- 1Department of Vascular Neurology, Faculty of Medicine, University Clinical Centre Ljubljana, University of Ljubljana, Ljubljana, Slovenia
- 2Department of Internal Medicine, Faculty of Medicine, School of Health Sciences, University of Thessaly, Larissa, Greece
- 3Department of Vascular Disorders, University Clinical Centre Ljubljana, Ljubljana, Slovenia
Background: Intracranial hemorrhage is a severe and possibly fatal consequence of anticoagulation therapy. Idarucizumab is used in dabigatran-treated patients suffering from intracranial hemorrhage (ICH) to reverse the anticoagulant effect of dabigatran. Systematic review of real-life mortality in these patients is missing.
Objectives: A review of all published dabigatran-related ICH cases treated with idarucizumab was performed. We aimed to estimate in-hospital mortality rate in these patients.
Method: We searched PubMed and Scopus for all published cases of ICH in idarucizumab/dabigatran-treated patients until May 15, 2021. The assessed outcome was in-hospital mortality.
Results: We identified six eligible studies (case series) with 386 patients and 54 single case reports. In-hospital mortality rate was 11.4% in the case series and 9.7% in the case reports.
Conclusions: Our analysis provides clinically relevant quantitative data regarding in-hospital mortality in idarucizumab/dabigatran-treated patients with ICH, which is estimated to be 9.7–11.4%.
Highlights
- Intracranial hemorrhage is a serious, life-threatening condition associated with anticoagulation therapy.
- Idarucizumab is a specific reversal agent of dabigatran, which achieves instantaneous reversal of anticoagulation after application.
- Idarucizumab is indicated in dabigatran-treated patients with life-threatening conditions, such as uncontrolled bleeding, or the need for urgent intervention.
- Our analysis revealed that the quantitative in-hospital mortality in idarucizumab/dabigatran-treated patients with ICH is estimated to be 9.7–11.4%.
Introduction
Intracranial hemorrhage is a serious, life-threatening condition associated with anticoagulation therapy. Idarucizumab is a specific reversal agent of dabigatran, which achieves instantaneous reversal of anticoagulation after application seemingly without prothrombotic or other serious side effects (1, 2). The use of idarucizumab is indicated in dabigatran-treated patients with life-threatening conditions, such as uncontrolled bleeding, or the need for urgent intervention (2, 3).
Intracranial hemorrhage in dabigatran-treated patients is a rare event, with estimated yearly incidences of 0.1% for the standard dose and 0.12% for the reduced dose (4). Due to very low frequency of intracranial hemorrhage (ICH), consequently, the number of reported cases is limited. The mortality rate in dabigatran-treated patient with ICH in the REVERSal Effects of idarucizumab on Active Dabigatran (REVERSE AD) study, which included only 98 patient with ICH, was 16.4% (2). A small number of patients did not allow for firm conclusions. On the other hand, a relatively high number of reported case series and case reports were published in the following years (1, 2, 5–24).
The aim of this systematic review was to summarize all published cases of dabigatran-treated patients with ICH who were treated with idarucizumab in order to quantitatively evaluate the in-hospital mortality rate.
Methodology
Search Strategy and Inclusion Criteria
We searched PubMed and Scopus until May 15, 2021 for studies reporting dabigatran reversal with idarucizumab in patients with ICH using the terms: “idarucizumab” or “reversal” or “dabigatran” and “hemorrhagic stroke” or “ICH” or “intracranial bleeding.” The search was limited from January 1, 2013 to May 15, 2021, since the term “idarucizumab” was first reported in 2014. In addition, we searched the references of related letters, reviews, and editorials to identify other potentially eligible studies. We also contacted experts in the field to identify studies that have been potentially missed. To be eligible for this analysis, all studies had to provide data about the in-hospital mortality in dabigatran-treated patients with ICH after reversal with idarucizumab. This study was reported according to the PRISMA statement (25).
Outcome and Data Extraction
The outcome assessed was in-hospital mortality. Eligible studies were assessed independently by the first two authors. Individual patient data were extracted from single case reports and case series whenever possible using prespecified forms. Quality evaluation of the included studies was conducted using a tool for the assessment of case series (26).
Methodology and Statistical Analysis
Patient characteristics and in-hospital mortality were described based on two different prespecified approaches: individual patient data from single case reports and case series that provided individual patient data were pooled together to form a unified group of patients. Case series that provided aggregate data rather than individual patient data were reported separately.
Results
Among 676 articles identified from the initial literature search, 22 studies were eligible and included in the analysis (1, 2, 5–24) (Supplementary Figure 1). Sixteen studies provided individual patient data of 53 patients with ICH (6–8, 10, 12, 13, 15–24), whereas six case series reported results on, overall, 394 patients (1, 2, 5, 9, 11, 14). Two studies (1, 24) included patient data that have been previously reported (7, 8). In order to avoid any duplication, in this study, we only included the data from the most recent reports (1, 24).
Quality assessment can be found in Supplementary Table 1.
Forty-four (11.4%) out of 386 patients in the case series and 4 (9.7%) out of 41 patients in the single case reports died. The characteristics of patients are summarized in Table 1.
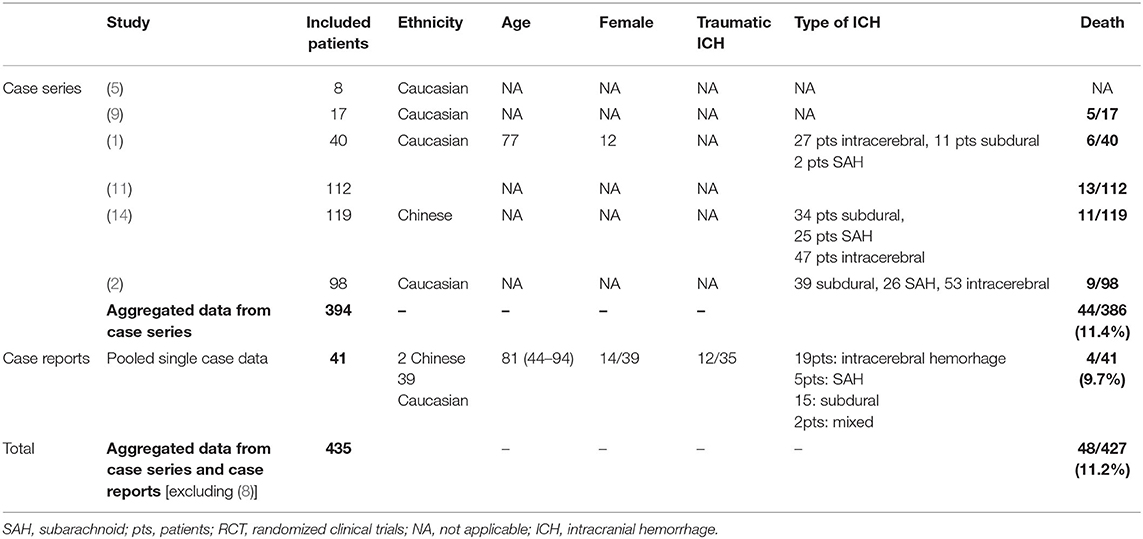
Table 1. Baseline characteristics and outcomes of idarucizumab/dabigatran-treated patients with ICH.
Out of 435 patients, individual data (age, sex, traumatic/non-traumatic type of ICH, time to idarucizumab treatment, etc.) were only available for 54, which did not allow us to perform an individual patient characteristic analysis.
Discussion
Our systematic review of data from published case reports and case series shows an estimated real-life in-hospital mortality of 9.7–11.4% in this patient population. Previous studies have reported the in-hospital mortality rate among warfarin- and direct oral anticoagulant (DOAC)-treated patients with ICH, which ranged from 25 to 33% and from 18 to 27%, respectively (27, 28). In the RE-VERSE AD study, which included dabigatran-treated patients with ICH who received idarucizumab, the described in-hospital mortality was 16.4% (2). This study, which includes data from case series and case reports, summarizes the evidence on the beneficial effect of idarucizumab in patients with ICH treated with dabigatran in order to present real-life quantitative data on in-hospital mortality.
Our review has several limitations. Heterogeneity of the cases (traumatic/non-traumatic, type of ICH: intraparenchymal/subdural hemorrhage) and missing data (age, gender, risk factors, comorbidities, timing of reversal agent application) are the main limitations of this study. Individual data were available for only 54 out of 435 patients, which could limit the assessment of how representative the cohort is. Comparison with patients with ICH who were not treated with idarucizumab would be valuable; however, there are no applicable published real-life data. Finally, we cannot exclude the presence of publication bias, particularly regarding the analysis of case reports. On the other hand, the strength of the study is consideration of all published cases.
In conclusion, our systematic review of all published cases of dabigatran-related patients with ICH treated with idarucizumab provides a real-life quantitative estimate for real-life in-hospital mortality. This estimate seems to suggest the clinically relevant efficacy and safety of dabigatran reversal in real-life treatment of patients with ICH. However, this needs to be assessed in a propensity-score matched analysis or, ideally, in a prospective study of appropriate size.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
Author Contributions
SF and DS: study design, data acquisition, statistical analysis and interpretation, and manuscript preparation. MŠ and GN: data acquisition, statistical analysis and interpretation, and critical revision of the manuscript. JO: study concept and design, statistical analysis and interpretation, manuscript preparation, and study supervision. All authors contributed to the article and approved the submitted version.
Conflict of Interest
SF, MŠ, and JO reported speaker fees from Bayer, Boehringer Ingelheim, and Pfizer. GN reported speaker fees/advisory boards/research support from Abbott, Amgen, Bayer, Boehringer Ingelheim, Elpen, and Pfizer outside the submitted study.
The remaining author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2021.727403/full#supplementary-material
Abbreviations
ICH, intracranial hemorrhage; DOAC, direct oral anticoagulant.
References
1. Kermer P, Eschenfelder CC, Diener HC, Grond M, Abdalla Y, Abraham A, et al. Antagonizing dabigatran by idarucizumab in cases of ischemic stroke or intracranial hemorrhage in germany—updated series of 120 cases. Int J Stroke. (2020) 12:383–91. doi: 10.1177/1747493019895654
2. Pollack CV, Reilly PA, van Ryn J, Eikelboom JW, Glund S, Bernstein RA, et al. Idarucizumab for dabigatran reversal—full cohort analysis. NE J Med. (2017) 377:431–41. doi: 10.1056/NEJMoa1707278
3. Thibault N, Morrill AM, Willett KC. Idarucizumab for reversing dabigatran-induced anticoagulation: a systematic review. Am J Ther. (2018) 25:e333–8. doi: 10.1097/MJT.0000000000000460
4. Connolly SJ, Ezekowitz MD, Yusuf S, Eikelboom J, Oldgren J, Parekh A, et al. Dabigatran versus warfarin in patients with atrial fibrillation. NE J Med. (2009) 361:1139–51. doi: 10.1056/NEJMoa0905561
5. Apostolaki-Hansson T, Ullberg T, Pihlsgård M, Noorving Bo, Petersson J. Reversal treatment in oral anticoagulant-related intracerebral hemorrhage-an observational study based on the swedish stroke register. Front Neurol. (2020) 11:760. doi: 10.3389/fneur.2020.00760
6. Bottaro FJ, Margan MM, Duboscq C, Ceresetto MJ. Experience with idarucizumab as a reverse agent of dabigatran]. Medicina. (2020) 80:405–410.
7. Frol S, PretnarOblak J. Early Outcome after Intracranial Hemorrhage Related to Non-Vitamin K Oral Anticoagulants. Interv Neurol. (2018) 7:19–25. doi: 10.1159/000480524
8. Kermer P, Eschenfelder CC, Diener HC, Grond M, Abdalla Y, Althaus K, et al. Antagonizing dabigatran by idarucizumab in cases of ischemic stroke or intracranial hemorrhage in Germany—a national case collection. Int J Stroke. (2017) 12:383–91. doi: 10.1177/1747493017701944
9. Küpper C, Feil K, Klein M, Feuerecker R, Lücking M, Thanbichler F, et al. Idarucizumab administration in emergency situations: The munich registry of reversal of pradaxa® in clinical routine (mr repair). J Neurol. (2019) 266:2807–11. doi: 10.1007/s00415-019-09492-w
10. Phua CS, Bonura A, Choong H. Hemorrhagic stroke complicated by cerebral venous sinus thrombosis with idarucizumab. NeurolClicPract. (2019) 9:e4–6. doi: 10.1212/CPJ.0000000000000555
11. Singh S, Nautyal A, Belk KW. Real World Outcomes Associated with Idarucizumab: Population-Based Retrospective Cohort Study. Am J Cardiovasc Drugs. (2020) 20:161–8. doi: 10.1007/s40256-019-00360-6
12. Vene N, Mavri A, Mijovski MB, Gregorič M, Uštar KK, Žerjav U, et al. Idarucizumab for dabigatran reversal in daily clinical practice: A case series. Eur J Anaesthesiol. (2019) 37:874–8. doi: 10.1097/EJA.0000000000001185
13. Vosko MR, Bocksrucker C, Drwiła R, Dulíček P, Hauer T, Mutzenbach J, et al. Real-life experience with the specific reversal agent idarucizumab for the management of emergency situations in dabigatran-treated patients: A series of 11 cases. J Thromb Thrombolysis. (2017) 43:306–17. doi: 10.1007/s11239-017-1476-2
14. Yasaka M, Yokota H, Suzuki M, Asakura H, Yamane T, Ogi Y, et al. Idarucizumab for emergency reversal of anticoagulant effects of dabigatran: interim results of a japanese post-marketing surveillance study. Cardiol Ther. (2020) 9:167–88. doi: 10.1007/s40119-020-00165-8
15. Arai N, Mine Y, Kagami H, Maruyama M, Daikoh A, Inaba M. Safe burr holesurgery for chronic subdural hematoma using dabigatran with idarucizumab. World Neurosurg. (2018) 109:432–5. doi: 10.1016/j.wneu.2017.10.043
16. Balakumar J, Santiago R, Supino M. Reversal of Dabigatran with idarucizumab in acute subarachnoid hemorrhage. Clin Pract Cases Emerg. (2017) 1:349–53. doi: 10.5811/cpcem.2017.6.34356
17. Edwards G, Roman C, Jithoo R, Mitra B. Use of Idarucizumab for dabigatran reversal: emergency department experience in two cases with subdural haematoma. Trauma Case Rep. (2018) 13:46–9. doi: 10.1016/j.tcr.2017.12.003
18. Gendron N, Feral-Pierssens AL, Jurcisin I, de Raucort E, Bouton V, Fischer AM, et al. Real-world use of idarucizumab for dabigatran reversal in three cases of serious bleeding. Clin Case Rep. (2017) 5:346–50. doi: 10.1002/ccr3.839
19. Goriacko P, Yaghdjian V, Koleilat I, Sinnett M, Shukla H. The use of idar-ucizumab for dabigatran reversal in clinical practice: a case series. J formulmanag. (2017) 42:699–703.
20. Quintavalla R, Lombardi M, Prandoni P, Manotti C, Tadonio I, Re F, et al. Increased dabigatran plasma concentration during Ibrutinib treatment: a case of cerebral hemorrhage and successful dabigatran reversal by idarucizumab. Aging Clin Exp Res. (2018) 30:93–5. doi: 10.1007/s40520-017-0752-5
21. Hieber M, Hollasch H, Heck D, Mächtel M, Geisen U, Niesen WD, et al. Reversal of dabigatran using idarucizumab: Single center experience in four acute stroke patients. J Thromb Thrombolysis. (2018) 46:12–5. doi: 10.1007/s11239-018-1658-6
22. Sheikh-Taha M. Idarucizumab for Reversal of dabigatran: single-center real-world experience. Am J Cardiovasc Drugs. (2019) 19:59–64. doi: 10.1007/s40256-018-0300-5
23. Krueger EM, Finneran MM, Smith M. Management strategies and outcomes of hemorrhagic traumatic brain injury on oral anticoagulants. Cureus. (2020) 12:e10508. doi: 10.7759/cureus.10508
24. Frol S, Sernec LP, Hudnik LK, Šabovič M, Oblak JP. Idarucizumab reversal of dabigatran in patients with acute ischemic stroke and intracranial hemorrhage: comparison with non-idarucizumab-treated patients. CNS Drugs. (2021) 35:233–42. doi: 10.1007/s40263-021-00792-2
25. Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analysis: The prisma statement. Ann Intern Med. (2009) 151:264–269. doi: 10.7326/0003-4819-151-4-200908180-00135
26. Murad MH, Sultan S, Haffar S, Bazerbachi F. Methodological quality and synthesis of case series and case reports. BMJ Evidence-Based Med. (2018) 23:60–63. doi: 10.1136/bmjebm-2017-110853
27. Inohara T, Xian Y, Liang L, Matsouaka RA, Saver JL, Smith EE, et al. Association of intracerebral hemorrhage among patients taking non-vitamin K antagonist vs vitamin K antagonist oral anticoagulants with in-hospital mortality. JAMA. (2018) 319:463–473. doi: 10.1001/jama.2017.21917
Keywords: dabigatran, idarucizumab, reversal agent, intracranial hemorrhage, in-hospital mortality
Citation: Frol S, Sagris D, Šabovič M, Ntaios G and Oblak JP (2021) Dabigatran Reversal With Idarucizumab and In-Hospital Mortality in Intracranial Hemorrhage: A Systematic Review of Real-Life Data From Case Reports and Case Series. Front. Neurol. 12:727403. doi: 10.3389/fneur.2021.727403
Received: 18 June 2021; Accepted: 15 October 2021;
Published: 24 November 2021.
Edited by:
Nishant K. Mishra, Yale University, United StatesReviewed by:
Peter E. Westerweel, Albert Schweitzer Ziekenhuis, NetherlandsKatherine Mun, University of California, Los Angeles, United States
Copyright © 2021 Frol, Sagris, Šabovič, Ntaios and Oblak. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Senta Frol, c2VudGFmcm9sQGdtYWlsLmNvbQ==
†These authors have contributed equally to this work
 Senta Frol
Senta Frol Dimitrios Sagris
Dimitrios Sagris Mišo Šabovič3
Mišo Šabovič3 George Ntaios
George Ntaios