- 1National Key Clinical Specialty/Engineering Technology Research Center of Education Ministry of China, Guangdong Provincial Key Laboratory on Brain Function Repair and Regeneration, Neurosurgery Institute, Department of Neurosurgery, Zhujiang Hospital, Southern Medical University, Guangzhou, China
- 2Department of Interventional Treatment, Southern Medical University, Guangzhou, China
- 3Department of Traditional Chinese Medicine, Southern Medical University, Guangzhou, China
Background: Previous studies have analyzed the association of aspect ratio (AR) on the ruptured intracranial aneurysm (IA), but the findings are inconclusive and controversial. Therefore, the study aimed to derive a more detailed estimation of this association between AR and ruptured IA in Chinese IA patients.
Methods: The present work was a cross-sectional study. We retrospectively collected 1,588 Chinese patients with a single IA from January 2010 to November 2017. The relationship was examined between AR at diagnosis and ruptured IA. Covariates included data of demographics, morphological parameters, lifestyle habits, clinical features, and comorbidities. Binary logistic regression and two-piecewise linear models were used to analyze independent associations of AR with ruptured IA.
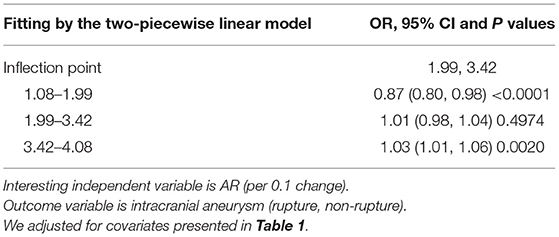
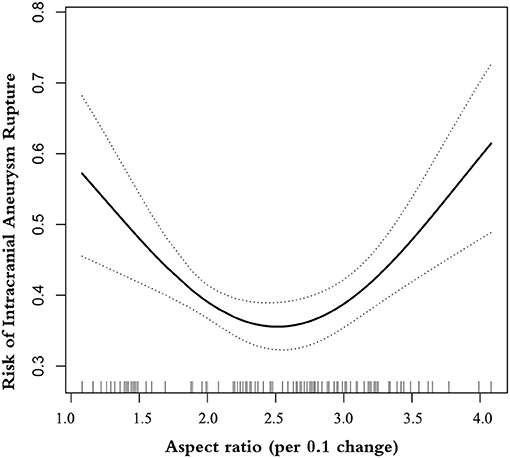
Results: The results suggest that the association between AR and IA rupture was U-shaped. In the AR range of 1.08–1.99, the prevalence of IA rupture was 13% lower for each 0.1-unit increment in AR [odds ratio 0.87, 95% confidence interval (CI) 0.80–0.98]. Conversely, for every 0.1-unit increase in AR, the prevalence of IA rupture increased by ~3% (odds ratio 1.03, 95% CI 1.01–1.06) in the AR range of 3.42–4.08.
Conclusion: The relationship between AR and ruptured IA was U-shaped, with the negative association at AR of 1.08–1.99 and positive association at AR of 3.42–4.08.
Introduction
Due to methodological limitations, the exact prevalence of intracranial aneurysms (IA) and the risk of rupture remains unclear (1). A current review suggests that guidelines (the American Heart Association/American Stroke Association (AHA/ASA) guideline) underestimate the incidence of aneurysm rupture (1). This review suggested that at least 1 in 20–30 adults is likely to carry an unruptured IA and approximately one-quarter of these UIAs rupture in a lifetime (1). In China, the prevalence of IA is roughly the same as in Western countries, but the exact incidence of IA rupture has not been reported (2). Based on China's huge population base, therefore, establishing an algorithm to stratify the risk of IA rupture is needed.
The prerequisites of predicted model development are based on the full clarification of ruptured IA-associated factors (3). Although the discovery of IA rupture-associated factors is still underway, it is widely accepted that the morphological parameters of IA are closely related to rupture. As an essential and the most used morphological indicator, aspect ratio (AR) was therefore incorporated as a candidate for constructing a model in most of these model-driven studies or IA rupture-associated factors discovery (4–8). However, a review reported that existing studies devoted to determining the correlations between IA rupture and AR yield mixed results (7). In addition, most studies have a small sample size, and rarely discuss the non-linear relationship and the interaction among AR and other morphological parameters. Because non-linear relationships have a significant impact on the evaluation of effect size or model development (3, 9, 10), we, therefore, present a large-scale cross-sectional study to investigate the association between AR and rupture risk of Chinese patients with single IA.
Participants and Methods
Study Design
We performed a cross-sectional study based on a large sample. The interesting independent variable is AR. The outcome variable is the rupture status of a single IA (dichotomous variable: 1 = rupture; 0 = unrupture).
Study Population
There were 1,786 patients with IA initially collected between January 2010 and November 2017 at the Department of Neurosurgery, Zhujiang Hospital. Diagnosis of IA for all involved patients was confirmed by magnetic resonance angiography (MRA) or computed tomography angiography (CTA) examination or by digital subtraction angiography (DSA) after admission. We excluded individuals with multiple IA (n = 55), feeding artery aneurysms-associated arteriovenous malformations (n = 67), fusiform IA (n = 29), or dissecting aneurysms (n = 47), leaving 1,588 individuals for the main analysis. Clinical information for each IA patient was obtained from our electronic medical record system. Participants' informed consent is not required in this study because of the nature of the retrospective study. The hospital institutional review board approved this study.
The Measurement of Morphological Parameters
All 1,588 IAs were examined by digital subtraction angiography (DSA). The patient-specific geometries were reconstructed from 3D-DSA images to confirm the morphological parameters (11, 12).
Aspect ratio: The aneurysm AR was determined as the ratio of the greatest perpendicular height to the mean neck diameter, where the mean neck diameter was evaluated as twice the mean distance from the neck centroid to the edge of the neck (12).
Size ratio: The aneurysm-to-vessel size ratio (SR) integrates the geometries of the IA and its parent vessel and was explained as SR = (maximum aneurysm height)/(average vessel diameter) (12).
IA size: IA size was defined as the largest perpendicular distance of the dome from the neck plane (12).
Outcome Variable
The outcome in this study was IA rupture. The diagnosis of subarachnoid hemorrhage was confirmed by computed tomography (CT) scan.
Other Involved Covariates
We collected information on covariates that are known risk factors for IA (13–22). Consequently, the following variables were included: age (year), cerebral microbleeds (yes, no), IA position (PcoA: posterior communicating artery; AcoA: anterior communicating artery; ICA: internal carotid artery; ACA: anterior cerebral artery; MCA: middle cerebral artery; VA: vertebrobasilar artery), sex (male, female), hypertension history (yes, no), diabetes history (yes, no), atherosclerosis (yes, no), hyperlipidemia (yes, no), coronary artery disease (CAD, yes, no), current smoker (yes, no), current alcohol consumption (yes, no), Willis variation (yes, no), the shape of IA (henceforth shape; regular or irregular), and neck status of IA (henceforth neck; wide or narrow). The history of comorbidities was self-reported by patients or blood relatives.
Missing Data Addressing
The proportion of missing data was low (<5%) for all variables, multiple imputation was not used.
Statistical Analysis
Continuous variables are presented as mean (SD) and categorical variables as No. (%). Differences between the unruptured and ruptured groups were evaluated using chi-square test for categorical variables and using t test for continuous variables. Multivariable logistic regression was performed to determine the association between AR and ruptured IA. We present both univariate and multivariate models. In the multivariate model, we included those variables that showed statistical differences between the two groups.
We also test the possibility of a non-linear relation of AR with ruptured IA. By the recurrence method, we calculated the inflection points of the U-shaped curve. Afterward, by the two-piecewise linear regression model, we divided the U-shaped curve according to the inflection point, and established the two-piecewise linear model on both sides of the inflection points, and calculated the OR and confidence intervals, respectively.
All the analyses were performed with the statistical software packages R (http://www.R-project.org, The R Foundation) and EmpowerStats (http://www.empowerstats.com, X&Y Solutions, Inc, Boston, MA). P < 0.05 (two-sided) were considered statistically significant.
Results
Baseline Characteristics of Selected Participants
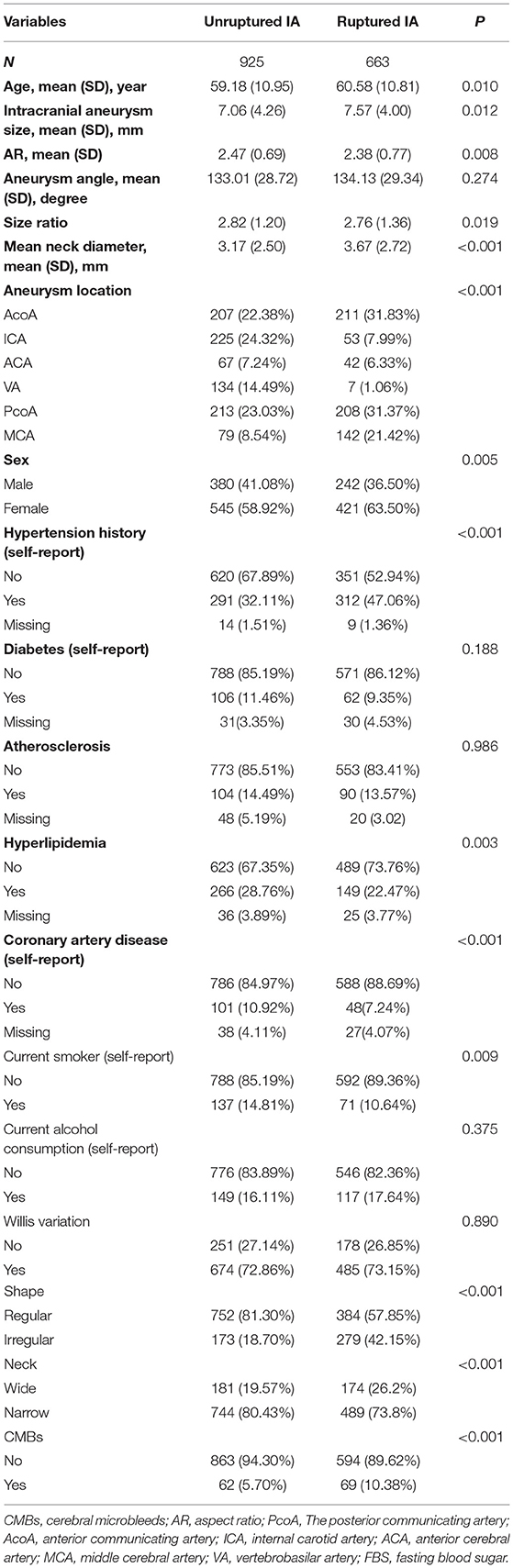
The overall prevalence of ruptured IA was 41.75% (n = 663). At baseline, the mean age of participants was 59.69 years, and the proportion of men was 40.0%. Table 1 describes the baseline characteristics of the patients. There were no statistically significant differences between non-ruptured and ruptured groups in terms of angle, diabetes history, atherosclerosis history, current alcohol consumption, or Willis variation (all P > 0.05). The ruptured group contained more patients who were older and had larger IA size, mean neck diameter, and comorbidity, more likely to be located in AcoA, PcoA, or MCA compared with patients with unruptured IA. In terms of morphological parameters, those in the ruptured group had lower SR, with a higher proportion of irregular shape, wide neck, and CMBs compared with the unruptured group.
Results of Multivariable Logistic Regression
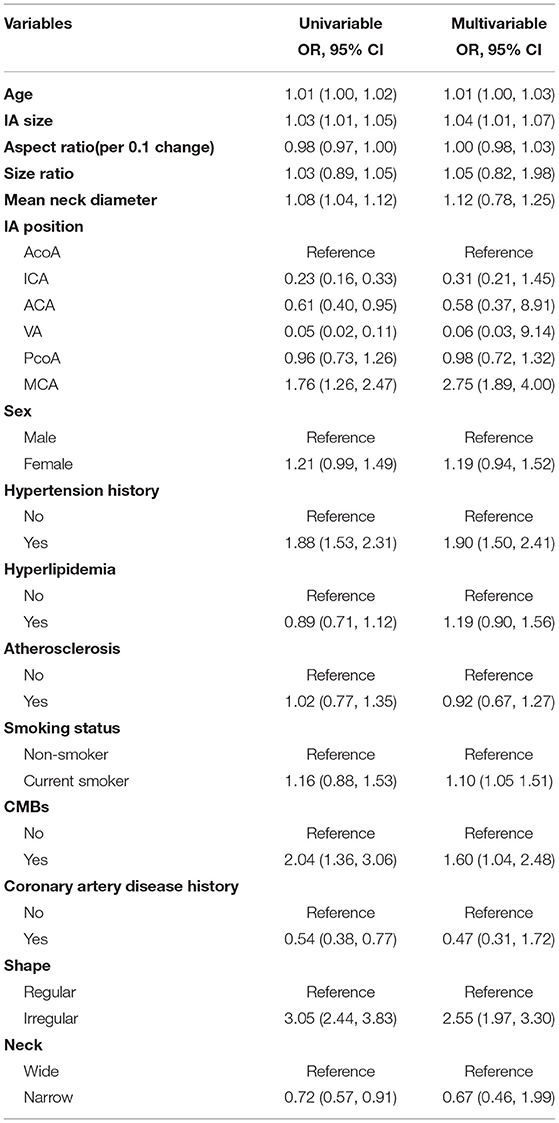
We put the variables with significant differences between the two groups in Table 1 into univariate and multivariate models (Table 2). The unadjusted OR for continuous AR (per 0.1-unit increase) and prevalence of ruptured IA was 0.98 (95% CI 0.97–1.00). This result means every 0.1 unit increase in AR was associated with 2% lower prevalence of ruptured IA. After adjusting for covariates presented in Table 1, a linear association of AR with ruptured IA was not observed (adjusted-OR: 1.19, 95% CI 1.04–1.35).
Results obtained from multivariable logistic regression have indicated that increasing age, higher IA size, patients with a history of hypertension, current smoker, higher size ratio, concurrent cerebral microbleeds, aneurysm located in MCA, and irregular shape IA are associated with a high risk of aneurysm rupture.
In addition, when the multivariate logistic regression model controls for confounding factors, we failed to observe the relationship between gender, history of hyperlipidemia, history of CAD, size ratio, mean neck diameter, and the state of aneurysm neck and rupture of an aneurysm.
The Results of Non-linearity of AR and Ruptured IA
A smooth curve-fitting analysis observed a U-shaped relationship of AR correlation to IA rupture (Table 3 and Figure 1). By recursive algorithm, we got the inflection points of 1.99 and 3.42. In the AR range of 1.08–1.99, the prevalence of IA rupture was 13% lower for each 0.1-unit increment in AR (odds ratio 0.87, 95% CI 0.80–0.98). Conversely, for every 0.1-unit increase in AR, the prevalence of IA rupture increased by ~ 3% (odds ratio 1.03, 95% CI 1.01–1.06) in the AR range of 3.42–4.08. It is worth mentioning that we observed no association between AR and the prevalence of IA rupture in the AR range of 1.99–3.42.
Discussion
In this study, we analyzed the relationship of AR with the prevalence of ruptured IA in a large sample set (N = 1588), including 663 patients with ruptured IA. The U-shaped relationship was seen here for AR with IA rupture. Interestingly, the non-linearity analysis by the two-piecewise linear model demonstrated here captures more variation than a single-slope logistic regression model: AR appears to follow a different pattern of association with ruptured IA in different ranges.
Previous studies focused only on the “linear” relationship and yielded conflicting results. Twelve studies (sample size ranging from 8 to 425) and two systematic reviews (19 studies, 1553 cases) found a positive correlation between AR and IA rupture (23–36). Eight studies (sample size ranging from 45 to 713) found AR was not associated with ruptured IA (17, 20, 37–42). However, none of these studies defined an applicability range for the associations of AR and ruptured IA (Supplementary Table 1). Indeed, we observed three distinct patterns of AR with ruptured IA in different AR ranges, including negative correlation (AR range 1.08–1.99), no correlation (AR range 1.99–3.42) or positive correlation (AR range 3.42–4.08). This interpretation might offer one explanation as to why the results obtained from previous studies are quite different but seem “reasonable.”
Greving et al. (43) pooled four prospective cohort studies and established PHASES score for prediction of the risk of rupture of incidental intracranial aneurysms. The study confirmed that older, a history of hypertension, current smoker, large-size IA, located in ACA/Pcom/posterior are independent risk factors for aneurysm rupture. This is consistent with our study. However, in this study, we only found that aneurysms located in the MCA are positively associated with the risk of rupture. The previous literature reported that in addition to MCA, there is also ACA/Pcom/posterior. We speculate that this is related to the small number of aneurysms located in ACA, Pcom, and posterior in our study.
We also found that patients with concurrent cerebral microbleeds or irregular IA are associated with a high risk of aneurysm rupture. This is consistent with the previously published literature. Two studies (44, 45) have confirmed that the bleeding rate of CMBs-related rupture is much higher than that in non-CMBs aneurysm patients. In addition, a review also suggested that compared with those with regular aneurysms, aneurysms with irregular shapes have a higher risk of rupture (46).
To investigate how these indicators could influence the differing impact of AR on bleeding risk, we conducted a series of sensitivity analyses. First, when we stratified by age (>60, ≤60), we found that the relationship between AR and IA bleeding risk is still a U-shaped relationship in patients older than 60 years (Supplementary Figure 1). Similarly, in non-smokers, we found a U-shaped relationship between AR and aneurysm rupture, but it was not found in current smokers (Supplementary Figure 2). Therefore, the two sensitivity analyses suggest that the U-shaped relationship between AR and aneurysm rupture in this study seems to be related to the older age and higher percentage of non-smokers of the study population. Our findings are more suitable for non-smokers and elderly patients over 60 years old.
Given that AR is the ratio of the greatest perpendicular height (IA size) to the mean neck diameter, we also evaluated the association between IA size and mean neck diameter and aneurysm rupture, respectively in this study. We observed a positive association between IA size and IA rupture. This is the same as the previous results. These studies found that the unruptured IAs were significantly smaller than the maximum size of ruptured IAs, and the presence of an unruptured IA of ≥7 mm was an independent risk factor for the lifetime occurrence of SAH (43, 47). We also found that the average neck diameter was not associated with bleeding risk. In a prospective large sample study, Mocco et al. (20) found that mean neck diameter was not a statistically significant predictor of rupture. This conclusion is the same as our study.
This research has several strengths of note. First, the sample size of this study is large, ensuring sufficient statistical power. Second, a case-control study design was not used in this study. Compared with previous studies, therefore, the selection bias is reduced. Third, compared to most previous studies that only adjust morphological parameters or demographic characteristics, the adjustment strategy of this study is sufficient and more complete. Fourth, to our best knowledge, it is first to address non-linearity and test interaction on the association of AR with IA rupture. Fifth, sensitivity analysis ensures the robustness of our findings. It roughly explains why the U-shaped relationship between AR and aneurysm rupture can be observed in this study.
Indeed, the interpretation of the findings of this study should be cautious. Several limitations are also noteworthy. First, we only include patients who have clinical symptoms to see a doctor. Therefore, the absence of patients without symptoms will result in selection bias. But such patients are non-ruptured IA, therefore, this would have biased results toward the null. Second, conclusions can be generalized to patients with single IA only, and the correlation of AR and ruptured IA may be different in multiple IA. Third, this study was designed for cross-section, so the causal association between AR and IA rupture could not be confirmed because there is no evidence that the morphological parameters before and after IA rupture are unchanged. Fourth, although we have adjusted for those of measurable confounders, as in all observational studies, there might have been still uncontrolled confounding due to unmeasured differences between IA with and without rupture.
Conclusion
The independent correlation between AR and ruptured IA is U-shaped. When clinically using AR to estimate the risk of IA bleeding, the different trends within different AR ranges should be judged, especially for the elderly or non-smokers. In addition, older, a history of hypertension, current smoker, large-size IA, located in MCA are independent risk factors for aneurysm rupture. Neurosurgeons can make more detailed and individualized clinical decisions for patients with aneurysms based on these characteristics.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by Ethics Committee of Zhujiang Hospital. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author Contributions
J-HY wrote the manuscript. S-XS is responsible for data analysis. XZ is responsible for study design and data analysis. Y-MB is responsible for data collection. C-ZD is responsible for the revision of the paper. W-mH and X-LW are responsible for data collection. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the National Natural Science Foundation Project (Grant Number: 81974177) and Initiation Plan Project of Clinical Research of Southern Medical University (Grant Number: QD2018N022).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
The authors thank Xiao-Yi Chen for statistical guidance and Ming for reviewing.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2021.731129/full#supplementary-material
References
1. Korja M, Kaprio J. Controversies in epidemiology of intracranial aneurysms and SAH. Nat Rev Neurol. (2016) 12:50–5. doi: 10.1038/nrneurol.2015.228
2. Editorial Board Member of Stroke Prevention Project of National Health and Family Planning Commission. Guidelines for diagnosis and treatment of aneurysmal subarachnoid hemorrhage in China. Zhong Guo Nao Xue Guan Bing Za Zhi. (2016) 13:384–92. doi: 10.3696/j.issn.1672-5921.2013.04.013
3. Steyerberg EW, Vergouwe Y. Towards better clinical prediction models: seven steps for development and an ABCD for validation. Eur Heart J. (2014) 35:1925–31. doi: 10.1093/eurheartj/ehu207
4. Jaja BNR, Saposnik G, Lingsma HF, Macdonald E, Thorpe KE, Mamdani M, et al. Development and validation of outcome prediction models for aneurysmal subarachnoid haemorrhage: the SAHIT multinational cohort study. BMJ. (2018) 360:j5745. doi: 10.1136/bmj.j5745
5. Ujiie H, Tamano Y, Sasaki K, Hori T. Is the aspect ratio a reliable index for predicting the rupture of a saccular aneurysm? Neurosurgery. (2001) 48:495–502, 502–3. doi: 10.1097/00006123-200103000-00007
6. Zheng Y, Xu F, Ren J, Xu Q, Liu Y, Tian Y, et al. Assessment of intracranial aneurysm rupture based on morphology parameters and anatomical locations. J Neurointerv Surg. (2016) 8:1240–6. doi: 10.1136/neurintsurg-2015-012112
7. Lall RR, Eddleman CS, Bendok BR, Batjer HH. Unruptured intracranial aneurysms and the assessment of rupture risk based on anatomical and morphological factors: sifting through the sands of data. Neurosurg Focus. (2009) 26:E2. doi: 10.3171/2009.2.FOCUS0921
8. Berg P, Voss S, Janiga G, Saalfeld S, Bergersen AW, Valen-Sendstad K, et al. Multiple Aneurysms AnaTomy CHallenge 2018 (MATCH)-phase II: rupture risk assessment. Int J Comput Assist Radiol Surg. (2019) 14:1795–804. doi: 10.1007/s11548-019-01986-2
9. von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet. (2007) 370:1453–7. doi: 10.1016/S0140-6736(07)61602-X
10. Collins GS, Reitsma JB, Altman DG, Moons KG. Transparent reporting of a multivariable prediction model for individual prognosis or diagnosis (TRIPOD). Ann Intern Med. (2015) 162:735–6. doi: 10.7326/L15-5093-2
11. Zhang X, Yao ZQ, Karuna T, Duan CZ, Wang XM, Li XF, et al. Cerebral microbleeds could be independently associated with intracranial aneurysm rupture: a cross-sectional population-based study. World Neurosurg. (2018) 115:e218–25. doi: 10.1016/j.wneu.2018.04.018
12. Dhar S, Tremmel M, Mocco J, Kim M, Yamamoto J, Siddiqui AH, et al. Morphology parameters for intracranial aneurysm rupture risk assessment. Neurosurgery. (2008) 63:185–96; discussion 196–97. doi: 10.1227/01.NEU.0000316847.64140.81
13. Can A, Castro VM, Dligach D, Finan S, Yu S, Gainer VS, et al. Lipid-lowering agents and high HDL (high-density lipoprotein) are inversely associated with intracranial aneurysm rupture. Stroke. (2018) 49:1148–54. doi: 10.1161/STROKEAHA.117.019972
14. Can A, Castro VM, Yu S, Dligach D, Finan S, Gainer VS, et al. Antihyperglycemic agents are inversely associated with intracranial aneurysm rupture. Stroke. (2018) 49:34–9. doi: 10.1161/STROKEAHA.117.019249
15. You SH, Kong DS, Kim JS, Jeon P, Kim KH, Roh HK, et al. Characteristic features of unruptured intracranial aneurysms: predictive risk factors for aneurysm rupture. J Neurol Neurosurg Psychiatry. (2010) 81:479–84. doi: 10.1136/jnnp.2008.169573
16. Backes D, Vergouwen MD, Velthuis BK, van der Schaaf IC, Bor AS, Algra A, et al. Difference in aneurysm characteristics between ruptured and unruptured aneurysms in patients with multiple intracranial aneurysms. Stroke. (2014) 45:1299–303. doi: 10.1161/STROKEAHA.113.004421
17. Björkman J, Frösen J, Tähtinen O, Backes D, Huttunen T, Harju J, et al. Irregular shape identifies ruptured intracranial aneurysm in subarachnoid hemorrhage patients with multiple aneurysms. Stroke. (2017) 48:1986–9. doi: 10.1161/STROKEAHA.117.017147
18. Millan RD, Dempere-Marco L, Pozo JM, Cebral JR, Frangi AF. Morphological characterization of intracranial aneurysms using 3-D moment invariants. IEEE Trans Med Imaging. (2007) 26:1270–82. doi: 10.1109/TMI.2007.901008
19. Elsharkawy A, Lehečka M, Niemelä M, Kivelev J, Billon-Grand R, Lehto H, et al. Anatomic risk factors for middle cerebral artery aneurysm rupture: computed tomography angiography study of 1009 consecutive patients. Neurosurgery. (2013) 73:825–37, 836–37. doi: 10.1227/NEU.0000000000000116
20. Mocco J, Brown RJ, Torner JC, Capuano AW, Fargen KM, Raghavanet ML. Aneurysm morphology and prediction of rupture: an international study of unruptured intracranial aneurysms analysis. Neurosurgery. (2018) 82:491–6. doi: 10.1093/neuros/nyx226
21. Ujiie H, Tachibana H, Hiramatsu O, Hazel AL, Matsumoto T, Ogasawara Y, et al. Effects of size and shape (aspect ratio) on the hemodynamics of saccular aneurysms: a possible index for surgical treatment of intracranial aneurysms. Neurosurgery. (1999) 45:119–29, 129–30. doi: 10.1097/00006123-199907000-00028
22. Matsukawa H, Kamiyama H, Kinoshita Y, Saito N, Hatano Y, Miyazaki T, et al. Morphological parameters as factors of 12-month neurological worsening in surgical treatment of patients with unruptured saccular intracranial aneurysms: importance of size ratio. J Neurosurg. (2018) 131:852–8. doi: 10.3171/2018.4.JNS173221
23. Kocur D, Przybylko N, Niedbala M, Rudniket A. Alternative definitions of cerebral aneurysm morphologic parameters have an impact on rupture risk determination. World Neurosurg. (2019) 126:e157–64. doi: 10.1016/j.wneu.2019.01.283
24. Liang L, Steinman DA, Brina O, Chnafa C, Cancelliere NM, Pereira VM. Towards the Clinical utility of CFD for assessment of intracranial aneurysm rupture - a systematic review and novel parameter-ranking tool. J Neurointerv Surg. (2019) 11:153–8. doi: 10.1136/neurintsurg-2018-014246
25. Wang GX, Zhang D, Wang ZP, Yang LQ, Yang H, Li W. Risk factors for ruptured intracranial aneurysms. Indian J Med Res. (2018) 147:51–7. doi: 10.4103/ijmr.IJMR_1665_15
26. Wang GX, Zhang D, Wang ZP, Yang LQ, Zhang L, Wen L. Risk factors for the rupture of bifurcation intracranial aneurysms using CT angiography. Yonsei Med J. (2016) 57:1178–84. doi: 10.3349/ymj.2016.57.5.1178
27. Lv N, Yu Y, Xu J, Karmonik C, Liu J, Huang Q. Hemodynamic and morphological characteristics of unruptured posterior communicating artery aneurysms with oculomotor nerve palsy. J Neurosurg. (2016) 125:264–8. doi: 10.3171/2015.6.JNS15267
28. Jing L, Fan J, Wang Y, Li HY, Wang SZ, Yang XJ, et al. Morphologic and hemodynamic analysis in the patients with multiple intracranial aneurysms: ruptured versus unruptured. PLoS ONE. (2015) 10:e132494. doi: 10.1371/journal.pone.0132494
29. Wang GX, Liu J, Chen YQ, Wen L, Yang MG, Gong MF, et al. Morphological characteristics associated with the rupture risk of mirror posterior communicating artery aneurysms. J Neurointerv Surg. (2018) 10:995–8. doi: 10.1136/neurintsurg-2017-013553
30. Gu Y, Xu L, Hu C, Zhang HW, Liu XG. Monitoring dynamic morphological changes with electrocardiography-gated dynamic 4-dimensional computed tomography angiography to predict intraoperative rupture of intracranial aneurysms. J Comput Assist Tomogr. (2018) 42:286–92. doi: 10.1097/RCT.0000000000000671
31. Wang GX, Liu LL, Wen L, Cao YX, Pei YC, Zhang D. Morphological characteristics associated with rupture risk of multiple intracranial aneurysms. Asian Pac J Trop Med. (2017) 10:1011–4. doi: 10.1016/j.apjtm.2017.09.015
32. Duan Z, Li Y, Guan S, Ma CM, Han YZ, Ren XY, et al. Morphological parameters and anatomical locations associated with rupture status of small intracranial aneurysms. Sci Rep. (2018) 8:6440. doi: 10.1038/s41598-018-24732-1
33. Jiang Y, Lan Q, Wang Q, Lu H, Ge F, Wang Y. Correlation between the rupture risk and 3D geometric parameters of saccular intracranial aneurysms. Cell Biochem Biophys. (2014) 70:1417–20. doi: 10.1007/s12013-014-0074-6
34. Bhogal P, AlMatter M, Hellstern V, Ganslandt O, Bäzner H, Henkes H, et al. Difference in aneurysm characteristics between ruptured and unruptured aneurysms in patients with multiple intracranial aneurysms. Surg Neurol Int. (2018) 9:1. doi: 10.4103/sni.sni_339_17
35. Lin N, Ho A, Charoenvimolphan N, Frerichs KU, Day AL, Du R. Analysis of morphological parameters to differentiate rupture status in anterior communicating artery aneurysms. PLoS ONE. (2013) 8:e79635. doi: 10.1371/journal.pone.0079635
36. Zheng Y, Zhou B, Wang X, Chen H, Fang X, Jiang P, et al. Size, Aspect ratio and anatomic location of ruptured intracranial aneurysms: consecutive series of 415 patients from a prospective, multicenter, observational study. Cell Transplant. (2018) 2136809931. doi: 10.1177/0963689718817227
37. Kang H, Ji W, Qian Z, Li Y, Jiang C, Wu Z, et al. Aneurysm characteristics associated with the rupture risk of intracranial aneurysms: a self-controlled study. PLoS ONE. (2015) 10:e142330. doi: 10.1371/journal.pone.0142330
38. Jiang H, Weng YX, Zhu Y, Shen J, Pan JW, Zhan RY. Patient and aneurysm characteristics associated with rupture risk of multiple intracranial aneurysms in the anterior circulation system. Acta Neurochir (Wien). (2016) 158:1367–75. doi: 10.1007/s00701-016-2826-0
39. Tateshima S, Chien A, Sayre J. Cebral J, Viñuela F. The effect of aneurysm geometry on the intra-aneurysmal flow condition. Neuroradiology. (2010) 52:1135–41. doi: 10.1007/s00234-010-0687-4
40. Lv N, Wang C, Karmonik C, Fang Y, Xu J, Yu Y, et al. Morphological and hemodynamic discriminators for rupture status in posterior communicating artery aneurysms. PLoS ONE. (2016) 11:e149906. doi: 10.1371/journal.pone.0149906
41. Lv N, Feng Z, Wang C, Cao W, Fang Y, Karmonik C, et al. Morphological risk factors for rupture of small (<7 mm) posterior communicating artery aneurysms. World Neurosurg. (2016) 87:311–5. doi: 10.1016/j.wneu.2015.12.055
42. Xiang J, Yu J, Choi H, Fox JMD, Snyder KV, Levy EI, et al. Rupture Resemblance Score (RRS): toward risk stratification of unruptured intracranial aneurysms using hemodynamic-morphological discriminants. J Neurointerv Surg. (2015) 7:490–5. doi: 10.1136/neurintsurg-2014-011218
43. Greving JP, Wermer MJ, Brown RD Jr, Morita A, Juvela S, Yonekura M, et al. Development of the PHASES score for prediction of risk of rupture of intracranial aneurysms: a pooled analysis of six prospective cohorts studies. Lancet Neurol. (2014) 13:59–66. doi: 10.1016/S1474-4422(13)70263-1
44. Takada S, Inoue T, Niizuma K, Shimizu H, Tominaga T. Hemosiderin detected by T2*-weighted magnetic resonance imaging in patients with unruptured cerebral aneurysms: indication of previous bleeding. Neurol Med Chir (Tokyo). (2011) 51:275–81. doi: 10.2176/nmc.51.275
45. Nussbaum ES, Defillo A, Zelensky A, Pulivarthi S, Nussbaum L. “Microbleeding” from intracranial aneurysms: Local hemosiderin deposition identified during microsurgical treatment of unruptured intracranial aneurysms. Surg Neurol Int. (2014) 5:28. doi: 10.4103/2152-7806.127967
46. Jirjees S, Htun ZM, Aldawudi I, Katwal PC, Khan S. role of morphological and hemodynamic factors in predicting intracranial aneurysm rupture: a review. Cureus. (2020) 12:e9178. doi: 10.7759/cureus.9178
Keywords: aspect ratio (AR), rupture of intracranial aneurysm, association, generalized additive model, subgroup analysis
Citation: Yin J-H, Su S-X, Zhang X, Bi Y-M, Duan C-Z, Huang W-m and Wang X-L (2021) U-Shaped Association of Aspect Ratio and Single Intracranial Aneurysm Rupture in Chinese Patients: A Cross-Sectional Study. Front. Neurol. 12:731129. doi: 10.3389/fneur.2021.731129
Received: 26 June 2021; Accepted: 27 September 2021;
Published: 03 November 2021.
Edited by:
Karsten Wrede, University of Duisburg-Essen, GermanyReviewed by:
Ulf Nestler, University Hospital Leipzig, GermanyQazi Zeeshan, University at Buffalo, United States
Copyright © 2021 Yin, Su, Zhang, Bi, Duan, Huang and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xin Zhang, emhhbmd4aW4xOTgzMDgxOEAxNjMuY29t
 Jia-He Yin
Jia-He Yin Shi-Xing Su
Shi-Xing Su Xin Zhang
Xin Zhang Yi-Ming Bi
Yi-Ming Bi Chuan-Zhi Duan
Chuan-Zhi Duan Wei-mei Huang
Wei-mei Huang Xi-Long Wang
Xi-Long Wang