima-media thickness in patients with systemic lupus erythematosus. Biomark Med. (2018) 12:359–64. doi: 10.2217/bmm-2016-0362
28. Wei X, Chen F, Huang J, He P, Wei Y, Tan N, et al. Novel risk biomarker for infective endocarditis patients with normal left ventricular ejection fraction - monocyte to high-density lipoprotein cholesterol ratio. Circ J. (2017) 82:283–8. doi: 10.1253/circj.CJ-17-0427
29. Sun M, Zhao D, Zhang Y, Zhai Y, Ye M, Wang X, et al. Prognostic utility of monocyte to high-density lipoprotein ratio in patients with acute coronary syndrome: a meta-analysis. Am J Med Sci. (2020) 359:281–6. doi: 10.1016/j.amjms.2020.01.018
30. Liu H, Liu K, Pei L, Gao Y, Zhao L, Sun S, et al. Monocyte-to-high-density lipoprotein ratio predicts the outcome of acute ischemic stroke. J Atheroscler Thromb. (2020) 27:959–68. doi: 10.5551/jat.51151
31. Ulusoy E, Bolattürk Ö, Göl M. Relation between the novel marker monocyte to high-density lipoprotein cholesterol ratio and severity in multiple sclerosis. Ann Indian Acad Neurol. (2020) 23:275–9. doi: 10.4103/aian.AIAN_249_19
32. Wingerchuk D, Banwell B, Bennett J, Cabre P, Carroll W, Chitnis T, et al. International consensus diagnostic criteria for neuromyelitis optica spectrum disorders. Neurology. (2015) 85:177–89. doi: 10.1212/WNL.0000000000001729
33. Wingerchuk D, Lennon V, Pittock S, Lucchinetti C, Weinshenker B. Revised diagnostic criteria for neuromyelitis optica. Neurology. (2006) 66:1485–9. doi: 10.1212/01.wnl.0000216139.44259.74
34. Conradsson D, Ytterberg C, von Koch L, Johansson S. Changes in disability in people with multiple sclerosis: a 10-year prospective study. J Neurol. (2018) 265:119–26. doi: 10.1007/s00415-017-8676-8
35. Sicras-Mainar A, Ruíz-Beato E, Navarro-Artieda R, Maurino J. Impact on healthcare resource utilization of multiple sclerosis in Spain. BMC Health Serv Res. (2017) 17:854. doi: 10.1186/s12913-017-2807-x
36. Harding K, Wardle M, Carruthers R, Robertson N, Zhu F, Kingwell E, et al. Socioeconomic status and disability progression in multiple sclerosis: a multinational study. Neurology. (2019) 92:e1497–506. doi: 10.1212/WNL.0000000000007190
37. McDonald W, Compston A, Edan G, Goodkin D, Hartung H, Lublin F, et al. Recommended diagnostic criteria for multiple sclerosis: guidelines from the International Panel on the diagnosis of multiple sclerosis. Ann Neurol. (2001) 50:121–7. doi: 10.1002/ana.1032
38. Varrin-Doyer M, Spencer C, Schulze-Topphoff U, Nelson P, Stroud R, Cree B, et al. Aquaporin 4-specific T cells in neuromyelitis optica exhibit a Th17 bias and recognize Clostridium ABC transporter. Ann Neurol. (2012) 72:53–64. doi: 10.1002/ana.23651
39. Linhares U, Schiavoni P, Barros P, Kasahara T, Teixeira B, Ferreira T, et al. The ex vivo production of IL-6 and IL-21 by CD4+ T cells is directly associated with neurological disability in neuromyelitis optica patients. J Clin Immunol. (2013) 33:179–89. doi: 10.1007/s10875-012-9780-2
40. Pentón-Rol G, Cervantes-Llanos M, Martínez-Sánchez G, Cabrera-Gómez J, Valenzuela-Silva C, Ramírez-Nuñez O, et al. TNF-alpha and IL-10 downregulation and marked oxidative stress in Neuromyelitis Optica. J Inflamm. (2009) 6:18. doi: 10.1186/1476-9255-6-18
41. Araki M, Aranami T, Matsuoka T, Nakamura M, Miyake S, Yamamura T. Clinical improvement in a patient with neuromyelitis optica following therapy with the anti-IL-6 receptor monoclonal antibody tocilizumab. Mod Rheumatol. (2013) 23:827–31. doi: 10.3109/s10165-012-0715-9
42. Waters P, Pittock S, Bennett J, Jarius S, Weinshenker B, Wingerchuk D. Evaluation of aquaporin-4 antibody assays. Clin Exp Neuroimmunol. (2014) 5:290–303. doi: 10.1111/cen3.12107
43. Dinarello C. Interleukin-1 in the pathogenesis and treatment of inflammatory diseases. Blood. (2011) 117:3720–32. doi: 10.1182/blood-2010-07-273417
44. Kitic M, Hochmeister S, Wimmer I, Bauer J, Misu T, Mader S, et al. Intrastriatal injection of interleukin-1 beta triggers the formation of neuromyelitis optica-like lesions in NMO-IgG seropositive rats. Acta Neuropathol Commun. (2013) 1:5. doi: 10.1186/2051-5960-1-5
45. Mitsdoerffer M, Kuchroo V, Korn T. Immunology of neuromyelitis optica: a T cell-B cell collaboration. Ann N Y Acad Sci. (2013) 1283:57–66. doi: 10.1111/nyas.12118
46. Murphy A, Chin-Dusting J, Sviridov D, Woollard K. The anti inflammatory effects of high density lipoproteins. Curr Med Chem. (2009) 16:667–75. doi: 10.2174/092986709787458425
47. Murphy A, Woollard K. High-density lipoprotein: a potent inhibitor of inflammation. Clin Exp Pharmacol Physiol. (2010) 37:710–8. doi: 10.1111/j.1440-1681.2009.05338.x
48. Murphy A, Woollard K, Hoang A, Mukhamedova N, Stirzaker R, McCormick S, et al. High-density lipoprotein reduces the human monocyte inflammatory response. Arterioscler Thromb Vasc Biol. (2008) 28:2071–7. doi: 10.1161/ATVBAHA.108.168690
49. Hyka N, Dayer J, Modoux C, Kohno T, Edwards C, Roux-Lombard P, et al. Apolipoprotein A-I inhibits the production of interleukin-1beta and tumor necrosis factor-alpha by blocking contact-mediated activation of monocytes by T lymphocytes. Blood. (2001) 97:2381–9. doi: 10.1182/blood.V97.8.2381
50. Bukhari W, Barnett M, Prain K, Broadley S. Molecular pathogenesis of neuromyelitis optica. Int J Mol Sci. (2012) 13:12970–93. doi: 10.3390/ijms131012970
51. Ito J, Nagayasu Y, Kato K, Sato R, Yokoyama S. Apolipoprotein A-I induces translocation of cholesterol, phospholipid, and caveolin-1 to cytosol in rat astrocytes. J Biol Chem. (2002) 277:7929–35. doi: 10.1074/jbc.M103878200
52. Li Y, Wang H, Hu X, Peng F, Yang Y. Serum lipoprotein levels in patients with neuromyelitis optica elevated but had little correlation with clinical presentations. Clin Neurol Neurosurg. (2010) 112:478–81. doi: 10.1016/j.clineuro.2010.03.017
53. Liu Z, Fan Q, Wu S, Wan Y, Lei Y. Compared with the monocyte to high-density lipoprotein ratio (MHR) and the neutrophil to lymphocyte ratio (NLR), the neutrophil to high-density lipoprotein ratio (NHR) is more valuable for assessing the inflammatory process in Parkinson's disease. Lipids Health Dis. (2021) 20:35. doi: 10.1186/s12944-021-01462-4
54. Omar T, Karakayali M, Yesin M, Alaydin H, Karabag Y, Gümüşdag A. Monocyte to high-density lipoprotein cholesterol ratio is associated with the presence of carotid artery disease in acute ischemic stroke. Biomark Med. (2021) 15:489–95. doi: 10.2217/bmm-2020-0705
55. Battaglia S, Scialpi N, Berardi E, Antonica G, Suppressa P, Diella F, et al. Gender, BMI and fasting hyperglycaemia influence Monocyte to-HDL ratio (MHR) index in metabolic subjects. PLoS ONE. (2020) 15:e0231927. doi: 10.1371/journal.pone.0231927
56. D'Amico E, Zanghì A, Romano A, Sciandra M, Palumbo G, Patti F. The neutrophil-to-lymphocyte ratio is related to disease activity in relapsing remitting multiple sclerosis. Cells. (2019) 8:1114. doi: 10.3390/cells8101114
57. Gelibter S, Pisa M, Croese T, Dalla Costa G, Orrico M, Preziosa P, et al. Neutrophil-to-lymphocyte ratio: a marker of neuro-inflammation in multiple sclerosis? J Neurol. (2021) 268:717–23. doi: 10.1007/s00415-020-10322-7
58. Zhou Y, Xie H, Zhao Y, Zhang J, Li Y, Duan R, et al. Neutrophil-to-lymphocyte ratio on admission is an independent risk factor for the severity of neurological impairment at disease onset in patients with a first episode of Neuromyelitis Optica spectrum disorder. Neuropsychiatr Dis Treat. (2021) 17:1493–503. doi: 10.2147/NDT.S311942
59. Bonnan M, Debeugny S, Mejdoubi M, Cabre P. Predictive value of conventional MRI parameters in first spinal attacks of neuromyelitis optica spectrum disorder. Mult Scler. (2020) 26:468–75. doi: 10.1177/1352458519834857
60. Shu Y, Li R, Qiu W, Chang Y, Sun X, Fang L, et al. Association of serum gamma-glutamyltransferase and C-reactive proteins with neuromyelitis optica and multiple sclerosis. Mult Scler Relat Disord. (2017) 18:65–70. doi: 10.1016/j.msard.2017.09.021
Keywords: neuromyelitis optica spectrum disorders, monocyte to high-density lipoprotein ratio, prognosis, severity, expanded disability status scale
Citation: Zhang J, Li Y, Zhou Y, Wang K, Pan C, Zhao Y, Xie H, Duan R, Gong Z and Jia Y (2021) Monocyte to High-Density Lipoprotein Ratio: A Novel Predictive Marker of Disease Severity and Prognosis in Patients With Neuromyelitis Optica Spectrum Disorders. Front. Neurol. 12:763793. doi: 10.3389/fneur.2021.763793
Received: 24 August 2021; Accepted: 28 September 2021;
Published: 27 October 2021.
Edited by:
Hans-Peter Hartung, Heinrich Heine University of Düsseldorf, GermanyReviewed by:
Emanuele D'amico, University of Catania, ItalyShuhei Nishiyama, Massachusetts General Hospital and Harvard Medical School, United States
Copyright © 2021 Zhang, Li, Zhou, Wang, Pan, Zhao, Xie, Duan, Gong and Jia. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yanjie Jia, amlheWFuamllMTk3MUB6enUuZWR1LmNu
†These authors have contributed equally to this work and share first authorship
 Jinwei Zhang
Jinwei Zhang Yanfei Li
Yanfei Li Yongyan Zhou
Yongyan Zhou Kaixin Wang
Kaixin Wang Chunyang Pan
Chunyang Pan Yi Zhao
Yi Zhao Haojie Xie
Haojie Xie Ranran Duan
Ranran Duan Yanjie Jia
Yanjie Jia