
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Neurol. , 09 December 2021
Sec. Stroke
Volume 12 - 2021 | https://doi.org/10.3389/fneur.2021.746567
This article is part of the Research Topic Understanding Stroke Recovery to Improve Outcomes: From Acute Care to Chronic Rehabilitation View all 31 articles
Objectives: To conduct a meta-analysis to assess the efficacy of scalp acupuncture (SA) in patients with stroke and consequent hemiparesis regardless of brain infarction or intracerebral hemorrhage.
Methods: A literature search of randomized controlled trials (RCTs) on SA for stroke was performed in five databases up to May 10, 2021. We investigated three types of outcome: motor function, sequelae of poststroke hemiparesis, and adverse effects. Methodological quality was assessed using the revised Cochrane risk of bias tool version 2.0.
Results: Of 1,063 papers, 30 RCTs involving Fugl–Meyer Assessment were selected, among which 10 and four RCTs were selected for evaluation of courses lasting of 1 and 3 months, respectively. The meta-analysis of 1- and 3-month courses revealed significant differences in the motor function of the SA plus Western standard treatment group vs. Western standard treatment only (medication plus rehabilitation; P < 0.001). A 3-month course tended to result in better outcomes than a 1-month course.
Conclusions: Our meta-analysis results reveal that SA improves motor function in patients with acute to chronic stroke, regardless of brain infarction or intracerebral hemorrhage. However, because of a lack of methodological quality, thoroughly planned clinical studies are still required.
Stroke is the sudden injury of neurons due to lack of blood supply to the brain, leading to the rapid development of a focal neurologic deficit. Globally, stroke is the second leading cause of death and a major cause of disability (1). It can be classified as ischemic (blood vessel occlusion) or hemorrhagic (blood vessel rupture). Common risk factors for ischemic and hemorrhagic stroke are age, race, sex, hypertension, diabetes, smoking, dyslipidemia, and alcohol use. For ischemic stroke, the specific risk factors are family history, atrial fibrillation, asymptomatic carotid stenosis, cardiac disease, sickle cell anemia, diet (high-sodium, low-potassium diet in overweight or elderly individuals), physical inactivity, obesity, hormone replacement therapy, hyperhomocysteinemia, hypercoagulability, lipoprotein(a), lipoprotein-associated phospholipase A2, inflammation, infection, and geography. For hemorrhagic stroke, the specific risk factors are antithrombolytic use, cerebral amyloid angiopathy, microbleeding, illicit drug use, dialysis, and tumors (2). Stroke incurs a considerable economic burden (3). Rehabilitation is a major part of stroke recovery (4). Furthermore, the use of sensory stimulation (transcutaneous electrical nerve stimulation or acupuncture) was reported to contribute to routine rehabilitation and improve poststroke hemiparesis (5).
Acupuncture has been a mainstream therapy in traditional and complementary medicine for >3,000 years for several diseases and also for poststroke recovery. Among the various methods, scalp acupuncture (SA) is a modern acupuncture technique integrating traditional Chinese needling methods with Western medical knowledge of representative areas of the cerebral cortex. SA is performed based on the functionality of brain areas to stimulate different scalp zones with needles; such stimulation improves the reflexivity of certain nerves and is mostly applied in individuals with acute and chronic central nervous system disorders, especially stroke (6). The mechanisms that are currently considered possible for SA include reducing brain edema, diminishing cerebral vessel permeability, promoting reparation of blood–brain barrier damage, decreasing inflammation, improving energy metabolism, and relieving the inhibitive generalization of the whole brain neuron function (7).
In the past decade, several systematic reviews and meta-analyses have been published that have assessed the effect of SA on acute hypertensive intracerebral hemorrhage (8) and postapoplectic aphasia (9). In addition, a meta-analysis of animal studies reported promising results, finding that SA improved infarct volume and neurological function score (10). Moreover, in Young-Nim et al. (11) obtained similar results in their meta-analysis; however, the intervention methods were mixed (SA and body acupuncture). Therefore, we decided to investigate whether additional SA has benefits for patients with stroke. The purpose of this study was to comprehensively review randomized controlled trials (RCTs) that evaluated the effectiveness of SA for motor dysfunction in stroke. We wanted to obtain a clinically meaningful synthesis of the existing evidence to determine whether SA may be a suitable complementary therapy for the motor sequelae of stroke.
Randomized controlled trials (RCTs) evaluating the effect of SA on motor function in stroke with the control group receiving modern standard treatment or conventional treatment were included in this study.
Randomized controlled trials (RCTs) that included patients of any age or sex with acute or chronic stroke were eligible. SA was administered to patients with stroke as diagnosed through CT or MRI. The control group in these RCTs received only standard Western treatment for stroke in a neurological ward.
Randomized controlled trials (RCTs) were included regardless of treatment duration and number of sessions, but acupoint selection was limited to the scalp; ears and body acupoints were excluded. The control group patients received Western standard treatment (medications and rehabilitation). Patients in the trial groups received SA therapy (SA of standard international acupuncture nomenclature) in addition to the same standard treatment as that in the control groups. Concerning SA, acupoints are mostly in the motor areas (the anterior oblique line of the vertex-temporal, MS 6) and sensory areas (the posterior oblique line of the vertex-temporal, MS 7) of the contralateral scalp of hemiplegic limbs. The needle retention time was mostly between 0.5 and 2 h, and the depth of needle insertion was 2–4 cm. The frequency was 5–7 days per week and once a day. The treatment courses ranged from 2 weeks to 6 months. Those who performed SA in these studies were experienced doctors.
We investigated three types of outcome: motor function, sequelae of poststroke hemiparesis, and adverse effects. We evaluated motor function in the upper and lower extremities at the end of the first and third months. The assessment was performed using the Fugl–Meyer Assessment (FMA), a stroke-specific, performance-based impairment index (12, 13). The FMA motor score ranges from 0 (hemiplegia) to 100 (normal motor performance) points, which were divided into 66 points for the upper extremity and 34 points for the lower extremity.
We searched databases, namely, PubMed, Embase, the Cochrane library, Airiti Library, and China National Knowledge Infrastructure (until May 2021) by two reviewers (YJH and CSH). The keywords used for the search were “scalp acupuncture” and “stroke.” The type of article searched for was the RCT. We focused on poststroke hemiparesis or hemiplegia and excluded unwanted outcomes (poststroke dysarthria, poststroke dysphagia, poststroke aphasia, central poststroke pain, poststroke depression, poststroke cognitive impairment, etc.). The reference lists of all relevant articles were searched for further studies. There was no language limitation.
Two reviewers (YJH and CSH) independently assessed all studies and independently extracted eligible data from the trial reports by using a data extraction form; data were cross-checked for accuracy before use. Disagreement was resolved through discussion with a third reviewer (SWC) if necessary. The authors of the trials were contacted and requested to provide missing data.
Quality assessment was conducted by revised Cochrane risk of bias tool 2.0 (RoB2.0) to determine whether the trials had the following concerns regarding internal validity: 1) risk of bias arising from the randomization process; 2) risk of bias due to deviation from intended intervention; 3) missing outcome data; 4) risk of bias in outcome measurements; or 5) risk of bias in the selection of the reported results. We conducted the risk of bias of summary and graph by using Review Manager 5.4.
Heterogeneity between trial results was tested using the standard I2 test. Results are reported as odds ratios with corresponding 95% CIs for dichotomous data. If continuous data were available, the weighted mean difference or standardized mean difference was calculated. Statistical analysis was performed using the statistical software R version 3.6.0 and the meta package.
Based on the search strategy, we manually retrieved 1,062 papers found in the five databases and one from another source (manual search using Google Scholar). These studies were published from 1975 to 2021, and 253 papers had duplicate titles. Of the remaining 810 papers, after screening the abstracts, 18 animal studies, six expert opinions, seven case studies, 57 cohort studies, nine protocols, six systematic reviews or meta-analyses, 56 narrative reviews, 326 relevant articles with an unwanted outcome (poststroke dysarthria, poststroke dysphagia, poststroke aphasia, central poststroke pain, poststroke depression, poststroke cognitive impairment, etc.), and 17 irrelevant articles were excluded; the remaining papers with full text available were selected. Among the 306 RCTs selected, 25 papers with incomplete or insufficient data, one paper regarding noninternational-standard SA (Yamamoto new SA), 212 papers regarding impure SA with rehabilitation, 21 papers comparing different SA types, and 17 papers not including FMA scores were excluded. A total of 30 RCT papers containing FMA scores were finally selected (14–43). The process used for the literature search for the application in the systematic review is summarized in Figure 1.
All these 30 RCTs were conducted in China, and the corresponding articles were published from 2007 to 2019 (Table 1). Of these RCTs, 15 mentioned 1-month results; five mentioned 6-week results; six mentioned 2-month results; and six mentioned 3-month results. We assessed both upper and lower extremity strengths, so we selected only papers with complete FMA scores (including the upper and lower extremities with a total score of 100). We selected treatment courses of 1 and 3 months and then conducted the meta-analysis. A total of 1,043 patients with acute or chronic stroke from 12 reports were included.
Based on Figure 2, we determined the overall quality of the included studies to be low by RoB2.0. For the first domain (risk of bias arising from the randomization process), the generally unclear risk was indicated. All trials used a randomization method, including a random number generator (calculator) and a sequencing method. One trial (28) described a grade-A level of adequate concealment of randomization in which the patients were allocated according to calculated random numbers sealed in opaque envelopes. For the second domain (risk of bias due to deviation from the intended intervention), the risk was generally high because only one trial (21) included a control group for which sham acupuncture was implemented. The remaining 29 trials did not mention blinding or intention-to-treat analysis. For the third domain (missing outcome data), the risk was generally high because only two trials (21, 28) recorded missing data. For the fourth domain (risk of bias in outcome measurement), the risk was generally unclear because only three trials (14, 28, 37) mentioned that the assessor did not participate in the therapy. For the fifth domain (risk of bias in the selection of the reported results), the risk was generally low. All trials conducted a comparative analysis between the SA group and control group.
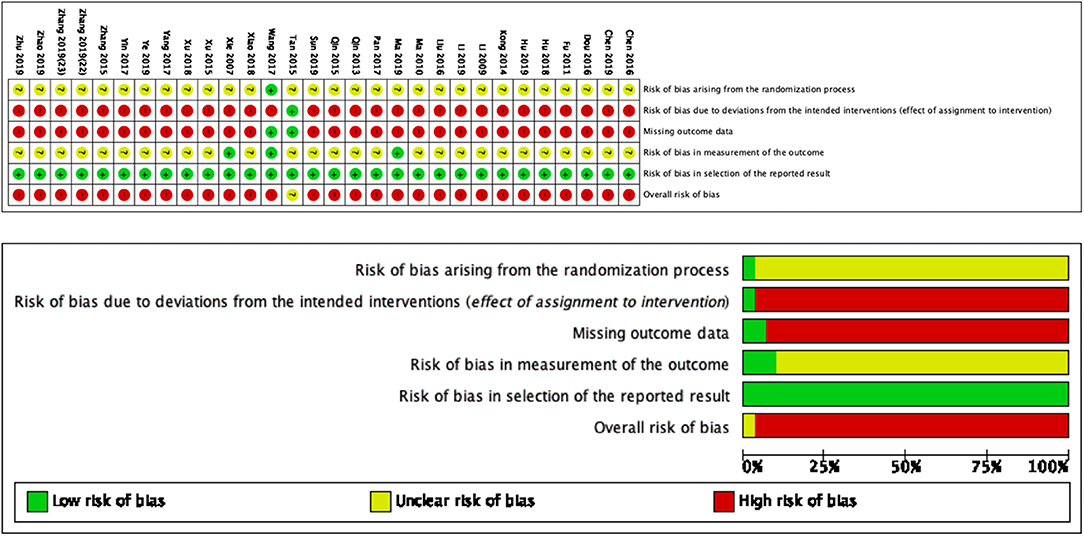
Figure 2. Risk of bias summary and graph for each risk of bias item presented as percentages across all included studies.
None of the trials used death or dependency as a primary outcome measure.
Among the papers on 30 independent trials, 10 reporting the complete FMA score described the 1-month outcome (Figure 3; mean difference, 10.3 [95% CI, 7.43–12.63]; P < 0.01), and four reporting the complete FMA score described the 3-month outcome (Figure 4; mean difference, 15.18 [95% CI, 8.06–22.31]; P < 0.01). Two papers (17, 29) with a 1-month course mentioned that special rehabilitation may diminish the effect of SA; therefore, we removed these papers and reconducted a sensitivity test of the meta-analysis (Figure 5; mean difference, 11.16 [95% CI, 8.09–14.23]; P < 0.01). There were no homogeneity in the consistency of the trial results (1 month: I2 = 78% and 3 months: I2 = 83%). SA was found to have significantly improved hemiparesis, as represented using the FMA score and when compared with the control group. The outcome after 3 months of SA (mean difference in FMA score: 15.18) seemed to be better than that after 1 month of SA (mean difference in FMA score: 11.16).
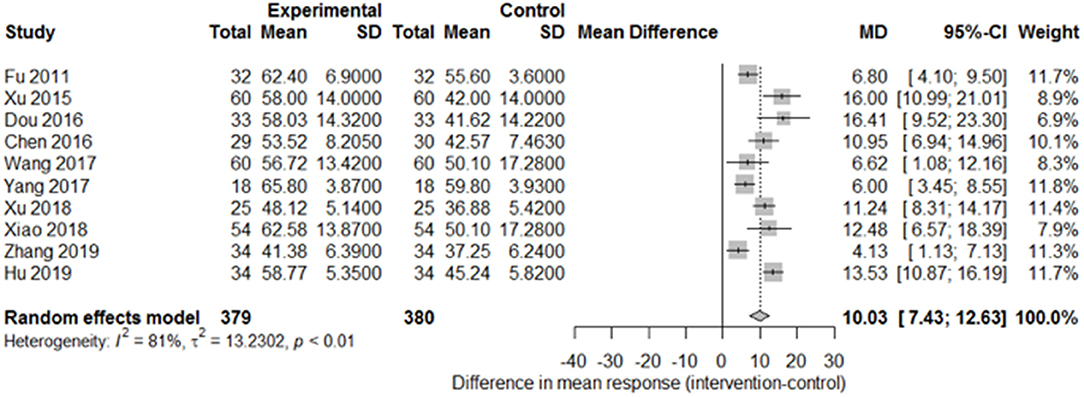
Figure 3. Forest plot of the comparison of mean difference in hemiparesis improvement after treatment with SA vs. control after a 1-month course.
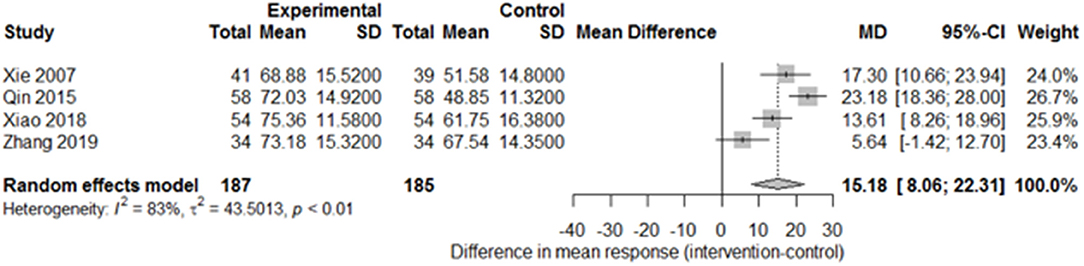
Figure 4. Forest plot of the comparison of mean difference in hemiparesis improvement after treatment with SA vs. control after a 3-month course.
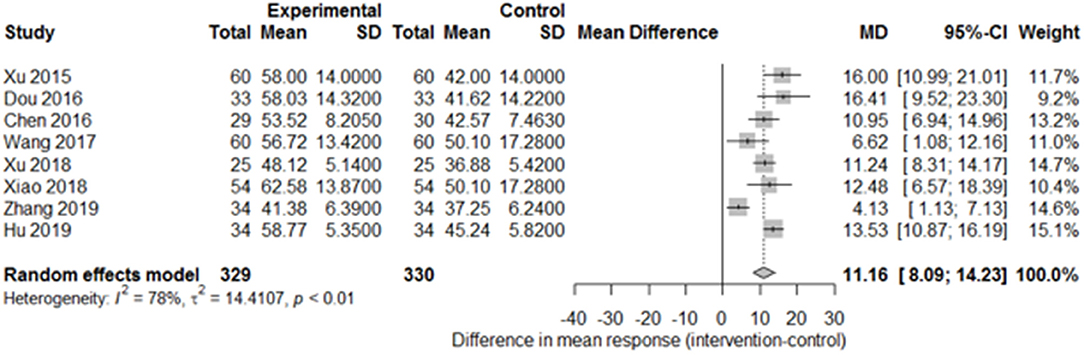
Figure 5. Forest plot of the comparison of mean difference in hemiparesis improvement after treatment with SA vs. control after a 1-month course and removal of two papers on special rehabilitation for the sensitivity test.
Only two studies (15, 27) recorded the sequelae of hemiparesis, such as shoulder–hand syndrome, shoulder pain, and muscle atrophy. Only one study (15) recorded complete muscle spasticity and bedsores. After pooling the results of the two papers, we found a significant difference in the incidence of shoulder–hand syndrome (Figure 6; odds ratio, 0.39 [95% CI, 0.16–0.94]) between the experimental and control groups, but no difference in that of shoulder pain (Figure 7; odds ratio, 0.43 [95% CI, 0.17–1.11]) or muscle atrophy (Figure 8; odds ratio, 0.39 [95% CI, 0.13–1.15]).
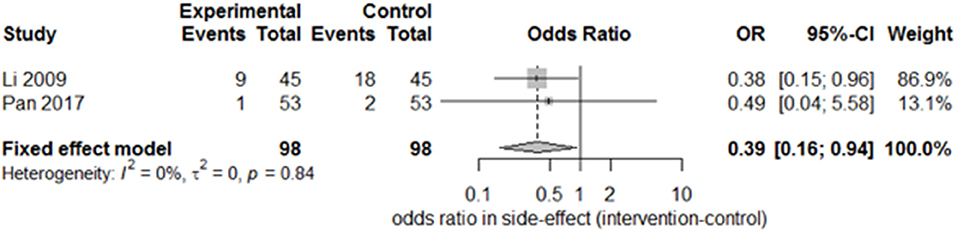
Figure 6. Forest plot of the odds ratio comparison for shoulder–hand syndrome in the SA group vs. the control group.
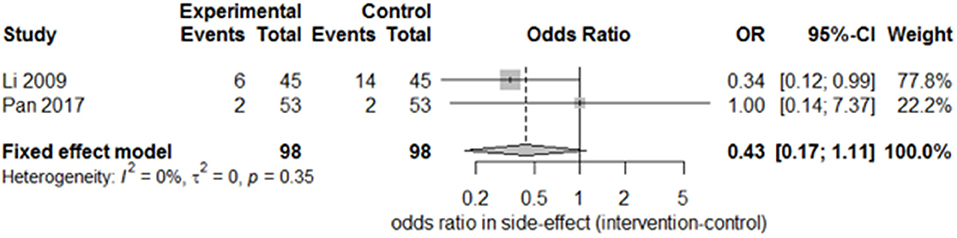
Figure 7. Forest plot of odds ratio comparison for shoulder pain in the SA group vs. the control group.
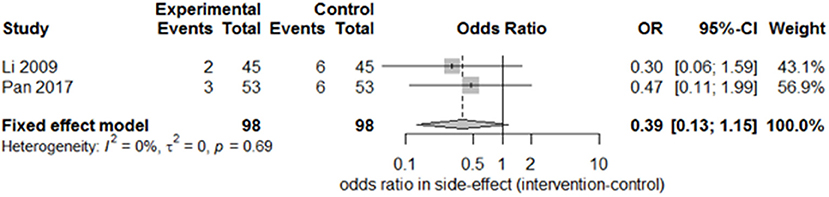
Figure 8. Forest plot of the odds ratio comparison for muscle atrophy in the SA group vs. the control group.
Adverse events were mentioned in one trial (38). In the SA groups, the abnormal response rate for dizziness and local skin redness was 6.67% (3/45), whereas it was 8.89% (4/45) in the control group. The difference was not significant.
We used a contour-enhanced funnel plot to differentiate between asymmetry due to publication bias and other reasons. Figure 9 demonstrates that almost all trials obtained a significant result favoring the experimental group, with P < 0.01 (light gray region) in nine trials and 0.01 < P < 0.05 (dark gray region) in one trial. However, publication bias was noted in five trials from the funnel plot.
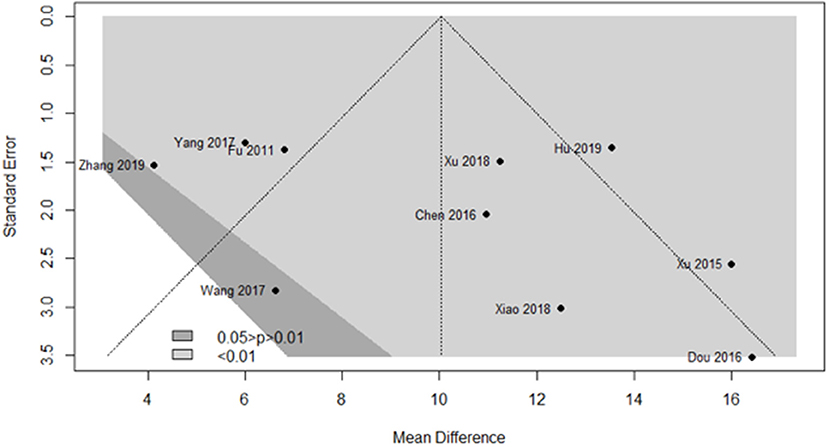
Figure 9. Funnel plot of the clinical effects in the SA group vs. the control group after a 1-month course.
This is the first meta-analysis to specifically evaluate the clinical outcomes of SA for poststroke hemiparesis, regardless of hemorrhage or infarction. The results indicate that SA is effective for improving motor dysfunction in patients with stroke.
Other clinical studies have suggested that SA therapy may be a suitable complementary treatment for poststroke neurological dysfunctions, namely, dysphagia (44), aphasia (45), central poststroke pain (46), depression (47), and cognitive impairment (48). In this meta-analysis, after searching five databases up to May 2021, 30 RCTs with FMA scores were selected; a systematic review of the clinical research on SA for stroke was conducted, and each study was qualitatively assessed. The disease period investigated in the meta-analysis varied from acute to chronic. We wished to focus only on motor function; therefore, we used the FMA score rather than the National Institutes of Health Stroke Score or Barthel index. Among the 30 RCT papers, 10 described the outcome after 1 month and 4 mentioned the outcome after 3 months. For the sensitivity test, two papers were removed from the 1-month course because of special rehabilitation, which may have influenced the effect of SA therapy. We observed that Zhang (40) reported markedly lower efficacy of SA therapy in contrast to other papers reporting 1- or 3-month courses; we speculated that this was related to the different areas of the scalp acupoints used, which were balance areas (MS 14) rather than the motor and sensory areas. When combined with Western standard medicine (medication and rehabilitation), SA obtained significantly better results in the meta-analysis on hemiparesis improvement during the stroke recovery period. A 3-month course tended to be better than a 1-month course. Regarding sequelae of hemiparesis-associated symptoms—namely, shoulder–hand syndrome, shoulder pain, and muscle atrophy—we observed additional SA benefits, although the difference was significant only in the incidence of shoulder–hand syndrome and not in the incidence of shoulder pain or muscle atrophy due to the small sample.
Almost all of the RCTs included in this meta-analysis were conducted without blinding due to the limitation inherent in the acupuncture procedure; thus, confounding factors, such as the placebo effect, may have been present. Only one paper (21) mentioned sham acupuncture as a control. Only two papers (21, 28) reported dropouts, and one paper (38) reported an adverse effect of SA; most papers did not mention these factors, which resulted in low assessment quality when the revised Cochrane risk of bias tool 2.0 was used.
There was no consistency in the rehabilitation method used in the studies in this meta-analysis, which may have affected the results on SA accuracy and efficacy. In most RCTs, physical therapy and occupational therapy were employed as rehabilitation interventions. One RCT added visual scanning and sensory integration training to the rehabilitation (17). Another study used special rehabilitation—constraint-induced movement therapy (29). The different rehabilitation types in these two studies weakened the effect of SA, which indicated that special rehabilitation may have a role in the improvement of poststroke hemiparesis. Different types of rehabilitation with integrated SA therapy as complementary treatment could be used in patients with stroke.
Not all RCTs compared the effect of SA between brain infarction and hemorrhage. The populations in these RCTs were all Asian, and there is a lack of data on Western populations; therefore, whether racial differences affect the outcome of SA must be determined in the future.
Only one study (38) reported the 6-month outcome of SA; significant improvement in poststroke hemiparesis was discovered, which indicated that longer SA duration may result in added benefits in patients with stroke. Another trial (49) assessed the degree of improvement of limb dysfunction by noting the scalp needle retention time in patients with stroke. The scalp needles in the treatment group were indwelled for 7–10 h and in the control group for 30 min. The results indicated greater improvement in limb dysfunction and activities of daily living after stroke when scalp needle retention was longer. The duration effect of SA therapy warrants further research.
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.
Ethical review and approval was not required for the study on human participants in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Category 1: Y-JH conception and design of study and acquisition of data. Y-JH, C-SH, K-FL, J-YS, and S-WC analysis and/or interpretation of data. Category 2: Y-JH drafting the manuscript. Category 3: C-SH, K-FL, J-YS, and S-WC revising the manuscript critically for important intellectual content. Y-JH, C-SH, K-FL, J-YS, and S-WC approval of the version of the manuscript to be published. All authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The authors would like to express their sincere thanks to colleagues and staff from the WanFang Hospital and the Taipei Medical University for their support. Besides, this manuscript was edited by the Wallace Academic Editing.
1. Katan M, Luft A. Global burden of stroke. Semin Neurol. (2018) 38:208–11. doi: 10.1055/s-0038-1649503
2. Grysiewicz RA., Thomas K, Pandey DK. Epidemiology of ischemic and hemorrhagic stroke: incidence, prevalence, mortality, and risk factors. Neurol Clin. (2008) 26:871–95. vii. doi: 10.1016/j.ncl.2008.07.003
3. Evers SM, Struijs JN, Ament AJ, van Genugten ML, Jager JH, van den Bos GA. International comparison of stroke cost studies. Stroke. (2004) 35:1209–15. doi: 10.1161/01.STR.0000125860.48180.48
4. Langhorne P, Bernhardt J, Kwakkel G. Stroke rehabilitation. Lancet. (2011) 377:1693–702. doi: 10.1016/S0140-6736(11)60325-5
5. Sharififar S, Shuster JJ, Bishop MD. Adding electrical stimulation during standard rehabilitation after stroke to improve motor function. A systematic review and meta-analysis. Ann Phys Rehabil Med. (2018) 61:339–44. doi: 10.1016/j.rehab.2018.06.005
6. Hao JJ, Hao LL. Review of clinical applications of scalp acupuncture for paralysis: an excerpt from chinese scalp acupuncture. Glob Adv Health Med. (2012) 1:102–21. doi: 10.7453/gahmj.2012.1.1.017
7. Liu Z, Guan L, Wang Y, Xie CL, Lin XM, Zheng GQ. History and mechanism for treatment of intracerebral hemorrhage with scalp acupuncture. Evid Based Complement Alternat Med. (2012) 2012:895032. doi: 10.1155/2012/895032
8. Zheng GQ, Zhao ZM, Wang Y, Gu Y, Li Y, Chen XM. Meta-analysis of scalp acupuncture for acute hypertensive intracerebral hemorrhage. J Altern Complement Med. (2011) 17:293–9. doi: 10.1089/acm.2010.0156
9. Tang HY, Tang W, Yang F, Wu WW, Shen GM. Efficacy of acupuncture in the management of post-apoplectic aphasia: a systematic review and meta-analysis of randomized controlled trials. BMC Complement Altern Med. (2019) 19:282. doi: 10.1186/s12906-019-2687-1
10. Wang WW, Xie CL, Lu L, Zheng GQ. A systematic review and meta-analysis of Baihui (GV20)-based scalp acupuncture in experimental ischemic stroke. Sci Rep. (2014) 4:3981. doi: 10.1038/srep03981
11. Young-Nim Y, Gwang-Cheon P, Myung-Rae C, Min-Yeong S, Chang-Su N, Jae-Young H. Meta-analysis on randomized controlled trials for scalp acupuncture treatment of stroke: a systematic review. J Tradit Chin Med. (2018) 38:465–79. doi: 10.1016/S0254-6272(18)30879-3
12. Fugl-Meyer AR, Jääskö L, Leyman I, Olsson S, Steglind S. The post-stroke hemiplegic patient. 1. a method for evaluation of physical performance. Scand J Rehabil Med. (1975) 7:13–31.
13. Gladstone DJ, Danells CJ, Black SE. The fugl-meyer assessment of motor recovery after stroke: a critical review of its measurement properties. Neurorehabil Neural Repair. (2002) 16:232–40. doi: 10.1177/154596802401105171
14. Xie, D. l., Zhu LF, Liu HY. Effect of scalp acupuncture on cognitive and motor functions of stroke patients in recovery stage. Chin J Rehabil Theory Pract. (2007) 13:542–3. doi: 10.3233/NRE-192942
15. Li XJ, Zheng B. Observations on the efficacy of early scalp acupuncture plus modern rehabilitation techniques in treating postapoplectic hemiplegia. Shanghai J Acu Mox. (2009) 28:380–2. doi: 10.13460/j.issn.1005-0957.2009.07.027
16. Ma CY, Gao GY, Wan WJ. Clinical effectiveness of scalp acupuncture and facilitation techniques in the treatment of hemiplegic patients with stroke. Chin J Rehabil Med. (2010) 25:432–3. doi: 10.3870/zgkf.2010.06.009
17. Fu JM, Gu XD, Yao YH. Observation on the effect of long-lasting scalp acupuncture combined with rehabilitation training on unilateral spatial neglect of stroke. Chin J Tradit Med Sci Technol. (2011) 18:53–4.
18. Qin H, Ma D, Luo F, Dong GR. Effect of scalp penetration needling at head points combined with motor relearning programme on the gait of hemiplegic patients. J Clin Acu Mox. (2013) 29:17–20.
19. Kong SJ, Chen QH, Yan WJ. Facilitated technique combined with scalp acupuncture for treatment of limb dysfunction in stroke patients. Prac J Med Pharm. (2014) 31:416–7. doi: 10.14172/j.cnki.issn1671-4008.2014.05.050
20. Qin D. The effect of scalp acupuncture on motor function and cognitive function of stroke patients in rehabilitation period. Chin J Gerontol. (2015) 35:5161–3. doi: 10.3969/j.issn.1005-9202.2015.18.054
21. Tan TC, Yu YM. Effect of extraction method of scalp acupuncture on hand function of stroke patients with hemiplegia. Chin J Integr Med. (2015) 35:1274–5. doi: 10.7661/CJIM.2015.10.1274
22. Xu XG, Han Y, Chen LR. Application of modified scalp acupuncture in rehabilitation treatment of stroke. Hebei Medical Journal. (2015) 37:374–6. doi: 10.3969/j.issn.1002-7386.2015.03.017
23. Zhang PJ, Mao GL, Guo QC, Guo J, Zhang M, Bai YJ. The effect observation of scalp acupuncture combined with E-Link system on hand function of hemiplegic patients after stroke. Chin Med Modern Distance Educ China. (2015) 13:84–6. doi: 10.3969/j.issn.1672-2779.2015.18.044
24. Chen QX, Su WL, Lin XZ. A randomized controlled trial of scalp acupuncture combined with rehabilitation exercise therapy on improving clinical efficacy of ischemic stroke. Pract Clin J Integr Tradit Chin Western Med. (2016) 16:20–2. doi: 10.13638/j.issn.1671-4040.2016.07.009
25. Dou JM, Huang CY, Ge YJ, Ji YY, Jiang ZD, Wan ZY. Analysis of the clinical value of modified scalp acupuncture in rehabilitation treatment of stroke. World Latest Med Inform. (2016) 16:175. doi: 10.3969/j.issn.1671-3141.2016.57.139
26. Liu YY, Meng DY, Zhou Y. Effect of head acupuncture combined with knee joint controlling exercise on walking ability in patients with hemiplegia after cerebral stroke. Mod J Integr Tradit Chin West Med. (2016) 25:3788–91. doi: 10.3969/j.issn.1008-8849.2016.34.009
27. Pan B, Shi J, Jia JJ, Zhu MQ. The efficacy of Zhu's scalp acupuncture combined with rehabilitation training in upper extremity function for stroke patients with hemiplegia. Inform Tradit Chin Med. (2017) 34:116–9. doi: 10.19656/j.cnki.1002-2406.2017.04.035
28. Wang J, Pei J, Cui X, Sun K, Fu Q, Xing C. Individualized scalp acupuncture for motor dysfunction in stroke: a randomized controlled trial. Zhongguo Zhen. (2017) 37:918–24. doi: 10.13703/j.0255-2930.2017.09.002
29. Yang W, Zhang WY, Li HJ, Guo HC, Wang LM, Jin YS. Study on the effect of constraint induced movement therapy combined with motor function of stroke patients improve scalp acupuncture. China Hlth Stand Manag. (2017) 8:116–7. doi: 10.3969/j.issn.1674-9316.2017.02.073
30. Yin YH. Effect analysis of scalp acupuncture therapy in treatment of cerebral apoplexy. China Hlth Stand Manag. (2017) 8:89–91. doi: 10.3969/j.issn.1674-9316.2017.15.053
31. Hu HY. Evaluation of scalp acupuncture combined with rehabilitation training in the treatment of motor dysfunction in patients with acute stroke. Asia-Pac Tradit Med. (2018) 14:153–4. doi: 10.11954/ytctyy.201801057
32. Xiao L. Effect of Jiao's scalp acupuncture on motor dysfunction in patients with ischemic stroke. Jilin J Chin Med. (2018) 38:597–600. doi: 10.13463/j.cnki.jlzyy.2018.05.029
33. Xu L, Hao Q, Ma ZQ. Clinical observation on scalp acupuncture combined with sling exercise in treating hemiplegia after stroke. Chin Med Mod Distance Educ China. (2018) 16:121–3. doi: 10.3969/j.issn.1672-2779.2018.11.056
34. Chen Y, Jiang YM. Comparative analysis of the effects of core muscle training and scalp acupuncture on balance dysfunction in stroke patients with hemiplegia. J Prev Med Chin PLA. (2019) 37:112–4. doi: 10.13704/j.cnki.jyyx.2019.08.051
35. Hu P, Yang XJ. Effect of scalp acupuncture plus task-oriented approach on somatosensory evoked potential and function recovery in cerebral stroke patients in flaccid paralysis stage. Shanghai J Acu-mox. (2019) 38:482–6. doi: 10.13460/j.issn.1005-0957.2019.05.0482
36. Li XQ, Zhang L. Effect of scalp acupuncture therapy on recovery of motor function in patients with stroke. China Mod Med. (2019) 26:81–3.
37. Ma ZY, Xu MF, Yu XF. Clinical study on scalp acupuncture combined with mirror therapy for spastic paralysis of upper limbs due to stroke. J New Chin Med. (2019) 51:182–5. doi: 10.13457/j.cnki.jncm.2019.01.048
38. Sun XQ. Interactive scalp acupuncture combined with PNF in the treatment of patients with ischemic stroke and spastic hemiplegia. Chin J Convalescent Med. (2019) 28:18–20. doi: 10.13517/j.cnki.ccm.2019.01.007
39. Ye JJ, Dong YW, Lin XF, Yang LD. Clinical study on scalp acupuncture combined with balance function training for post-stroke balance dysfunction. J New Chin Med. (2019) 51:232–5. doi: 10.13457/j.cnki.jncm.2019.07.069
40. Zhang XY, Ren JM, Lu LM. Effect of scalp acupuncture combined with rehabilitation exercise on improving spasm status, neurological deficit and daily living ability in patients with stroke recovery. Clin Educ Gen Pract. (2019) 17:898–900. doi: 10.13558/j.cnki.issn1672-3686.2019.010.009
41. Zhang HL, Che WS, Chu NN. Effect of scalp acupuncture combined with virtual situational interactive training on functional rehabilitation of stroke patients. Chin J Gerontol. (2019) 39:4902–6. doi: 10.3969/j.issn.1005-9202.2019.20.008
42. Zhao GY, Yang T. Analysis of interactive scalp acupuncture in improving physical and physiological functions of stroke patients with hemiplegia. Jilin J Chin Med. (2019) 39:404–6. doi: 10.13463/j.cnki.jlzyy.2019.03.036
43. Zhu D, Zhu KY, Wang LX, Zhan JL, Tan TC. The effect of the mirror therapy combined with scalp acupuncture used in training lower extremity function of stroke patients. Chin J Rehabil Med. (2019) 34:533–8. doi: 10.3969/j.issn.1001-1242.2019.05.007
44. Zou N, Ding CY. Clinical effect of scalp acupuncture combined with low-frequency pulse electrical stimulation in the treatment of swallowing dysfunction in patients with cerebral apoplexy during convalescence. China Modern Med. (2019) 26:78–80.
45. Zhang RM, Sun SB. Treatment of motor aphasia after stroke with scalp acupuncture and speech rehabilitation. J Changchun Univer Chin Med. (2020) 36:339–42. doi: 10.13463/j.cnki.cczyy.2020.02.038
46. Qiao HZ, Dong AQ. Analysis of clinical effect of scalp acupuncture on central post stroke pain. Home Med. (2018) 101:101–2.
47. Zhang HJ, Xu LL, Xu TS. Scalp acupuncture therapy for post-stroke depression:a systematic review. J Liaoning Univer Tradit Chin Med. (2017) 19:163–6. doi: 10.13194/j.issn.1673-842x.2017.03.047
48. Sun YJ, Liang XN. The effect of scalp acupuncture on the cognitive function of stroke patients in recovery stage. Chin Rural Health. (2019) 7:7.
Keywords: stroke, meta-analysis, randomized controlled trial, scalp acupuncture, revised Cochrane risk of bias assessment
Citation: Huang Y-J, Huang C-S, Leng K-F, Sung J-Y and Cheng S-W (2021) Efficacy of Scalp Acupuncture in Patients With Post-stroke Hemiparesis: Meta-Analysis of Randomized Controlled Trials. Front. Neurol. 12:746567. doi: 10.3389/fneur.2021.746567
Received: 24 July 2021; Accepted: 29 October 2021;
Published: 09 December 2021.
Edited by:
Andreas R. Luft, University of Zurich, SwitzerlandReviewed by:
Anna Christina Alegiani, University Medical Center Hamburg-Eppendorf, GermanyCopyright © 2021 Huang, Huang, Leng, Sung and Cheng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jia-Ying Sung, OTYzMTdAdy50bXUuZWR1LnR3; Sheng-Wei Cheng, OTc0MjdAdy50bXUuZWR1LnR3
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.