
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
MINI REVIEW article
Front. Neurol. , 13 October 2021
Sec. Neuro-Otology
Volume 12 - 2021 | https://doi.org/10.3389/fneur.2021.747887
This article is part of the Research Topic The Clinical Role of Auditory Implants View all 8 articles
 Jack Shohet*
Jack Shohet* Jacqueline Bibee
Jacqueline BibeeTotally implantable active middle ear implants (AMEI) provide full-time hearing amplification to those with moderate to severe sensorineural hearing loss. While technology in conventional hearing aids (CHA) has advanced greatly, limitations remain for people with active lifestyles, limited vision or dexterity, and hearing aid fit issues. Furthermore, direct-drive properties of AMEI are thought to provide those with inefficient middle ear transfer functions a distinct advantage in delivering prescribed sound to the cochlea, ultimately improving speech understanding with less distortion. AMEI safety, stability, and efficacy outcomes are well documented and fitting strategies continue to improve. Recent studies show how simple aided speech testing can help predict whether a patient struggling with CHA may instead benefit from an AMEI. Totally implantable AMEI continue to be a viable option for patients who cannot or will not utilize traditional hearing aids.
Conventional hearing aids (CHA) are the standard treatment recommendation for 90–95% of people with sensorineural, conductive, and mixed hearing loss (1). However, despite advances in CHA digital technology, hearing aid adoption rate remains low. Recent reports in the U.S. indicate only 34% of people with hearing loss wear hearing aids (2) and rates are still <50% in European countries where hearing aids are provided at no cost (3). Of those that do use hearing aids, an average of 8.9 years lapses from candidacy to adoption (4). Several factors have been identified as contributing to limited CHA use and can primarily be grouped into a few categories:
1. Fit: discomfort, otitis externa, occlusion effect, poor retention.
2. Use: limited vision, dexterity, self-efficacy, lost devices, frequent battery changes.
3. Lifestyle: Removal for sleep, water wear, athletic endeavors with heavy perspiration.
Concurrently, risks of untreated hearing loss are becoming clearer. Accelerated cognitive decline and dementia (5), increased fall risk (6) and emergency room visits (7), and mental health challenges (8) have all been linked to hearing loss. Clearly, the need for alternative hearing treatment options is higher than ever.
Active middle ear implants (AMEI) were developed to provide solutions to the above CHA limitations. While four AMEI are commercially available worldwide, only two are totally implantable. This mini review will report the long term device outcomes and updated candidacy considerations for one of these totally implantable AMEI systems.
The Envoy Medical Corporation, originally called St. Croix Medical, was founded in 1995 in St. Paul, MN, quickly laying groundwork for a totally implantable hearing aid. What developed was the Envoy Esteem® device, which underwent clinical trials starting in 2000. European CU mark cleared in 2006 and the device was FDA approved in 2010. Uniquely, this AMEI utilizes the tympanic membrane as the natural hearing aid microphone. A piezoelectric sensor on the incus sends ossicular vibration information to a sound processor/battery combination unit, implanted subcutaneously in the postauricular region. The filtered and amplified signal is delivered back to a driver connected to the stapes capitulum, which directly drives the oval window for transduction into the cochlea. This direct drive system contrasts traditional CHA mechanics, which do not account for individual variance in middle ear mechanics affecting the efficiency with which sound reaches the cochlea.
Ongoing device maintenance includes an outpatient surgery on average every 4.9 years (10) to replace the battery. Annual device programming and testing with an Audiologist is recommended. No day-to-day maintenance is required, though some people turn the device volume down or off at night to save battery. Patients receive a remote control for optional volume and programming adjustments.
Adults with moderate to severe sensorineural hearing loss in one or both ears, ≥40% maximum word understanding, and some experience with CHA are the best candidates for this device. AMEI is not a replacement for those with severe to profound hearing loss needing cochlear implant(s). Additional considerations for the Esteem are anatomical and surgical; Imaging is needed to determine if there is ample middle ear space to insert the device and medical consultation is required to ensure patients are healthy enough to undergo an outpatient surgery lasting on average 4.5 h (11). Contraindications include abnormal middle ear anatomy, recurrent otitis media, mastoiditis, Eustachian tube dysfunction, fluctuating hearing loss, Meniere's disease, retrocochlear pathologies, and disabling tinnitus. Motivated patients are often those who have not tolerated CHA well or desire freedom from routine maintenance of hearing aids i.e. batteries, daily insertion and removal.
After establishing audiologic and radiologic candidacy, patients are counseled extensively as to expectations of hearing with a middle ear implant. Existing limitations of current hearing technologies to fully rehabilitate hearing loss must be impressed on the patient who needs to understand that their hearing will not be “normal” and that they will continue to experience difficulties in certain listening situations. The goal of facilitating hearing rehabilitation without an external device and the conveniences, and the conveyed lifestyle benefits (12) are communicated. In addition, the patient is made aware that battery changes will require future surgery and up to 16% of patients will require some type of second surgery other than battery change (10). These revision procedures include transcanal or transmastoid revisions for removal of scar tissue or transducer misalignment or explanation for infection or inadequate benefit. Accommodations are made for the patient to be able to communicate effectively during the 6–8 weeks postoperatively when they will not be able to hear from the implanted ear due to a temporary middle ear serous effusion. Patients are counseled that the ossicular chain is permanently disrupted and although reversible, they are not likely to achieve complete middle ear transmission properties with a reconstructed ossicular chain should the implant have to be removed.
The Esteem surgical procedure is planned as an outpatient procedure unless medically indicated to observe the patient in hospital postoperatively. It requires unique pieces of equipment including a second microscope for laser doppler vibrometry measurements of ossicular movement, a cutting laser and an intracanal microphone assembly to facilitate intraoperative testing. The surgery requires many steps, each of which builds upon earlier ones such that sufficient achievement of each step is required before proceeding. A field clinical engineer performs intraoperative measurements and provides feedback to the surgeon at various steps of the procedure to ensure system integrity. Failure to achieve adequate exposure or transducer alignment can add considerable surgical time. Indeed, a surgeon's first implants often take 6–8 h (10) requiring urinary catheterization. With experience, operative times can be brought down to 2.5–3 h on average (13).
The surgery is done through a postauricular incision large enough to accommodate the amply sized battery/sound processor (SP). A bony trough is drilled through the outer occipitoparietal skull cortex for the SP. A sufficient mastoidectomy is performed to allow the body of the transducers to reside within the mastoid cavity. Adequate exposure requires that bone lateral to the body of the incus is removed and a posterior tympanotomy through the facial recess is performed to facilitate exposure of the lenticular process of the incus and as much of the stapes as is possible to visualize given the anatomic restrictions of the facial nerve and fibrous tympanic annulus.
After ensuring adequate native ossicular movement with laser doppler vibrometry, the incudostapedial joint is disarticulated and the lenticular process of the incus is removed with a laser. The transducers are affixed to the skull using flexible stabilizing bars that facilitate adequate positioning of the sensor transducer with the tip over the incus body and the driver transducer tip to the stapes capitulum. The transducer bodies are affixed to the mastoid using a hydroxyapatite cement and the tips of the transducers are attached to the ossicles using a glass ionomer cement (Figure 1). Measurements are made to ensure adequate ossicular displacement and lack of acoustic feedback before attaching the transducer leads to the SP and closing the incision. Patients are brought back to the clinic for activation approximately 8 weeks after surgery to allow time for the middle ear serous effusion which can impact device function to resolve.
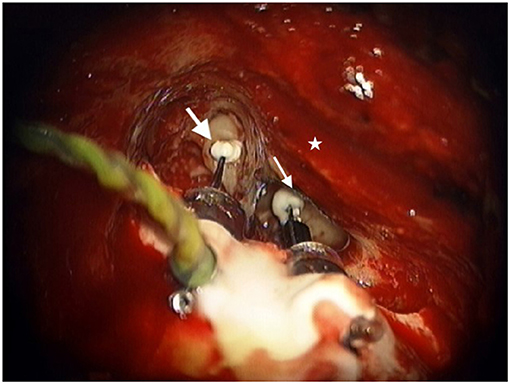
Figure 1. Sensor transducer (large white arrow) and Driver transducer (small white arrow) bodies cemented in mastoid with tips attached to the incus and stapes respectively as viewed through the right-side mastoid exposure. Star represents the posterior external auditory canal.
Ongoing data collection pre and post FDA approval has shown device longevity and programmability over time for proper candidates (13–16). Banakis et al. (17) additionally outlined 16 studies across 18 years showing implantable hearing aid performance benefits. Four of these studies evaluated the Envoy Esteem specifically (11, 16, 18, 19) all of which showed better AMEI objective outcomes compared to CHA as determined by speech recognition scores. Two of these four studies included subjective comparison using the standardized abbreviated profile of hearing aid benefit (APHAB) questionnaire which also showed AMEI preference. These results contrasted with other AMEIs which had more variable outcomes. Common findings amongst Envoy Esteem outcome studies include excellent mid-frequency gain, improved word understanding at conversational levels, and patient preference on validated rating scales. Interestingly, while not the intent of AMEI, one study showed 56.1% of users reported reduction or elimination of tinnitus with the device (14).
Data from 231 ears among these four studies along with extensive phase I and II clinical trial efficacy data (13, 18) demonstrate that AMEIs are no longer experimental; Rather, there is plenty of data showing consistently improved outcomes with AMEI over baseline hearing and CHA treatment. Single-cohort surgeon studies corroborate the importance of surgeon experience to the success of the Esteem recipient, showing declining rates of revision surgery (20) and shorter surgery length (10) over time. During those same multi-year intervals, rates of device explant were fairly stable, between 5 and 11%.
With a wide candidacy range and variable outcomes, it is reasonable to ask if metrics are available to help predict which patients have the most to gain by changing from CHA to AMEI. One metric that has been proposed as predictive of AMEI advantage is aptly named the speech perception gap (SPgap) by Dyer et al. (21) or earphone to aided difference by McRackan et al. (22). This performance gap describes the difference between a patient's CHA aided speech score at conversational level (i.e., 50 dB HL) and their maximum unaided word recognition score (WRS) already obtained under earphones during the standard audiogram.
In the Shohet et al. (9) retrospective study, 86% of the 133 subjects demonstrated a SPgap pre-operatively with their appropriately fit CHA, reinforcing that CHA users often do not reach their cochlear potential. The average SPgap of CHA users pre-operatively in this study was 24.7% compared to 3.0% once they received their AMEI. Individual data and trends are plotted in Figure 2. Importantly, it was discovered that the larger the pre-operative SPgap, the greater the performance improvement with AMEI over CHA. Similarly, Chang et al. (23) found an average SPgap of 48.2% with CHA vs. 6.6% with a semi-implantable AMEI. All subjects in that study showed smaller SPgaps with their AMEI compared to their previous CHA. Franks and Jacob show that the SPgap is common in patients with moderate to severe hearing loss and suggest that aided speech testing should be integrated into hearing aid verification for all patients, especially those with maximum unaided word recognition between 40 and 70% (24).
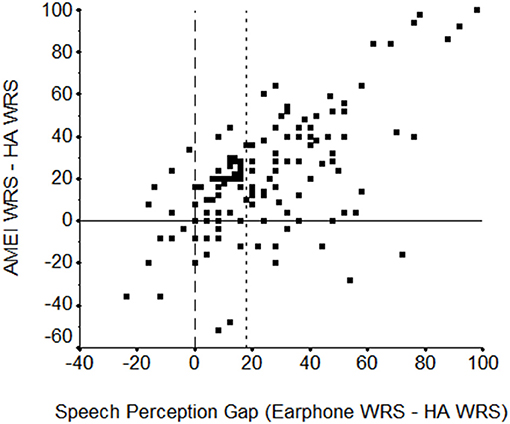
Figure 2. Scatterplot of the earphone (max-WRS) minus hearing aid (HA, at 50 dB HL) word recognition score (WRS) difference in percent correct (“speech perception gap”) by the WRS difference in percent correct between the active middle ear implant (AMEI) and the HA (both at 50 dB HL). The vertical reference line at zero indicates the point of no difference between earphone and HA word recognition scores. To the left, the HA performed better while to the right, earphone performance was better. The horizontal reference line indicates the point of no difference between word recognition scores with the AMEI and HA, below which the HA performance was better and above which the AMEI was better. A second vertical reference line at 18 shows the Earphone-HA speech perception gap at and beyond which >85% of subjects perform better with the AMEI than with their HA. From Shohet et al. (9).
The SPgap metric seems to be sensitive to distinct AMEIs audibility advantages, so it is gaining popularity amongst hearing implant manufacturers who argue they can close this performance gap using direct drive properties. It may also be useful to clinicians who want a simple yet sensitive test to identify good audiometric AMEI candidates.
Initially, gain prescription for the Esteem required some trial and error, estimating input gain to achieve a certain amount of output aided gain measured in soundfield. Sound processing options have improved since the first generation Esteem setup, and Envoy describes its current sound processing as hybrid: analog signal processing controlled digitally. Also, in 2014 the prescriptive algorithm called SoundFit became available to help clinicians better prescribe Esteem gain based on baseline audiograms. This statistical NAL-RP based formula was developed using data from 540 users' profile settings and correlating gain outcomes. From this prescriptive starting point, clinicians can now fine-tune different program options into the Esteem, just like a CHA. Software developments have also allowed for more transparent device diagnostic testing and parameter programming over time.
Widespread adoption of middle ear implants in the United States has met many hurdles some of which have severely limited their adoption. First, and most impactful, has been the absence of Currently Procedural Terminology (CPT) coding that appropriately describes the services being provided which has prevented payment considerations by Centers for Medicare and Medicaid Services (CMS) as well as commercial payers. Second, with limited third-party reimbursement, many surgeons have given up on implanting or even offering these devices to their patients due to device unfamiliarity and discomfort in performing the complex surgical procedure. Lastly, as CHA sound processing technology advances, the perceived sound advantage of direct drive technology over conventional acoustic stimulation through the ear canal may become less perceivable.
1. Standardize audiometric test metrics pre and post intervention to allow meta-analyses of AMEI compared to CHA. Individual clinics may also use these metrics to help identify good AMEI candidates and change treatment course if CHA are not effective.
2. Develop a validated questionnaire for assessing AMEI hearing aid lifestyle benefit. Amongst the many benefits of fully implantable AMEI, freedom from daily device maintenance and confidence gained from 24/7 hearing is described empirically but not measured.
3. Determine if direct drive AMEIs are a suitable, less invasive alternative to hybrid cochlear implants.
4. Re-examine if digital processing mechanisms could be supported by today's implantable battery technology.
5. Compare modern extended bandwidth hearing devices to AMEI output. While AMEI provided more gain than open-fit CHA in the past (25, 26), this should be revisited with modern feedback cancellation systems and non-conventional hearing aids.
6. Consider integrating SPgap testing into standard hearing aid care to help identify patients that may be better served with an AMEI.
Envoy Esteem totally implantable AMEI device stability and clinical outcomes have been verified through clinical trials and independent analyses for over 20 years to date. Results consistently show aided hearing thresholds similar to and often better than CHA with notable advantages in mid-frequency gain and superior speech understanding at conversational levels. AMEI should be discussed as an alternative treatment to CHA for proper candidates during hearing device consultations. A simple speech testing metric called the SPgap can be incorporated into busy clinics to help identify good audiometric candidates in addition to those who simply cannot or will not wear CHA. While questionnaires show overall satisfaction with Esteem vs. CHA, opportunities remain to further illuminate why patients prefer the lifestyle advantage of a fully implantable AMEI and “absence of a daily reminder of the disability” (Barbara) (11).
JS and JB contributed to conception and design of the study. JB wrote the clinical aspects and JS the surgical details of the manuscript. Both authors contributed to manuscript revision, read, and approved the submitted version.
JS is a member of the Envoy Medical Advisory Board. None of the authors has a financial relationship with Envoy Medical.
The remaining author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Kochkin S. The Impact of Untreated Hearing Loss on Household Income (2007). Available online at: https://betterhearing.org/HIA/assets/File/public/marketrak/MarkeTrak_VII_The_Impact_of_Untreated_Hearing_Loss_on_Household_Income.pdf (accessed Aug 24, 2021).
2. Picou EM. MarkeTrak 10 (MT10) Survey results demonstrate high satisfaction with and benefits from hearing aids. Semin Hear. (2020) 41:21–36. doi: 10.1055/s-0040-1701243
3. EHIMA. EuroTrak Country Market Surveys (2020. Available online at: https://www.ehima.com/surveys/ (accessed July 20, 2021).
4. Simpson AN, Matthews LJ, Cassarly C. Dubno JR. Time From Hearing Aid Candidacy to Hearing Aid Adoption: A Longitudinal Cohort Study. Ear Hear. (2019) 40:468–76. doi: 10.1097/AUD.0000000000000641
5. Lin FR, Yaffe K, Xia J, et al. Hearing loss and cognitive decline in older adults. JAMA Intern Med. (2013) 173:293–299. doi: 10.1001/jamainternmed.2013.1868
6. Lin FR, Ferrucci L. Hearing loss and falls among older adults in the United States. Arch Intern Med. (2012) 172:369–71. doi: 10.1001/archinternmed.2011.728
7. Genther DJ, Frick KD, Chen D, Betz J, Lin FR. Association of hearing loss with hospitalization and burden of disease in older adults. JAMA. (2013) 309:2322–4. doi: 10.1001/jama.2013.5912
8. Lawrence BJ, Jayakody DMP, Bennett RJ, Eikelboom RH, Gasson N, Friedland PL. Hearing loss and depression in older adults: a systematic review and meta-analysis. The Gerontologist. (2020) 60:137–54. doi: 10.1093/geront/gnz009
9. Shohet JA, Gende DM, Bibee JB, Patzkowski G, Tanita C. Speech perception gap is predictive of an active middle ear implant advantage. Otology & Neurotology. (2020) 41:663–8. doi: 10.1097/MAO.0000000000002589
10. Shohet JA, Kraus EM, Catalano PJ. Toh E. Totally implantable hearing system: Five-year hearing results. Laryngoscope. (2018) 128:210–6. doi: 10.1002/lary.26602
11. Barbara M, Filippi C, Covelli E, Volpini L, Monini S. Ten years of active middle ear implantation for sensorineural hearing loss. Acta Otolaryngol. (2018) 138:807–14. doi: 10.1080/00016489.2018.1472807
12. Kahue CN, Carlson ML, Daugherty JA, Haynes DS, Glasscock ME. 3rd. Middle ear implants for rehabilitation of sensorineural hearing loss: a systematic review of FDA approved devices. Otol Neurotol. (2014) 35:1228–37. doi: 10.1097/MAO.0000000000000341
13. Kraus EM, Shohet JA. Catalano PJ. Envoy esteem totally implantable hearing system: phase 2 trial, 1-year hearing results. Otolaryngology–Head and Neck Surgery. (2011) 145:100–9. doi: 10.1177/0194599811401709
14. Barbara M, Biagini M. Monini S. The totally implantable middle ear device ‘Esteem’ for rehabilitation of severe sensorineural hearing loss. Acta Otolaryngol. (2011) 131:399–404. doi: 10.3109/00016489.2010.536994
15. Zwartenkot JW, Mulder JJ, Snik AF, Cremers CWRJ, Mylanus EAM. Active middle ear implantation: long-term medical and technical follow-up, implant survival, and complications. Otol Neurotol. (2016) 37: 513–9. doi: 10.1097/MAO.0000000000001015
16. Shohet JA, Gende DM, Tanita CS. Totally implantable active middle ear implant: Hearing and safety results in a large series. Laryngoscope. (2018) 128:2872–8. doi: 10.1002/lary.27246
17. Banakis Hartl RM, Jenkins HA. Implantable hearing aids: where are we in 2020? Laryngoscope Investigative Otolaryngology. (2020) 5:1184–91. doi: 10.1002/lio2.495
18. Chen DA, Backous DD, Arriaga MA, Garvin R, Kobylek D, Littman L, et al. Phase 1 clinical trial results of the envoy system: a totally implantable middle ear device for sensorineural hearing loss. Otolaryngol Head Neck Surg. (2004) 131:904–16. doi: 10.1016/j.otohns.2004.05.027
19. Boeheim K, Pok SM, Schloegel M, Filzmoser P. Active middle ear implant compared with open-fit hearing aid in sloping high-frequency sensorineural hearing loss. Otol Neurotol. (2010 A) 31:424–9. doi: 10.1097/MAO.0b013e3181cabd42
20. McCarty Walsh E, Morrison, D, McFeely W. Totally implantable active middle-ear implants: A large, single-surgeon cohort. The Journal of Laryngology & Otology. (2021) 135:304–9. doi: 10.1017/S0022215121000335
21. Dyer RK, Spearman M, Spearman B, McCraney A. Evaluating speech perception of the MAXUM middle ear implant versus speech perception under inserts. Laryngoscope. (2018) 128:456–60. doi: 10.1002/lary.26605
22. McRackan TR, Clinkscales WB, Ahlstrom JB, Nguyen SA, Dubno JR. Factors associated with benefit of active middle ear implants compared to conventional hearing aids. Laryngoscope. (2018) 128:2133–8. doi: 10.1002/lary.27109
23. Chang CYJ, Spearman M, Spearman B, McCraney A, Glasscock ME. 3rd. Comparison of an electromagnetic middle ear implant and hearing aid word recognition performance to word recognition performance obtained under earphones. Otol Neurotol. (2017) 38:1308–14. doi: 10.1097/MAO.0000000000001554
24. Franks ZG. Jacob A. The speech perception gap in cochlear implant patients. Cochlear Implants Int. (2019) 20:176–81. doi: 10.1080/14670100.2019.1582165
25. Gerard JM, Thill MP, Chantrain G, Gersdorff M, Deggouj N. Esteem 2 middle ear implant: our experience. Audiol Neurotol. (2012) 17:267–74. doi: 10.1159/000338689
Keywords: implants, otology, active middle ear implant, hearing loss, direct drive, speech perception gap
Citation: Shohet J and Bibee J (2021) New Considerations for a Totally Implantable Active Middle Ear Implant. Front. Neurol. 12:747887. doi: 10.3389/fneur.2021.747887
Received: 27 July 2021; Accepted: 15 September 2021;
Published: 13 October 2021.
Edited by:
Maurizio Barbara, Sapienza University of Rome, ItalyReviewed by:
Takeshi Tsutsumi, Tokyo Medical and Dental University, JapanCopyright © 2021 Shohet and Bibee. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jack Shohet, anNob2hldEBlYXJkb2N0b3Iub3Jn
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.