- 1Department of Radiology and Imaging Sciences, University of Utah, Salt Lake City, UT, United States
- 2Department of Radiology, Weill Cornell Medicine, New York, NY, United States
- 3Feil Family Brain and Mind Research Institute, Weill Cornell Medicine, New York, NY, United States
The development of cognitive dysfunction and dementia is a complex, multifactorial process. One of the contributors to various types of cognitive dysfunction is carotid atherosclerosis which can frequently be seen in asymptomatic individuals. There are a number of different manifestations of asymptomatic carotid atherosclerosis including arterial stiffness, carotid intima-media thickening, flow-limiting stenosis, and complex, atherosclerotic plaque. Each of these forms of atherosclerosis may contribute to cerebral parenchymal damage, contributing to cognitive dysfunction. In this review article, we will discuss each of these forms of carotid atherosclerosis, present the potential mechanistic underpinnings behind an association, and then review the scientific evidence supporting potential associations to cognitive dysfunction and dementia.
Introduction
With an ever-increasing aging world population, there is increasing demand for identifying effective preventative and treatment strategies for the development of dementia and cognitive dysfunction (1). Early identification and effective treatment of dementia and cognitive dysfunction has been an ongoing challenge due to the multifactorial nature of disease development. One of the factors that appears to be contributing to the development of cognitive dysfunction and dementia is carotid atherosclerotic disease, including carotid stiffness, increased carotid intima media thickness, flow-limiting carotid stenosis, and high-risk carotid plaque features (2, 3). While traditionally thought to primarily contribute to ischemic stroke, there is increasing evidence of the contribution of carotid atherosclerotic disease to the development of cognitive impairment and dementia. Though the exact mechanisms by which each of these manifestations of carotid atherosclerosis contribute to the development of dementia is still under investigation, they each appear to contribute in unique but perhaps overlapping ways. In this article, we will review the scientific evidence supporting the links between each of these disease processes and the development of cognitive dysfunction.
Though there are many contributing factors in the development of dementia, in this review, we will focus on the role of asymptomatic carotid artery atherosclerosis in contributing to cognitive dysfunction and dementia (Figure 1). Asymptomatic carotid artery disease, which is more commonly seen in male patients, is frequently associated with vascular risk factors such has hyperlipidemia, diabetes, smoking, and hypertension. Manifestations vary, often starting as minimal wall thickening and then ultimately leading to flow-limiting stenosis and/or vulnerable plaque components which may rupture leading to cerebral ischemia. Currently, there is clinical equipoise with regard to optimal treatment for asymptomatic carotid atherosclerotic disease due to difficulties in balancing the risk and benefit calculations with treatment. Though the primary concern with carotid atherosclerosis is ischemic stroke, we will review some of the associations of asymptomatic carotid atherosclerosis to cognitive dysfunction and dementia.
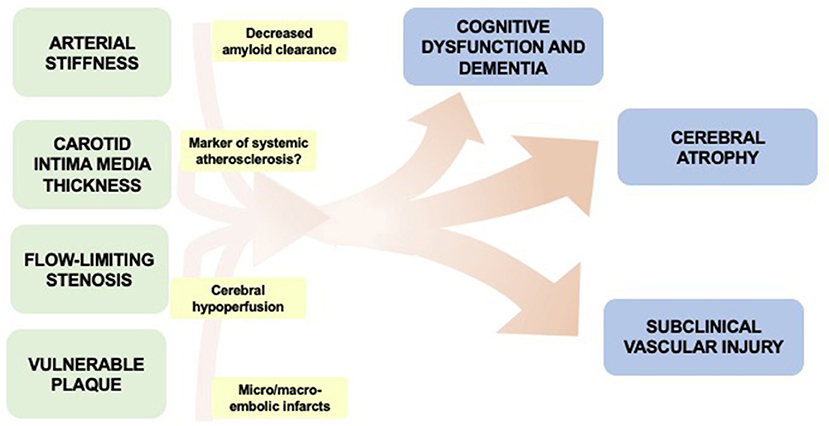
Figure 1. Overview of potential associations between manifestations of asymptomatic carotid disease and cognitive impairment.
First, we will discuss the often clinically silent presence of carotid stiffness and its association with cognitive impairment. We will then review a common finding indicative of subclinical atherosclerosis—carotid intima-media thickening. Then we will discuss the evidence supporting an association between flow-limiting extracranial carotid stenosis and cognitive impairment. Finally, we will review the relevant evidence behind specific carotid plaque features in the development of mild cognitive impairment and dementia.
Search Methods for Review
We performed a robust search of the available medical literature searching for manuscripts with key terms related to carotid atherosclerosis, carotid stenosis, arterial stiffness, and carotid plaque along with any terms related to cognitive impairment, dysfunction, or dementia. The primary search was performed using PubMed and included the use of MeSH terms. In addition, we evaluated cited references in each of the manuscripts we evaluated.
Carotid Stiffness and Cognitive Impairment
Definition and Measurement
Stiffening of the carotid artery or other elastic arteries is the gradual loss of elastin fibers and accumulation of stiffer collagen fibers in the media over time (4, 5). This process, which can occur independent of the development of atherosclerosis, leads to loss of the ability of vasculature to appropriately accommodate to changes in blood pressure variation (6). This loss of responsive distensibility leads to higher pulsatile pressures and eventually increased flow load experienced by cerebral microvasculature and ultimately the brain parenchyma (4).
Arterial stiffness is not routinely measured in clinical practice but has been well-studied in several epidemiologic cohort studies. It is most commonly measured via indirect methods by measurement of pulse wave velocity (PWV) (7). Pulse wave velocity is an estimation of central arterial stiffness via measurement of pressure waves in two different vascular beds, commonly the carotid and femoral arterial beds (8). This indirect measure is a surrogate for aortic stiffness and has been widely used in multiple cohort studies. In addition to these indirect measures of central arterial stiffness, there are additional methods to directly measure vascular stiffness, specifically in the carotid artery (7, 9). These are most commonly performed via ultrasound measurement techniques.
Potential Mechanisms
Since there are many shared risk factors for the development of arterial stiffness and other common cardiovascular diseases, it can be difficult to determine the specific effects of arterial stiffness on the downstream cerebral parenchyma. One of the major vascular risk factors contributing to increased arterial stiffness is increasing age. In addition to age, hypertension, diabetes, and smoking are additional factors that seem to accelerate the development of arterial stiffness (10). A major proposed mechanism by which arterial stiffness contributes to cognitive dysfunction is through the increased flow load experienced by the cerebral parenchyma leading to end-organ damage (11, 12). The resultant damage may manifest as cerebral small vessel disease evident on brain imaging, including white matter hyperintensities, covert brain infarctions, or cerebral microbleeds. These findings of cerebral small vessel disease are also independently associated with cognitive impairment and dementia, in addition to stroke and overall mortality (13–16).
Stiffness and Imaging Markers Associated With Cognitive Impairment
Arterial stiffness has been associated with several imaging findings which are in turn also associated with cognitive dysfunction and dementia (Table 1). Specifically, there have been several studies showing that decreased carotid compliance (or increased carotid stiffness), is associated with decreased total brain and cortical gray matter volumes and also decreased volume in the hippocampal and parahippocampal regions (11, 17, 18). In the Age, Gene/Environment Susceptibility (AGES)-Reykjavik Study in 422 participants free from cerebrovascular disease and dementia, the authors found that carotid stiffness was associated with lower whole brain (-0.127 ± 0.037 SD/SD, p < 0.001), gray matter (−0.079 ± 0.038 SD/SD, p = 0.038), and white matter volumes (−0.128 ± 0.039 SD/SD, p = 0.028) along with lower memory scores (11). In the SMART-MR study of 526 participants, the authors found a cross-sectional association with increased carotid stiffness and lower total brain and cortical gray matter volume (B = −0.24%, 95% confidence interval [CI] −0.44 to −0.04%, and B = −0.47%, 95% CI −0.75 to −0.19%) but this association was no longer significant when evaluated prospectively after a mean of 4 years (17). When evaluating 614 participants in the Atherosclerosis Risk in the Community Study (ARIC), authors found a significant association between decreased carotid stiffness and lower parahippocampal and hippocampal volumes 20 years later [R = 0.218(0.144–0.291), p < 0.001 and R = 0.181 (0.105–0.255), P < 0.001], even after adjusting for confounders. Decreased total brain volumes and decreased volumes in the parahippocampal and hippocampal regions are independently associated with cognitive dysfunction and dementia (19).

Table 1. Overview of feature of asymptomatic carotid atherosclerosis and their potential association with cognitive dysfunction and dementia.
In addition to decreased cerebral parenchymal volumes, there is evidence of the association between carotid stiffness to imaging findings of cerebral small vessel disease, including white matter hyperintensities, covert brain infarctions, and cerebral microbleeds, including in the AGES-Reykjavik, SMART-MR, and ARIC epidemiologic cohort studies (20–22). In the SMART-MR study, there was a significant association between carotid stiffness and larger volume of white matter hyperintensities (B = 0.09%, 95% CI −0.01 to 0.19%) as well as cortical and subcortical brain infarcts (RR = 1.44, 95% CI 1.14–1.81). In the ARIC study, this association was found to be significant 20 years after the carotid stiffness measures (20). These imaging markers are also independently associated with mortality, stroke, cognitive dysfunction, and dementia (14, 16, 23).
Stiffness and Cognitive Impairment
Though there is fairly robust evidence of an association between carotid stiffness to brain imaging markers that are associated with dementia, the association of stiffness to cognitive impairment and dementia are more clinically relevant. There are several strong studies demonstrating that central arterial stiffness is an independent predictor of cognitive dysfunction and dementia (24–28). These studies, many of which were performed in epidemiologic cohorts including the Framingham Heart Study, the Maastricht study, and the PARTAGE study, found strong associations between stiffness and cognitive impairment. Interestingly, in the Maastricht study, the authors found a strong association with central stiffness (−0.018 SD [95% CI, −0.036 to −0.000]), but not with carotid stiffness (24). In addition, there are multiple studies establishing a cross-sectional association of central arterial stiffness to cognitive decline (29–32). Though there is relatively strong data supporting a link between central arterial stiffening to cognitive dysfunction, there is less supporting evidence between carotid stiffness and dementia with some studies supporting this association (Table 2) (33–38), and other studies showing no association, after adjusting for other confounders (24, 67, 68). This is certainly an area worthy of future investigation to further our understanding of the role of carotid stiffness, an asymptomatic marker of vascular aging, to the development of dementia. Future longitudinal prospective cohort studies may be able to aid in clarifying this potential association.
Carotid Intima-Media Thickening and Cognitive Impairment
Definition
Another marker of asymptomatic carotid atherosclerosis is thickening of the carotid intima-media. This subtle thickening of the arterial wall measured in either the distal common carotid artery or proximal internal carotid artery using ultrasound is a marker of subclinical atherosclerosis. Similar to arterial stiffness, both hypertension and smoking, along with other vascular risk factors, are known associations to CIMT. Since carotid intima-media thickening (CIMT) is a precursor for the development of atherosclerotic plaque, it is often considered an imaging marker of generalized cardiovascular risk. Though this thickening is asymptomatic, it is associated with cognitive dysfunction and dementia, along with stroke and overall increased mortality risk (47, 60, 69, 70).
CIMT and Imaging Markers Associated With Cognitive Impairment
There is evidence of an association of baseline increased CIMT to brain imaging markers of cerebral small vessel disease including white matter hyperintensities, covert brain infarctions, cerebral microbleeds, and also lower total brain volumes (71–76) (Figure 2). Specifically, a systematic review and meta-analysis of nine studies found that CIMT was associated with white matter hyperintensities [odds ratio (OR) 1.42, 95% CI 1.22–1.66, p < 0.0001], covert brain infarctions (OR 1.89, CI 1.46–2.45, p < 0.0001) (74). As for the association of CIMT with brain volume, there is evidence of lower total brain volume (−0.05 per SD, P < 0.05) in the Framingham Heart Study (77) and lower total brain and cortical gray matter (−0.29 per SD) in the SMART-MR study cohort (72). In addition, there is evidence that the progression of CIMT over time is also associated with lower hippocampal volumes in the Framingham Heart Study (78). As discussed previously, these imaging markers of cerebral small vessel disease and brain aging are independently associated with cognitive dysfunction and dementia.
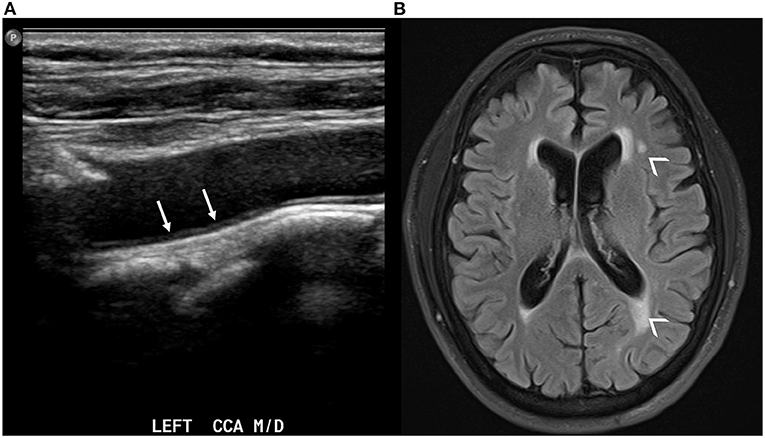
Figure 2. Carotid ultrasound demonstrating thickening of the intima-media in the left common carotid artery [(A), white arrows]. The same patient also has evidence of white matter hyperintensities in the periventricular white matter on axial T2 FLAIR brain MRI [(B), small arrowheads].
CIMT and Cognitive Impairment
Several studies have evaluated the association between CIMT on ultrasound and the future development of dementia and cross-sectional association with cognitive function. Several studies from epidemiologic cohorts have found a positive association between increased CIMT at baseline and future development of cognitive decline (44–46). These studies followed cohorts of participants over time and found that having higher baseline CIMT on ultrasound was correlated with poorer future performance on cognitive testing and increased rates of dementia diagnoses. Hazard ratio for development of cognitive impairment based on elevated CIMT was 1.251 (95% CI 1.006–1.555, p = 0.044) in the Korean Longitudinal Study on Health and Aging after adjusting for basic demographics and baseline cognition (44). Further, in the Northern Manhattan Study, a cohort study of stroke-free participants, found that those with greater CIMT had worse performance on cognitive testing (ß = −0.60, p = 0.04 for episodic memory) (46). There are also many studies demonstrating a cross-sectional association between increased CIMT and poorer cognitive function (39–43).
Potential Mechanism
The exact pathophysiologic mechanism underpinning this association is unclear at this time, though it may be similar to the proposed mechanisms for arterial stiffness. Since CIMT is thought to be marker for systemic atherosclerosis, it may not necessarily be a direct contributor to cognitive dysfunction, but rather reflect general cardiovascular risk. Further evaluation of this association is necessary in order to identify potentially modifiable vascular contributions to cognitive dysfunction.
Carotid Stenosis and Cognitive Impairment
Definition
Flow-limiting carotid stenosis is a well-documented risk factor for stroke and is an established indication for carotid revascularization. Severe carotid stenosis is traditionally defined as 70–99% narrowing of the vessel lumen by various measuring methods, most commonly the North American Symptomatic Carotid Endarterectomy Trial (NASCET) method in the United States (79). Moderate stenosis is defined as 50–70% stenosis. The degree of stenosis is a leading risk factor in the development of ischemic stroke with annual rates of ischemic stroke estimated to be around 1% (80). Though not nearly as well-studied, some have found that the annual rate of dementia in the presence of severe carotid stenosis to be around 1 (55).
Potential Mechanisms
The main mechanism by which carotid stenosis is hypothesized to contribute to cognitive impairment is via hypoperfusion (81). By limiting the flow to the brain parenchyma, some hypothesize that this may lead to end-organ damage, parenchymal atrophy, and neurodegneration. Cerebral hypoperfusion is thought to accelerate amyloid and tau deposition, which is a potential link between flow-limitation and cognitive dysfunction (82, 83). Other potential mechanistic explanations include via covert brain infarctions from embolization, which may act as an intermediate step between stenosis and cognitive dysfunction. Similar to many other features of carotid atherosclerosis, it is difficult to disentangle the complex associations between carotid stenosis, other vascular risk factors, and other findings of cerebral parenchymal damage. One potential method for clarifying these associations is by evaluating individuals with unilateral stenosis to determine if there are varying degrees of parenchymal damage downstream from the affected side. Currently, the exact mechanism by which flow-limiting stenosis contributes to cognitive dysfunction is not clearly established.
Stenosis and Cognition
Several studies have shown an association with carotid stenosis and poorer performance on cognitive testing (47–52), while others have failed to find an association. There are several studies finding that asymptomatic individuals with severe carotid stenosis with evidence of altered perfusion are more likely to develop cognitive impairment (51, 84, 85). In one of the largest studies evaluating this association in over 4,000 participants, the authors found that high-grade carotid artery stenosis was associated with cognitive impairment (OR 6.7, 95% CI 2.4 to 18.1) and cognitive decline (OR 2.6, CI, 1.1 to 6.3) (48). Some have found that left sided carotid stenosis is more likely to result in cognitive dysfunction than right-sided carotid stenosis, indicating that carotid stenosis may be an independent risk factor for cognitive impairment (48, 53). A systematic review and meta-analysis including over 760 subjects with asymptomatic carotid stenosis found an association between the presence of carotid stenosis and cognitive impairment (54).
Several studies have attempted to isolate the effect of carotid stenosis by assessing for changes in cognitive function after carotid endarterectomy with conflicting results (56). Overall, the majority of studies have found an improvement in cognition after CEA, but there are many studies showing either no change, and even a deterioration in cognitive function. In a large study of patients with asymptomatic carotid stenosis, there was no difference in mini-mental status examination scores in those who received medical therapy compared to those who underwent CEA (57). Another study based on patients from a randomized controlled trial with severe carotid stenosis without history of stroke or known dementia (ACST-1) found that carotid endarterectomy had no significant effect on the incidence of dementia (55). There is less data regarding cognitive changes after CAS, however, there are similarly mixed results with some studies showing an improvement in cognition after undergoing CAS (56, 58, 59).
Stenosis and Imaging Markers Associated With Cognitive Impairment
There is strong evidence that the presence of flow-limiting stenosis results in both cerebral atrophy as well as other markers of subclinical vascular injury, such as white matter hypterintensities and CBIs. There is evidence that severe carotid stenosis is associated with progression of brain atrophy, while the same link was not as pronounced in those with moderate stenosis (72). Further, there is strong evidence that carotid stenosis is associated with markers of parenchymal damage, including white matter hyperintensities and microstructural damage to both gray and white matter (86–88). In addition, there is strong evidence of an association between carotid stenosis and downstream CBI with a systematic review and meta-analysis of 11 studies reporting an OR of 2.78 (95% CI, 2.19 to 3.52, p < 0.0001) (89). Further, studies demonstrate that there are asymmetries in prevalence of CBI in cerebral hemispheres downstream from severe carotid stenosis, specifically with more cortical CBIs downstream from stenosis (90).
This role of flow-limiting stenosis in the development of dementia is an area worthy of further investigation. Though CEA and CAS procedures are primarily performed for stroke risk reduction, the potential added bonus of improved cognition may alter the risk calculus when identifying patients for carotid revascularization.
Carotid Plaque and Cognitive Impairment
Definition
Carotid plaque is a specific marker of advanced atherosclerosis usually found in the carotid bifurcation. There are many different features of the plaque itself that carry varying degrees of associated embolic risk. There has been increased attention on individual plaque components and plaque volume in addition to the degree of stenosis when assessing stroke risk from carotid atherosclerotic disease. There is strong evidence that certain plaque components are more strongly associated with future and recurrent stroke, including intraplaque hemorrhage, lipid-rich necrotic core, and plaque ulceration (91). Many of these specific plaque elements are often more strongly associated with stroke than degree of stenosis which has led to a paradigm shift in stroke risk assessment (92). Though the major concern for carotid plaques is their leading to symptomatic strokes and/or transient ischemic attacks, many of these high-risk plaque features are also seen in asymptomatic individuals.
Though there is a strong association with high-risk plaque elements to stroke, there are fewer studies evaluating the association of plaque with the development of cognitive dysfunction and dementia. The current literature has shown mixed results with respect to the association of vulnerable plaque components and cognitive dysfunction.
Plaque and Cognition
Some studies have evaluated the association of increased plaque volume to performance on cognitive examinations and have found that there is worse performance on cognitive testing with higher plaque volume, even when accounting for education level and other confounding (60–66). For example, a study evaluating high risk plaque features on ultrasound found that those with more plaques were more likely to have poor performance on cognitive testing, including mini-mental status examinations (OR 1.72, 1.00–2.96) (61). Most of the studies evaluating this association used either ultrasound or CT imaging techniques to evaluate plaque. Other studies have shown no significant difference in cognitive function when accounting for other cardiovascular risk factors (93).
Plaque and Imaging Markers Associated With Cognitive Impairment
There is evidence that vulnerable plaque features are associated with other markers of neurodegeneration and subclinical vascular injury. For instance, there is evidence that vulnerable plaque features contribute to cortical micro-infarcts detected on MR which are in turn associated with poor cognitive function (94). There are other studies which demonstrate an association with white matter hyperintensities and CBIs as well (74), though there are few studies explicitly looking at specific plaque features (Figure 3).
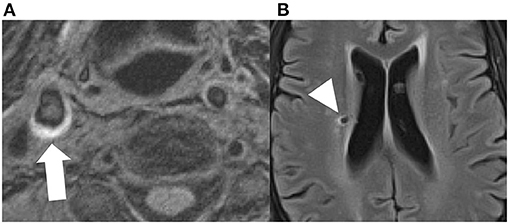
Figure 3. Axial MPRAGE sequence demonstrating T1 hyperintensity in the right carotid bifurcation, compatible with intraplque hemorrhage [(A), white arrow]. The same patient had a small covert brain infarction in the right periventricular white matter [(B), arrowhead].
Potential Mechanisms and Future Directions
The exact mechanism behind this potential association is unclear at this time. Whether high risk plaque is directly associated with dementia due to repeated microembolic phenomena is not well established. Though there is evidence that high-risk plaque lead to increased risk of stroke, it is unclear if these plaque features may also contribute to microembolic phenomenon contributing to cognitive dysfunction. Other studies have found that there are increased markers of subclinical vascular injury which are associated with high-risk plaque features, including covert brain infarctions, cerebral microbleeds, and white matter hyperintensities which are independently associated with cognitive dysfunction. At this time, this exact association is unclear and further studies would be helpful in elucidating the exact contribution of high-risk plaque features to cognitive dysfunction and dementia.
Conclusion
Ranging from increased stiffness of the arterial wall to complex atherosclerotic plaques prone to rupture, there is a wide variety of manifestations of carotid disease. All of these manifestations can be seen in asymptomatic individuals. Though there has been a strong association between many features of carotid atherosclerosis and stroke, there has been less attention on the link to cognitive dysfunction. In this review, we present the existing evidence supporting this potential link.
There are many risk factors associated with the development of the presented types of carotid atherosclerosis, ranging from carotid stiffness to complex, vulnerable plaque components, including smoking and hypertension. A potential method for mitigating the association of asymptomatic carotid atherosclerosis and the development of cognitive impairment is to target these known contributors to atherosclerosis.
In the studies cited in this review, there are many definitions and diagnostic criteria for various types of cognitive dysfunction and dementia including mild cognitive impairment, vascular dementia, and Alzheimer's and Alzheimer's-related dementia. Future studies are needed with more streamlined diagnostic criteria and biomarker validation for the various forms of cognitive dysfunction. Future prospective longitudinal studies are necessary to further elucidate this relationship. As more studies confirm this link, we may expect changes to the risk-benefit assessment of pursuing surgical intervention in asymptomatic individuals with certain types of carotid artery atherosclerosis. In addition, with stronger scientific support, more targeted preventative strategies, including stringent medical management, could be considered directed at the development of carotid atherosclerosis as a means of dementia prevention. Currently, there is formal guidance not to perform routine screening for extracranial carotid plaque in individuals who are asymptomatic from the US Preventive Task Force. Though there is evidence of a potential link between asymptomatic carotid disease and cognitive impairment, the existing data is not strong enough at this time to reverse this recommendation. Further prospective observational studies confirming the link between carotid disease and cognitive impairment are necessary before making this recommendation. Additional evidence could warrant altering this recommendation including a randomized controlled trial comparing stringent medical management to those with less standard of care treatment with asymptomatic carotid artery disease showing that changes to medical management may decrease risk of cognitive impairment.
Author Contributions
AG made substantial contributions to the conception or design of the work, drafted the work and revised it critically for important intellectual content, provided approval for publication of the content, and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All authors contributed to the article and approved the submitted version.
Funding
HB is in part supported by the University of Utah Program in Personalized Health and National Center for Advancing Translational Sciences of the National Institutes of Health under Award Number 1UL1TR002538.
Author Disclaimer
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. International AsD. World Alzheimer Report 2010: The Global Economic Impact of Dementia: Alzheimer's Disease International (2010).
2. Gorelick PB, Scuteri A, Black SE, DeCarli C, Greenberg SM, Iadecola C, et al. Vascular contributions to cognitive impairment and dementia. Stroke. (2011) 42:2672. doi: 10.1161/STR.0b013e3182299496
3. van Oijen M, Jan de. Jong F, Witteman J, Hofman A, Koudstaal PJ, Breteler M. Atherosclerosis and risk for dementia. Ann Neurol. (2007) 61:403–10. doi: 10.1002/ana.21073
4. van Sloten TT, Stehouwer CD. Carotid stiffness: a novel cerebrovascular disease risk factor. Pulse (Basel). (2016) 4:24–7. doi: 10.1159/000445354
5. Mikael LR, Paiva AMG, Gomes MM, Sousa ALL, Jardim P, Vitorino PVO, et al. Vascular aging and arterial stiffness. Arq Bras Cardiol. (2017) 109:253–8. doi: 10.5935/abc.20170091
6. Rabkin SW. Arterial stiffness: detection and consequences in cognitive impairment and dementia of the elderly. J Alzheimers Dis. (2012) 32:541–9. doi: 10.3233/JAD-2012-120757
7. Townsend RR, Wilkinson IB, Schiffrin EL, Avolio AP, Chirinos JA, Cockcroft JR, et al. Recommendations for improving and standardizing vascular research on arterial stiffness: a scientific statement from the American Heart Association. Hypertension. (2015) 66:698–722. doi: 10.1161/HYP.0000000000000033
8. O'Rourke MF, Staessen JA, Vlachopoulos C, Duprez D. Clinical applications of arterial stiffness; definitions and reference values. Am J Hypertens. (2002) 15:426–44. doi: 10.1016/S0895-7061(01)02319-6
9. Zieman SJ, Melenovsky V, Kass DA. Mechanisms, pathophysiology, and therapy of arterial stiffness. Arterioscler Thromb Vasc Biol. (2005) 25:932–43. doi: 10.1161/01.ATV.0000160548.78317.29
10. Benetos A, Waeber B, Izzo J, Mitchell G, Resnick L, Asmar R, et al. Influence of age, risk factors, and cardiovascular and renal disease on arterial stiffness: clinical applications. Am J Hypertens. (2002) 15:1101–8. doi: 10.1016/S0895-7061(02)03029-7
11. Mitchell GF, van Buchem MA, Sigurdsson S, Gotal JD, Jonsdottir MK, Kjartansson Ó, et al. Arterial stiffness, pressure and flow pulsatility and brain structure and function: the age, gene/environment susceptibility–Reykjavik study. Brain. (2011) 134:3398–407. doi: 10.1093/brain/awr253
12. Mitchell GF. Effects of central arterial aging on the structure and function of the peripheral vasculature: implications for end-organ damage. J Appl Physiol. (2008) 105:1652–60. doi: 10.1152/japplphysiol.90549.2008
13. Wardlaw JM, Smith EE, Biessels GJ, Cordonnier C, Fazekas F, Frayne R, et al. Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration. Lancet Neurol. (2013) 12:822–38. doi: 10.1016/S1474-4422(13)70124-8
14. Vermeer SE, Prins ND, den Heijer T, Hofman A, Koudstaal PJ, Breteler MM. Silent brain infarcts and the risk of dementia and cognitive decline. N Engl J Med. (2003) 348:1215–22. doi: 10.1056/NEJMoa022066
15. Debette S, Markus HS. The clinical importance of white matter hyperintensities on brain magnetic resonance imaging: systematic review and meta-analysis. BMJ. (2010). 341:c3666. doi: 10.1136/bmj.c3666
16. Debette S, Beiser A, DeCarli C, Au R, Himali JJ, Kelly-Hayes M, et al. Association of MRI markers of vascular brain injury with incident stroke, mild cognitive impairment, dementia, and mortality. Stroke. (2010) 41:600–6. doi: 10.1161/STROKEAHA.109.570044
17. Jochemsen HM, Muller M, Bots ML, Scheltens P, Vincken KL, Mali WP, et al. Arterial stiffness and progression of structural brain changes: the SMART-MR study. Neurology. (2015) 84:448–55. doi: 10.1212/WNL.0000000000001201
18. Baradaran A, McNally JS, Alexander M, Majersik J, Parker DL, de Havenon A. carotid compliance and parahippocampal and hippocampal volume over a 20-year period. Dement Geriatr Cogn Disord Extra. (2021) 11:227–34. doi: 10.1159/000518234
19. Chao L, Mueller S, Buckley S, Peek K, Raptentsetseng S, Elman J, et al. Evidence of neurodegeneration in brains of older adults who do not yet fulfill MCI criteria. Neurobiol Aging. (2010) 31:368–77. doi: 10.1016/j.neurobiolaging.2008.05.004
20. de Havenon A, Wong K-H, Elkhetali A, McNally J, Majersik J, Rost N. Carotid artery stiffness accurately predicts white matter hyperintensity volume 20 years later: a secondary analysis of the atherosclerosis risk in the community study. Amer J Neuroradiol. (2019) 40:1369–73. doi: 10.3174/ajnr.A6115
21. Huang X, Kang X, Xue J, Kang C, Lv H, Li Z. Evaluation of carotid artery elasticity changes in patients with cerebral small vessel disease. Int J Clin Exp Med. (2015) 8:18825.
22. Ding J, Mitchell GF, Bots ML, Sigurdsson S, Harris TB, Garcia M, et al. Carotid arterial stiffness and risk of incident cerebral microbleeds in older people: the age, gene/environment susceptibility (AGES)-Reykjavik study. Arterioscler Thromb Vasc Biol. (2015) 35:1889–95. doi: 10.1161/ATVBAHA.115.305451
23. Bernick C, Kuller L, Dulberg C, Longstreth Jr W, Manolio T, Beauchamp N, et al. Silent MRI infarcts and the risk of future stroke: the cardiovascular health study. Neurology. (2001) 57:1222–9. doi: 10.1212/WNL.57.7.1222
24. Rensma SP, Stehouwer CD, Van Boxtel MP, Houben AJ, Berendschot TT, Jansen JF, et al. Associations of arterial stiffness with cognitive performance, and the role of microvascular dysfunction: the maastricht study. Hypertension. (2020) 75:1607–14. doi: 10.1161/HYPERTENSIONAHA.119.14307
25. Pase MP, Beiser A, Himali JJ, Tsao C, Satizabal CL, Vasan RS, et al. Aortic stiffness and the risk of incident mild cognitive impairment and dementia. Stroke. (2016) 47:2256–61. doi: 10.1161/STROKEAHA.116.013508
26. Benetos A, Watfa G, Hanon O, Salvi P, Fantin F, Toulza O, et al. Pulse wave velocity is associated with 1-year cognitive decline in the elderly older than 80 years: the PARTAGE study. J Am Med Dir Assoc. (2012) 13:239–43. doi: 10.1016/j.jamda.2010.08.014
27. Watson NL, Sutton-Tyrrell K, Rosano C, Boudreau RM, Hardy SE, Simonsick EM, et al. Arterial stiffness and cognitive decline in well-functioning older adults. J Gerontol A Biomed Sci Med Sci. (2011) 66:1336–42. doi: 10.1093/gerona/glr119
28. Elias MF, Robbins MA, Budge MM, Abhayaratna WP, Dore GA, Elias PK. Arterial pulse wave velocity and cognition with advancing age. Hypertension. (2009) 53:668–73. doi: 10.1161/HYPERTENSIONAHA.108.126342
29. Muela HC, Costa-Hong VA, Yassuda MS, Moraes NC, Memória CM, Machado MF, et al. Higher arterial stiffness is associated with lower cognitive performance in patients with hypertension. J Clin Hypertens. (2018) 20:22–30. doi: 10.1111/jch.13129
30. Mehrabian S, Raycheva M, Gateva A, Todorova G, Angelova P, Traykova M, et al. Cognitive dysfunction profile and arterial stiffness in type 2 diabetes. J Neurol Sci. (2012) 322:152–6. doi: 10.1016/j.jns.2012.07.046
31. Triantafyllidi H, Arvaniti C, Lekakis J, Ikonomidis I, Siafakas N, Tzortzis S, et al. Cognitive impairment is related to increased arterial stiffness and microvascular damage in patients with never-treated essential hypertension. Am J Hypertens. (2009) 22:525–30. doi: 10.1038/ajh.2009.35
32. Scuteri A, Brancati AM, Gianni W, Assisi A, Volpe M. Arterial stiffness is an independent risk factor for cognitive impairment in the elderly: a pilot study. J Hypertens. (2005) 23:1211–6. doi: 10.1097/01.hjh.0000170384.38708.b7
33. Huck DM, Hanna DB, Rubin LH, Maki P, Valcour V, Springer G, et al. Carotid artery stiffness and cognitive decline among women with or at risk for HIV infection. J Acquir Immune Defic Syndr. (2018). 78:338. doi: 10.1097/QAI.0000000000001685
34. DuBose LE, Voss MW, Weng TB, Kent JD, Dubishar KM, Lane-Cordova A, et al. Carotid β-stiffness index is associated with slower processing speed but not working memory or white matter integrity in healthy middle-aged/older adults. J Appl Physiol. (2017) 122:868–76. doi: 10.1152/japplphysiol.00769.2016
35. Lim SL, Gao Q, Nyunt MSZ, Gong L, Lunaria JB, Lim ML, et al. Vascular health indices and cognitive domain function: Singapore longitudinal ageing studies. J Alzheimers Dis. (2016) 50:27–40. doi: 10.3233/JAD-150516
36. Tarumi T, Gonzales MM, Fallow B, Nualnim N, Pyron M, Tanaka H, et al. Central artery stiffness, neuropsychological function, and cerebral perfusion in sedentary and endurance-trained middle-aged adults. J Hypertens. (2013) 31:2400–9. doi: 10.1097/HJH.0b013e328364decc
37. Hoth KF, Moreau KL, Weinberger HD, Holm KE, Meschede K, Crapo JD, et al. Carotid artery stiffness is associated with cognitive performance in former smokers with and without chronic obstructive pulmonary disease. J Am Heart Assoc. (2020) 9:e014862. doi: 10.1161/JAHA.119.014862
38. Geijselaers SL, Sep SJ, Schram MT, van Boxtel MP, van Sloten TT, Henry RM, et al. Carotid stiffness is associated with impairment of cognitive performance in individuals with and without type 2 diabetes. The Maastricht Study. Atherosclerosis. (2016) 253:186–93. doi: 10.1016/j.atherosclerosis.2016.07.912
39. Suemoto CK, Santos IS, Bittencourt MS, Pereira AC, Goulart AC, Rundek T. Subclinical carotid artery atherosclerosis and performance on cognitive tests in middle-aged adults: baseline results from the ELSA-Brasil. Atherosclerosis. (2015) 243:510–5. doi: 10.1016/j.atherosclerosis.2015.10.008
40. Fabbiani M, Ciccarelli N, Tana M, Farina S, Baldonero E, Di Cristo V, et al. Cardiovascular risk factors and carotid intima-media thickness are associated with lower cognitive performance in HIV-infected patients. HIV Med. (2013) 14:136–44. doi: 10.1111/j.1468-1293.2012.01044.x
41. Wang A, Chen G, Su Z, Liu X, Yuan X, Jiang R, et al. Carotid intima-media thickness and cognitive function in a middle-aged and older adult community: a cross-sectional study. J Neurol. (2016) 263:2097–104. doi: 10.1007/s00415-016-8234-9
42. Park J, Park J-H, Park H. Association between carotid artery intima-media thickness and combinations of mild cognitive impairment and pre-frailty in older adults. Int J Environ Res Public Health. (2019) 16:2978. doi: 10.3390/ijerph16162978
43. Yue W, Wang A, Liang H, Hu F, Zhang Y, Deng M, et al. Association between carotid intima-media thickness and cognitive impairment in a Chinese stroke population: a cross-sectional study. Sci Rep. (2016) 6:1–6. doi: 10.1038/srep19556
44. Moon JH, Lim S, Han JW, Kim KM, Choi SH, Park KS, et al. Carotid intima-media thickness is associated with the progression of cognitive impairment in older adults. Stroke. (2015) 46:1024–30. doi: 10.1161/STROKEAHA.114.008170
45. Frazier DT, Seider T, Bettcher BM, Mack WJ, Jastrzab L, Chao L, et al. The role of carotid intima-media thickness in predicting longitudinal cognitive function in an older adult cohort. Cerebrovasc Dis. (2014) 38:441–7. doi: 10.1159/000366469
46. Gardener H, Caunca MR, Dong C, Cheung YK, Elkind MSV, Sacco RL, et al. Ultrasound markers of carotid atherosclerosis and cognition. Stroke. (2017) 48:1855–61. doi: 10.1161/STROKEAHA.117.016921
47. Mathiesen E, Waterloo K, Joakimsen O, Bakke S, Jacobsen E, Bønaa K. Reduced neuropsychological test performance in asymptomatic carotid stenosis: The Tromsø Study. Neurology. (2004) 62:695–701. doi: 10.1212/01.WNL.0000113759.80877.1F
48. Johnston SC, O'Meara ES, Manolio TA, Lefkowitz D, O'Leary DH, Goldstein S, et al. Cognitive impairment and decline are associated with carotid artery disease in patients without clinically evident cerebrovascular disease. Ann Intern Med. (2004) 140:237–47. doi: 10.7326/0003-4819-140-4-200402170-00005
49. Cheng H-L, Lin C-J, Soong B-W, Wang P-N, Chang F-C, Wu Y-T, et al. Impairments in cognitive function and brain connectivity in severe asymptomatic carotid stenosis. Stroke. (2012) 43:2567–73. doi: 10.1161/STROKEAHA.111.645614
50. Paraskevas KI, Faggioli G, Ancetti S, Naylor AR. Editor's choice s sic005dementia: 15-year follow-up of the asymptomatic carotid surgery trial-iEur J Vasc Endovasc Surg. (2021) 61:888–99. doi: 10.1016/j.ejvs.2021.03.024
51. Buratti L, Viticchi G, Falsetti L, Balucani C, Altamura C, Petrelli C, et al. Thresholds of impaired cerebral hemodynamics that predict short-term cognitive decline in asymptomatic carotid stenosis. J Cereb Blood Flow Metab. (2016) 36:1804–12. doi: 10.1177/0271678X15613526
52. Buratti L, Balucani C, Viticchi G, Falsetti L, Altamura C, Avitabile E, et al. Cognitive deterioration in bilateral asymptomatic severe carotid stenosis. Stroke. (2014) 45:2072–7. doi: 10.1161/STROKEAHA.114.005645
53. Silvestrini M, Paolino I, Vernieri F, Pedone C, Baruffaldi R, Gobbi B, et al. Cerebral hemodynamics and cognitive performance in patients with asymptomatic carotid stenosis. Neurology. (2009) 72:1062–8. doi: 10.1212/01.wnl.0000345015.35520.52
54. Chang X-L, Zhou H-Q, Lei C-Y, Wu B, Chen Y-C, Hao Z-L, et al. Association between asymptomatic carotid stenosis and cognitive function: a systematic review. Neurosci Biobehav Rev. (2013) 37:1493–9. doi: 10.1016/j.neubiorev.2013.05.011
55. Halliday A, Gottsäter A, Sneade M, Whiteley W, Llewellyn-Bennett R, Parish S, et al. Stroke prevention and dementia: 15-year follow-up of the asymptomatic carotid surgery trial-1 (ACST-1). Eur J Vasc Endovasc Surg. (2019) 58:e721–2. doi: 10.1016/j.ejvs.2019.09.259
56. De Rango P, Caso V, Leys D, Paciaroni M, Lenti M, Cao P. The role of carotid artery stenting and carotid endarterectomy in cognitive performance: a systematic review. Stroke. (2008) 39:3116–27. doi: 10.1161/STROKEAHA.108.518357
57. Pettigrew LC, Thomas N, Howard V, Veltkamp R, Toole J, investigators ACAS. Low mini-mental status predicts mortality in asymptomatic carotid arterial stenosis. Neurology. (2000) 55:30–4. doi: 10.1212/WNL.55.1.30
58. Cheng Y, Wang YJ, Yan JC, Zhou R, Zhou HD. Effects of carotid artery stenting on cognitive function in patients with mild cognitive impairment and carotid stenosis. Exp Ther Med. (2013) 5:1019–24. doi: 10.3892/etm.2013.954
59. Whooley JL, David BC, Woo HH, Hoh BL, Raftery KB, Siddiqui AH, et al. Carotid revascularization and its effect on cognitive function: a prospective nonrandomized multicenter clinical study. J Stroke Cerebrovasc Dis. (2020) 29:104702. doi: 10.1016/j.jstrokecerebrovasdis.2020.104702
60. Hofman A, Ott A, Breteler MM, Bots ML, Slooter AJ, van Harskamp F, et al. Atherosclerosis, apolipoprotein E, and prevalence of dementia and Alzheimer's disease in the Rotterdam Study. Lancet. (1997) 349:151–4. doi: 10.1016/S0140-6736(96)09328-2
61. Auperin A, Berr C, Bonithon-Kopp C, Touboul P-J, Ruelland I, Ducimetiere P, et al. Ultrasonographic assessment of carotid wall characteristics and cognitive functions in a community sample of 59-to 71-year-olds. Stroke. (1996) 27:1290–5. doi: 10.1161/01.STR.27.8.1290
62. Buratti L, Balestrini S, Altamura C, Viticchi G, Falsetti L, Luzzi S, et al. Markers for the risk of progression from mild cognitive impairment to Alzheimer's disease. J Alzheimers Dis. (2015) 45:883–90. doi: 10.3233/JAD-143135
63. Mawet J, Vahedi K, Aout M, Vicaut E, Duering M, Touboul PJ, et al. Carotid atherosclerotic markers in CADASIL. Cerebrovasc Dis. (2011) 31:246–52. doi: 10.1159/000321932
64. Mworozi K, Ameda F, Byanyima RK, Nakasujja N. Carotid artery plaque detected on ultrasound is associated with impaired cognitive state in the elderly: a population-based study in Wakiso district, Uganda. J Clin Neurosci. (2019) 68:194–200. doi: 10.1016/j.jocn.2019.06.011
65. Talelli P, Ellul J, Terzis G, Lekka N, Gioldasis G, Chrysanthopoulou A, et al. Common carotid artery intima media thickness and post-stroke cognitive impairment. J Neurol Sci. (2004) 223:129–34. doi: 10.1016/j.jns.2004.05.013
66. Xiang J, Zhang T., Yang Q-w, Liu J, Chen Y, Cui M, et al. Carotid artery atherosclerosis is correlated with cognitive impairment in an elderly urban Chinese non-stroke population. J Clin Neurosci. (2013) 20:1571–5. doi: 10.1016/j.jocn.2013.02.026
67. Chiesa ST, Masi S, Shipley MJ, Ellins EA, Fraser AG, Hughes AD, et al. Carotid artery wave intensity in mid-to late-life predicts cognitive decline: the Whitehall II study. Eur Heart J. (2019) 40:2300–9. doi: 10.1093/eurheartj/ehz189
68. Poels MM, van Oijen M, Mattace-Raso FU, Hofman A, Koudstaal PJ, Witteman JC, et al. Arterial stiffness, cognitive decline, and risk of dementia: the Rotterdam study. Stroke. (2007) 38:888–92. doi: 10.1161/01.STR.0000257998.33768.87
69. O'Leary DH, Polak JF, Kronmal RA, Manolio TA, Burke GL, Wolfson SK Jr. Carotid-artery intima and media thickness as a risk factor for myocardial infarction and stroke in older adults. N Engl J Med. (1999) 340:14–22. doi: 10.1056/NEJM199901073400103
70. van den Oord SC, Sijbrands EJ, Gerrit L, van Klaveren D, van Domburg RT, van der Steen AF, et al. Carotid intima-media thickness for cardiovascular risk assessment: systematic review and meta-analysis. Atherosclerosis. (2013) 228:1–11. doi: 10.1016/j.atherosclerosis.2013.01.025
71. Romero JR, Beiser A, Seshadri S, Benjamin EJ, Polak JF, Vasan RS, et al. Carotid artery atherosclerosis, MRI indices of brain ischemia, aging, and cognitive impairment. Stroke. (2009) 40:1590. doi: 10.1161/STROKEAHA.108.535245
72. Muller M, van der Graaf Y, Algra A, Hendrikse J, Mali WP, Geerlings MI. Carotid atherosclerosis and progressions of brain atrophy: the SMART-MR study. Ann Neurol. (2011) 70:237–44. doi: 10.1002/ana.22392
73. Pico F, Dufouil C, Lévy C, Besançon V, de Kersaint-Gilly A, Bonithon-Kopp C, et al. Longitudinal study of carotid atherosclerosis and white matter hyperintensities: the EVA-MRI cohort. Cerebrovasc Dis. (2002) 14:109–15. doi: 10.1159/000064741
74. Moroni F, Ammirati E, Magnoni M, D'Ascenzo F, Anselmino M, Anzalone N, et al. Carotid atherosclerosis, silent ischemic brain damage and brain atrophy: a systematic review and meta-analysis. Int J Cardiol. (2016) 223:681–7. doi: 10.1016/j.ijcard.2016.08.234
75. Chung C-P, Chou K-H, Chen W-T, Liu L-K, Lee W-J, Huang A-C, et al. Location of cerebral microbleeds and their association with carotid intima-media thickness: a community-based study. Sci Rep. (2017) 7:12058. doi: 10.1038/s41598-017-12176-y
76. Zhao F-f, Gao H-y, Gao Y, Zhao Z, Li J, Ning F-b, et al. A correlational study on cerebral microbleeds and carotid atherosclerosis in patients with ischemic stroke. J Stroke Cerebrovasc Dis. (2018). 27:2228–34. doi: 10.1016/j.jstrokecerebrovasdis.2018.04.009
77. Romero JR, Preis SR, Beiser A, DeCarli C, D'Agostino RB, Wolf PA, et al. Carotid atherosclerosis and cerebral microbleeds: the Framingham Heart Study. J Am Heart Assoc. (2016) 5:e002377. doi: 10.1161/JAHA.115.002377
78. Baradaran H, Demissie S, Himali JJ, Beiser A, Gupta A, Polak JF, et al. The progression of carotid atherosclerosis and imaging markers of dementia. Alzheimers Dement. (2020). 6:e12015. doi: 10.1002/trc2.12015
79. Collaborators* NASCET. Beneficial effect of carotid endarterectomy in symptomatic patients with high-grade carotid stenosis. N Engl J Med. (1991). 325:445–53. doi: 10.1056/NEJM199108153250701
80. Meschia JF, Klaas JP, Brown Jr RD, Brott TG editors. Evaluation and management of atherosclerotic carotid stenosis. Mayo Clin Proc. (2017) 92:1144–57. doi: 10.1016/j.mayocp.2017.02.020
81. Ruitenberg A, den Heijer T, Bakker SL, van Swieten JC, Koudstaal PJ, Hofman A, et al. Cerebral hypoperfusion and clinical onset of dementia: the Rotterdam Study. Ann Neurol. (2005) 57:789–94. doi: 10.1002/ana.20493
82. Koike MA, Green KN, Blurton-Jones M, LaFerla FM. Oligemic hypoperfusion differentially affects tau and amyloid-β. Am J Pathol. (2010) 177:300–10. doi: 10.2353/ajpath.2010.090750
83. Kitaguchi H, Tomimoto H, Ihara M, Shibata M, Uemura K, Kalaria RN, et al. Chronic cerebral hypoperfusion accelerates amyloid β deposition in APPSwInd transgenic mice. Brain Res. (2009) 1294:202–10. doi: 10.1016/j.brainres.2009.07.078
84. Silvestrini M, Viticchi G, Falsetti L, Balucani C, Vernieri F, Cerqua R, et al. The role of carotid atherosclerosis in Alzheimer's disease progression. J Alzheimers Dis. (2011) 25:719–26. doi: 10.3233/JAD-2011-101968
85. Balestrini S, Perozzi C, Altamura C, Vernieri F, Luzzi S, Bartolini M, et al. Severe carotid stenosis and impaired cerebral hemodynamics can influence cognitive deterioration. Neurology. (2013) 80:2145–50. doi: 10.1212/WNL.0b013e318295d71a
86. Baradaran H, Mtui EE, Richardson JE, Delgado D, Dunning A, Marshall RS, et al. White matter diffusion abnormalities in carotid artery disease: a systematic review and metarmnalysis. J Neuroimaging. (2016) 26:481–8. doi: 10.1111/jon.12347
87. Baradaran H, Mtui E, Richardson J, Delgado D, Gupta A. Hemispheric differences in leukoaraiosis in patients with carotid artery stenosis: a systematic review. Clin Neuroradiol. (2017) 27:7–13. doi: 10.1007/s00062-015-0402-2
88. Avelar WM, D'Abreu A, Coan AC, Lima FO, Guimarães R, Yassuda CL, et al. Asymptomatic carotid stenosis is associated with gray and white matter damage. Int J Stroke. (2015) 10:1197–203. doi: 10.1111/ijs.12574
89. Finn C, Giambrone AE, Gialdini G, Delgado D, Baradaran H, Kamel H, et al. The association between carotid artery atherosclerosis and silent brain infarction: a systematic review and meta-analysis. J Cerebrovasc Dis. (2017) 26:1594–601. doi: 10.1016/j.jstrokecerebrovasdis.2017.02.028
90. Baradaran H, Gialdini G, Mtui E, Askin G, Kamel H, Gupta A. Silent brain infarction in patients with asymptomatic carotid artery atherosclerotic disease. Stroke. (2016) 47:1368–70. doi: 10.1161/STROKEAHA.116.013193
91. Gupta A, Baradaran H, Schweitzer AD, Kamel H, Pandya A, Delgado D, et al. Carotid plaque MRI and stroke risk. Stroke. (2013) 44:3071–7. doi: 10.1161/STROKEAHA.113.002551
92. Baradaran H, Eisenmenger LB, Hinckley PJ, de Havenon AH, Stoddard GJ, Treiman LS, et al. Optimal carotid plaque features on computed tomography angiography associated with ischemic stroke. J Am Heart Assoc. (2021) 10:e019462. doi: 10.1161/JAHA.120.019462
93. Chen W-H, Jin W, Lyu P-Y, Liu Y, Li R, Hu M, et al. Carotid atherosclerosis and cognitive impairment in nonstroke patients. Chin Med J. (2017) 130:2375. doi: 10.4103/0366-6999.215331
Keywords: carotid atherosclerosis, cognitive impairment (CI), dementia, carotid stenosis, carotid plaque (CP)
Citation: Baradaran H, Sarrami AH and Gupta A (2021) Asymptomatic Carotid Disease and Cognitive Impairment: What Is the Evidence? Front. Neurol. 12:741500. doi: 10.3389/fneur.2021.741500
Received: 14 July 2021; Accepted: 25 October 2021;
Published: 18 November 2021.
Edited by:
Seemant Chaturvedi, University of Maryland, United StatesReviewed by:
Frank J. Wolters, Erasmus Medical Center, NetherlandsRaffaele Ornello, University of L'Aquila, Italy
Copyright © 2021 Baradaran, Sarrami and Gupta. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hediyeh Baradaran, aGVkaXllaC5iYXJhZGFyYW5AaHNjLnV0YWguZWR1
 Hediyeh Baradaran
Hediyeh Baradaran Amir Hossein Sarrami
Amir Hossein Sarrami Ajay Gupta
Ajay Gupta