
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Neurol., 10 September 2021
Sec. Stroke
Volume 12 - 2021 | https://doi.org/10.3389/fneur.2021.727962
This article is part of the Research TopicPrecision of Minimally Invasive Surgery for Intracerebral Hemorrhage TreatmentView all 13 articles
 Danyang Chen1
Danyang Chen1 Yingxin Tang1
Yingxin Tang1 Hao Nie2
Hao Nie2 Ping Zhang1
Ping Zhang1 Wenzhi Wang3
Wenzhi Wang3 Qiang Dong4
Qiang Dong4 Guofeng Wu5
Guofeng Wu5 Mengzhou Xue6
Mengzhou Xue6 Yuping Tang4
Yuping Tang4 Wenjie Liu7
Wenjie Liu7 Chao Pan1*
Chao Pan1* Zhouping Tang1*
Zhouping Tang1*Primary brainstem hemorrhage (PBSH) is the most fatal subtype of intracerebral hemorrhage and is invariably associated with poor prognosis. Several prognostic factors are involved, of which the two most predominant and consistent are the initial level of consciousness and hemorrhage size. Other predictors, such as age, hyperthermia, and hydrocephalus, are generally not dependable indicators for making prognoses. Scoring systems have now been developed that can predict mortality and functional outcomes in patients suffering from PBSH, which can thus guide treatment decision-making. A novel grading scale, entitled “the new primary pontine hemorrhage (PPH) score,” represents the latest approach in scoring systems. In this system, patients with a score of 2–3 points appear to benefit from surgical management, although this claim requires further verification. The four main surgical options for the treatment of PBSH are craniotomy, stereotactic hematoma puncture and drainage, endoscopic hematoma removal, and external ventricular drainage. Nevertheless, the management of PBSH still primarily involves conservative treatment methods and surgery is generally not recommended, according to current practice. However, the ongoing clinical trial, entitled Safety and Efficacy of Surgical Treatment in Severe Primary Pontine Hemorrhage Evacuation (STIPE), should provide additional evidence to support the surgical treatment of PBSH. Therefore, we advocate the update of epidemiological data and re-evaluation of PBSH treatment in a contemporary context.
Primary brainstem hemorrhage (PBSH) is a type of spontaneous brainstem hemorrhage that is particularly relevant to chronic hypertension but is not associated with definite or objective lesions such as cavernomas and arteriovenous malformations. PBSH is the most fatal subtype of intracerebral hemorrhage (ICH) and invariably has a bleak prognosis (1–3). It has the clinical characteristics of sudden onset, rapid evolution, and high morbidity and mortality (4, 5). Multiple studies have investigated the correlation between the prognosis of PBSH and its clinical features, neuroradiological presentation and neurophysiological properties (6–8). The identification of prognostic factors contributes to the development of a specific scoring system for PBSH, and a fast and accurate prognostic assessment in the emergency room plays a key role in the selection of reasonable therapeutic strategies (9). The new primary pontine hemorrhage (PPH) score represents the very latest approach in scoring systems, which will be explained below (3). It is suggested to spare medical resources for patients with a maximum score (3). However, the availability of the new PPH score for determining the surgical indications needs to be further investigated.
Actually, PBSH is currently mainly subjected to conservative treatment, and the efficacy of surgical procedures such as hematoma clearance remains questionable (4, 10–13). However, surgical interventions promise to become attractive options to manage PBSH with growing knowledge of safe entry zones into the brainstem and advances in new technologies as well as equipment in the fields of neuroimaging, microsurgery, neuronavigation, neuroendoscopy, intraoperative monitoring, and neurological rehabilitation. In this review, we aimed to analyze the identification of prognostic factors and scoring systems in PBSH and to discuss the current status and future prospects of controversial surgical management. Specifically, because PPH accounts for the vast majority (60–80%) of PBSH (5), both of these terms are used in our review, depending on the actual situation.
According to different localization of bleeding, ICH falls into two types-supratentorial and infratentorial ICH. Supratentorial ICH mainly involves basal ganglion and spontaneous infratentorial hemorrhage consists primarily of spontaneous cerebellar ICH and PBSH. PBSH occurs most frequently in the region of pontine, constituting 6 to 10% of ICH with an incidence of about 2 to 4 in 100,000 people per year and a mortality rate varying between 30 and 90% in different reports (4, 6, 14–16). PBSH occurs most often in patients aged 40 to 60, showing trends toward younger age compared with supratentorial and cerebellar ICH (17, 18). The incidence is higher in men than in women, probably because of personal living habits and health conditions prior to their illness. Hypertension is the most important risk factor of PBSH and other relative factors include anticoagulation therapy, amyloid angiopathy, etc. (16).
Researches, that carried out multivariate logistic regression analysis to identify independent predictors for PBSH, were shown in Table 1.
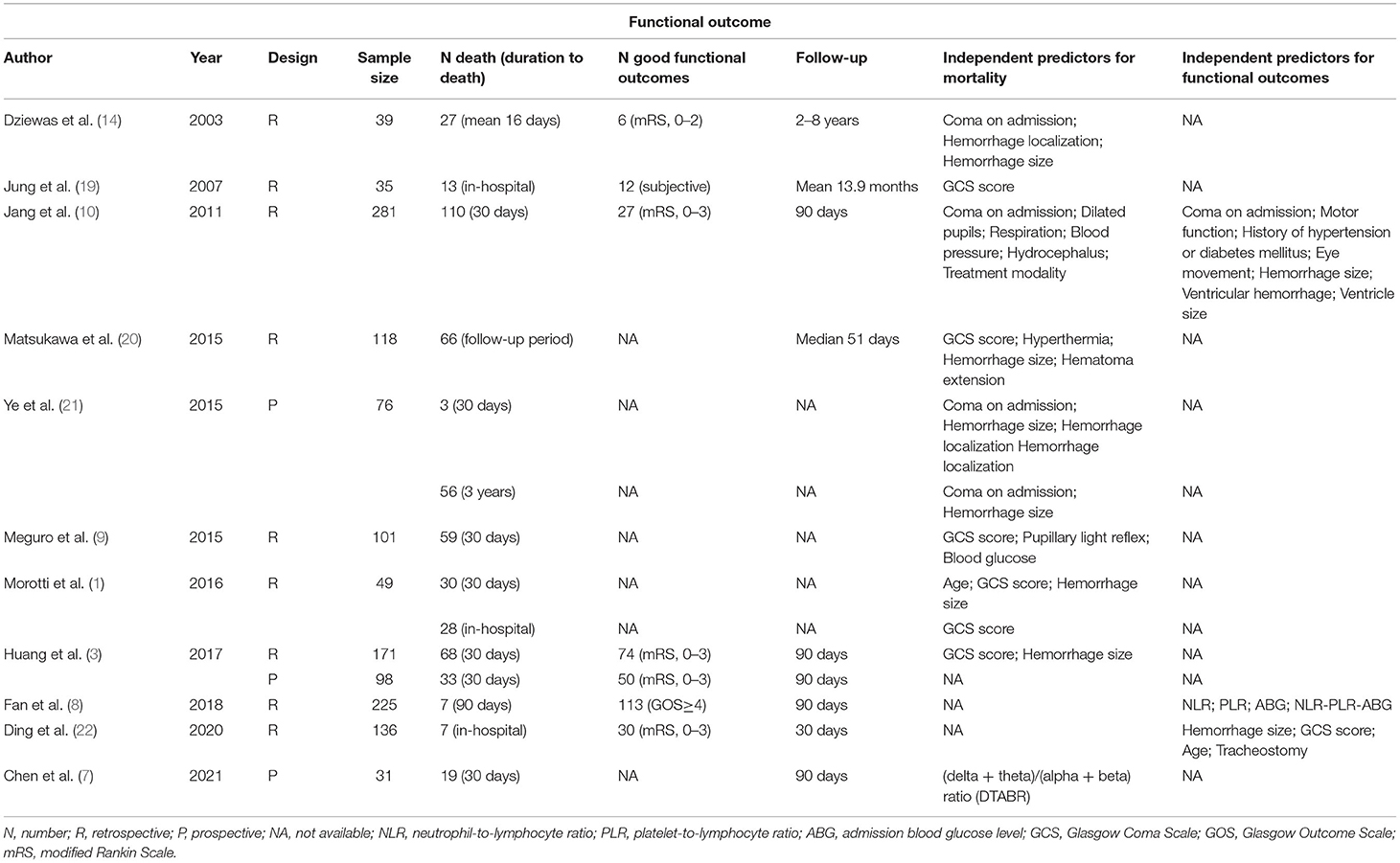
Table 1. Researches that used multivariate logistic regression analysis to identify independent predictors for PBSH.
The incidence of ICH continues to increase as people age (4, 23). Age plays a vital prognostic role in ICH and is an important part of the ICH score (23, 24). Furthermore, previous studies showed that ICH appears to be more common in men, while women show better survival (23, 25). However, whether age or sex affects patients with PBSH remains an unresolved problem. Patient age was found to independently affect 30-day mortality or functional outcomes by Morotti et al. and Ding et al. by multivariate logistic regression analysis (1, 22). Intriguingly, no study has demonstrated that sex is a predictor of the outcomes of PBSH.
Coma is one of the typical symptoms of PBSH. In previous studies, depressed and poor initial levels of consciousness were usually described as “coma on admission” or measured by different GCS score critical thresholds in the range of <4 to ≤ 9 (3, 9, 16, 20, 21). In agreement, both of these descriptions could independently and reliably predict death and an unfavorable functional outcome of PBSH (6). According to Table 1, the initial level of consciousness has been identified as an independent predictor in 9 different studies, which presents the most consistent and influential predictor for PBSH. The initial level of consciousness is also simple to judge and has the potential to be part of a future PBSH-related scoring system.
Central hyperthermia is a complication after PBSH that is characterized by a core temperature of ≥39°C and is unresponsive to conventional antipyretic treatments due to an unchanged thermoregulatory setpoint (26–28). Central hyperthermia was proven to be independently related to death in PPH by Matsukawa et al., but was not identified as an independent predictive factor of 30-day outcomes after PBSH in another study (20, 22). Although central hyperthermia associated with PBSH is supposed to be injurious to patients, it remains unknown whether a positive pursuit of a normal body temperature contributes to a more favorable clinical prognosis in the absence of evidence. Therefore, future studies of patients suffering from PBSH-related central hyperthermia are absolutely essential to reveal its mechanism and preventive and treatment measures.
Additionally, patients with severe PBSH present with a high risk of neurological complications and in desperate need of measures to protect the airway, especially within the acute phase. A study revealed that early tracheostomy (≤ 7 days after admission) was significantly associated with a favorable 30-day functional outcome (prognostic benefits) and was also able to reduce the length of hospitalization and intensive care unit stay (financial benefits) (22). However, there are potential risks that should not be neglected when performing a tracheostomy, such as skin breakdown, tracheomalacia and so on (29).
In addition, other factors, such as tachycardia (>110 beat/min), absence of a pupillary light reflex, the necessity for mechanical ventilation, and pupillary abnormalities, systolic blood pressure <100 mmHg, intact motor function, and a history of diabetes mellitus, have also been identified to significantly affect death or functional outcomes after PBSH (9, 16, 28, 30).
In a retrospective study enrolling 225 patients with PBSH, Fan et al. found that elevated platelet-to-lymphocyte ratio, neutrophil-to-lymphocyte ratio, and admission blood glucose level were independently correlated with unfavorable 90-day functional outcomes of PBSH, with critical thresholds defined as 59.3, 6.65, and 7.81 mmol/L, respectively (8). Moreover, a combination of the aforementioned three factors showed a better predictive value than a single factor (8). In another study, plasma glucose with a threshold value ≥180 mmol/L (10 mmol/L) was identified to independently predict 30-day mortality in patients with PPH (9). Hyperglycemia reflects a stress-response level in the setting of the acute phase of PBSH, which could result in heightened susceptibility to complications connected with hospitalization and ultimately lead to unfavorable outcomes (31, 32).
MRI findings are less relevant to prognosis after PBSH and only certain case report-level evidences have examined the potential relationship between functional prognosis after PBSH and findings derived from diffusion tensor imaging (DTI) and diffusion tensor tractography (DTT) (33–35). Instead, CT scanning is routinely deemed the method of preference for assessing PBSH owing to its general accessibility and rapid availability. Moreover, some CT findings closely correlate with prognosis in PBSH.
1) Hemorrhage size. In view of the small size of the brainstem, the average hemorrhage size of PBSH is less than that of supratentorial hemorrhage but might be more fatal (36). Hemorrhage size is a reliable and significant independent prognostic factor for PBSH, of which the threshold values are found to fluctuate between 4–5 ml and 20–31.5 mm for hemorrhage volume and transverse diameter, respectively (6, 37). Hemorrhage size is another most important predictor besides the initial level of consciousness, which has been demonstrated as an independent predictor in 7 different studies in Table 1. Given the importance of hemorrhage size, inaccurate data might exert a certain adverse influence on the judgment of the prognosis. Therefore, it is probably pertinent to discuss studies on the measurement of hemorrhage volume. Computer-assisted volumetric analysis and 3D slices are typically viewed as the “gold standard” to measure hemorrhage volume (38, 39). However, the calculation process is time-consuming and tedious, hindering their clinical application. Among the formula methods, 1/2ABC is the most common and convenient way to calculate hemorrhage volume of ICH in clinical work. Nevertheless, as it may underestimate or overestimate the volume of irregularly shaped hemorrhage and small hemorrhage in the brainstem, some researchers began to question its accuracy (38, 40, 41). Through a review of 147 CT results of patients with infratentorial hemorrhage, Yang et al. found that 2/3SH was more accurate than 1/2ABC for the volume calculation of brainstem hemorrhage and irregular hemorrhage (42). As for formula 2/3SH, S represents the area of largest axial hemorrhagic slice and H represents the height of hematoma which is derived from the number of slices times the slice thickness. Overall, a more precise and simple method to measure brainstem hemorrhage size remains to be developed.
2) Hemorrhage classification, localization, extension and hydrocephalus. There is currently no unified classification for PBSH (5). PBSH can be divided into three subtypes of medullary hemorrhage, pontine hemorrhage, and midbrain hemorrhage in clinical practice (43). Among them, pontine hemorrhage is the most frequent type, and isolated medullary and midbrain hemorrhages have a lower incidence (44). The medullary type may lead to ataxic respiration and cause rapid death (45). In addition, based on the axial CT findings of the exact anatomical location and spread direction, various sorts of classifications have been established (4). All of the studies are consistent with the view that unilateral tegmental hemorrhage is related to a good outcome, while massive hemorrhage (located in bilateral basal and anterior segments) is closely associated with the most unfavorable outcomes (6, 10, 14, 16, 20, 21, 26–46). For patients with neuroradiological results that fall between the two, it is difficult to predict the survival outcome according to CT findings alone (4). Intraventricular extension is an important predictive factor in ICH but is not an independent determinant of early death for PBSH patients (6, 24, 47). Jang et al. ascribed this phenomenon to the active use of external ventricular drainage (EVD), which could be conducive to the reduction of mortality and short-term prognosis in ICH (10, 48). Moreover, hemorrhage vertically extending from the pontine to the midbrain and/or thalamus could predict adverse outcomes (20). According to a systematic review, 30.3% of patients developed hydrocephalus after PPH (6). However, only one study has identified it as an independent prognostic factor of mortality for PBSH (6, 10).
3) Hemorrhage expansion. In recent years, hematoma expansion has attracted wide attention in clinical practice and has been identified to independently predict mortality and functional prognosis in patients with ICH (49, 50). Hematoma shape-related signs, such as the CTA spot sign and some non-contrast computed tomography markers, have been demonstrated to be potential markers for screening out patients at high risk of hematoma expansion among ICH patients (51–53). Nevertheless, few studies have focused on hematoma expansion and relevant signs in PBSH. Even so, due to the small size but vital role of the brainstem, hematoma expansion at this site was presumed to wreak havoc on survival and prognosis. Therefore, it is an indicator to which we should attach importance in patients with PBSH. Hematoma expansion and CTA spot signs also exist in patients with PPH. In this retrospective analysis of forty-nine PPH cases, Andrea et al. found that the spot sign showed good accuracy for the prediction of in-hospital mortality (61%) and 30-day mortality (57%) but was not an independent predictor (1). In addition, the presence of spot signs was not significantly associated with hematoma expansion rates (1). However, the lack of statistical significance is ascribed to a deficient number of cases, and a clear association between spot signs and hematoma expansion rates remains uncertain.
Different from the hemorrhage size and localization, hematoma expansion has a characteristic of preventability to a certain extent. Virtual measures to restrict hematoma expansion seem beneficial to PBSH patients due to their function in reducing the ultimate hemorrhage size. Patients with PBSH are often in an urgent and high-risk state, and only a routine CT scan could be acquired. Moreover, because CTA is not available routinely in many emergency departments, the application of spot signs to predict early hematoma expansion is subject to certain restrictions (54). Under such circumstances, NCCT markers, such as the island sign (55), satellite sign (56), black hole sign (57), and blend sign (58), seem to have a clear advantage. However, as patients with PBSH are excluded from almost all relevant studies, the application value of these markers in PBSH remains unclear and needs further verification. Furthermore, the definite correlation on between hematoma expansion and PBSH and the exact mechanisms of hematoma expansion remain to be clarified. Future studies with large sample sizes are also needed to determine whether there are differences in the incidence of hematoma expansion between supratentorial hemorrhage and PBSH.
Although neuromonitoring is generally deemed a predictive tool for functional recovery in stroke patients, few articles have focused on the same topic in patients with PBSH (59, 60). In an analysis of 31 consecutive comatose patients with acute severe brainstem hemorrhage, Chen et al. found that a quantitative electroencephalography parameter [i.e., (delta + theta)/(alpha + beta) ratio, DTABR] could independently predict 90-day mortality, whereas no transcranial Doppler (TCD) variables showed prognostic value (7). However, that study only focused on mortality and did not attach importance to the correlation between neurophysiological parameters and functional recovery. An abnormal brainstem auditory evoked potentials (BAEPs) may predict hearing loss in PBSH as well as a poor prognosis (61). Furthermore, Seong et al. confirmed that using somatosensory evoked potentials (SEPs) and motor evoked potentials (MEPs) in combination was a reliable predictor for functional recovery in PBSH patients (62). In summary, the potential of neurophysiological parameters for predicting functional recovery still needs to be fully tapped in patients with PBSH, who often have a tendency toward severe disability.
A scoring system plays an important role in the risk stratification of patients with brainstem hemorrhage, which also contributes to a consensus on their management (3, 9, 36, 63). Therefore, we discuss the development and present status of scoring systems for brainstem hemorrhage in detail.
The ICH score and its modified version are reliable and convenient and have been extensively used to predict mortality and functional recovery in ICH (24, 64). Subsequently, Del Brutto et al. revealed that both the original and modified ICH scores proved accurate for predicting the risk of 30-day mortality in PPH (63). Nevertheless, there are still some concerns. First, in the cohort used for the development of the original ICH, less than one-tenth (15 of 152, 9.87%) of all subjects were diagnosed with brainstem hemorrhage (24). Second, in light of its content, the original and modified ICH scores lead to infratentorial hemorrhage being regarded as an independent predictor of a poor outcome. Third, the cut-off value of hemorrhage size and GCS score should be different in the scoring systems for ICH and PPH. Last, a comparative study conducted by Huang et al. revealed that the original ICH score lacked discrimination and ought to be revised specifically for PPH (36). Taken together, the original and modified ICH scores may not apply well to PPH.
To solve this problem, Meguro et al. proposed the first specific grading scale (entitled the PPH score) for predicting 30-day mortality of PPH and validated it in a retrospective review of a cohort of 101 consecutive patients with PPH (9). However, the study had several flaws. The researchers did not carry out external validation and did not take into account early do not resuscitate orders (DNRs). As demonstrated by Zahuranec et al., an illusion of model accuracy may be generated when DNRs are ignored (65). Consequently, Huang et al. established and validated a new PPH score for predicting short-term outcome (30-day mortality) and long-term outcome (90-day functional prognosis) in PPH patients and demonstrated that it had a higher discrimination (area under the curve for 30-day mortality was 0.902 and that for 90-day good outcome was 0.927) and calibration than the original ICH score and the PPH score in their study cohort (3). This is the largest study with the best evidence for scoring systems to date, including a total of 269 cases (171 cases as the training set for scale development and the other 98 cases as the prediction set for external validation) (3). The detailed grading standards of these two scoring systems are shown in Table 2. Significantly, variables in the new PPH score are precisely the two most influential predictors we proposed above.
In terms of registered clinical studies, an ongoing trial based on the application of radiomics methods, entitled “a new prognostic scoring system for patients with primary pontine hemorrhage: medical records-based study” (URL: http://www.chictr.org.cn. Unique identifier: ChiCTR2100042705) aims to construct a new grading scale for PPH to determine the prognosis and guide therapeutic decisions.
The next step for research in scoring systems will focus on the question whether the existing system is applicable to determine the surgical indications, thereby stratifying patients and guiding treatment decision-making.
Chinese researchers developed and issued the first guideline for brainstem hemorrhage in 2020 (5). However, there are no definite specifications focusing exclusively on the diagnosis and treatment of PBSH in widely recognized guidelines issued by the American Heart Association/American Stroke Association (AHA/ASA) and European Stroke Organization (66, 67). The AHA/ASA guidelines are explicitly against surgical interventions for brainstem hematomas (66). Moreover, conservative treatment of PBSH is widely accepted, whereas surgical management remains questionable because the complex anatomical structures and critical functions of the brainstem have potential risks during surgery (4, 10–13). However, conservative treatment may do little to prevent fatal outcomes in many cases and with new surgical and neuroimaging technological advances, surgical procedures are expected to be more optimistic options for the treatment of PBSH. Therefore, it is meaningful to discuss the controversial but promising surgical management of PBSH in further detail below based on the available evidence.
Identifying optimal candidates for surgery is an essential question. Surgical prognostic factors after PBSH conduce to the identification of ideal candidates for PBSH. Through analyses of prognostic factors, Tao et al. concluded that patients with a smaller hematoma (>5 ml and <10 ml), a greater GCS score (>6 and <8), age <65 years, unilateral tegmental hemorrhage, and without extrapontine extension might benefit from surgical treatment (2). Furthermore, based on their experience with five severe cases of surgical treatment, Shrestha et al. proposed their indication for surgery: (1) hemorrhage volume >5 ml (concentrated relatively), (2) GCS score <8 with progressive neural dysfunction, (3) unstable basic vital signs, especially for patients who require mechanical ventilation, (4) location of the hematoma <1 cm from the brainstem surface, and (5) time of hemorrhage <24 h (68).
The indicator by Tao et al. is the equivalent of 2 points in the new PPH score. Four cases in the study by Shrestha et al. scored 2 or 3 points. All the 4 cases survived during their hospital stay and one of them even could go about all daily tasks and walk with minimal help after surgery. According to the findings of Huang et al., a score of 4 points in the new PPH score is the contraindication for both surgery and medical treatments (3). Also, Huang et al. suggested sparing medical resources for patients with a score of 4 points (3). Notably, prompt evacuation of hematoma remains contraindicated in the absence of all brainstem reflexes (68). To sum up, we made the assumption that PBSH patients with a score of 2–3 points in the new PPH score might benefit from surgical management. However, because surgery is not recommended for PBSH based on the current evidence, the assumption requires further verification and should be treated with caution.
As a result of the entirely different anatomical features, blood supply system and the possible distinct cell reactions to hemorrhages (69, 70), the findings and experience of the timing of surgery for supratentorial ICH could not be applied to PBSH directly. Pathological changes observed in animal experiments show that brain edema and arterial necrosis generally appear 6 h after PBSH onset (71). Therefore, in theory, surgery carried out in the super early phase (within a 6 h time window) seems to be the best choice. According to a study by Lan et al., patients with PBSH in the early operative group (≤ 6 h) had a better neurologic recovery than those in the late operative group (>6 h), and this difference was statistically significant (P = 0.02) (72). However, based on their experience with 52 cases of surgical treatment, Chen et al. proposed that 12–48 h after ictus may be the optimal surgical timing for PBSH (73).
Overall, as with supratentorial ICH, the exact indicators and the optimal surgical timing for PBSH remain controversial and undetermined (74).
The ideal surgical approaches often depend on the location and size of the hematoma. The two-point rule by Brown et al. (namely, one point at the center of the hematoma and the other at the point on the brainstem surface to which the hematoma is closest) is frequently used as a means to enter a brainstem hematoma while minimizing disruption of the normal structure (75). However, with the widening knowledge of the anatomy of the brainstem, safe entry zones are considered to have an advantage over the simple two-point rule (76). Various safe entry zones and surgical approaches for the brainstem have been designed to reduce, as much as possible, damage to any eloquent or essential structures. Yang et al. identified 21 different safe entry zones according to the existing literature and endowed each of them with an evidence level (Table 3) (76). Endoscopic endonasal transclival approach (EETA) is a useful approach for endoscopic hematoma removal to provide adequate exposure of the ventral brainstem structure (77). The routine surgical approaches for microsurgery in different brainstem divisions are shown in Table 3 (68).
Patients with PBSH should be given proper type of surgical options based on the concrete states. Craniotomy is a classic surgical procedure used for PBSH, with advantage of definite hemostasis effect; stereotactic hematoma puncture and drainage is particularly useful for patients who are reluctant to accept craniectomy or are old and feeble; endoscopic hematoma removal could provide adequate exposure of the ventral brainstem lesion when used in a special approach; EVD could be used in emergency medical treatment especially in primary hospitals. We discuss these four main surgical options below and summarize their advantages and disadvantages in Table 4. Major milestones for research on the surgical management of PBSH are presented in Figure 1.
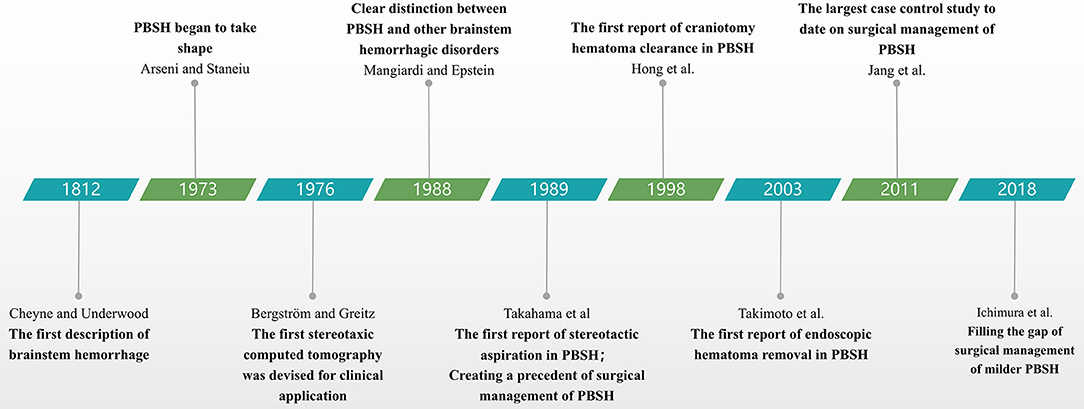
Figure 1. Timeline diagram depicting major milestones for surgical management of PBSH. PBSH, primary brainstem hemorrhage.
1) Craniotomy
Since suboccipital craniectomy was first used for brainstem hematoma clearance by Hong et al. (85), craniotomy has become one of the most important surgical treatments for PBSH. Lan et al. conducted a case-control study including 286 patients with severe PBSH (GCS ≤ 8), and 46 patients underwent craniotomy under microscope for hematoma clearance (72). Compared with the conservative group, the surgical group had a lower mortality rate (30.4% vs. 70.45%) and a higher good recovery rate (13.1% vs. 5.9%) at the expense of a higher rate of a vegetative state (4.3% vs. 2.5%), severe disability (32.6% vs. 13.3%), and moderate disability (19.6% vs. 7.9%) (72). Ichimura et al. reported the surgical results of five patients with relatively mild PBSH (patients without low initial consciousness and bilateral pupil dilation) (86). All of them had ameliorations in consciousness, motor performance, and mRS grades after surgery (86). Moreover, the authors suggested that the half-sitting position could greatly lower the risk of injury to normal tissue in surgical treatment of brainstem lesions (86). With growing knowledge of safe entry zones and continuing advances in microsurgical techniques (87), satisfactory results could be obtained in a minimally invasive way. Empirical evidence from 52 patients with PBSH indicated that minimally invasive microsurgery for hematoma clearance was very rapid, effective, and safe and was especially suitable for patients with hemorrhage volume <10 ml (73).
2) Stereotactic hematoma puncture and drainage
Stereotactic hematoma puncture and drainage was the earliest surgery performed to treat PBSH by Takahama et al. (12). This surgical procedure is easy to perform and has many advantages, such as minimally invasive characteristics and a short surgery time. With the use of stereotactic equipment, anticoagulant urokinase, and rt-PA, it is endowed with high precision and a high hematoma clearance rate. A study by Shitamichi et al. of 45 patients with PPH showed that CT-guided stereotaxic aspiration could improve the prognosis, especially for severe cases (88). In another study enrolling 37 PPH patients, Hara et al. found that 72% (13 of 18) of subjects undergoing CT-guided stereotaxic aspiration showed a dramatic improvement, whereas only 42% (8 of 19) of subjects treated conservatively did (11).
The application of three-dimensional (3D) printing technology is achieving great success in various medical fields, including surgical intraoperative navigation (89, 90). Recently, Wang et al. successfully tested the application of a 3D-printed navigation template for puncture drainage in patients with severe brainstem hemorrhage (78). The actual puncture end was located precisely in the hematoma cavity in all cases, and the postoperative outcomes were satisfactory in all 7 included patients (78). 3D print-assisted hematoma puncture and drainage provides a highly promising new modality for the surgical treatment of PBSH and achieves precision medicine in a completely personalized manner.
With the application of various advanced stereotactic techniques, such as the ROSA (Robotized Stereotactic Assistant) device, stereoscopic virtual reality system, and augmented reality interactive neuronavigation, the surgical procedure would be increasingly safe and precise (91–93).
3) Endoscopic hematoma removal
Takimoto et al. were the first to evacuate a pontine hemorrhage with the aid of neuroendoscopy, which provided a new method for the surgical treatment of PBSH (80). However, ventrally located brainstem lesions are still surgically challenging due to their inaccessibility through traditional transcranial approaches. With several advancements, the EETA of neuroendoscopy has gradually become a feasible alternative to treat well-selected ventral brainstem lesions with the advantages of direct visualization and less injury (77). Both Essayed et al. and Weiss et al. proposed the potential feasibility and surgical limitations of EETA to remove ventral brainstem lesions based on cadaveric anatomical studies, and the combination of fiber dissection and 7T-MRI neuronavigation may help us to better understand the clear internal anatomical structure of the brainstem to enter the site of the lesion in a safer manner (94, 95). Topczewski et al. conducted a single-center study of 5 patients undergoing endoscopic endonasal surgery and concluded that EETA could provide enough access to the ventral brainstem (83). Adept operative techniques, the assistance of neuronavigation and intraoperative neurophysiological monitoring are critical for achieving better surgical results. Liu et al. reported a successful case of EETA used in the surgical treatment of a man with severe PBSH (77). An immediate improvement was found in his spontaneous respiration, and his GCS score improved significantly from 3 to 11 1 month after surgery (77). However, there are no other reports of EETA for PBSH. Therefore, EETA used in PBSH remains a surgical challenge that requires further verification of feasibility and surgical limitations based on a large sample.
4) EVD
Intraventricular hemorrhage occurs as a rupture of a hematoma into the ventricular system in approximately 39.5% of PBSH patients (6), and it is very frequently involved in elevated intracranial pressure and acute obstructive hydrocephalus due to its physical effect and mass effect (96, 97). EVD is conducive to the clearance of intraventricular blood and the normalization of intracranial pressure (82). Currently, EVD has been used extensively to rescue acute obstructive hydrocephalus and prevent the potential risk of brain herniation induced by high intracranial pressure in the setting of PBSH because no special equipment is required (82, 98). Intraventricular thrombolytics are widely used to dissolve the casting of a hematoma, whereas the recent CLEAR III trial failed to prove a significant improvement in functional outcome with irrigation with alteplase in adult intraventricular hemorrhage (5, 99).
Due to the low incidence of PBSH (accounting for 6–10% of spontaneous ICH cases), it is difficult to collect large sample size surgical data within a short time (4). Moreover, in consideration of the high risk, various complications, high treatment costs and uncertain efficacy, the current treatment of PBSH is still mainly conservative. As a consequence, almost all of these previous studies were performed retrospectively with a small sample size, and no high-level evidence is available to support surgical management of PBSH to date.
One ongoing clinical trial, entitled Safety and Efficacy of Surgical Treatment in Severe Primary Pontine Hemorrhage Evacuation (STIPE) (URL: https://clinicaltrials.gov. Unique identifier: NCT04647162), is designed to fill this gap. The STIPE trial is sponsored by West China Hospital and is currently recruiting with an estimated enrollment of 345 participants. Furthermore, it is a multicentric, prospective, randomized, controlled, open-label, clinical study with the objective of evaluating the safety and efficacy of surgical treatment in patients with primary severe PPH (defined as GCS <8 and hemorrhage volume ≥5 ml, the equivalent of 2–4 points in the new PPH score). Patients in the experimental group will receive surgical intervention, such as craniotomy, stereotactic hematoma puncture and drainage or endoscopic hematoma removal, and the control group will only receive conservative medical treatment. The primary outcome measures include the mortality rate and intracranial infection rate at 30 days as well as the rebleeding rate within 3 days after the operation. The secondary outcome measures included the mRS and EQ-5D-5 L questionnaire results at 90, 180, and 365 days after surgery. The study started on January 1, 2021, and the estimated completion date is May 2025.
In conclusion, PBSH has a low incidence but high mortality compared to other forms of ICH in which various prognostic factors are involved. Initial level of consciousness and hemorrhage size are the two most important and consistent predictors and present the two variables in the new PPH score. The new PPH score represents the latest developments in scoring systems and patients with a score of 2–3 points might benefit from surgical management. Therefore, the future direction of scoring systems should verify the availability of the new PPHl score for determining the surgical indications. However, conservative treatment still plays a major role in the management of PBSH and surgery is not recommended for PBSH based on the current evidence. PBSH is always excluded from previous surgical intervention trials for spontaneous ICH, such as the MISTIE III trial, the ICES trial, and the STICH I-II trials (100–103). Besides, in consideration of the complex structures and critical functions of the brainstem, more attention should be paid to potential risks during surgery. Because the number of cases is insufficient, there remains a lack of high-level evidence to prove the efficacy and safety of surgical intervention. The ongoing STIPE trial may fill this gap and provide additional evidence for the surgical treatment of PBSH.
From another point, the plight of clinical studies highlights that animal studies of brainstem hemorrhage are also essential to understand pathophysiological mechanism of PBSH and provide some reference to determining surgical timing, exploring surgical approaches and evaluating surgical efficacy. Though rat models of PPH by autologous blood or collagenase infusion have been established successfully (104–106), there is insufficient evidence to determine whether the pathophysiological difference between supratentorial ICH and PBSH exists. Furthermore, scant attention has been given to PBSH based on the perspective of translational stroke research (106). More efforts should be made in the future to explore pathophysiological features of PBSH and for better translational research.
In summary, we advocate the establishment of a worldwide registry and expert cooperative group to update the epidemiological data (incidence, mortality, etc.), re-evaluate prognostic factors, and re-investigate the surgical indication and timing. Prevention of PBSH also cannot be ignored. Recognizing and controlling risk factors actively are recommended to prevent PBSH.
DC, YiT, HN, and PZ conceived the review. DC wrote the paper, with major contributions from WW, QD, GW, MX, YuT, and WL. CP and ZT take responsibility for the manuscript as a whole. All authors contributed to the article and approved the submitted version.
This work was supported by grant 81901219 and 82071330 from National Natural Science Foundation of China.
WL is employed by Beijing WanTeFu Medical Apparatus Co., Ltd.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Our deepest gratitude goes to the editors and reviewers for their careful work and thoughtful suggestions that have helped improve this manuscript substantially.
1. Morotti A, Jessel MJ, Brouwers HB, Falcone GJ, Schwab K, Ayres AM, et al. CT angiography spot sign, hematoma expansion, and outcome in primary pontine intracerebral hemorrhage. Neurocrit Care. (2016) 25:79–85. doi: 10.1007/s12028-016-0241-2
2. Tao C, Li H, Wang J, You C. Predictors of surgical results in patients with primary pontine hemorrhage. Turk Neurosurg. (2016) 26:77–83. doi: 10.5137/1019-5149.JTN.12634-14.1
3. Huang K, Ji Z, Sun L, Gao X, Lin S, Liu T, et al. Development and validation of a grading scale for primary pontine hemorrhage. Stroke. (2017) 48:63–9. doi: 10.1161/STROKEAHA.116.015326
4. Wang SS, Yang Y, Velz J, Keller E, Luft AR, Regli L, et al. Management of brainstem haemorrhages. Swiss Med Wkly. (2019) 149:w20062. doi: 10.4414/smw.2019.20062
5. Chen L, Chen T, Mao GS, Chen BD, Li MC, Zhang HB, et al. Clinical neurorestorative therapeutic guideline for brainstem hemorrhage (2020 China version). J Neurorestoratol. (2020) 8:232–40. doi: 10.26599/JNR.2020.9040024
6. Behrouz R. Prognostic factors in pontine haemorrhage: a systematic review. Eur Stroke J. (2018) 3:101–9. doi: 10.1177/2396987317752729
7. Chen Y, Wang L, Zhang J, Wang S, Qi Y, Cao J, et al. Monitoring of patients with brainstem hemorrhage: a simultaneous study of quantitative electroencephalography and transcranial Doppler. Clin Neurophysiol. (2021) 132:946–52. doi: 10.1016/j.clinph.2020.12.026
8. Fan Z, Hao L, Chuanyuan T, Jun Z, Xin H, Sen L, et al. Neutrophil and platelet to lymphocyte ratios in associating with blood glucose admission predict the functional outcomes of patients with primary brainstem hemorrhage. World Neurosurg. (2018) 116:e100-7. doi: 10.1016/j.wneu.2018.04.089
9. Meguro T, Kuwahara K, Tomita Y, Okuma Y, Tanabe T, Muraoka K, et al. Primary pontine hemorrhage in the acute stage: clinical features and a proposed new simple scoring system. J Stroke Cerebrovasc Dis. (2015) 24:860–5. doi: 10.1016/j.jstrokecerebrovasdis.2014.12.006
10. Jang JH, Song YG, Kim YZ. Predictors of 30-day mortality and 90-day functional recovery after primary pontine hemorrhage. J Korean Med Sci. (2011) 26:100–7. doi: 10.3346/jkms.2011.26.1.100
11. Hara T, Nagata K, Kawamoto S, Sashida J, Abe T, Wada A, et al. [Functional outcome of primary pontine hemorrhage: conservative treatment or stereotaxic surgery]. No Shinkei Geka. (2001) 29:823–9. doi: 10.2176/nmc.41.466
12. Takahama H, Morii K, Sato M, Sekiguchi K, Sato S. [Stereotactic aspiration in hypertensive pontine hemorrhage: comparative study with conservative therapy]. No Shinkei Geka. (1989) 17:733–9.
13. Manno EM, Atkinson JL, Fulgham JR, Wijdicks EF. Emerging medical and surgical management strategies in the evaluation and treatment of intracerebral hemorrhage. Mayo Clin Proc. (2005) 80:420–33. doi: 10.4065/80.3.420
14. Dziewas R, Kremer M, Ludemann P, Nabavi DG, Drager B, Ringelstein B. The prognostic impact of clinical and CT parameters in patients with pontine hemorrhage. Cerebrovasc Dis. (2003) 16:224–9. doi: 10.1159/000071120
15. Balci K, Asil T, Kerimoglu M, Celik Y, Utku U. Clinical and neuroradiological predictors of mortality in patients with primary pontine hemorrhage. Clin Neurol Neurosurg. (2005) 108:36–9. doi: 10.1016/j.clineuro.2005.02.007
16. Wessels T, Moller-Hartmann W, Noth J, Klotzsch C. CT findings and clinical features as markers for patient outcome in primary pontine hemorrhage. AJNR Am J Neuroradiol. (2004) 25:257–60. doi: 10.1080/02841850410000782
17. Dinsdale HB. Spontaneous hemorrhage in the posterior fossa. A study of primary cerebellar and pontine hemorrhages with observations on their pathogenesis. Arch Neurol. (1964) 10:200–17. doi: 10.1001/archneur.1964.00460140086011
18. van Asch CJ, Luitse MJ, Rinkel GJ, van der Tweel I, Algra A, Klijn CJ. Incidence, case fatality, and functional outcome of intracerebral haemorrhage over time, according to age, sex, and ethnic origin: a systematic review and meta-analysis. Lancet Neurol. (2010) 9:167–76. doi: 10.1016/S1474-4422(09)70340-0
19. Jung DS, Jeon BC, Park YS, Oh HS, Kim NK. The predictors of survival and functional outcome in patients with pontine hemorrhage. J Korean Neurosurg Soc. (2007) 41:82–7. doi: 10.1111/j.1365-2788.2006.00946.x
20. Matsukawa H, Shinoda M, Fujii M, Takahashi O, Murakata A. Risk factors for mortality in patients with non-traumatic pontine hemorrhage. Acta Neurol Scand. (2015) 131:240–5. doi: 10.1111/ane.12312
21. Ye Z, Huang X, Han Z, Shao B, Cheng J, Wang Z, et al. Three-year prognosis of first-ever primary pontine hemorrhage in a hospital-based registry. J Clin Neurosci. (2015) 22:1133–8. doi: 10.1016/j.jocn.2014.12.024
22. Ding WL, Xiang YS, Liao JC, Wang SY, Wang XY. Early tracheostomy is associated with better prognosis in patients with brainstem hemorrhage. J Integr Neurosci. (2020) 19:437–42. doi: 10.31083/j.jin.2020.03.25
23. An SJ, Kim TJ, Yoon BW. Epidemiology, risk factors, and clinical features of intracerebral hemorrhage: an update. J Stroke. (2017) 19:3–10. doi: 10.5853/jos.2016.00864
24. Hemphill JC III, Bonovich DC, Besmertis L, Manley GT, Johnston SC. The ICH score: a simple, reliable grading scale for intracerebral hemorrhage. Stroke. (2001) 32:891–7. doi: 10.1161/01.STR.32.4.891
25. Zia E, Engstrom G, Svensson PJ, Norrving B, Pessah-Rasmussen H. Three-year survival and stroke recurrence rates in patients with primary intracerebral hemorrhage. Stroke. (2009) 40:3567–73. doi: 10.1161/STROKEAHA.109.556324
26. Morales-Ortiz A, Jimenez-Pascual M, Perez-Vicente JA, Monge-Arguiles A, Bautista-Prados J. [Fever of central origin during stroke]. Rev Neurol. (2001) 32:1111–4.
27. Lee HC, Kim JM, Lim JK, Jo YS, Kim SK. Central hyperthermia treated with baclofen for patient with pontine hemorrhage. Ann Rehabil Med. (2014) 38:269–72. doi: 10.5535/arm.2014.38.2.269
28. Wijdicks EF, St Louis E. Clinical profiles predictive of outcome in pontine hemorrhage. Neurology. (1997) 49:1342–6. doi: 10.1212/WNL.49.5.1342
29. Morris LL, Whitmer A, McIntosh E. Tracheostomy care and complications in the intensive care unit. Crit Care Nurse. (2013) 33:18–30. doi: 10.4037/ccn2013518
30. Murata Y, Yamaguchi S, Kajikawa H, Yamamura K, Sumioka S, Nakamura S. Relationship between the clinical manifestations, computed tomographic findings and the outcome in 80 patients with primary pontine hemorrhage. J Neurol Sci. (1999) 167:107–11. doi: 10.1016/S0022-510X(99)00150-1
31. Kruyt ND, Biessels GJ, DeVries JH, Luitse MJ, Vermeulen M, Rinkel GJ, et al. Hyperglycemia in aneurysmal subarachnoid hemorrhage: a potentially modifiable risk factor for poor outcome. J Cereb Blood Flow Metab. (2010) 30:1577–87. doi: 10.1038/jcbfm.2010.102
32. Capes SE, Hunt D, Malmberg K, Pathak P, Gerstein HC. Stress hyperglycemia and prognosis of stroke in nondiabetic and diabetic patients: a systematic overview. Stroke. (2001) 32:2426–32. doi: 10.1161/hs1001.096194
33. Jang SH, Chang MC. Recovery of an injured corticoreticulospinal tract in a patient with pontine hemorrhage. Int J Stroke. (2016) 11:NP18–9. doi: 10.1177/1747493015609933
34. Yeo SS, Jang SH, Park GY, Oh S. Effects of injuries to descending motor pathways on restoration of gait in patients with pontine hemorrhage. J Stroke Cerebrovasc Dis. (2020) 29:104857. doi: 10.1016/j.jstrokecerebrovasdis.2020.104857
35. Jang SH, Kwon HG. Injury of the ipsilateral vestibulothalamic tract in a patient with pontine hemorrhage. Acta Neurol Belg. (2020) 120:951–4. doi: 10.1007/s13760-018-01073-4
36. Huang KB, Ji Z, Wu YM, Wang SN, Lin ZZ, Pan SY. The prediction of 30-day mortality in patients with primary pontine hemorrhage: a scoring system comparison. Eur J Neurol. (2012) 19:1245–50. doi: 10.1111/j.1468-1331.2012.03724.x
38. Divani AA, Majidi S, Luo X, Souslian FG, Zhang J, Abosch A, et al. The ABCs of accurate volumetric measurement of cerebral hematoma. Stroke. (2011) 42:1569–74. doi: 10.1161/STROKEAHA.110.607861
39. Strik HM, Borchert H, Fels C, Knauth M, Rienhoff O, Bahr M, et al. Three-dimensional reconstruction and volumetry of intracranial haemorrhage and its mass effect. Neuroradiology. (2005) 47:417–24. doi: 10.1007/s00234-005-1373-9
40. Huttner HB, Steiner T, Hartmann M, Kohrmann M, Juettler E, Mueller S, et al. Comparison of ABC/2 estimation technique to computer-assisted planimetric analysis in warfarin-related intracerebral parenchymal hemorrhage. Stroke. (2006) 37:404–8. doi: 10.1161/01.STR.0000198806.67472.5c
41. Kothari RU, Brott T, Broderick JP, Barsan WG, Sauerbeck LR, Zuccarello M, et al. The ABCs of measuring intracerebral hemorrhage volumes. Stroke. (1996) 27:1304–5. doi: 10.1161/01.STR.27.8.1304
42. Yang W, Feng Y, Zhang Y, Yan J, Fu Y, Chen S. Volume quantification of acute infratentorial hemorrhage with computed tomography: validation of the formula 1/2ABC and 2/3SH. PLoS ONE. (2013) 8:e62286. doi: 10.1371/journal.pone.0062286
43. AlMohammedi RM, AlMutairi H, AlHoussien RO, AlOtayan MT, AlMutairi AK, Bafail WO, et al. Brainstem hemorrhage is uncommon and is associated with high morbidity, mortality, and prolonged hospitalization. Neurosciences. (2020) 25:91–6. doi: 10.17712/nsj.2020.2.20190102
44. Kumral E, Bayam FE, Ozerol R, Orman M. Predictors of outcome in patients with medullary hemorrhage. J Stroke Cerebrovasc Dis. (2020) 29:105337. doi: 10.1016/j.jstrokecerebrovasdis.2020.105337
45. Kwon HM, Park JM, Lee JY, Yoon BW. Primary medullary hemorrhage associated with hypertension. J Clin Neurol. (2005) 1:177–9. doi: 10.3988/jcn.2005.1.2.177
46. Russell B, Rengachary SS, McGregor D. Primary pontine hematoma presenting as a cerebellopontine angle mass. Neurosurgery. (1986) 19:129–33. doi: 10.1227/00006123-198607000-00023
47. Hemphill JC III, Farrant M, Neill TA Jr. Prospective validation of the ICH Score for 12-month functional outcome. Neurology. (2009) 73:1088–94. doi: 10.1212/WNL.0b013e3181b8b332
48. Herrick DB, Ullman N, Nekoovaght-Tak S, Hanley DF, Awad I, LeDroux S, et al. Determinants of external ventricular drain placement and associated outcomes in patients with spontaneous intraventricular hemorrhage. Neurocrit Care. (2014) 21:426–34. doi: 10.1007/s12028-014-9959-x
49. Li Z, You M, Long C, Bi R, Xu H, He Q, et al. Hematoma expansion in intracerebral hemorrhage: an update on prediction and treatment. Front Neurol. (2020) 11:702. doi: 10.3389/fneur.2020.00702
50. Davis SM, Broderick J, Hennerici M, Brun NC, Diringer MN, Mayer SA, et al. Hematoma growth is a determinant of mortality and poor outcome after intracerebral hemorrhage. Neurology. (2006) 66:1175–81. doi: 10.1212/01.wnl.0000208408.98482.99
51. Morotti A, Boulouis G, Dowlatshahi D, Li Q, Barras CD, Delcourt C, et al. Standards for detecting, interpreting, and reporting noncontrast computed tomographic markers of intracerebral hemorrhage expansion. Ann Neurol. (2019) 86:480–92. doi: 10.1002/ana.25563
52. Delgado Almandoz JE, Yoo AJ, Stone MJ, Schaefer PW, Goldstein JN, Rosand J, et al. Systematic characterization of the computed tomography angiography spot sign in primary intracerebral hemorrhage identifies patients at highest risk for hematoma expansion: the spot sign score. Stroke. (2009) 40:2994–3000. doi: 10.1161/STROKEAHA.109.554667
53. Lv XN, Deng L, Yang WS, Wei X, Li Q. Computed tomography imaging predictors of intracerebral hemorrhage expansion. Curr Neurol Neurosci Rep. (2021) 21:22. doi: 10.1007/s11910-021-01108-z
54. Caplan LR. Recognizing and preventing intracerebral hematoma expansion. JAMA Neurol. (2016) 73:914–5. doi: 10.1001/jamaneurol.2016.1899
55. Li Q, Liu QJ, Yang WS, Wang XC, Zhao LB, Xiong X, et al. Island sign: an imaging predictor for early hematoma expansion and poor outcome in patients with intracerebral hemorrhage. Stroke. (2017) 48:3019–25. doi: 10.1161/STROKEAHA.117.017985
56. Shimoda Y, Ohtomo S, Arai H, Okada K, Tominaga T. Satellite sign: a poor outcome predictor in intracerebral hemorrhage. Cerebrovasc Dis. (2017) 44:105–12. doi: 10.1159/000477179
57. Li Q, Zhang G, Xiong X, Wang XC, Yang WS, Li KW, et al. Black hole sign: novel imaging marker that predicts hematoma growth in patients with intracerebral hemorrhage. Stroke. (2016) 47:1777–81. doi: 10.1161/STROKEAHA.116.013186
58. Li Q, Zhang G, Huang YJ, Dong MX, Lv FJ, Wei X, et al. Blend sign on computed tomography: novel and reliable predictor for early hematoma growth in patients with intracerebral hemorrhage. Stroke. (2015) 46:2119–23. doi: 10.1161/STROKEAHA.115.009185
59. Lee SY, Lim JY, Kang EK, Han MK, Bae HJ, Paik NJ. Prediction of good functional recovery after stroke based on combined motor and somatosensory evoked potential findings. J Rehabil Med. (2010) 42:16–20. doi: 10.2340/16501977-0475
60. Guerit JM. Neurophysiological testing in neurocritical care. Curr Opin Crit Care. (2010) 16:98–104. doi: 10.1097/MCC.0b013e328337541a
61. Kim SK, Kim AR, Kim JY, Kim DY. A long-term follow-up of pontine hemorrhage with hearing loss. Ann Rehabil Med. (2015) 39:634–9. doi: 10.5535/arm.2015.39.4.634
62. Seong JW, Kim MH, Shin HK, Lee HD, Park JB, Yang DS. Usefulness of the combined motor evoked and somatosensory evoked potentials for the predictive index of functional recovery after primary pontine hemorrhage. Ann Rehabil Med. (2014) 38:13–8. doi: 10.5535/arm.2014.38.1.13
63. Del Brutto OH, Campos X. Validation of intracerebral hemorrhage scores for patients with pontine hemorrhage. Neurology. (2004) 62:515–6. doi: 10.1212/WNL.62.3.515
64. Cheung RT, Zou LY. Use of the original, modified, or new intracerebral hemorrhage score to predict mortality and morbidity after intracerebral hemorrhage. Stroke. (2003) 34:1717–22. doi: 10.1161/01.STR.0000078657.22835.B9
65. Zahuranec DB, Morgenstern LB, Sanchez BN, Resnicow K, White DB, Hemphill JC III. Do-not-resuscitate orders and predictive models after intracerebral hemorrhage. Neurology. (2010) 75:626–33. doi: 10.1212/WNL.0b013e3181ed9cc9
66. Hemphill JC 3rd, Greenberg SM, Anderson CS, Becker K, Bendok BR, Cushman M, et al. Guidelines for the management of spontaneous intracerebral hemorrhage: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. (2015) 46:2032–60. doi: 10.1161/STR.0000000000000069
67. Steiner T, Al-Shahi Salman R, Beer R, Christensen H, Cordonnier C, Csiba L, et al. European Stroke Organisation (ESO) guidelines for the management of spontaneous intracerebral hemorrhage. Int J Stroke. (2014) 9:840–55. doi: 10.1111/ijs.12309
68. Shrestha BK, Ma L, Lan ZG, Li H, You C. Surgical management of spontaneous hypertensive brainstem hemorrhage. Interdiscip Neurosurg. (2015) 2:145–8. doi: 10.1016/j.inat.2015.06.005
69. Perko D, Pretnar-Oblak J, Sabovic M, Zvan B, Zaletel M. Differences between cerebrovascular reactivity to L-arginine in the anterior and posterior cerebral circulation. Cerebrovasc Dis. (2011) 31:358–64. doi: 10.1159/000322562
70. Kim JS, Nah HW, Park SM, Kim SK, Cho KH, Lee J, et al. Risk factors and stroke mechanisms in atherosclerotic stroke: intracranial compared with extracranial and anterior compared with posterior circulation disease. Stroke. (2012) 43:3313–8. doi: 10.1161/STROKEAHA.112.658500
71. Matsuoka K, Sakaki S, Okamoto E. Experimental study on hemorrhage in the brain stem induced by an intracranial space occupyting lesion. Folia Psychiatr Neurol Jpn. (1968) 22:245–58. doi: 10.1111/j.1440-1819.1968.tb01423.x
72. Lan Z, Richard SA, Li H, Chen M, Chao Y. Spontaneous hypertensive brainstem hemorrhage: does surgery benefit the severe cases? Interdiscip Neurosur Adv Tech Case Manage. (2018) 15:66–70. doi: 10.1016/j.inat.2018.10.015
73. Chen LH, Li FJ, Zhang HT, Chen WJ, Sun K, Xu RX. The microsurgical treatment for primary hypertensive brainstem hemorrhage: experience with 52 patients. Asian J Surg. (2021) 44:123–30. doi: 10.1016/j.asjsur.2020.04.016
74. de Oliveira Manoel AL. Surgery for spontaneous intracerebral hemorrhage. Crit Care. (2020) 24:45. doi: 10.1186/s13054-020-2749-2
75. Brown AP, Thompson BG, Spetzler RF. The two-point method: evaluating brain stem lesions. BNI Q. (1996). 12:20–4.
76. Yang Y, van Niftrik B, Ma X, Velz J, Wang S, Regli L, et al. Analysis of safe entry zones into the brainstem. Neurosurg Rev. (2019) 42:721–9. doi: 10.1007/s10143-019-01081-9
77. Liu B, Zheng T, Mao Y, Bian K, He S, Lv W. Endoscopic endonasal transclival approach to spontaneous hypertensive brainstem hemorrhage. J Craniofac Surg. (2020) 31:e503–6. doi: 10.1097/SCS.0000000000006599
78. Wang Q, Guo W, Liu Y, Shao W, Li M, Li Z, et al. Application of a 3D-printed navigation mold in puncture drainage for brainstem hemorrhage. J Surg Res. (2020) 245:99–106. doi: 10.1016/j.jss.2019.07.026
79. Ganguli A, Pagan-Diaz GJ, Grant L, Cvetkovic C, Bramlet M, Vozenilek J, et al. 3D printing for preoperative planning and surgical training: a review. Biomed Microdevices. (2018) 20:65. doi: 10.1007/s10544-018-0301-9
80. Takimoto H, Iwaisako K, Kubo S, Yamanaka K, Karasawa J, Yoshimine T. Transaqueductal aspiration of pontine hemorrhage with the aid of a neuroendoscope. Technical note. J Neurosurg. (2003) 98:917–9. doi: 10.3171/jns.2003.98.4.0917
81. Nagata Y, Watanabe T, Nagatani T, Takeuchi K, Chu J, Wakabayashi T. The multiscope technique for microvascular decompression. World Neurosurg. (2017) 103:310–4. doi: 10.1016/j.wneu.2017.04.059
82. Dey M, Jaffe J, Stadnik A, Awad IA. External ventricular drainage for intraventricular hemorrhage. Curr Neurol Neurosci Rep. (2012) 12:24–33. doi: 10.1007/s11910-011-0231-x
83. Topczewski TE, Somma AD, Culebras D, Reyes L, Torales J, Tercero A, et al. Endoscopic endonasal surgery to treat intrinsic brainstem lesions: correlation between anatomy and surgery. Rhinology. (2021) 59:191–204. doi: 10.4193/Rhin20.064
84. Panigrahi M, Gupta B, Reddy R. Neuroendoscopy - is it safe? Asian J Neurosurg. (2017) 12:17–21. doi: 10.4103/1793-5482.145567
85. Hong JT, Choi SJ, Kye DK, Park CK, Lee SW, Kang JK. Surgical Outcome of Hypertensive Pontine Hemorrhages: Experience of 13 Cases. J Korean Neurosurg Soc. (1998) 27:59–65.
86. Ichimura S, Bertalanffy H, Nakaya M, Mochizuki Y, Moriwaki G, Sakamoto R, et al. Surgical treatment for primary brainstem hemorrhage to improve postoperative functional outcomes. World Neurosurg. (2018) 120:e1289–94. doi: 10.1016/j.wneu.2018.09.055
87. Cavalcanti DD, Preul MC, Kalani MY, Spetzler RF. Microsurgical anatomy of safe entry zones to the brainstem. J Neurosurg. (2016) 124:1359–76. doi: 10.3171/2015.4.JNS141945
88. Shitamichi M, Nakamura J, Sasaki T, Suematsu K, Tokuda S. Computed tomography guided stereotactic aspiration of pontine hemorrhages. Stereotact Funct Neurosurg. (1990) 54–55:453–6. doi: 10.1159/000100252
89. Pugliese L, Marconi S, Negrello E, Mauri V, Peri A, Gallo V, et al. The clinical use of 3D printing in surgery. Updates Surg. (2018) 70:381–8. doi: 10.1007/s13304-018-0586-5
90. Li K, Ding X, Wang Q, Fan G, Guo W, Li C, et al. Low-cost, accurate, effective treatment of hypertensive cerebral hemorrhage with three-dimensional printing technology. Front Neurol. (2021) 12:608403. doi: 10.3389/fneur.2021.608403
91. Leger E, Reyes J, Drouin S, Popa T, Hall JA, Collins DL, et al. MARIN: an open-source mobile augmented reality interactive neuronavigation system. Int J Comput Assist Radiol Surg. (2020) 15:1013–21. doi: 10.1007/s11548-020-02155-6
92. Xu F, Jin H, Yang X, Sun X, Wang Y, Xu M, et al. Improved accuracy using a modified registration method of ROSA in deep brain stimulation surgery. Neurosurg Focus. (2018) 45:E18. doi: 10.3171/2018.4.FOCUS1815
93. Bernardo A. Virtual reality and simulation in neurosurgical training. World Neurosurg. (2017) 106:1015–29. doi: 10.1016/j.wneu.2017.06.140
94. Essayed WI, Singh H, Lapadula G, Almodovar-Mercado GJ, Anand VK, Schwartz TH. Endoscopic endonasal approach to the ventral brainstem: anatomical feasibility and surgical limitations. J Neurosurg. (2017) 127:1139–46. doi: 10.3171/2016.9.JNS161503
95. Weiss A, Perrini P, De Notaris M, Soria G, Carlos A, Castagna M, et al. Endoscopic endonasal transclival approach to the ventral brainstem: anatomic study of the safe entry zones combining fiber dissection technique with 7 tesla magnetic resonance guided neuronavigation. Oper Neurosurg. (2019) 16:239–49. doi: 10.1093/ons/opy080
96. Garton T, Hua Y, Xiang J, Xi G, Keep RF. Challenges for intraventricular hemorrhage research and emerging therapeutic targets. Expert Opin Ther Targets. (2017) 21:1111–22. doi: 10.1080/14728222.2017.1397628
97. Maller VV, Gray RI. Noncommunicating hydrocephalus. Semin Ultrasound CT MR. (2016) 37:109–19. doi: 10.1053/j.sult.2015.12.004
98. Fu C, Liu L, Chen B, Wang N, Tan Z, Chen H, et al. Risk factors for poor outcome in hypertensive intraventricular hemorrhage treated by external ventricular drainage with intraventricular fibrinolysis. World Neurosurg. (2017) 102:240–5. doi: 10.1016/j.wneu.2017.03.029
99. Hanley DF, Lane K, McBee N, Ziai W, Tuhrim S, Lees KR, et al. Thrombolytic removal of intraventricular haemorrhage in treatment of severe stroke: results of the randomised, multicentre, multiregion, placebo-controlled CLEAR III trial. Lancet. (2017) 389:603–11. doi: 10.1016/S0140-6736(16)32410-2
100. Hanley DF, Thompson RE, Rosenblum M, Yenokyan G, Lane K, McBee N, et al. Efficacy and safety of minimally invasive surgery with thrombolysis in intracerebral haemorrhage evacuation (MISTIE III): a randomised, controlled, open-label, blinded endpoint phase 3 trial. Lancet. (2019) 393:1021–32. doi: 10.1016/S0140-6736(19)30195-3
101. Mendelow AD, Gregson BA, Rowan EN, Murray GD, Gholkar A, Mitchell PM, et al. Early surgery versus initial conservative treatment in patients with spontaneous supratentorial lobar intracerebral haematomas (STICH II): a randomised trial. Lancet. (2013) 382:397–408. doi: 10.1016/S0140-6736(13)60986-1
102. Mendelow AD, Gregson BA, Fernandes HM, Murray GD, Teasdale GM, Hope DT, et al. Early surgery versus initial conservative treatment in patients with spontaneous supratentorial intracerebral haematomas in the International Surgical Trial in Intracerebral Haemorrhage (STICH): a randomised trial. Lancet. (2005) 365:387–97. doi: 10.1016/S0140-6736(05)70233-6
103. Vespa P, Hanley D, Betz J, Hoffer A, Engh J, Carter R, et al. ICES (intraoperative stereotactic computed tomography-guided endoscopic surgery) for brain hemorrhage: a multicenter randomized controlled trial. Stroke. (2016) 47:2749–55. doi: 10.1161/STROKEAHA.116.013837
104. Shrestha BK, Guo X, Ma L, Qi X, Sun T, Li H, et al. Rat brainstem hemorrhage model: key points to success in modeling. World Neurosurg. (2018) 117:e106–16. doi: 10.1016/j.wneu.2018.05.195
105. Tao C, Zhang R, Hu X, Song L, Wang C, Gao F, et al. A novel brainstem hemorrhage model by autologous blood infusion in rat: white matter injury, magnetic resonance imaging, and neurobehavioral features. J Stroke Cerebrovasc Dis. (2016) 25:1102–9. doi: 10.1016/j.jstrokecerebrovasdis.2016.01.025
Keywords: primary brainstem hemorrhage, prognostic factors, scoring system, surgical management, surgical options
Citation: Chen D, Tang Y, Nie H, Zhang P, Wang W, Dong Q, Wu G, Xue M, Tang Y, Liu W, Pan C and Tang Z (2021) Primary Brainstem Hemorrhage: A Review of Prognostic Factors and Surgical Management. Front. Neurol. 12:727962. doi: 10.3389/fneur.2021.727962
Received: 20 June 2021; Accepted: 19 August 2021;
Published: 10 September 2021.
Edited by:
Yasushi Takagi, Tokushima University, JapanCopyright © 2021 Chen, Tang, Nie, Zhang, Wang, Dong, Wu, Xue, Tang, Liu, Pan and Tang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chao Pan, cHVuY3R1YWxwY0AxNjMuY29t; Zhouping Tang, ZGRqdHpwQDE2My5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.