
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Neurol. , 22 October 2021
Sec. Stroke
Volume 12 - 2021 | https://doi.org/10.3389/fneur.2021.754204
 Amy Brodtmann1,2,3*
Amy Brodtmann1,2,3* Emilio Werden1
Emilio Werden1 Mohamed Salah Khlif1
Mohamed Salah Khlif1 Laura J. Bird1
Laura J. Bird1 Natalia Egorova1,2,4
Natalia Egorova1,2,4 Michele Veldsman1,5
Michele Veldsman1,5 Heath Pardoe6
Heath Pardoe6 Graeme Jackson1,3
Graeme Jackson1,3 Jennifer Bradshaw7
Jennifer Bradshaw7 David Darby1,2
David Darby1,2 Toby Cumming1
Toby Cumming1 Leonid Churilov1,3†
Leonid Churilov1,3† Geoffrey Donnan1,3†
Geoffrey Donnan1,3†Background: Stroke survivors are at high risk of dementia, associated with increasing age and vascular burden and with pre-existing cognitive impairment, older age. Brain atrophy patterns are recognised as signatures of neurodegenerative conditions, but the natural history of brain atrophy after stroke remains poorly described. We sought to determine whether stroke survivors who were cognitively normal at time of stroke had greater total brain (TBV) and hippocampal volume (HV) loss over 3 years than controls. We examined whether stroke survivors who were cognitively impaired (CI) at 3 months following their stroke had greater brain volume loss than cognitively normal (CN) stroke participants over the next 3 years.
Methods: Cognition And Neocortical Volume After Stroke (CANVAS) study is a multi-centre cohort study of first-ever or recurrent adult ischaemic stroke participants compared to age- and sex-matched community controls. Participants were followed with MRI and cognitive assessments over 3 years and were free of a history of cognitive impairment or decline at inclusion. Our primary outcome measure was TBV change between 3 months and 3 years; secondary outcomes were TBV and HV change comparing CI and CN participants. We investigated associations between group status and brain volume change using a baseline-volume adjusted linear regression model with robust standard error.
Results: Ninety-three stroke (26 women, 66.7 ± 12 years) and 39 control participants (15 women, 68.7 ± 7 years) were available at 3 years. TBV loss in stroke patients was greater than controls: stroke mean (M) = 20.3 cm3 ± SD 14.8 cm3; controls M = 14.2 cm3 ± SD 13.2 cm3; [adjusted mean difference 7.88 95%CI (2.84, 12.91) p-value = 0.002]. TBV decline was greater in those stroke participants who were cognitively impaired (M = 30.7 cm3; SD = 14.2 cm3) at 3 months (M = 19.6 cm3; SD = 13.8 cm3); [adjusted mean difference 10.42; 95%CI (3.04, 17.80), p-value = 0.006]. No statistically significant differences in HV change were observed.
Conclusions: Ischaemic stroke survivors exhibit greater neurodegeneration compared to stroke-free controls. Brain atrophy is greater in stroke participants who were cognitively impaired early after their stroke. Early cognitive impairment was associated greater subsequent atrophy, reflecting the combined impacts of stroke and vascular brain burden. Atrophy rates could serve as a useful biomarker for trials testing interventions to reduce post-stroke secondary neurodegeneration.
Clinical Trail Registration: http://www.clinicaltrials.gov, identifier: NCT02205424.
Stroke care has been transformed in the last three decades by improved reperfusion treatments and rehabilitation therapies. Despite this, the global burden of stroke remains high, in part due to long-term cognitive impairments and increased risk of dementia (1). Cumulative vascular risk factors increase dementia risk (2, 3); conversely, good cardiovascular health reduces this risk (4). Risk of stroke and of cognitive decline are also conflated: cognitive impairment can anticipate the development of incident stroke (5) and be a manifestation of this risk (6). One of the strongest risk factors for post-stroke cognitive impairment is a history of pre-stroke cognitive or functional decline (7).
Brain atrophy precedes and predicts cognitive decline in many neurodegenerative syndromes, but the trajectories of brain volume loss and cognitive impairment in stroke survivors are poorly understood. These have been difficult to disentangle given relatively few post-stroke longitudinal cohort studies with high-quality serial imaging and cognitive data. Prior studies also often included people with pre-stroke cognitive impairment and dementia, further complicating associations. Structural brain changes are already present at the time of stroke, including smaller hippocampal volumes and increased white matter hyperintensity (WMH) volumes (8). It has been demonstrated that both greater brain atrophy and increased WMH are associated with risk of future stroke (9). We have demonstrated that stroke survivors have greater predicted brain age (10), extensive white matter degeneration at 3 months after stroke (11), and hippocampal, thalamic and ipsilesional hemispheric atrophy that continues over the first post-stroke year (12).
Brain atrophy rates are now being used as biomarkers for clinical trials in a number of neurological disorders (13, 14), including dementia (15). Regional atrophy rates have been shown to correlate with other markers of neurodegeneration, including CSF and blood biomarkers (16). Annualised hippocampal and total brain atrophy rates have been described in normal ageing and dementia syndromes (17–20), as well as being used as outcome measures for treatment response in clinical trials (15, 21). While the neuroimaging signatures of AD and other neurodegenerative diseases are now well-described (22–25), there are few data in ischaemic stroke survivors.
Understanding the trajectories of brain volume loss in people with vascular contributions to their cognitive profile has important clinical implications. Mixed neuropathologies are ubiquitous in late-life dementias (26), and a greater understanding of vascular neurodegenerative imaging signatures would assist in diagnostic assignment and clinical treatment. Total and regional brain atrophy rates may serve as useful tools to assess treatment response for interventions to reduce post-stroke secondary degeneration and vascular cognitive impairment, especially as most recent multimodal intervention trials for dementia prevention largely targeted cardiovascular risk (27, 28). An imaging biomarker has several advantages over more traditionally used cognitive assessments, as atrophy rates are not as dependent on language, educational attainment, culture, or socio-economic status as canonical cognitive tests (29, 30).
We aimed to determine whether ischaemic stroke was associated with progressive neurodegeneration via a prospective, 3-year cohort study comparing participants with ischaemic stroke to their age- and sex-matched healthy controls. Our aim in this study was to test for associations between group status: total brain volume in stroke vs. control participants for our primary hypothesis, brain volume change in those stroke participants with cognitive impairment vs. normal cognition at 3 months in our secondary hypothesis. We included participants with no history of cognitive impairment or decline, cross-checked via interview with family members and treating health practitioners. In addition, we aimed to determine whether stroke survivors with cognitive impairment at 3 months exhibited greater brain atrophy over the subsequent 3 years compared to those who were cognitively normal. We hypothesised that stroke survivors would exhibit greater total brain volume loss than controls, and that those who were cognitively impaired at 3 months after stroke would exhibit greater brain volume loss than those who were cognitively normal.
We received ethics approval from Human Research Ethics Committees at each of the participating hospitals. All participants provided written consent in person in accordance with the Declaration of Helsinki.
The Cognition And Neocortical Volume Study (CANVAS) study is a multi-centre cohort study including patients with ischaemic stroke and healthy controls and tested with serial MRI scanning and cognitive testing over 3 years (31).
Patients were recruited between April 2012 and July 2015 from the Stroke Units at three University teaching hospitals in Melbourne, Australia: Austin Hospital, Box Hill Hospital, and Royal Melbourne Hospital. Control participants were recruited until September 2015 to optimise age- and sex-matching. All testing and brain imaging were done at the Melbourne Brain Centre, Austin Hospital campus.
Stroke survivors with first-ever clinical or recurrent ischaemic stroke and no history of dementia were recruited within the first 3 months of their stroke. They were approached for recruitment as inpatients or via phone interview once discharged. They were included if they had an ischaemic stroke of any stroke subtype (7), and excluded if there was pre-existing cognitive impairment (based on participant, primary care practitioner, and informant); could not have MRI (e.g., implanted metal, severe orthopnoea); had primary haemorrhagic stroke, TIA, or no clinically confirmed stroke; or were unlikely to survive 3 years due to severe medical illness.
Healthy age- and sex-matched control participants with no history of cognitive impairment were recruited from a pool of community participants who had previously volunteered in MRI studies and from local community groups. Spouses and age-appropriate family members of stroke participants were also approached to attempt socioeconomic matching. Inclusion and exclusion criteria were the same as for stroke patients, except for the stroke diagnosis.
We obtained information via interview: age, years of education, handedness, marital status, stroke and dementia family history, smoking pack years (number of cigarettes per day multiplied by years of smoking), alcohol consumption (low ≤14; high > standard drinks/week); history of depression, hypertension, type 2 diabetes mellitus (T2DM), hypercholesterolemia and atrial fibrillation (AF) given either via physician diagnosis or medication use. Body mass index [BMI; kg/m2; low <25, high (≥25)] was calculated using weight and height measurements.
We obtained information of stroke side, use of thrombolysis, admission stroke severity [National Institute of Health Stroke Scale (NIHSS)] score, aetiology (32) and subtype (Oxfordshire criteria) (33). We used the modified Rankin Scale (34) (mRS) as an estimate of neurological disability and the Charlson Comorbidity Index (CCI) (35), a validated co-morbidity score, as an estimate of general medical comorbidity, removing the stroke score for people with stroke.
Venous blood was drawn for APOE genotype determination on participants who consented to DNA analyses and storage. Individuals were categorised as APOE ε4 carriers or non-carriers.
All participants were scanned on the same MRI scanner which did not undergo significant hardware or software upgrades over the study period. Whole brain images were acquired on a single 3T Siemens Tim Trio Scanner with a 12-channel head coil (Siemens, Erlangen, Germany) (please see https://www.ahajournals.org/journal/str for details). All images were visually inspected for quality control before processing using automated pipelines and excluded if degraded by motion or other artefacts. Cortical reconstruction and volumetric segmentation on MPRAGE images were performed using the longitudinal stream (http://surfer.nmr.mgh.harvard.edu/fswiki/LongitudinalProcessing) in FreeSurfer V6.0 (36). Hippocampal results were based on averages of left and right hippocampal volumes.
Stroke lesions were traced by our imaging analyst (MSK) and cross-checked by a stroke neurologist (AB). Stroke lesion sites were also cross-checked with acute inpatient imaging, particularly their DWI lesion site on acute MRI where available. We did not adjust for stroke lesion volume as its association with post-stroke brain atrophy rates and cognition is not known (7), and because it was fully correlated with stroke status (zero volume in controls); therefore, a feature not an artefact. We understand that there are vigorous arguments both for and against lesion volume adjustment. WMH probability maps were obtained from FLAIR images using the lesion prediction algorithm (37) from the lesion segmentation toolbox included in SPM12.
We used the National Adult Reading Test [NART (38)] to estimate pre-morbid IQ and the Informant Questionnaire on Cognitive Decline in the Elderly [IQCODE-Short Form (39)] to estimate pre-morbid general cognitive functioning and to probe for the presence of preceding undiagnosed cognitive decline. Symptoms of anxiety and depression were examined at each session using the Generalised Anxiety Disorder-7 [GAD-7 (40)] scale and Patient Health Questionnaire-9 [PHQ-9 (41)] together with a clinical interview.
Neuropsychological testing was done in a single session allowing time for breaks. The cognitive testing protocol has been previously described (31), and included: Hopkins Verbal Learning Test-Revised [HVLT-R (42)]; Detection, Identification and One-Back computerised tests from the CogState Battery (43); Rey-Osterrieth Complex Figure; Star Cancellation Task; Verbal Fluency Task (FAS and Animals); Trail-Making Test A and B; Digit Span and Digit-Symbol Tasks from the Weschler Adult Intelligence Scale-Third Edition [WAIS-III (44)]; Token Test 16-item version; Boston Naming Test (45); and Clock Drawing Test. Age-appropriate normative values (mean, standard deviation) were used where available to create z-scores for each cognitive task.
A composite z-score was created for each cognitive domain by averaging z-scores across tasks: attention (focused attention, working memory, processing speed), executive function, memory, language, and visuospatial function – see Table 1. These groupings were via consensus decision after round-table discussions and input from our cognitive neurologists (A.B., D.D.), psychologist (L.B.) and neuropsychologists (J.B., E.W., T.C.).
A weighted Global Clinical Dementia Rating (CDR) Score (47) was performed at the 3-year post-stroke assessment to examine for functional decline.
Participants were assigned their status of normal cognition (CN), cognitively impaired (CI), or dementia, in these panel meetings. We also allowed the assignment of unclassifiable if the participant was unable to complete sufficient cognitive testing and/or no history was obtained from an informant.
A participant was assigned cognitively normal (CN) if z-scores in all cognitive domains were within accepted age- and years of education-adjusted norms and there was no evidence of functional decline due to cognitive impairment (i.e., activities of daily living (ADLs) were unaffected).
A participant was judged to be CI if (1) the z-score for at least one cognitive domain was lower than −1.5, and (2) there was no evidence of functional decline due to cognitive impairment (ADLs were unaffected).
A participant was judged to have dementia if (1) z-scores for two or more cognitive domains were lower than −1.5 and (2) there was evidence of functional decline (i.e., significant impact on activities of daily living between time-points due to cognitive impairment, as demonstrated by (a) discussions with next-of-kin and the Short-Form IQCODE, and/or (b) CDR score of 1 or greater.
We note that a threshold of z-score >−1.5 is a standard cut-point for most neuropsychological tests, as an accepted threshold for decline or impairment for those tests with extensive normative data. While continuous z-scores may be better approach for assessing cognitive trajectories and longitudinal change, we emphasise that the 3-month allocation was a cross-sectional one to divide the groups into impaired/not impaired, therefore allowing examination of the association between early cognitive impairment and 3-year brain volume change.
Our primary outcome measure was total brain volume (TBV) change between the 3-month and 3-year time-points compared between stroke patients and controls. TBV and CCI were chosen a priori as the adjustment covariates, as male sex is associated with larger heads (48), and both medical co-morbidities (2) and age (48) are associated with brain atrophy.
Secondary outcome 1 was TBV change between 3-months and 3-years comparing CN and CI stroke participants. We adjusted for TBV at 3-months, CCI scores, and years of education; the latter as it is correlated with cognitive performance and post-stroke dementia risk (7). Cognitive status at 3 months, not 3 years, was included in our model. Three-year cognitive outcomes were not included in this model as we did not perform predictive modelling for 3-year cognitive outcomes. These outcomes will be utilised in future analyses examining associations between imaging metrics and risk factors and will be available for future pooled analyses to allow adequate power for predictive modelling.
Secondary outcome 2 was hippocampal volume (HV) change between 3-months and 3-years in stroke patients and controls with adjustments identical to primary outcome.
Secondary outcome 3 was the comparison of HV change between 3-months and 3-years comparing CN and CI stroke participants with adjustments identical to secondary outcome 1.
We calculated our sample size to include sufficient participants for both the primary outcome and secondary outcome 1. We based our brain volume loss estimates on pilot (49) and published data (48, 50). We estimated 35 participants per group yielding power of 0.8 assuming two-sided alpha of 0.05. For the CI vs. CN comparison, we estimated an expected prevalence of cognitive impairment of 30% at 3 months (7). We used an ANCOVA method to estimate sample size for two samples with repeated measures (CN vs. CI stroke in a ratio of 2:1), including a correlation score between baseline and follow-up. Using an alpha level of 0.05 (two-sided), power = 0.8, and correlation = 0.1, we estimated that 108 participants would be required (36 CI and 72 CN), with predicted 20% attrition due to death or non-participation, giving a total 135 stroke and 40 control participants.
Our statistical analysis plan was formulated prior to study database lock. All missing data were assumed to be missing-at-random following deliberations by the Cognitive Outcome Evaluation Committee prior to statistical analysis. After cognitive group allocation and analyses, we compared demographic and stroke characteristics in stroke patients who completed the 3-month and 3-year review sessions with those who withdrew from the study or were unable to attend study visits (Supplementary Tables II, III).
Patient characteristics were summarised as either means and SD, or medians and interquartile ranges (IQRs), for continuous variables; and counts (proportions) for categorical variables. They were compared between groups using t-test, Mann-Whitney-test, or chi-square/Fisher Exact-test depending on the distribution. We conducted our primary complete case analysis on the sub-sample of participants with data available at 3 months and 3 years.
We used a linear regression model with robust standard error estimation to investigate the primary outcome for the complete case analysis: TBV change between 3-months and 3-years as the dependent variable, group (stroke vs. control) as independent variable, and TBV and CCI at 3 months as covariates. The same approach was used for Secondary Outcome 1 using CN vs. CI group, and the inclusion of years of education as covariate.
We applied the same models for Secondary Outcomes 2 and 3 but used HV change as our dependent variable.
We conducted further sensitivity analysis on the sub-sample of participants who had 3-month data irrespective of 3-year data availability under the assumption of missing-at-random. This sensitivity analysis was conducted using linear mixed-effects modelling with specific outcomes of interest as output variables; individual group, time-points, and multiplicative group-by-time interaction term as independent variables; adjustment covariates as specified above; and individual participants as random effects.
Analyses were done in Stata version 15.0 (StataCorp, College Station, TX, USA). Two-sided p-values < 0.05 were regarded as indicative of statistical significance. No correction for multiplicity of comparisons to limit family-wise Type I error rate was undertaken.
Over the study period, 3,037 stroke patients were admitted to the participating stroke units and screened for potential study recruitment; 2,659 did not meet study criteria (i.e., haemorrhagic stroke, prior history of dementia, severe stroke making 3-year survival unlikely, etc.). Of the 378 potential participants, 203 declined study participation, 40 were not included for other reasons (e.g., rural or regional participant unable to travel to the scanner). We recruited 135 stroke participants (Supplementary Figure I).
Nine were not available at the 3-month study visit (two returned at 3 years). One-hundred and 26 stroke patients attended their testing session at 3 months: four had incomplete or non-evaluable MRI scans; one had incomplete cognitive testing. A total of 122 were available for primary outcome analyses and a total of 121 participants had complete data sets at 3 months (Table 2).
At 3 years, 15 stroke participants had died, and 14 had moved interstate, were uncontactable, or withdrew for other reasons (n = 29; Supplementary Tables I, III); 102 stroke participants returned for their study visit but nine had non-evaluable scans, meaning a total of 93 were included for the primary outcome analysis at 3 years; 92 were included for secondary outcomes 1 and 3 as one stroke participant had an incomplete cognitive assessment at 3 months (Table 3; Supplementary Tables II, III). Our attrition rate was 19% for stroke participants (102/126), in line with the 20% predicted for our analyses.
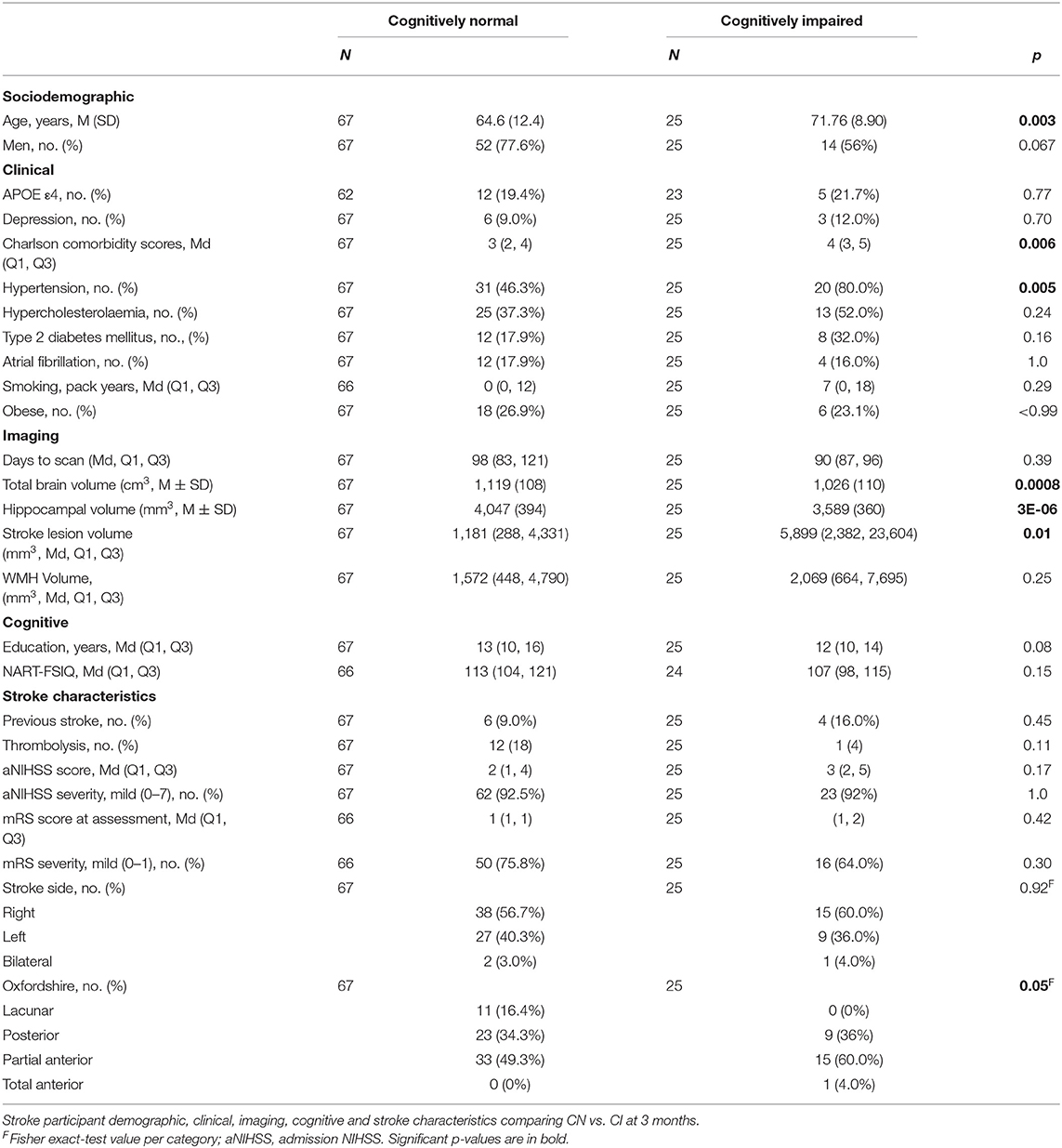
Table 3. Characteristics of stroke participants who were cognitively normal vs. cognitively impaired at 3-month visit.
We examined for attendance and evaluable scan bias following database lock. Incomplete data sets were associated with obesity at both time-points, and with older age and history of hypertension at 3 years (Supplementary Tables II, III), but not with admission stroke severity.
One-hundred and forty-six control participants were screened for study participation: 18 did not meet inclusion criteria, 22 declined participation and 66 did not respond to our invitation to participate. Forty control participants were included in the 3-month assessment and 39 were available for the 3-year assessment as one participant withdrew. Two participants had silent lesions noted on their research MRI scans, but no stroke diagnosis after clinical review, remaining in the study.
All control participants were cognitively normal at 3 months and 3 years. At 3 months, there were 80 CN and 41 CI stroke participants (Table 3). At 3 years, there were 73 CN, 16 CI and 4 dementia stroke participants, which included 67 CN and 25 CI assigned at the 3-month visit. Significantly more (p = 0.016) 3-month CI participants died or did not attend their 3-year visit (34%) than CN (14%). Note that cognitive status groupings were done at the 3-month timepoint, not at 3 years, when there were 4 participants with dementia.
No differences in age and sex were observed between groups at 3 months and 3 years.
Strokes were in all vascular territories (Supplementary Figure II). No differences in stroke characteristics were observed between stroke participants available at 3 months and 3 years (Supplementary Tables I, III).
At 3 months, stroke participants had higher CCI scores than controls and were more likely to have a history of T2DM, hypertension, smoking and atrial fibrillation (Table 2). At 3 years, stroke participants had more atrial fibrillation than controls.
Cognitive impairment at 3 months was associated with greater age (71.8 ± 8.9 years CI vs. 64.6 ± 12.4 CN), higher CCI (4 CI vs. 3 CN) and hypertension (80% CI vs. 46% CN; Table 3).
Control participants had significantly greater years of education and higher NART-FSIQ scores compared to stroke participants (Table 2). No associations were observed between years of education and NART-FSIQ and cognitive impairment in stroke participants (Table 3).
Stroke participants had smaller HV than controls at 3 months (3,862 ± 490 mm3 vs. 4,032 ± 343 mm3, Table 2) and higher WMH volumes than controls at both time-points (e.g., 3-month median stroke = 866 mm3, controls = 684 mm3, p = 0.0001; Table 2; Supplementary Tables).
Cognitively impaired stroke participants had smaller TBV (CN = 1,119 ± 108 cm3, CI = 1,026 ± 110 cm3) and HV (CN = 4,047 ± 394 mm3, CI = 3,589 ± 360 mm3) and larger stroke lesion volumes (CN = 1,181 mm3, CI = 5,899 mm3) but not WMH volume (median CN = 1,572 mm3, CI = 2,069 mm3) than cognitively normal participants. Cognitive impairment was not associated with admission NIHSS, prior stroke, or side of stroke (Table 3). We observed more large-artery embolic and less lacunar strokes in the CI group.
Figure 1 and Table 4 demonstrate that both groups lost TBV between 3-months and 3-years, but TBV loss in stroke participants (n = 93, 26 women, 66.7 ± 12 years) was significantly greater than controls (n = 39, 15 women, 68.7 ± 6.7 years): stroke mean 20.31 cm3 ± SD 14.8 cm3; controls mean 14.22 cm3 ± SD 13.21 cm3; adjusted mean difference 7.88 95% CI (2.84, 12.91) p-value 0.002. This was confirmed on sensitivity analysis (n = 122 stroke, n = 40 controls, p-value for group-by-time interaction 0.002).
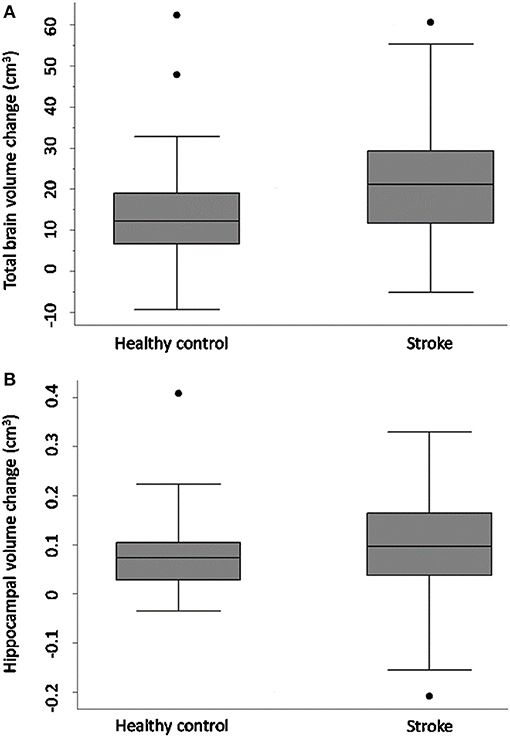
Figure 1. Total brain and hippocampal volume change stroke vs. controls. Total brain volume (A) and hippocampal volume (B) change between 3-months and 3-years in stroke patients and healthy controls. Y-axis = change in brain volume (cm3). Positive values represent decline in brain volumes: i.e., greater brain volume loss. Midline = median brain volume change. X-axis: n = 93 stroke, n = 39 healthy controls.
Figure 2 shows that TBV decline was greater in the CI group (mean = 30.67 ± SD 14.18 cm3) relative to the CN group (mean = 19.63 ± 13.84 cm3); adjusted mean difference [10.42; 95% CI (3.04, 17.80), p-value = 0.006; Figure 2], confirmed on sensitivity analysis (CN n = 80, CI n = 41, p-value for group-by-time interaction 0.001).
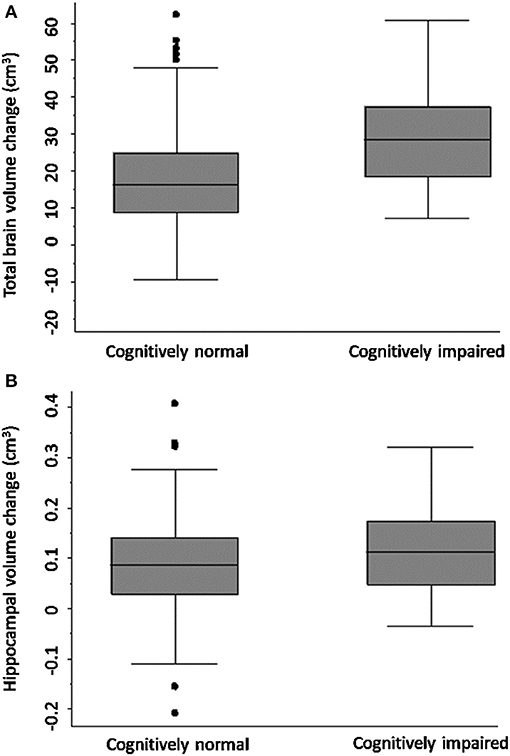
Figure 2. Total brain and hippocampal volume change cognitively normal vs. impaired. Total brain volume (A) and hippocampal volume (B) change between 3-months and 3-years post-stroke in CN and CI stroke groups determined at 3-months. Y-axis = change in brain volume (cm3). Positive values represent decline in brain volume, negative values represent an increase in brain volume. Midline = median brain volume change.
HV change was comparable in both stroke and control participants [stroke n = 93, controls n = 39, complete case analysis adjusted mean difference 0.02, 95% CI (−0.02, 0.05), p = 0.32; sensitivity analysis: n = 40 controls, n = 122 strokes, p-value for group-by-time interaction 0.26]. No significant difference in HV change was observed between CN and CI stroke participants [complete case analysis adjusted mean difference 0.01 (−0.04, 0.06), p-value = 0.61; sensitivity analysis: CN n = 80 CI, n = 41, p-value for group-by-time interaction 0.41].
We report the primary and secondary outcomes from CANVAS, a prospective cohort study of ischaemic stroke patients compared to age- and sex-matched controls where all participants were cognitively normal at recruitment and all ischaemic stroke subtypes were included. We found that TBV loss in stroke participants over 3 years was significantly greater than controls. Further, TBV loss over 3 years was greater in stroke patients who were cognitively impaired at 3 months compared to those without cognitive impairment. We did not find these associations for hippocampal volume loss, although cognitively impaired participants had smaller hippocampi than those who were cognitively normal.
No participants had a history of cognitive decline prior to study entry. One-third of our stroke participants were cognitively impaired 3 months after stroke, and 26% were impaired at 3 years, but more participants who were cognitively impaired at 3 months died or did not attend the 3-year visit. In addition, we found an association with higher BMI and incomplete data sets. Cognitively impaired stroke participants at 3 months had smaller brain volumes, larger stroke lesion volumes, more vascular risk factors, and higher CCI scores, but not WMH volume, than those who were cognitively normal.
The lack of difference between WMH volume in cognitively normal and cognitively impaired stroke participants at 3 months is perhaps a surprising null finding. There are several possible explanations. Our stroke participants are already at high risk of greater WMH burden due to their vascular risk factors, and we demonstrated that they have greater WMH volume than control participants. In addition, our study was powered for our primary hypothesis and first secondary hypothesis using TBV, and it is likely that we were underpowered to detect difference in WMH volume between the cognitively normal and cognitively impaired groups. We would argue that WMH volume is perhaps better than visual rating scales of WMH severity, due to its objectivity and more quantitative nature. We have reported on WMH estimation in prior papers, and note that even using current methods, the impact of WMH on white matter integrity may be underestimated (51).
Hippocampal atrophy is strongly associated with vascular risk factors. We have previously reported smaller hippocampi in stroke than control participants at baseline (8), and shown that atrophy rates are greater in 3 months after stroke (12) than the ensuing 9 months. However, we did not find that stroke hippocampal atrophy rates were greater over 3 years than controls, nor did we find an association with cognitive impairment. This could be because most of the hippocampal atrophy had already occurred – a potential floor effect. Many authors have proposed that incipient Alzheimer's pathology is contributing to the hippocampal atrophy, and that pre-existing protein deposition leads to the observed atrophy. However, this has not been borne out on PET amyloid imaging studies of stroke (52, 53), nor did we find an association between hippocampal volume and amyloid status in a small PET amyloid sub-study at 3 years (54). Vascular risk factors are increasingly considered as primary drivers of hippocampal degeneration (11, 12).
Also, the lack of association between hippocampal atrophy and post-stroke cognitive impairment may not be surprising when viewed in the clinical context of vascular cognitive deficits. We did not use paired associate measures as our verbal memory test, which are more sensitive to hippocampal dysfunction. Vascular cognitive impairment is not characterised by the primary amnestic deficits seen in clinical Alzheimer's dementia. Rather, it is characterised by slowed speed of processing, memory retrieval problems, behavioural, attention and executive dysfunction, which are dependent on normal thalamic and frontal white matter tract and cortical function. Thalamic dysfunction has been implicated in the clinical impairments seen after stroke, with ipsi- and contralesional thalamic atrophy over the first year reported (12). In addition, we have previously reported that hippocampal subfield atrophy correlated better with verbal memory impairment in this cohort, suggesting that whole hippocampal atrophy may not be the metric of choice in post-stroke cognitive impairment (55).
The observed reduction in TBV in stroke survivors and the strong association with greater TBV loss in those who were cognitively impaired at 3 months are novel. Imaging measures of global brain volume necessarily capture both grey and white matter atrophy. The latter is often underestimated in histopathological studies, as white matter atrophy is hard to estimate histologically. Advances in neuroimaging have allowed us to chart white matter loss after stroke (11). The frontal lobes are especially vulnerable to vascular brain burden (56), and attention and executive function are dependent on the integrity of these frontal networks. We have demonstrated degeneration of the frontal distributed brain networks subserving attention and executive function 1 year after stroke (56, 57). The deficits associated with vascular cognitive impairments could be posited as disconnection syndromes, arising both from the stroke lesions and from concomitant white matter disease.
It is likely that there are multiple mechanisms underlying this white matter degeneration in stroke populations, but we know that brain infarction triggers extensive, pervasive neuroinflammation (58). Microglial activation occurs within brain networks involving the infarct, including the thalami bilaterally (58). These sustained inflammatory responses may contribute to the Wallerian degeneration in tracts connected to the stroke. This ongoing grey and white matter degeneration (11, 12), captured in our study by the increased TBV loss seen in the stroke group, may manifest clinically as cognitive decline.
Our findings further our understanding of post-stroke neurodegeneration and open a window into potential mechanisms. They also have important clinical implications. Post-stroke outcomes are usually measured at 3 months after stroke. The focus of stroke research in the last two decades has been on penumbral salvage and therapies that enhance reperfusion and recanalisation – especially thrombolytic therapies and endovascular clot retrieval (59–62). There is no doubt that these treatments have transformed stroke care. However, stroke clinicians are now shifting their focus to improvements in rehabilitation and post-acute care (63–66). Post-stroke cognitive impairment is identified as a key priority area for research and support by stroke survivors, but predictive models for individualised risk factors for cognitive decline are sorely needed (67). In other neurodegenerative diseases causing dementia, there is a strong association between brain atrophy and cognitive decline: neurodegeneration precedes and predicts the onset of cognitive impairment (68–71). Researchers are now seeking interventions to prevent post-stroke cognitive decline (14, 72, 73). The description of brain atrophy rates following stroke allows the use of atrophy rates as a potentially modifiable imaging biomarker that could be used in association with other more traditionally used outcome measures of function.
Study strengths include the strongly positive primary hypothesis, and our prospective, observational control-cohort design with identical study visits, imaging protocols and cognitive tasks for all participants. Our high-quality imaging, multi-domain approach to cognition, expected attrition rate and documented missing data are also strengths. Study weaknesses include the small numbers compared to large community-based studies, incomplete data sets associated with older age, relatively mild, mainly male, stroke participants, and our healthier, more educated control participants – a common problem with volunteer bias, especially imaging studies (74). The mismatch between years of education and NART is an important limitation, as it is well-described that less education is associated with higher risk of dementia; more education is associated with a lower risk and greater cognitive reserve (75). Educational attainment is also impacted by geography and socioeconomic status. However, we would emphasise that while overall our stroke participants had less years of education than our controls (stroke 12 years vs. controls 17 years, p = 0.0005), there was no difference in educational attainment between those stroke participants with cognitive impairment vs. those who were cognitively normal (cognitively normal 13 years vs. 12 years for impaired, p = 0.08).
Like many cohort studies with long follow-up, our exclusion criteria were biased towards participants who would be likely to be alive in 3 years. In addition, the rigorous exclusion of prior cognitive decline or impairment immediately reduced a third of stroke ward admissions, especially with the exclusion of prior psychiatric or neurological disease. Another group of potential participants was excluded on the basis of insufficient English proficiency (all participants had to have English proficiency as the administered cognitive tests were all normed on English speakers). This is a limitation in our study and biased our recruitment towards milder stroke in people without severe medical illness. In addition, it can be very difficult to match vascular risk factors in stroke-free controls, as these same risk factors obviously increase their chances of incidental or concomitant stroke. Unlike some other, larger studies, our primary objective was not to examine determinants of post-stroke dementia, and therefore we were not powered to look at predictors of cognitive decline and dementia at 3 years, but plan to pool these data with larger prospective studies including stroke participants alone.
Ischaemic stroke survivors exhibit greater neurodegeneration compared to stroke-free controls. Atrophy is greater in those who are cognitively impaired early after stroke. The coupling of early cognitive impairment and greater subsequent atrophy likely reflects the combined impacts of stroke and vascular brain burden. Our results help to disentangle the complex interactions between incipient cognitive decline, the cumulative impact of vascular risk factors, and the effect of the super-added stroke lesion. These brain atrophy rates can be used for future intervention trials to reduce post-stroke neurodegeneration.
The data collected for this study is available on request to the authors. The study protocol was published in 2014 and is freely available. Data requests should be sent to the corresponding author and will be evaluated and approved after consultation with CANVAS chief investigators.
The studies involving human participants were reviewed and approved by Austin Health HREC H2012-04650; also approved at RMH and Eastern Health HREC. The patients/participants provided their written informed consent to participate in this study.
All authors have contributed to the design, data acquisition, curation and analyses, statistical analyses, manuscript writing and figure creation, and editing.
This work was supported by: NHMRC GNT1020526, GNT1045617 (AB), GNT1094974; Brain Foundation; Wicking Trust; Collie Trust; Sidney and Fiona Myer Family Foundation; Australian Research Council DE180100893 (NE); and Heart Foundation Future Leader Fellowship 100784 (AB).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The authors thank the University of Melbourne Victorian Life Sciences Computation Initiative, Florey Node National Imaging Facility, Melbourne Brain Centre radiographers, and all our participants, who so generously contributed their time to the study.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2021.754204/full#supplementary-material
CANVAS, Cognition And Neocortical Volume After Stroke; CCI, Charlson Comorbidity Index; CDR, Clinical Dementia Rating; CI, cognitively impaired; CN, cognitively normal; HV, hippocampal volume; IQCODE, Informant Questionnaire on Cognitive Decline in the Elderly Short Form; NART, National Adult Reading Test; TBV, total brain volume.
1. Norrving B, Davis SM, Feigin VL, Mensah GA, Sacco RL, Varghese C. Stroke prevention worldwide–what could make it work? Neuroepidemiology. (2015) 45:215–20. doi: 10.1159/000441104
2. Satizabal C, Beiser AS, Seshadri S. Incidence of dementia over three decades in the Framingham heart study. N Engl J Med. (2016) 375:93–4. doi: 10.1056/NEJMc1604823
3. Debette S, Seshadri S, Beiser A, Au R, Himali JJ, Palumbo C, et al. Midlife vascular risk factor exposure accelerates structural brain aging and cognitive decline. Neurology. (2011) 77:461–8. doi: 10.1212/WNL.0b013e318227b227
4. Pase MP, Beiser A, Enserro D, Xanthakis V, Aparicio H, Satizabal CL, et al. Association of ideal cardiovascular health with vascular brain injury and incident dementia. Stroke. (2016) 47:1201–6. doi: 10.1161/STROKEAHA.115.012608
5. Zheng F, Yan L, Zhong B, Yang Z, Xie W. Progression of cognitive decline before and after incident stroke. Neurology. (2019) 93:e20–8. doi: 10.1212/WNL.0000000000007716
6. Levine DA, Galecki AT, Langa KM, Unverzagt FW, Kabeto MU, Giordani B, et al. Trajectory of cognitive decline after incident stroke. JAMA. (2015) 314:41–51. doi: 10.1001/jama.2015.6968
7. Pendlebury ST, Rothwell PM. Prevalence, incidence, and factors associated with pre-stroke and post-stroke dementia: a systematic review and meta-analysis. Lancet Neurol. (2009) 8:1006–18. doi: 10.1016/S1474-4422(09)70236-4
8. Werden E, Cumming T, Li Q, Bird L, Veldsman M, Pardoe HR, et al. Structural MRI markers of brain aging early after ischemic stroke. Neurology. (2017) 89:116–24. doi: 10.1212/WNL.0000000000004086
9. Weinstein G, Beiser AS, Decarli C, Au R, Wolf PA, Seshadri S. Brain imaging and cognitive predictors of stroke and Alzheimer disease in the Framingham heart study. Stroke. (2013) 44:2787–94. doi: 10.1161/STROKEAHA.113.000947
10. Egorova N, Liem F, Hachinski V, Brodtmann A. Predicted brain age after stroke. Front Aging Neurosci. (2019) 11:348. doi: 10.3389/fnagi.2019.00348
11. Egorova N, Dhollander T, Khlif MS, Khan W, Werden E, Brodtmann A. Pervasive white matter fiber degeneration in ischemic stroke. Stroke. (2020) 51:1507–13. doi: 10.1161/STROKEAHA.119.028143
12. Brodtmann A, Khlif MS, Egorova N, Veldsman M, Bird LJ, Werden E. Dynamic regional brain atrophy rates in the first year after ischemic stroke. Stroke. (2020) 51:e183–e92. doi: 10.1161/STROKEAHA.120.030256
13. Langeskov-Christensen M, Grondahl Hvid L, Nygaard MKE, Ringgaard S, Jensen HB, Nielsen HH, et al. Efficacy of high-intensity aerobic exercise on brain mri measures in multiple sclerosis. Neurology. (2021) 96:e203–e13. doi: 10.1212/WNL.0000000000011241
14. Johnson L, Werden E, Shirbin C, Bird L, Landau E, Cumming T, et al. The post ischaemic stroke cardiovascular exercise study: protocol for a randomised controlled trial of fitness training for brain health. Eur Stroke J. (2018) 3:379–86. doi: 10.1177/2396987318785845
15. Dubois B, Chupin M, Hampel H, Lista S, Cavedo E, Croisile B, et al. Donepezil decreases annual rate of hippocampal atrophy in suspected prodromal Alzheimer's disease. Alzheimers Dement. (2015) 11:1041–9. doi: 10.1016/j.jalz.2014.10.003
16. Rojas JC, Karydas A, Bang J, Tsai RM, Blennow K, Liman V, et al. Plasma neurofilament light chain predicts progression in progressive supranuclear palsy. Ann Clin Transl Neurol. (2016) 3:216–25. doi: 10.1002/acn3.290
17. Pini L, Pievani M, Bocchetta M, Altomare D, Bosco P, Cavedo E, et al. Brain atrophy in Alzheimer's Disease and aging. Ageing Res Rev. (2016) 30:25–48. doi: 10.1016/j.arr.2016.01.002
18. Salat DH, Buckner RL, Snyder AZ, Greve DN, Desikan RS, Busa E, et al. Thinning of the cerebral cortex in aging. Cereb Cortex. (2004) 14:721–30. doi: 10.1093/cercor/bhh032
19. Salat DH, Greve DN, Pacheco JL, Quinn BT, Helmer KG, Buckner RL, et al. Regional white matter volume differences in nondemented aging and Alzheimer's disease. Neuroimage. (2009) 44:1247–58. doi: 10.1016/j.neuroimage.2008.10.030
20. Chen Q, Boeve BF, Senjem M, Tosakulwong N, Lesnick T, Brushaber D, et al. Trajectory of lobar atrophy in asymptomatic and symptomatic GRN mutation carriers: a longitudinal MRI study. Neurobiol Aging. (2020) 88:42–50. doi: 10.1016/j.neurobiolaging.2019.12.004
21. Cavedo E, Grothe MJ, Colliot O, Lista S, Chupin M, Dormont D, et al. Reduced basal forebrain atrophy progression in a randomized Donepezil trial in prodromal Alzheimer's disease. Sci Rep. (2017) 7:11706. doi: 10.1038/s41598-017-09780-3
22. Dickerson BC, Bakkour A, Salat DH, Feczko E, Pacheco J, Greve DN, et al. The cortical signature of Alzheimer's disease: regionally specific cortical thinning relates to symptom severity in very mild to mild AD dementia and is detectable in asymptomatic amyloid-positive individuals. Cereb Cortex. (2009) 19:497–510. doi: 10.1093/cercor/bhn113
23. Greicius MD, Srivastava G, Reiss AL, Menon V. Default-mode network activity distinguishes Alzheimer's disease from healthy aging: evidence from functional MRI. Proc Natl Acad Sci USA. (2004) 101:4637–42. doi: 10.1073/pnas.0308627101
24. Seeley WW, Crawford RK, Zhou J, Miller BL, Greicius MD. Neurodegenerative diseases target large-scale human brain networks. Neuron. (2009) 62:42–52. doi: 10.1016/j.neuron.2009.03.024
25. Zhou J, Greicius MD, Gennatas ED, Growdon ME, Jang JY, Rabinovici GD, et al. Divergent network connectivity changes in behavioural variant frontotemporal dementia and Alzheimer's disease. Brain. (2010) 133:1352–67. doi: 10.1093/brain/awq075
26. Boyle PA, Yu L, Leurgans SE, Wilson RS, Brookmeyer R, Schneider JA, et al. Attributable risk of Alzheimer's dementia attributed to age-related neuropathologies. Ann Neurol. (2019) 85:114–24. doi: 10.1002/ana.25380
27. Kivipelto M, Solomon A, Ahtiluoto S, Ngandu T, Lehtisalo J, Antikainen R, et al. The Finnish geriatric intervention study to prevent cognitive impairment and disability (FINGER): study design and progress. Alzheimers Dement. (2013) 9:657–65. doi: 10.1016/j.jalz.2012.09.012
28. Ngandu T, Lehtisalo J, Solomon A, Levalahti E, Ahtiluoto S, Antikainen R, et al. A 2 year multidomain intervention of diet, exercise, cognitive training, and vascular risk monitoring versus control to prevent cognitive decline in at-risk elderly people (FINGER): a randomised controlled trial. Lancet. (2015) 385:2255–63. doi: 10.1016/S0140-6736(15)60461-5
29. Makin SD, Doubal FN, Shuler K, Chappell FM, Staals J, Dennis MS, et al. The impact of early-life intelligence quotient on post stroke cognitive impairment. Eur Stroke J. (2018) 3:145–56. doi: 10.1177/2396987317750517
30. Harrison SL, Sajjad A, Bramer WM, Ikram MA, Tiemeier H, Stephan BC. Exploring strategies to operationalize cognitive reserve: a systematic review of reviews. J Clin Exp Neuropsychol. (2015) 37:253–64. doi: 10.1080/13803395.2014.1002759
31. Brodtmann A, Werden E, Pardoe H, Li Q, Jackson G, Donnan G, et al. Charting cognitive and volumetric trajectories after stroke: protocol for the cognition and neocortical volume after stroke (CANVAS) study. Int J Stroke. (2014) 9:824–8. doi: 10.1111/ijs.12301
32. Adams HP Jr., Bendixen BH, Kappelle LJ, Biller J, Love BB, Gordon DL, et al. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke. (1993) 24:35–41. doi: 10.1161/01.STR.24.1.35
33. Bamford J, Sandercock P, Dennis M, Warlow C, Jones LN, McPherson K, et al. A prospective study of acute cerebrovascular disease in the community: the Oxfordshire Community Stroke Project 1981-86, 1: methodology, demography, and incident cases of first-ever stroke. J Neurol Neurosurg Psychiatry. (1988) 51:1373–80. doi: 10.1136/jnnp.51.11.1373
34. van Swieten JC, Koudstaal PJ, Visser MC, Schouten HJ, van Gijn J. Interobserver agreement for the assessment of handicap in stroke patients. Stroke. (1988) 19:604–7. doi: 10.1161/01.STR.19.5.604
35. D'Hoore W, Sicotte C, Tilquin C. Risk adjustment in outcome assessment: the Charlson comorbidity index. Methods Inf Med. (1993) 32:382–7. doi: 10.1055/s-0038-1634956
36. Reuter M, Schmansky NJ, Rosas HD, Fischl B. Within-subject template estimation for unbiased longitudinal image analysis. Neuroimage. (2012) 61:1402–18. doi: 10.1016/j.neuroimage.2012.02.084
37. Schmidt P. Bayesian Inference for Structured Additive Regression Models for Large-Scale Problems with Applications to Medical Imaging. Munich: LMU München (2017).
38. Nelson HE. The National Adult Reading Test (NART): Test Manual. Windsor, ON: Berks, PA: NFER-Nelson (1982).
39. Jorm AF. A short form of the Informant Questionnaire on Cognitive Decline in the Elderly (IQCODE): development and cross-validation. Psychol Med. (1994) 24:145–53. doi: 10.1017/S003329170002691X
40. Spitzer RL, Kroenke K, Williams JB, Lowe B. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med. (2006) 166:1092–7. doi: 10.1001/archinte.166.10.1092
41. Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. (2001) 16:606–13. doi: 10.1046/j.1525-1497.2001.016009606.x
43. Lim YY, Jaeger J, Harrington K, Ashwood T, Ellis KA, Stoffler A, et al. Three-month stability of the CogState brief battery in healthy older adults, mild cognitive impairment, and Alzheimer's disease: results from the Australian Imaging, Biomarkers, and Lifestyle-rate of change substudy (AIBL-ROCS). Arch Clin Neuropsychol. (2013) 28:320–30. doi: 10.1093/arclin/act021
44. Wechsler D. Wechsler Adult Intelligence Scale®-Third Edition (WAIS®-III). London: Pearson. p. 197.
46. Hachinski V, Iadecola C, Petersen RC, Breteler MM, Nyenhuis DL, Black SE, et al. National institute of neurological disorders and stroke-canadian stroke network vascular cognitive impairment harmonization standards. Stroke. (2006) 37:2220–41. doi: 10.1161/01.STR.0000237236.88823.47
47. Morris JC. The clinical dementia rating (CDR): current version and scoring rules. Neurology. (1993) 43:2412–4. doi: 10.1212/WNL.43.11.2412-a
48. Fjell AM, Walhovd KB, Fennema-Notestine C, McEvoy LK, Hagler DJ, Holland D, et al. One-year brain atrophy evident in healthy aging. J Neurosci. (2009) 29:15223–31. doi: 10.1523/JNEUROSCI.3252-09.2009
49. Brodtmann A, Puce A, Darby D, Donnan G. Regional fMRI brain activation does correlate with global brain volume. Brain Res. (2009) 1259:17–25. doi: 10.1016/j.brainres.2008.12.044
50. Nitkunan A, Lanfranconi S, Charlton RA, Barrick TR, Markus HS. Brain atrophy and cerebral small vessel disease: a prospective follow-up study. Stroke. (2011) 42:133–8. doi: 10.1161/STROKEAHA.110.594267
51. Khan W, Egorova N, Khlif MS, Mito R, Dhollander T, Brodtmann A. Three-tissue compositional analysis reveals in-vivo microstructural heterogeneity of white matter hyperintensities following stroke. Neuroimage. (2020) 218:116869. doi: 10.1016/j.neuroimage.2020.116869
52. Hagberg G, Ihle-Hansen H, Fure B, Thommessen B, Ihle-Hansen H, Oksengard AR, et al. No evidence for amyloid pathology as a key mediator of neurodegeneration post-stroke - a seven-year follow-up study. BMC Neurol. (2020) 20:174. doi: 10.1186/s12883-020-01753-w
53. Han JW, Maillard P, Harvey D, Fletcher E, Martinez O, Johnson DK, et al. Association of vascular brain injury, neurodegeneration, amyloid and cognitive trajectory. Neurology. (2020) 95:e2622–34. doi: 10.1212/WNL.0000000000010531
54. Brodtmann A, Khlif MS, Bird LJ, Cumming T, Werden E. Hippocampal volume and amyloid PET status three years after ischemic stroke: a pilot study. J Alzheimers Dis. (2021) 80:527–32. doi: 10.3233/JAD-201525
55. Khlif MS, Bird LJ, Restrepo C, Khan W, Werden E, Egorova-Brumley N, et al. Hippocampal subfield volumes are associated with verbal memory after first-ever ischemic stroke. Alzheimers Dement (Amst). (2021) 13:e12195. doi: 10.1002/dad2.12195
56. Veldsman M, Werden E, Egorova N, Khlif MS, Brodtmann A. Microstructural degeneration and cerebrovascular risk burden underlying executive dysfunction after stroke. Sci Rep. (2020) 10:17911. doi: 10.1038/s41598-020-75074-w
57. Veldsman M, Cheng H-J, Fang JY, Werden E, Khlif MS, Ng KK, et al. Degeneration of structural brain networks is associated with cognitive decline after ischaemic stroke. Brain Commun. (2020) 2:fcaa155. doi: 10.1093/braincomms/fcaa155
58. Weishaupt N, Zhang A, Deziel RA, Tasker RA, Whitehead SN. Prefrontal ischemia in the rat leads to secondary damage and inflammation in remote gray and white matter regions. Front Neurosci. (2016) 10:81. doi: 10.3389/fnins.2016.00081
59. Campbell BC, Mitchell PJ, Kleinig TJ, Dewey HM, Churilov L, Yassi N, et al. Endovascular therapy for ischemic stroke with perfusion-imaging selection. N Engl J Med. (2015) 372:1009–18. doi: 10.1056/NEJMoa1414792
60. Campbell BCV, Ma H, Parsons MW, Churilov L, Yassi N, Kleinig TJ, et al. Association of reperfusion after thrombolysis with clinical outcome across the 45- to 9-hours and wake-up stroke time window: a meta-analysis of the EXTEND and EPITHET randomized clinical trials. JAMA Neurol. (2021) 78:236–40. doi: 10.1001/jamaneurol.2020.4123
61. Campbell BCV, Mitchell PJ, Churilov L, Yassi N, Kleinig TJ, Dowling RJ, et al. Tenecteplase versus alteplase before thrombectomy for ischemic stroke. N Engl J Med. (2018) 378:1573–82. doi: 10.1056/NEJMoa1716405
62. Parsons M, Spratt N, Bivard A, Campbell B, Chung K, Miteff F, et al. A randomized trial of tenecteplase versus alteplase for acute ischemic stroke. N Engl J Med. (2012) 366:1099–107. doi: 10.1056/NEJMoa1109842
63. Bernhardt J, Hayward KS, Dancause N, Lannin NA, Ward NS, Nudo RJ, et al. A stroke recovery trial development framework: consensus-based core recommendations from the Second Stroke Recovery and Rehabilitation Roundtable. Int J Stroke. (2019) 14:792–802. doi: 10.1177/1747493019879657
64. Borschmann K, Hayward KS, Raffelt A, Churilov L, Kramer S, Bernhardt J. Rationale for intervention and dose is lacking in stroke recovery trials: a systematic review. Stroke Res Treat. (2018) 2018:8087372. doi: 10.1155/2018/8087372
65. Hayward KS, Churilov L, Dalton EJ, Brodtmann A, Campbell BCV, Copland D, et al. Advancing stroke recovery through improved articulation of nonpharmacological intervention dose. Stroke. (2021) 52:761–9. doi: 10.1161/STROKEAHA.120.032496
66. Rethnam V, Bernhardt J, Johns H, Hayward KS, Collier JM, Ellery F, et al. Look closer: The multidimensional patterns of post-stroke burden behind the modified Rankin Scale. Int J Stroke. (2021) 16:420–8. doi: 10.1177/1747493020951941
67. Drozdowska BA, McGill K, McKay M, Bartlam R, Langhorne P, Quinn TJ. Prognostic rules for predicting cognitive syndromes following stroke: a systematic review. Eur Stroke J. (2021) 6:18–27. doi: 10.1177/2396987321997045
68. Jack CR Jr., Shiung MM, Gunter JL, O'Brien PC, Weigand SD, Knopman DS, et al. Comparison of different MRI brain atrophy rate measures with clinical disease progression in AD. Neurology. (2004) 62:591–600. doi: 10.1212/01.WNL.0000110315.26026.EF
69. Jack CR Jr., Shiung MM, Weigand SD, O'Brien PC, Gunter JL, Boeve BF, et al. Brain atrophy rates predict subsequent clinical conversion in normal elderly and amnestic MCI. Neurology. (2005) 65:1227–31. doi: 10.1212/01.wnl.0000180958.22678.91
70. Jack CR Jr., Weigand SD, Shiung MM, Przybelski SA, O'Brien PC, Gunter JL, et al. Atrophy rates accelerate in amnestic mild cognitive impairment. Neurology. (2008) 70:1740–52. doi: 10.1212/01.wnl.0000281688.77598.35
71. Jack CR Jr., Wiste HJ, Therneau TM, Weigand SD, Knopman DS, Mielke MM, et al. Associations of amyloid, tau, and neurodegeneration biomarker profiles with rates of memory decline among individuals without dementia. JAMA. (2019) 321:2316–25. doi: 10.1001/jama.2019.7437
72. Quinn TJ, Richard E, Teuschl Y, Gattringer T, Hafdi M, O'Brien JT, et al. European stroke organisation (ESO) and European academy neurology (EAN) joint guidelines on post stroke cognitive impairment. Eur J Neurol. (2021). doi: 10.1111/ene.15068. [Epub ahead of print].
73. Wardlaw JM, Doubal F, Brown R, Backhouse E, Woodhouse L, Bath P, et al. Rates, risks and routes to reduce vascular dementia (R4vad), a UK-wide multicentre prospective observational cohort study of cognition after stroke: protocol. Eur Stroke J. (2021) 6:89–101. doi: 10.1177/2396987320953312
74. Ganguli M, Lee CW, Hughes T, Snitz BE, Jakubcak J, Duara R, et al. Who wants a free brain scan? Assessing and correcting for recruitment biases in a population-based sMRI pilot study. Brain Imaging Behav. (2015) 9:204–12. doi: 10.1007/s11682-014-9297-9
Keywords: stroke, neurodegeneration, brain atrophy, post-stroke cognition, cognitive impairment (CI)
Citation: Brodtmann A, Werden E, Khlif MS, Bird LJ, Egorova N, Veldsman M, Pardoe H, Jackson G, Bradshaw J, Darby D, Cumming T, Churilov L and Donnan G (2021) Neurodegeneration Over 3 Years Following Ischaemic Stroke: Findings From the Cognition and Neocortical Volume After Stroke Study. Front. Neurol. 12:754204. doi: 10.3389/fneur.2021.754204
Received: 06 August 2021; Accepted: 27 September 2021;
Published: 22 October 2021.
Edited by:
Swathi Kiran, Boston University, United StatesReviewed by:
Maria Varkanitsa, Boston University, United StatesCopyright © 2021 Brodtmann, Werden, Khlif, Bird, Egorova, Veldsman, Pardoe, Jackson, Bradshaw, Darby, Cumming, Churilov and Donnan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Amy Brodtmann, YWdicm9kQHVuaW1lbGIuZWR1LmF1
†These authors have contributed equally to this work and share last authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.