
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Neurol., 08 October 2021
Sec. Pediatric Neurology
Volume 12 - 2021 | https://doi.org/10.3389/fneur.2021.744041
 Haixia Zhu†
Haixia Zhu† Wenlin Wu†
Wenlin Wu† Lianfeng Chen†
Lianfeng Chen† Chi Hou
Chi Hou Yiru Zeng
Yiru Zeng Yang Tian
Yang Tian Huiling Shen
Huiling Shen Yuanyuan Gao
Yuanyuan Gao Yani Zhang
Yani Zhang Bingwei Peng
Bingwei Peng Wen-Xiong Chen*
Wen-Xiong Chen* Xiaojing Li*
Xiaojing Li*Objective: To study the clinical characteristics and treatment of pediatric opsoclonus-myoclonus syndrome (OMS).
Methods: We analyzed the clinical data of nine children OMS between June 2017 and Nov 2020.
Results: Nine children (M/F = 3:6, median onset age was 18 months) diagnosed with OMS were included in the study. Before onset, human rhinovirus and respiratory syncytial virus were seen in one patient, respectively. And one patient received Japanese encephalitis vaccination. Three patients had neuroblastoma, and one patient had ganglioneuroblastoma. All patients' symptoms were improved after receiving surgery (for four patients with tumor), intravenous human immunoglobulin and pulsed methylprednisolone. However, four patients without mass relapsed and became relapse free after rituximab treatment. The relapse rate was 44.4% (4/9). The OMS severity score at the last follow-up was significantly lower than the OMS severity score at onset (3.0 ± 1.0 vs. 11.0 ± 2.2, paired-samples t-test, P < 0.001). All patients had at least one item of neurological symptoms or neuropsychological disturbances.
Conclusion: For pediatric OMS, human rhinovirus infection and respiratory syncytial virus infection can be seen before onset. Rituximab is effective in reducing relapse. Improving recognition and long-term prognosis in OMS is urgent.
Opsoclonus-myoclonus syndrome (OMS) is a rare neurological disease. The incidence of OMS was 0.18 cases per million population per year in a prospective survey of United Kingdom pediatric neurology centers and 0.27 to 0.40 cases per million in Japanese children (1, 2). It usually affects children with a median age of 18–22 months (3). The disease is characterized by acute or subacute episodes of ataxia, opsoclonus (bursts of high-frequency oscillations of the eyes with horizontal, vertical, and torsional saccades), myoclonus (non-epileptic involuntary jerks of the limbs and trunk), irritability, sleep disturbances, and behavioral disruption (3). The absence of one or some distinctive features in the early stages of the disease may delay the diagnosis of OMS by weeks or months. OMS is a rare paraneoplastic or possibly post-viral severe neurologic syndrome (3). In adults with paraneoplastic OMS, small cell lung cancer, breast carcinoma, and ovarian teratoma were the most frequently reported tumors. In contrast, more than half of pediatric OMS cases are associated with neuroblastoma (NB) (3). Although the etiology of OMS is thought to be immune mediated, disease-associated autoantibodies have yet to be identified. OMS is an important differential diagnosis of acute post-infection cerebellar ataxia, with significant differences in treatment and prognosis. About 70% of children with OMS suffer from motor, cognitive, and language impairment, so early diagnosis and timely treatment are crucial (4, 5). In order to improve recognition and determine the optimal treatment approach for children with OMS, we conducted a single-center retrospective review of children with OMS at Guangzhou Women and Children's Medical Center in China between June 2017 and Nov 2020.
Children with OMS were retrospectively recruited from June 2017 and Nov 2020 in the department of neurology of Guangzhou Women and Children's Medical Center, one of the National Children's Medical centers in China. Diagnostic criteria for OMS was defined as requiring three of the four following features (3): (1) opsoclonus, (2) myoclonus or ataxia, (3) behavioral change or sleep disturbance, and (4) NB. The relapse of OMS was defined as worsening of OMS symptoms lasting for at least 72 h after a period of stability or improvement for at least 30 days, or the escalation of immunotherapy as a proxy measure (6). This study was approved by the Ethics Committee of Guangzhou Women and Children's Medical Center. Written and signed consent was obtained from the patient's parents or guardians, who also explicitly consented to publish their personal details, clinical data and images that could identify them.
Clinical data, including the history of prodromal infection and vaccination, clinical manifestations, laboratory tests, neuroelectrophysiological examination, imaging data, treatments, and prognosis, were retrieved from electronic medical records and electronic medical record-assisted telephone follow-up. A standardized 0–15 point grading scale for OMS symptom severity, developed by Drs. Wendy Mitchell and Michael Pike, was used. And symptoms assessed in the scale included stance, gait, hand and arm function, opsoclonus, mood and behavior (7). The development quotient (DQ) of patients was calculated by Gesell development schedules at last follow-up visit.
Statistical analysis was performed with IBM SPSS 20.0 for windows (SPSS Inc., Chicago IL, USA). Quantitative data with normal distribution were described by mean ± SD and compared by independent t-test or paired samples t-test. The correlation between two Quantitative data with normal distribution was analyzed by the Pearson correlation coefficient analysis. Categorical data were described as frequency and percentage otherwise as median with the range. P < 0.05 (two-sided) was considered statistically significant. Figures were graphed using GraphPad Prism 7.01 (GraphPad Software Inc., US).
A total of nine children (male: female was 3:6) diagnosed with OMS were involved. The median age of symptom onset was 18 months (ranged from 10 to 20 months), while the median age of diagnosis was 22 months (ranged from 13 to 35 months). All patients normally developed before the onset of OMS. At the initial evaluation in our hospital, opsoclonus, myoclonus, and ataxia were seen in all nine patients. Five patients had sleep disturbances (#2,3,5,8,9), seven patients (#1,2,3,4,7,8,9) displayed irritability and five patients (#3,5,7,8,9) exhibited speech disorders. The OMS severity score at the initial evaluation in our hospital was 11 ± 2.2. During the whole disease course, ataxia, opsoclonus, myoclonus, motor and sleep disturbances were seen in all patients, while irritation (#1,2,3,4,5,7,8,9) and speech disorders (#1,2,3,5,6,7,8,9) were found in eight patients.
Seven patients (#1,2,3,4,5,8,9) had prodromal events 1 week before symptom onset. Six of them had upper respiratory infection disease (#1,3,4,5,8,9), and one patient (#2) had vomiting and diarrhea, while this patient also received an anti-Japanese encephalitis vaccination 1 week before onset.
Blood lactic acid/pyruvic acid, blood ammonia, blood urine organic acids, amino acids and blood acylcarnitine were tested normal in all patients.
Seven patients (#1,2,3,4,6,7,9) were tested for human chlamydia psittaci, mycoplasma pneumonia, respiratory adenovirus, enterovirus, influenza A, influenza B, human rhinovirus, respiratory syncytial virus with throat swab samples. Patient #1's was positive for human rhinovirus and patient #2's was positive for respiratory syncytial virus. Cerebrospinal fluid (CSF) was tested negative for the nucleic acid of enterovirus, influenza A, influenza B, Herpes simplex virus, Epstein-Barr virus, Cytomegalovirus and antibodies of human chlamydia psittaci and mycoplasma pneumonia in all patients.
All patients underwent tumor markers tests, including 24-h urinary vanillylmandelic acid, serum neuron enolase, alpha-fetoprotein and other examinations. All findings are within normal ranges, except mild increase of serum neuron enolase in three patients (#1, 2, 4).
All patients underwent chest and abdomen computed tomography for tumor detection. Chest mass was in three patients (#1,4,6), while abdominal mass was found in two patients (#5,9). Four (#1, 4, 6, 9) of these five patients underwent bone marrow examination through bone marrow puncture, and the results were normal. These four patients received tumor removal surgical treatment. According to the pathology results of the tumor, three patients (#1,4,9) were diagnosed as NB (all their tumor were at stage 2 assessed by international NB staging system) and one patient (#6) was diagnosed as ganglioneuroblastoma (stage 1 assessed by international NB staging system, more details seen in Table 1). One patient (#5) declined surgery treatment.
All patients underwent brain magnetic resonance image and electroencephalography examination. Eight patients showed no abnormalities in brain magnetic resonance image and one patient (#1) showed the lateral ventricle slightly enlarged. Electroencephalography in two patients (#7, 9) showed non-specific slow wave background and electroencephalography in the others were normal.
Four patients (#1,4,6,9) with tumor were treated with surgery, intravenous human immunoglobulin (IVIG) (a dose of 2 g/kg over a period of 2–5 days) combined with intravenous pulsed methylprednisolone (IVMP, 10–15 mg/kg/day infusion for 3 days) and chemotherapy (for patient #1,4,9). Their Symptoms were improved without relapse and OMS severity score decreased (details seen in Table 2; Figure 1). One patient (#5) with mass declined surgery treatment and only received IVIG treatment (a dose of 2 g/kg over a period of 5 days). Her OMS scores decreased from 11 to 2 in the last follow-up (details seen in Table 2; Figure 1).
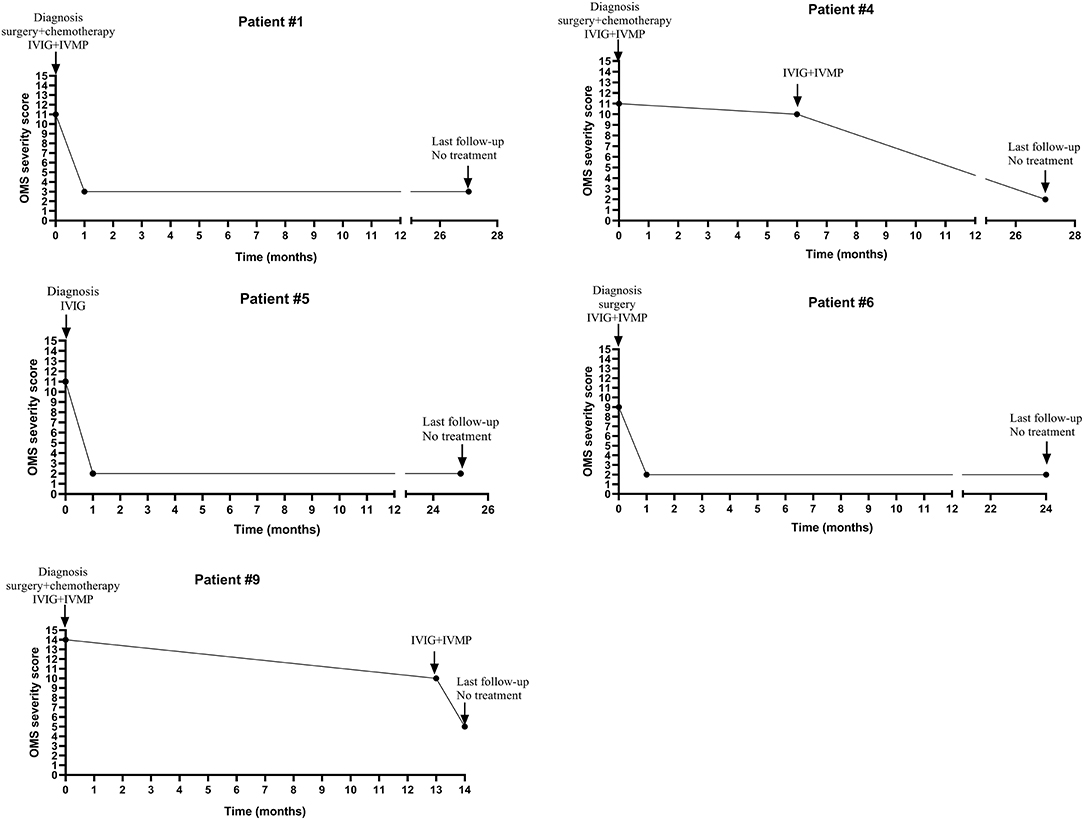
Figure 1. The change of OMS severity score and immunotherapy of five patients with tumor or mass during the clinical course.
Four patients (#2,3,7,8) without tumor or mass received initial IVIG and IVMP treatment tapered off within 6 months. Symptoms in these four patients were improved, but they had several relapses during the weaning off steroids (the dose of prednisone ranged from 0.42 to 1.25 mg/kg/d when relapsed). During relapses, IVIG combined with IVMP treatment could still improve OMS symptoms. These four patients experienced one to the three times relapses, so they received Rituximab (RTX) treatment (375 mg/m2 weekly for 4 weeks). After RTX treatment, the patients' condition was improved and they did not relapse during a follow-up ranging from 6 to 29 months (more details seen in Table 2; Figure 2).
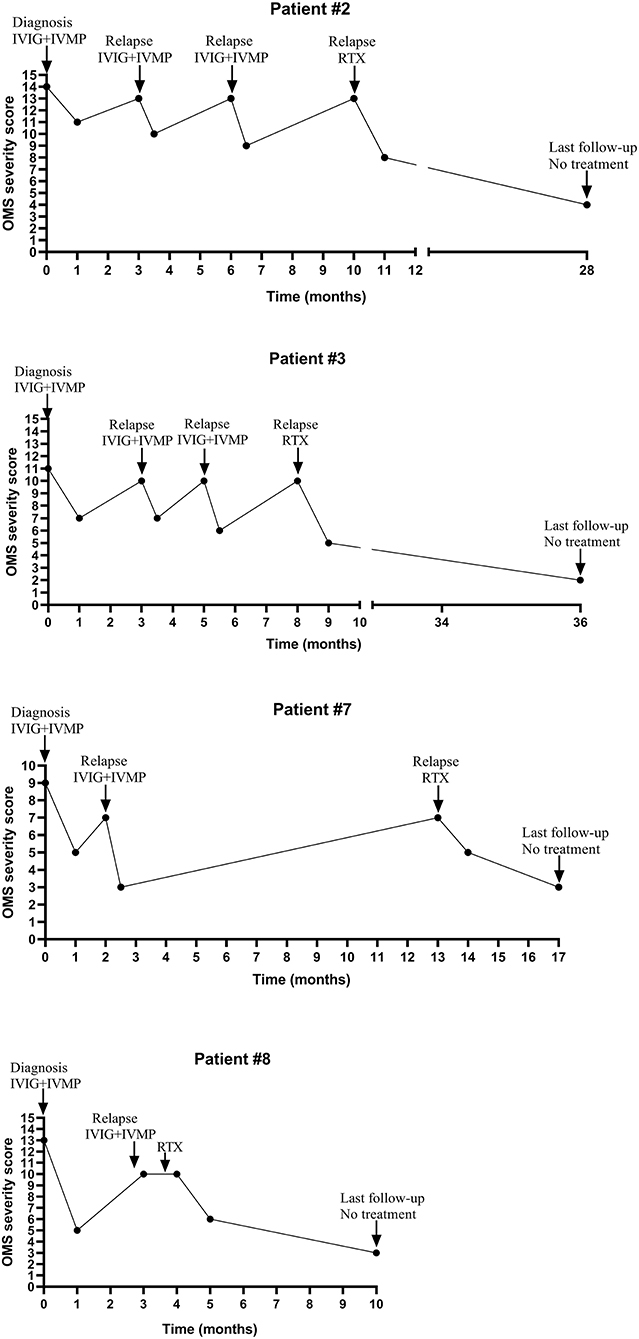
Figure 2. The change of OMS severity score and immunotherapy of four relapsed patients during the clinical course.
The median interval between onset and diagnosis was 4.5 months (ranged from 4 weeks to 17 months). The relapse rate was 44.4% (4/9). The relapse rate in patients with tumor or mass was 0%, while in patients without tumor or mass was 100% (more details seen in Table 2; Figure 1).
Up to Nov 2020, none was lost to follow-up. The median follow-up interval was 25 months (ranging from 10 to 36 months). The OMS severity score at the last follow-up was significantly lower than the OMS severity score at onset (3.0 ± 1.0 vs. 11.0 ± 2.2, paired-samples t-test, P < 0.001). The DQ of patients at the last follow-up was 77.6 ± 9.8 (ranged from 65 to 94, more details seen in Table 2). The DQ of patients at the last follow-up was not correlated with the age at onset, the OMS severity score of onset or last follow-up, the number of relapses, the duration of follow-up (P > 0.05, Pearson correlation coefficient analysis). There was no statistical difference in the DQ at the last follow-up between patients with tumor or not (76.8 ± 11.3 vs. 78.5 ± 9.1, t = 0.244, independent t-test, P = 0.814). There was no statistically difference in the DQ at the last follow-up between patients receiving RTX treatment or not (78.5 ± 9.1 vs. 76.8 ± 11.3, t = −0.244, independent t-test, P = 0.814). All patients had at least one item of neurological symptoms or neuropsychological disturbances at the most recent evaluation. Eight patients (88.9%) had delayed speech development, typically present with lack of vocabulary. Five patients (55.6%) had mild ataxia, 2 (22.2%) had mild opsoclonus, and 3 (33.3%) had behavioral disorders characterized by irritability, hyperactivity, and restlessness in sleep. None of the children with NB had tumor recurrence during the follow-up period.
Our study described nine pediatric patients with OMS retrospectively. Their median age at onset was 18 months (ranged from 10 to 20 months), similar to the onset age in other reports, ranging from 6 months to 4 years (2, 5, 8–13). The ratio of male to female is 3:6, which was also consistent with other reports showing OMS with a female predominance (8, 9).
Though Marcel Kinsbourne first described OMS in 1962, the pathogenesis of OMS is still unclear. Before OMS onset, some patients could have infectious events and a few children with NB could develop OMS. In Pranzatelli et al.'s study, 31% of patients had prodromal symptoms of a possible non-specific upper respiratory illness, vomiting in 26%, diarrhea in 14%. In our study, six patients had symptoms of upper respiratory tract infection, and one patient had both vomiting and diarrhea. In many case reports, the virus was the most common infectious seen in OMS, including west-Nile virus (14, 15), human herpes virus-6 (16, 17), Epstein-Barr virus (18), adenovirus (19), influenza A virus (20), varicella-zoster virus (21), rotavirus (22) and herpes simplex virus (23). OMS onset followed mycoplasma pneumonia has also been reported (24–27). In our study, two patients had rhinovirus infection and respiratory syncytial virus infection, respectively. Rhinovirus infection and respiratory syncytial virus infection were not reported in previous studies. Viral illnesses are prevalent among patients and healthy children at the age of OMS onset. Whether these infections are occasionally presented in OMS or play a role in the pathogenesis of OMS needs further investigation. Moreover, it is also important to note that the presence of infection does not exclude a NB (as patient #1, 4, and 9 in our study), and therefore one always needs to look for NB. Interestingly, one female patient in our study received Japanese encephalitis vaccination before OMS onset. OMS onset followed by receiving vaccination was rare. In previous studies, two OMS female patients (one was 11 years old and the other one was 30 years old) received human papilloma virus vaccination and anti-Rubella vaccination about 2 weeks before OMS onset (28, 29). Japanese encephalitis vaccination was associated with some autoimmune diseases in the central nervous system like anti-N-Methy-D-aspartate receptor encephalitis and acute disseminated encephalomyelitis (30). Though there was no direct evidence about how the Japanese encephalitis vaccination triggered these autoimmune diseases, Wang et al. found some miRNAs associated with the Japanese encephalitis vaccination, were closer to let-7 family which was related to anti-N-Methy-D-aspartate receptor encephalitis by phylogenetic tree analysis (30). And Wang et al.'s study also provided a protocol for investigating the association of vaccination and anti-N-Methy-D-aspartate receptor encephalitis. This method may be helpful to study the association of infection or vaccination with OMS in further study.
The classic clinical manifestations of OMS are opsoclonus (conjugation, non-phasic, fast and multi-direction), myoclonus (non-epileptic limb convulsions), ataxia, sleep disturbances, cognitive dysfunction, and behavioral (3). The earlier appearance of ataxia than opsoclonus or myoclonus in OMS may provide one reason why acute cerebellar ataxia is such a diagnostic pitfall. In Pranzatelli et al.'s studies, the interval between OMS onset and diagnosis was 2.8 months in 2005, while it was 1.2 months in 2017. In Mitchell et al.'s study, the interval between OMS onset and diagnosis ranged from 2 days to 14 months. In our study, this interval ranged from 1 to 17 months, with a median interval of 3 months. Evidence suggests that at least 20% of OMS cases have an atypical presentation. For example, opsoclonus may occur 1–18 months after onset, so the diagnosis is often delayed by weeks or months (31). Opsoclonus is a vital symptom to distinguish OMS from acute ataxia. Though some of the patients in our study presented ataxia, myoclonus, and opsoclonus at first, were misdiagnosed by their primary care doctors as acute cerebellar ataxia before admission to our hospital. These might be caused by neglecting the opsoclonus symptom, or the OMS was a rare disorder and not be well-known by doctors who were not neurologist. OMS was rarely resolved spontaneously without immunotherapy (acute cerebellar ataxia does). For patients with ataxia presentation, if opsoclonus is observed, OMS diagnosis should be taken into consideration.
Some of the OMS is associated with malignancies. In adults, OMS is primarily associated with breast cancer and lung cancer. In children, OMS is associated in more than half of cases with NB (3). Interestingly, for reasons as yet unknown, the survival rate of children with OMS and NB is far better than that of the general population of children with NB. NB with OMS symptoms has a lower grade and no MYCN oncogene amplification and tends to have a better prognosis. OMS-associated NB is typical of low risk and carries an excellent prognosis. Low-grade tumors are often metabolically inactive, resulting in an increased urinary catecholamine uncommon. Increased vanillylmandelic acid levels can be detected only in 21% of OMS children with NB (11). In this study, four children with OMS diagnosed with NB showed normal urinary vanillylmandelic acid and no amplification of MYCN oncogene in the tumor tissues, similar to the results reported by Brunklaus et al. (32). Autoimmune pathophysiology is thought to be possible in OMS disease. And patients appear to respond to immunosuppressive treatment such as IVIG, methylprednisolone, dexamethasone, adrenocorticotropic hormone, RTX and cyclophosphamide pulses. International shared guidelines stating the optimal combination and duration of different immunomodulatory drugs are still lacking. In the beginning, the treatment protocol for OMS in our center was that when patients still relapsed after three cycles of IVIG combined with IVMP, then RTX treatment should be considered (#2, 3). Later, based on the good response of RTX treatment in case 2 and case 3, and more international studies have suggested that the effect of RTX in OMS, especially when given early in the disease course, improved OMS outcome. So we advanced the time of initiating RTX treatment when the patients still relapsed after two cycles of IVIG combined with IVMP treatment (#7, 8). In our study, neurological symptoms in patients with or without tumors were improved after treatment with IVIG combined with IVMP or IVIG only (2, 5, 9–13) (More details seen in Supplementary Material). Moreover, a randomized, open-label, phase 3 trial study from de Alarcon et al. showed that even for children with OMS associated with neuroblastoma, the addition of IVIG to prednisone and risk-adapted chemotherapy improved treatment response rate significantly (33). However, some patients would experience relapse. In our study, all patients without tumor or mass experienced relapse during weaning of steroid. This result was similar to previous studies (34). After treatment with RTX, all these four patients without tumor or mass in our study became relapse free. RTX is an anti-CD20 monoclonal antibody that depletes circulating B cells. Pranzatelli et al. found that the CD19+ B cell recruitment in the CSF of the OMS patients was correlated with their neurological severity (35). Besides, some autoantibodies were found in OMS patients, such as anti-Hu autoantibody, anti-Ru and so on and B-cell activating factor BAFF increased in the CSF in childhood OMS (34). This evidence suggested the B-cell has participated in the autoimmune pathophysiology of OMS. Furthermore, RTX effectively reduced relapse in our study and suggested this relapse might be B-cell associated. The first successful treatment of pediatric OMS patients using RTX was done by Pranzatelli et al. in 2005 (36). Subsequently, other studies also confirm the beneficial effect of RTX in OMS patients, even in otherwise treatment refractory patients or in relapsed patients (8, 9, 34). Consistently, we found RTX was effective in preventing relapse in OMS patients.
Though neurological symptoms in patients with OMS show at least some improvement under current treatment, neuropsychological disturbances became a more considerable part of the clinical course at long-term follow-up. Over 70% of OMS patients have neurological sequelae, especially intellectual disability and language developmental delay (2, 4, 5, 9–13, 34, 37) (More details seen in Supplementary Material). Similarly, in our study, eight patients (88.9%) had delayed speech development, and three patients (33.3%) had behavioral disorders characterized by irritability, hyperactivity and restlessness in sleep. We found that the DQ of patients at the last follow-up was 77.6 ± 9.8. And three OMS patients in En Lin et al.'s study had evolving cognitive dysfunction despite being in remission and been followed up for a long time (ranged from 5 to 10 years) (38). In our study, we found the DQ of patients at the last follow-up was not correlated with the age at onset, the OMS severity score onset or last follow-up, the number of relapses, the duration of follow-up. In addition, there was no statistical difference in the DQ at the last follow-up between patients with tumor or not, as well as patients receiving RTX treatment or not. A recent study conducted by Sheridan et al. showed that the number of relapses negatively correlates with full-scale IQ in pediatric OMS (6). We failed to found the correlation between the relapse and DQ in our patients might be caused by the small number of patients in our study. More active immunoregulatory strategies to reduce OMS relapses may improve the prognosis. In Michell et al.'s study from Children's Hospital Los Angeles, RTX treatment was initiated after one or more relapses for OMS patients. More recently, it became a part of initial therapy. Their study found that using such a more aggressive immunosuppression treatment improved adaptive behavior, cognitive and motor scores. Although this study did not meet the standards of a randomized clinical trial, it suggested that more aggressive treatment with RTX might be a possible way to achieve the aim that is not only the improvement of neurological symptoms but also the improvement of neuropsychological disturbances. Further study with a large-scale clinical trial is needed to investigate whether a more aggressive immunosuppression treatment can improve neuropsychological disturbances.
Our study has several limitations. The first limitation is that we were unable to test the lymphocyte subset in the CSF. Neurological severity of OMS was positively related to the percentage of CD19+ B lymphocytes in CSF and was negatively related to CD4+ T lymphocytes in CSF (35, 39). The second limitation is that our study is a retrospective study, and we did not test the oligoclonal bands in CSF which was also a marker of disease activity (40).
For pediatric OMS, human rhinovirus infection and respiratory syncytial virus infection can be seen before onset. Rituximab is effective in reducing relapse. Improving recognition and long-term prognosis in OMS is urgent.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
The studies involving human participants were reviewed and approved by the Ethics Committee of Guangzhou Women and Children's Medical Center. Written informed consent to participate in this study was provided by the participants' legal guardian/next of kin.
HZ, WW, and LC study concept, acquisition of data, and draft the manuscript. CH, YZe, YT, HS, YG, and YZh study concept and acquisition of data. BP analyzed the data. XL and W-XC study concept and critical revision of manuscript for intellectual content. All authors gave final approval of the version to be published.
The present study was supported by the Health and family planning technological project foundation of Guangzhou city (Grant No. 20181A011038). The funders had no role in the study concept, study design, data analysis, interpretation or reporting of the results. The authors had full control of the data and information submitted for publication.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2021.744041/full#supplementary-material
CSF, cerebrospinal fluid; DQ, development quotient; IVIG, intravenous human immunoglobulin; IVMP, intravenous pulsed methylprednisolone; NB, neuroblastoma; OMS, opsoclonus-myoclonus syndrome; RTX, Rituximab.
1. Pang KK, de Sousa C, Lang B, Pike MG. A prospective study of the presentation and management of dancing eye syndrome/opsoclonus-myoclonus syndrome in the United Kingdom. Eur J Paediatr Neurol. (2010) 14:156–61. doi: 10.1016/j.ejpn.2009.03.002
2. Hasegawa S, Matsushige T, Kajimoto M, Inoue H, Momonaka H, Oka M, et al. A nationwide survey of opsoclonus-myoclonus syndrome in Japanese children. Brain Dev. (2015) 37:656–60. doi: 10.1016/j.braindev.2014.10.010
3. Matthay KK, Blaes F, Hero B, Plantaz D, De Alarcon P, Mitchell WG, et al. Opsoclonus myoclonus syndrome in neuroblastoma a report from a workshop on the dancing eyes syndrome at the advances in neuroblastoma meeting in Genoa, Italy, 2004. Cancer Lett. (2005) 228:275–82. doi: 10.1016/j.canlet.2005.01.051
4. Catsman-Berrevoets CE, Aarsen FK, van Hemsbergen ML, van Noesel MM, Hakvoort-Cammel FG, van den Heuvel-Eibrink MM. Improvement of neurological status and quality of life in children with opsoclonus myoclonus syndrome at long-term follow-up. Pediatr Blood Cancer. (2009) 53:1048–53. doi: 10.1002/pbc.22226
5. Brunklaus A, Pohl K, Zuberi SM, de Sousa C. Outcome and prognostic features in opsoclonus-myoclonus syndrome from infancy to adult life. Pediatrics. (2011) 128:e388–94. doi: 10.1542/peds.2010-3114
6. Sheridan A, Kapur K, Pinard F, Dietrich Alber F, Camposano S, Pike MG, et al. IQ predictors in pediatric opsoclonus myoclonus syndrome. A large international cohort study. Dev Med Child Neurol. (2020) 62:1444–9. doi: 10.1111/dmcn.14628
7. Gorman MP. Update on diagnosis, treatment, prognosis in opsoclonus-myoclonus-ataxia syndrome. Curr Opin Pediatr. (2010) 22:745–50. doi: 10.1097/MOP.0b013e32833fde3f
8. Mitchell WG, Wooten AA, O'Neil SH, Rodriguez JG, Cruz RE, Wittern R. Effect of increased immunosuppression on developmental outcome of Opsoclonus Myoclonus Syndrome (OMS). J Child Neurol. (2015) 30:976–82. doi: 10.1177/0883073814549581
9. Pranzatelli MR, Tate ED, McGee NDemographic R. Clinical immunologic features of 389 children with opsoclonus-myoclonus syndrome: a cross-sectional study. Front Neurol. (2017) 8:468. doi: 10.3389/fneur.2017.00468
10. Tate ED, Allison TJ, Pranzatelli MR, Verhulst SJ. Neuroepidemiologic trends in 105 US cases of pediatric opsoclonus-myoclonus syndrome. J Pediatr Oncol Nurs. (2005) 22:8–19. doi: 10.1177/1043454204272560
11. Krug P, Schleiermacher G, Michon J, Valteau-Couanet D, Brisse H, Peuchmaur M, et al. Opsoclonus-myoclonus in children associated or not with neuroblastoma. Eur J Paediatr Neurol. (2010) 14:400–9. doi: 10.1016/j.ejpn.2009.12.005
12. Tate ED, Pranzatelli MR, Verhulst SJ, Markwell SJ, Franz DN, Graf WD, et al. Active comparator-controlled, rater-blinded study of corticotropin-based immunotherapies for opsoclonus-myoclonus syndrome. J Child Neurol. (2012) 27:875–84. doi: 10.1177/0883073811428816
13. Dale RC, Brilot F, Duffy LV, Twilt M, Waldman AT, Narula S, et al. Utility safety of rituximab in pediatric autoimmune inflammatory CNS disease. Neurology. (2014) 83:142–50. doi: 10.1212/WNL.0000000000000570
14. Radu RA, Terecoasă EO, Ene A, Băjenaru OA, Tiu C. Opsoclonus-myoclonus syndrome associated with west-nile virus infection: case report and review of the literature. Front Neurol. (2018) 9:864. doi: 10.3389/fneur.2018.00864
15. Hébert J, Armstrong D, Daneman N, Jain JD, Perry J. Adult-onset opsoclonus-myoclonus syndrome due to West Nile Virus treated with intravenous immunoglobulin. J Neurovirol. (2017) 23:158–9. doi: 10.1007/s13365-016-0470-3
16. Simon T, Cheuret E, Fiedler L, Mengelle C, Baudou E, Deiva K. Acute transverse myelitis following an opsoclonus-myoclonus syndrome: an unusual presentation. Eur J Paediatr Neurol. (2018) 22:878–81. doi: 10.1016/j.ejpn.2018.05.002
17. Belcastro V, Piola M, Binda S, Santoro D, Rezzonico M, Arnaboldi M. Opsoclonus-myoclonus syndrome associated with human herpes virus-6 rhomboencephalitis. J Neurol Sci. (2014) 341:165–6. doi: 10.1016/j.jns.2014.04.013
18. Verma A, Brozman B. Opsoclonus-myoclonus syndrome following Epstein-Barr virus infection. Neurology. (2002) 58:1131–2. doi: 10.1212/WNL.58.7.1131
19. Syrbe S, Merkenschlager A, Bernhard MK, Grosche J, Liebert UG W., Hirsch, et al. Opsoclonus-myoclonus syndrome after adenovirus infection. Springerplus. (2015) 4:636. doi: 10.1186/s40064-015-1429-1
20. Morita A, Ishihara M, Kamei S, Ishikawa H. Opsoclonus-myoclonus syndrome following influenza a infection. Intern Med. (2012) 51:2429–31. doi: 10.2169/internalmedicine.51.7627
21. Singh D, Sinha M, Kumar R, Shukla R, Ahuja RC. Opsoclonus-myoclonus syndrome caused by varicella-zoster virus. Ann Indian Acad Neurol. (2010) 13:211–2. doi: 10.4103/0972-2327.70876
22. Gurkas E, Gucuyener K, Yilmaz U, Havali C, Demir E. Opsoclonus-myoclonus syndrome following rotavirus gastroenteritis. Pediatr Int. (2014) 56:e86–7. doi: 10.1111/ped.12433
23. Chen Y, Chen D, Zhou X, Zhang H, Liao S, Xu Z, et al. Opsoclonus-myoclonus syndrome associated with herpes simplex virus infection: a case report. Int J Neurosci. (2020) 131:307–11. doi: 10.1080/00207454.2020.1737531
24. Shiihara T, Takahashi Y. Correspondence: a further case of opsoclonus-myoclonus syndrome associated with Mycoplasma pneumoniae infection. Eur J Pediatr. (2010) 169:639. doi: 10.1007/s00431-009-1105-y
25. Huber BM, Strozzi S, Steinlin M, Aebi C, Fluri S. Mycoplasma pneumoniae associated opsoclonus-myoclonus syndrome in three cases. Eur J Pediatr. (2010) 169:441–5. doi: 10.1007/s00431-009-1048-3
26. Nunes JC, Bruscato AM, Walz R, Lin K. Opsoclonus-myoclonus syndrome associated with Mycoplasma pneumoniae infection in an elderly patient. J Neurol Sci. (2011) 305:147–8. doi: 10.1016/j.jns.2011.03.012
27. Mesraoua B, Abbas M, D'Souza A, Miyares FR, Hashem M, Osman Y, et al. Adult opsoclonus-myoclonus syndrome following Mycoplasma pneumoniae infection with dramatic response to plasmapheresis. Acta Neurol Belg. (2011) 111:136–8. doi: 10.1055/s-0031-1283109
28. McCarthy JE, Filiano J. Opsoclonus Myoclonus after human papilloma virus vaccine in a pediatric patient. Parkinsonism Relat Disord. (2009) 15:792–4. doi: 10.1016/j.parkreldis.2009.04.002
29. Lapenna F, Lochi L, de Mari M, Iliceto G, Lamberti P. Post-vaccinic opsoclonus-myoclonus syndrome: a case report. Parkinsonism Relat Disord. (2000) 6:241–2. doi: 10.1016/S1353-8020(00)00020-1
30. Wang H. A protocol for investigating the association of vaccination and anti-NMDA receptor encephalitis. Front Biosci (Schol Ed). (2018) 10:229–37. doi: 10.2741/s511
31. Mitchell WG, Snodgrass SR. Opsoclonus-ataxia due to childhood neural crest tumors: a chronic neurologic syndrome. J Child Neurol. (1990) 5:153–8. doi: 10.1177/088307389000500217
32. Brunklaus A, Pohl K, Zuberi SM, de Sousa C. Investigating neuroblastoma in childhood opsoclonus-myoclonus syndrome. Arch Dis Child. (2012) 97:461–3. doi: 10.1136/adc.2010.204792
33. de Alarcon PA, Matthay KK, London WB, Naranjo A, Tenney SC, Panzer JA, et al. Intravenous immunoglobulin with prednisone and risk-adapted chemotherapy for children with opsoclonus myoclonus ataxia syndrome associated with neuroblastoma (ANBL00P3): a randomised, open-label, phase 3 trial. Lancet Child Adolesc Health. (2018) 2:25–34. doi: 10.1016/S2352-4642(17)30130-X
34. Blaes F, Dharmalingam B. Childhood opsoclonus-myoclonus syndrome: diagnosis and treatment. Expert Rev Neurother. (2016) 16:641–8. doi: 10.1080/14737175.2016.1176914
35. Pranzatelli MR, Travelstead AL, Tate ED, Allison TJ, Moticka EJ, Franz DN, et al. B- and T-cell markers in opsoclonus-myoclonus syndrome: immunophenotyping of CSF lymphocytes. Neurology. (2004) 62:1526–32. doi: 10.1212/WNL.62.9.1526
36. Pranzatelli MR, Tate ED, Travelstead AL, Longee D. Immunologic and clinical responses to rituximab in a child with opsoclonus-myoclonus syndrome. Pediatrics. (2005) 115:e115–9. doi: 10.1542/peds.2004-0845
37. Klein A, Schmitt B, Boltshauser E. Long-term outcome of ten children with opsoclonus-myoclonus syndrome. Eur J Pediatr. (2007) 166:359–63. doi: 10.1007/s00431-006-0247-4
38. Goh EL, Scarff K, Satariano S, Lim M, Anand G. Evolving cognitive dysfunction in children with neurologically stable opsoclonus-myoclonus syndrome. Children (Basel). (2020) 7:103. doi: 10.3390/children7090103
39. Pranzatelli MR, Travelstead AL, Tate ED, Allison TJ, Verhulst SJ. CSF B-cell expansion in opsoclonus-myoclonus syndrome: a biomarker of disease activity. Mov Disord. (2004) 19:770–7. doi: 10.1002/mds.20125
Keywords: opsoclonus-myoclonus syndrome, paraneoplastic syndrome, ataxia, rituximab, pediatric
Citation: Zhu H, Wu W, Chen L, Hou C, Zeng Y, Tian Y, Shen H, Gao Y, Zhang Y, Peng B, Chen W-X and Li X (2021) Clinical Analysis of Pediatric Opsoclonus-Myoclonus Syndrome in One of the National Children's Medical Center in China. Front. Neurol. 12:744041. doi: 10.3389/fneur.2021.744041
Received: 19 July 2021; Accepted: 17 September 2021;
Published: 08 October 2021.
Edited by:
Vincenzo Belcastro, Lodi Hospital, ItalyReviewed by:
Thea Giacomini, Istituto Giannina Gaslini (IRCCS), ItalyCopyright © 2021 Zhu, Wu, Chen, Hou, Zeng, Tian, Shen, Gao, Zhang, Peng, Chen and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wen-Xiong Chen, Z3pjaGN3eEAxMjYuY29t; Xiaojing Li, bGl4aWFvamluZ2Z5QDE2My5jb20=
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.