- 1Department of Neurology, Changhua Christian Hospital, Changhua, Taiwan
- 2Department of Neurology, National Taiwan University Hospital, Taipei, Taiwan
Background and Purpose: Cases of acute pesticide poisoning account for significant morbidity and mortality in developing countries; however, its burden in Taiwan remains unknown. The study examined acute pesticide poisoning (APP) involving adults in the central region of Taiwan, which is a mainly agricultural sub-urban area.
Methods: The retrospective study evaluated the outcome and neurological sequelae of patients with APP in a Taiwanese cohort between April 2002 and February 2019. The pesticides were classified according to the Insecticide Resistance Action Committee Mode of Action (MoA) classification. The clinical characteristics, duration of hospitalization (days), follow-up duration (years), in-hospital mortality, neurological sequela, and imaging findings were recorded. Furthermore, multivariate logistic regression analyses were performed.
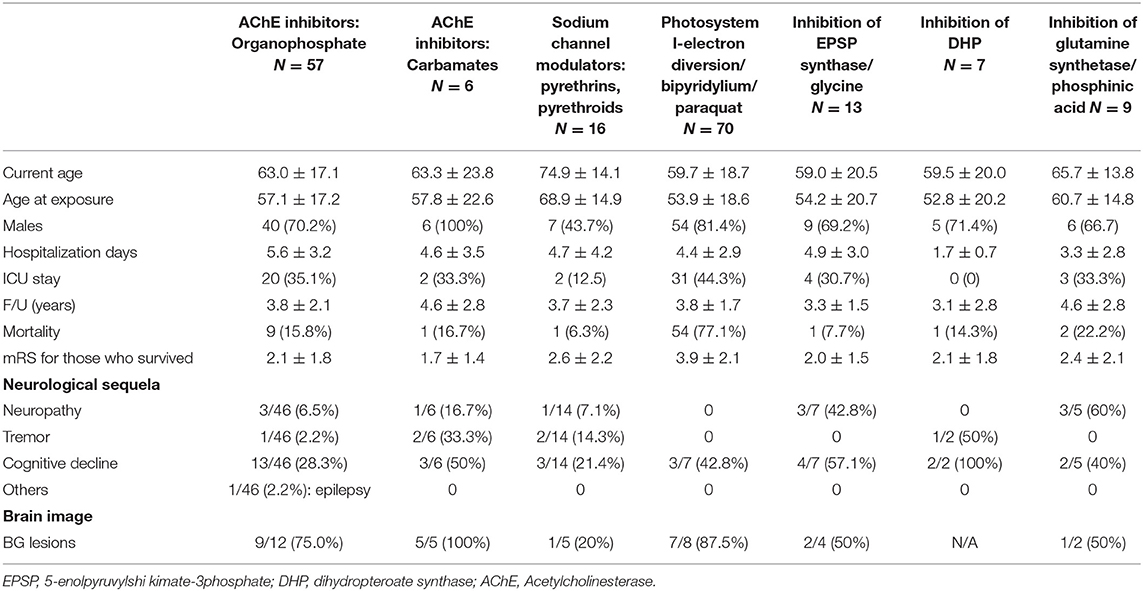
Results: We identified 299 patients with APP comprising 206 (68.9%) adult men with a mean exposure age of 56.4 ± 16.8 years. Paraquat, organophosphates, pyrethroids, carmabates, and phosphinic acid were the most commonly known reported poisoning agents. The mortality rate was highest in users with paraquat (77.1%), followed by phosphinic acid (22.2%), carbamates (16.7%), and organophosphates (15.8%). After a mean follows up of 3.69 ± 2.26 years, the most common neurological sequela was a cognitive decline (56 among 225 survivors, 24.89%), peripheral neuropathy (11 among 225 survivors, 4.89%), tremor (10 among 225 survivors, 4.44%), ataxia (3/225, 1.33%), and parkinsonism feature (2/225, 0.89%). Brain imaging studies revealed basal ganglion lesions on CT or hyperintensity on T2-weighted MRI images in 26 among 46 patients (56.5%). The basal ganglion lesions on brain imaging had a positive correlation with neurological sequelae.
Conclusion: Acute pesticide poisoning (APP)-related mortality is high especially paraquat intoxication, and cognitive decline, as well as peripheral neuropathy, were the most common neurological sequelae among survivors, which is highly correlated with basal ganglia lesions on brain imaging.
Introduction
Acute pesticide poisoning is a global health concern with acute manifestations of cardiorespiratory, central nervous system, and gastrointestinal illness (1). Pesticides cause an estimated 200,000 acute poisoning deaths each year, 99% of which occur in developing countries (2). According to the report of the United Nations, pesticide poisoning inflicts substantial costs on Governments and has catastrophic impacts on the environment, human health, and society (3). Even people who survived a single acute pesticide poisoning (APP) event could have potential psychological and neurological deficits after years (4, 5). More and more studies have demonstrated that exposure to hazardous pesticides is strongly associated with growth problems, endocrine disruption, malignancy, nervous system disorders (especially neuropathy and neurodegenerative diseases like Alzheimer's and Parkinson's diseases), and sterility (6–16).
For nervous system sequelae, basal ganglia are vulnerable to toxic damage in the absence of a sufficient detoxification pathway, so brain imaging findings on computed tomography or magnetic resonance imaging are helpful tools for prognostic evaluation and possible pathophysiology studies based on different localizations (17–19).
Since pesticides are functionally divided into various types with different mechanisms such as insecticides, disinfectants, herbicides, fumigants, repellents or combined, they could have diverse acute symptoms and long-term impacts on exposed people (20). However, the different impacts on neurological sequelae and brain imaging presentations between different types of APP are limited.
We aimed to investigate the burden (especially mortality), chronic neurological sequela, and brain imaging patterns associated with the different subtypes of APP involving adults in the central region of Taiwan, which is a major agricultural sub-urban county.
Methods
Data Source
This retrospective cohort study was conducted at a poison center of a tertiary care hospital located in an important agricultural district in central Taiwan and received institutional review board approval from the Changhua Christian Hospital committee for the Human Subjects Protection (200316).
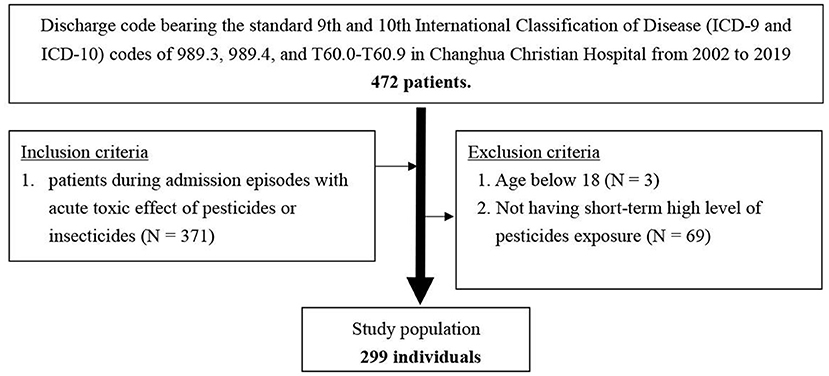
Consecutive hospital admissions of patients ≥18 years old from April 2002 to February 2019 with a discharge code bearing the standard 9th and 10th International Classification of Disease (ICD-9 and ICD-10) codes of 989.3, 989.4, and T60.0–T60.9, and admission episodes with a confirmed diagnosis of the acute toxic effect of pesticides or insecticides were included. We excluded patients younger than 18 years and those that did not have short-term high-level pesticide exposure (Figure 1).
Demographic and comorbidity data, including age at admission, gender, exposure substances classification, underlying diseases such as metabolic diseases, cardiovascular diseases, malignancy, and neuropsychological diseases were retrieved. The pesticides were classified according to the Insecticide Resistance Action Committee Mode of Action (MoA) classification (21).
Outcomes Measures
The major outcome was in-hospital mortality. Minor outcomes included neurological sequelae and basal ganglion lesions on CT or hyperintensity on T2-weighted MRI images. The neurological sequela was screened through a basic neurological examination at the office and compared with previous medical records to confirm whether they occurred after this episode of acute poisoning. For cognitive declination, we diagnosed based on clinical visiting data whether the patients' have an impaired ability to acquire and remember new information or impaired reasoning and handling of complex tasks, poor judgment or impaired visuospatial abilities or impaired language functions or changes in personality, behavior, or comportment. The clinical characteristics record the duration of hospitalization (days), follow-up duration (years), ICU admission for ventilator support, mortality, neurological sequela, and imaging findings.
Data Analysis
Baseline characteristics were reported using frequencies and proportions for categorical variables and using mean values and standard deviations for continuous variables. A multiple logistic regression model was developed to determine the factors associated with neurological sequelae. The model included exposure age, gender, admission to ICU for ventilator support or not, basal ganglion lesions on brain imaging, and the most common poisoned pesticides and insecticides.
Overall survival for all groups (different pesticides or insecticides) was defined from the time of admission to the time of death or discharge. Survival time was censored for patients alive at the end of the discharge day. Estimates and 95% CIs of overall survival proportions were computed using the Cox proportional hazards regression, and survival distributions were compared across groups using the log-rank test.
A multivariate Cox proportional hazards model was developed for all patients who were poisoned to predict factors associated with survival, shown as hazard ratios (HRs) with 95% CIs. The model was adjusted for exposure age, present basal ganglion lesions on brain imaging, sex, and pesticides subgroups. Two-sided statistical tests were specified in all analyses and p < 0.05 were considered statistically significant. Statistical analysis was conducted using the procedures available in the licensed MedCalc Statistical Software version 19.5.3.
Results
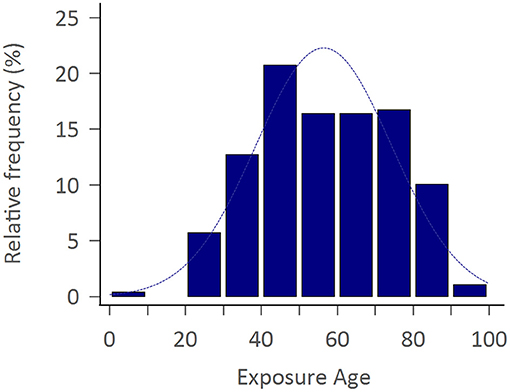
The study consisted of 299 APP cases of whom 206 (68.9%) were adult men with the mean exposure age of 56.4 ± 16.8 years. Figure 2 shows the distribution of exposure age. Paraquat, organophosphates, pyrethroids, carbamates, and phosphinic acid were the most common amongst known reported poisoning agents. The mortality rate was highest with paraquat users (77.1%), followed by phosphinic acid users (22.2%), carbamates users (16.7%), and organophosphates users (15.8%) (Table 1). After a mean follows up of 3.69 ± 2.26 years, the most common neurological sequelae were cognitive decline (56 among 225 survivors, 24.89%), peripheral neuropathy (11 among 225 survivors, 4.89%), tremor (10 among 225 survivors, 4.44%), ataxia (3/225, 1.33%) and parkinsonism feature (2/225, 0.89%). Brain imaging studies revealed basal ganglion lesions on CT or T2 weighted MRI images in 26 among 46 patients (56.5%) (Figure 3). All patients' features, distinct by subgroup, are listed in Table 1. For peripheral neuropathy, 6 of the 11 patients were diagnosed with sensorimotor polyneuropathy through nerve conduction examination. Others were diagnosed clinically by neurologists. All patients with tremors presented with action tremors in their hands. The two Parkinsonism patients with bradykinesia under different pesticide poisoning poorly responded to dopaminergic therapy. Subgroups with fewer than 3 are not listed in the table.
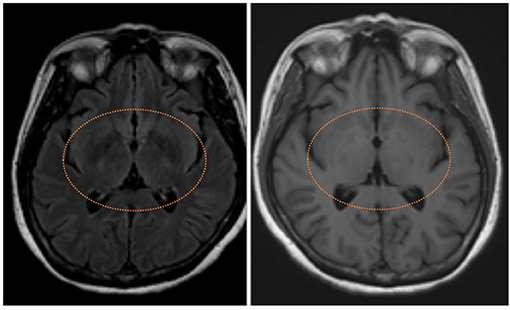
Figure 3. T2 fluid-attenuated inversion recovery magnetic resonance image showing symmetrical hyperintensities in bilateral basal ganglion (left) with T1 hypointensities (right) of neonicotinoids intoxication patient.
Multivariate logistic regression analysis showed that only present basal ganglion lesions on brain imaging (odds ratio [OR]: 4.16, 95% CI: 1.48–11.74, p = 0.007) were found to be significantly associated with neurological sequelae (Table 2).
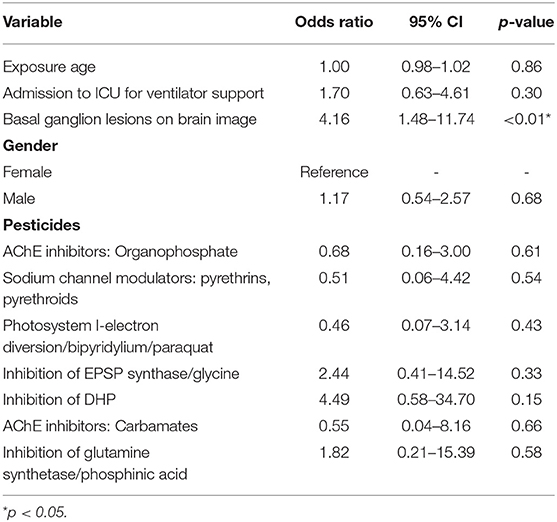
Table 2. Multiple logistic regression analysis of risk factors associated with neurological sequela.
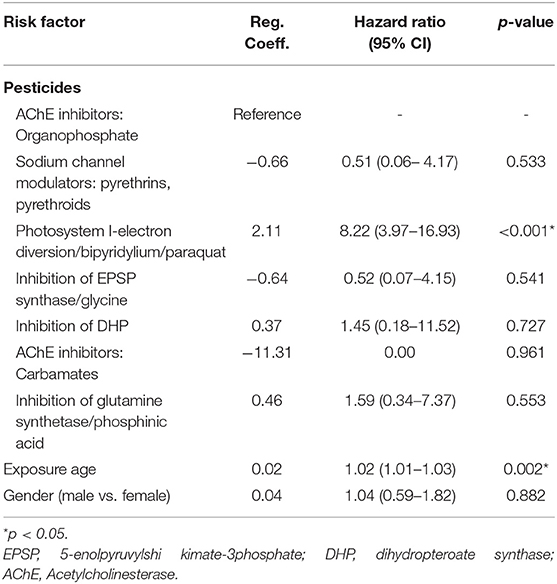
Upon Cox regression for survival analysis during hospitalization, paraquat poisoning was confirmed as an independent predictor (p < 0.0001) (Figure 4). After adjustment, we found that advanced exposure age (OR 1.03, 95% CI 1.01–1.04, p = 0.0001) was significantly associated with mortality (Table 3).
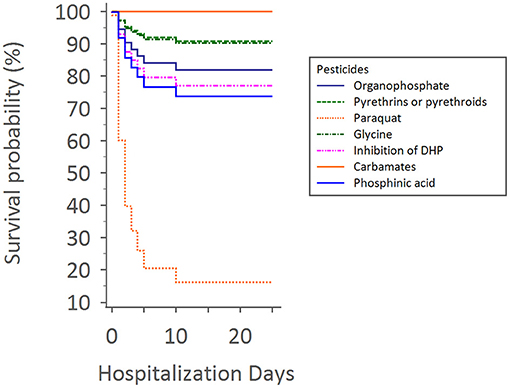
Figure 4. Cox regression for survival analysis during hospitalization times with different pesticides.
Discussion
This retrospective study showed that patients with acute pesticide poisoning have a high associated mortality rate, especially when the pesticide involved is paraquat; also, most people with neurological sequelae who survived APP had cognitive function impairment as well as peripheral neuropathy. Bilateral basal ganglion signal abnormalities' involvement on CT or MRI was positively correlated with neurological sequelae.
Earlier nationwide acute pesticide poisoning research in Taiwan mostly focus on organophosphate pesticides (22). Our study estimated the burden of acute poisoning by different pesticides or insecticides in the suburban of central Taiwan. From our study, most exposures were acute ingestion with organophosphate pesticide, which is an agent of acetylcholinesterase inhibitor to attempt suicide, and our result is compatible with global systemic review data (23). Males were more numerous (68.9%) in our study. Most published pesticide exposures in Asia involve acute ingestions in male adults' intent on self-harm (22, 24). Acute intoxication by pesticides or insecticides or herbicides causes neurotoxicity mostly by inhibiting the normal functioning of neurotransmitters or inducing oxidative stress; however, most of their effects are minor to moderate effects (25, 26). We followed up these patients for chronic neurological sequelae and found that cognitive function impairment and peripheral neuropathy constitute the majority of these sequelae, which is in line with the findings of previous studies (25). More than half of our patients with chronic neurological sequela were poisoned by acetylcholinesterase inhibitors. Nevertheless, significant morbidity and mortality rates were recorded in over three-quarters of all paraquat exposed areas from our investigation. Indika et al. suggested that acute paraquat poisoning could lead to multi-organ failure within a couple of hours to days, causing extremely high mortality rates (27).
The mortality rates reported in this study are similar to the previously reported acetylcholinesterase inhibitor poisoning mortality rate of 10–20% in many Asian and developing countries throughout the world (22, 28), and the rates of survival from paraquat poisoning are as low, as previous similar studies have reported (29).
The basal ganglia are a bunch of nuclei in the subcortical area that are responsible for crucial functions such as motor control and learning, executive function, emotion, and behaviors. A large number of robust pieces of evidence show that basal ganglia are vulnerable to toxic, metabolic, or ischemic damage; so, many studies show that bilateral basal ganglion image signal abnormalities are seen after intoxication, including acute pesticides poisoning (18, 30). From our study, it can be seen that basal ganglion lesions on brain imaging have a positive correlation with neurological sequelae which is compatible with earlier studies.
The main strength of this study is its detailed classification of pesticides using real-world data. However, the result should be interpreted in the context of the following limitations. First, it is a retrospective study without a normal control group, and the diagnosis of APP in this study relied mostly on administrative diagnosis codes. Not every patient was diagnosed directly through a neurologist. However, we had traced back every single patient's medical record to confirm which kind of pesticide they were exposed to, and the diagnosis correlated with the patient's present illness, and we also reviewed all the medical records case by case before the exposure to make sure the sequelae are not relevant to their earlier illness. Second, although we adjusted for as much bias as we could by using multivariable analysis to balance the baseline differences between the groups and eliminate residual confounding effects, biases related to unmeasured confounders remain a potential issue given the nature of this study. Third, patients may not have been followed up at a neurology clinic; so, subtle or mild neurological problems may have been underdiagnosed. Finally, this was a single-institution study, which means there might have been selection bias. Nevertheless, our burden of the neurological sequelae or mortality is consistent with previous studies in Asia even worldwide.
In conclusion, the mortality rate associated with acute pesticide intoxication is high and strongly correlated with paraquat. Bilateral basal ganglion signal abnormalities findings on imaging are significantly associated with neurological sequelae. Cognitive function impairment and peripheral neuropathy are the most commonly encountered residual neurological symptoms. Our results shed light on the burden and influences of acute pesticide poisoning. Further public health regulations should be put in place.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.
Ethics Statement
The studies involving human participants were reviewed and approved by Changhua Christian Hospital Committee for the Human Subjects Protection (200316). The patients/participants provided their written informed consent to participate in this study.
Author Contributions
S-LW contributed to the conception and design of the study. Y-CC organized the database and wrote the first draft of the manuscript. Y-CC and C-HL performed the statistical analysis. All authors contributed to manuscript revision, read, and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We would like to thank the www.enago.tw for reviewing English language and grammar.
References
1. Choi PT-L, Quinonez LG, Cook DJ. Acute organophosphate insecticide poisoning. Clin Intensive Care. (1995) 6:5. doi: 10.3109/tcic.6.5.228.235
2. Svensson M, Urinboyev R, Wigerfelt Svensson A, Lundqvist P, Littorin M, Albin M. Migrant agricultural workers and their socio-economic, occupational and health conditions-a literature review. Occupat Health Conditions-A Literature Rev. (2013). doi: 10.2139/ssrn.2297559. [Epub ahead of print].
3. Council UNHR. Report of the Special Rapporteur on the Right to Food. Geneva: United Nations (2017).
4. Muñoz-Quezada MT, Lucero BA, Iglesias VP, Muñoz MP, Cornejo CA, Achu E, et al. Chronic exposure to organophosphate (OP) pesticides and neuropsychological functioning in farm workers: a review. Int J Occupat Environ Health. (2016) 22:68–79. doi: 10.1080/10773525.2015.1123848
5. Savage EP, Keefe TJ, Mounce LM, Heaton RK, Lewis JA, Burcar PJ. Chronic neurological sequelae of acute organophosphate pesticide poisoning. Arch Environ Health: An Int J. (1988) 43:38–45. doi: 10.1080/00039896.1988.9934372
6. Yu CJ, Du JC, Chiou HC, Chung MY, Yang W, Chen YS, et al. Increased risk of attention-deficit/hyperactivity disorder associated with exposure to organophosphate pesticide in Taiwanese children. Andrology. (2016) 4:695–705. doi: 10.1111/andr.12183
7. Tang BL. Neuropathological mechanisms associated with pesticides in Alzheimer's disease. Toxics. (2020) 8:21. doi: 10.3390/toxics8020021
8. Moshou H, Karakitsou A, Yfanti F, Hela D, Vlastos D, Paschalidou AK, et al. Assessment of genetic effects and pesticide exposure of farmers in NW Greece. Environ Res. (2020) 186:109558. doi: 10.1016/j.envres.2020.109558
9. Ling C, Liew Z, von Ehrenstein OS, Heck JE, Park AS, Cui X, et al. Prenatal exposure to ambient pesticides and preterm birth and term low birthweight in agricultural regions of California. Toxics. (2018) 6:41. doi: 10.3390/toxics6030041
10. Koutros S, Silverman DT, Alavanja MC, Andreotti G, Lerro CC, Heltshe S, et al. Occupational exposure to pesticides and bladder cancer risk. Int J Epidemiol. (2016) 45:792–805. doi: 10.1093/ije/dyv195
11. Hu Y, Zhang Y, Vinturache A, Wang Y, Shi R, Chen L, et al. Effects of environmental pyrethroids exposure on semen quality in reproductive-age men in Shanghai, China. Chemosphere. (2020) 245:125580. doi: 10.1016/j.chemosphere.2019.125580
12. Delamarre A, Meissner WG. Epidemiology, environmental risk factors and genetics of Parkinson's disease. Presse Med. (2017) 46:175–81. doi: 10.1016/j.lpm.2017.01.001
13. Dardiotis E, Aloizou AM, Sakalakis E, Siokas V, Koureas M, Xiromerisiou G, et al. Organochlorine pesticide levels in Greek patients with Parkinson's disease. Toxicol Rep. (2020) 7:596–601. doi: 10.1016/j.toxrep.2020.03.011
14. Brouwer M, Huss A, van der Mark M, Nijssen PCG, Mulleners WM, Sas AMG, et al. Environmental exposure to pesticides and the risk of Parkinson's disease in the Netherlands. Environ Int. (2017) 107:100–10. doi: 10.1016/j.envint.2017.07.001
15. Boulanger M, Tual S, Lemarchand C, Guizard AV, Velten M, Marcotullio E, et al. Agricultural exposure and risk of bladder cancer in the AGRIculture and CANcer cohort. Int Arch Occup Environ Health. (2017) 90:169–78. doi: 10.1007/s00420-016-1182-y
16. Aloizou AM, Siokas V, Vogiatzi C, Peristeri E, Docea AO, Petrakis D, et al. Pesticides, cognitive functions and dementia: A review. Toxicol Lett. (2020) 326:31–51. doi: 10.1016/j.toxlet.2020.03.005
17. Bhanu KU, Khandelwal N, Vyas S, Singh P, Prabhakar A, Mittal BR, et al. Evaluation of MR perfusion abnormalities in organophosphorus poisoning and its correlation with SPECT. Indian J Radiol Imaging. (2017) 27:36–42. doi: 10.4103/0971-3026.202961
18. Ravikanth R. Role of magnetic resonance imaging in diagnosing neurological complications in intermediate syndrome of organophosphate poisoning. Indian J Crit Care Med. (2017) 21:105–7. doi: 10.4103/ijccm.IJCCM_357_16
19. Yang Y, Liu H, Li S, Wang Y, Huang B, Chi C, et al. The brain imaging study of the organophosphorus pesticides poisoning. Chin J Radiol. (2004) 38:820–3.
20. Alarcon WA, Calvert GM, Blondell JM, Mehler LN, Sievert J, Propeck M, et al. Acute illnesses associated with pesticide exposure at schools. JAMA. (2005) 294:455. doi: 10.1001/jama.294.4.455
22. TJ, Walter FG, Hung DZ, Tsai JL, Hu SC, Chang JS, et al. Epidemiology of organophosphate pesticide poisoning in Taiwan. Clin Toxicol (Phila). (2008) 46:794–801. doi: 10.1080/15563650801986695
23. Boedeker W, Watts M, Clausing P, Marquez E. The global distribution of acute unintentional pesticide poisoning: estimations based on a systematic review. BMC Public Health. (2020) 20:1875. doi: 10.1186/s12889-020-09939-0
24. Ko S, Cha ES, Choi Y, Kim J, Kim JH, Lee WJ. The burden of acute pesticide poisoning and pesticide regulation in Korea. J Korean Med Sci. (2018) 33:e208. doi: 10.3346/jkms.2018.33.e208
25. Kamel F, Hoppin JA. Association of pesticide exposure with neurologic dysfunction and disease. Environ Health Perspect. (2004) 112:950–8. doi: 10.1289/ehp.7135
26. Franco R, Li S, Rodriguez-Rocha H, Burns M, Panayiotidis MI. Molecular mechanisms of pesticide-induced neurotoxicity: Relevance to Parkinson's disease. Chem Biol Interact. (2010) 188:289–300. doi: 10.1016/j.cbi.2010.06.003
27. Gawarammana IB, Buckley NA. Medical management of paraquat ingestion. Br J Clin Pharmacol. (2011) 72:745–57. doi: 10.1111/j.1365-2125.2011.04026.x
28. Eddleston M, Phillips MR. Self poisoning with pesticides. Bmj. (2004) 328:42–4. doi: 10.1136/bmj.328.7430.42
29. Koo JR, Yoon JW, Han SJ, Choi MJ, Park II. Rapid analysis of plasma paraquat using sodium dithionite as a predictor of outcome in acute paraquat poisoning. Am J Med Sci. (2009) 338:373–7. doi: 10.1097/MAJ.0b013e3181b4deee
Keywords: neurological sequela, mortality, basal ganglion lesions on brain images, insecticide resistance action committee mode of action (MoA) classification, acute pesticide poisoning
Citation: Chen Y-C, Lin C-H and Wu S-L (2021) Neurological Sequela of Acute Pesticide Poisoning Among Adults in Central Taiwan. Front. Neurol. 12:745265. doi: 10.3389/fneur.2021.745265
Received: 21 July 2021; Accepted: 04 November 2021;
Published: 10 December 2021.
Edited by:
Santiago Perez-Lloret, Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET), ArgentinaReviewed by:
Malco Rossi, Fundación Para la Lucha Contra las Enfermedades Neurológicas de la Infancia (FLENI), ArgentinaWillian Garcia Birolli, Federal University of São Carlos, Brazil
Copyright © 2021 Chen, Lin and Wu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shey-Lin Wu, c2hleWxpbkBjY2gub3JnLnR3
 Yen-Chung Chen
Yen-Chung Chen Chin-Hsien Lin
Chin-Hsien Lin Shey-Lin Wu
Shey-Lin Wu