- 1Department of Neurology, Peking University First Hospital, Beijing, China
- 2Department of Neurology, Second Hospital of Hebei Medical University, Shijiazhuang, China
- 3Department of Neurology, First Affiliated Hospital of Harbin Medical University, Harbin, China
- 4Department of Neurology, General Hospital of Shenyang Military Command, Shenyang, China
- 5Department of Neurology, Penglai People's Hospital, Penglai, China
- 6Department of Neurology, Fifth Affiliated Hospital of Sun Yat-sen University, Zhuhai, China
- 7Department of Neurology, Qindao University Medical College Affiliated Yantai Yuhuangding Hospital, Yantai, China
- 8Department of Neurology, Huizhou First Hospital, Huizhou, China
- 9Department of Neurology, Harrison International Peace Hospital, Hengshui, China
Background and Purpose: There is limited information on symptomatic intracranial hemorrhage (sICH) in stroke patients without thrombolysis. This study aimed to evaluate the risk factors of sICH and the association between sICH and the prognosis at 3 and 12 months in acute ischemic stroke patients without thrombolysis.
Methods: Data originated from the Chinese Acute Ischemic Stroke Treatment Outcome Registry. Univariate analysis and multivariate logistic regression were used to screen the risk factors of sICH. Multivariable logistic regression models were used to assess the association of sICH with poor outcome and all-cause mortality.
Results: Totally, 9,484 patients were included, of which 69 (0.73%) had sICH. Atrial fibrillation (odds ratio [OR], 3.682; 95% confidence interval [CI], 1.945–6.971; p < 0.001), history of tumors (OR, 2.956; 95% CI, 1.115–7.593; p = 0.024), and the National Institutes of Health Stroke Scale (NIHSS) score on admission ([6–15: OR, 2.344; 95% CI, 1.365–4.024; p = 0.002] [>15: OR, 4.731; 95% CI, 1.648–13.583; p = 0.004]) were independently associated with sICH. After adjustment of the confounders, patients with sICH had a higher risk of poor outcome (OR, 1.983; 95% CI, 1.117–3.521; p = 0.018) at 3 months and that of all-cause mortality at 3 (OR, 6.135; 95% CI, 2.328–16.169; p < 0.001) and 12 months (OR, 3.720; 95% CI, 1.513–9.148; p = 0.004).
Conclusion: sICH occurred in 0.73% of acute ischemic stroke patients without thrombolysis and was associated with a worse prognosis at 3 and 12 months. Atrial fibrillation, history of tumors, and NIHSS score at admission were independent risk factors of sICH.
Introduction
Stroke is the second leading cause of disability-adjusted life years worldwide in people over 50 years of age (1), which has brought a heavy burden on society and families. Until now, intravenous thrombolysis with recombinant tissue plasminogen activator (rt-PA) remains the first line treatment for patients with acute ischemic stroke. Symptomatic intracranial hemorrhage (sICH) is recognized as a devastating complication of thrombolysis treatment, which occurs in 0.4–10.3% patients depending on the varied diagnostic criteria and is consistently associated with an increased mortality and a worse functional outcome (2–16). Many studies have reported the predictors of sICH after intravenous thrombolysis, including stroke severity, age, onset-to-treatment time, baseline glucose, hyperdense cerebral artery sign, and early infarct signs on baseline imaging (2, 3, 7, 17). However, there is limited information on sICH in stroke patients without intravenous thrombolysis.
Although the incidence of sICH was lower in the patients who received placebo compared to those given t-PA in the National Institute of Neurological Disorders and Stroke (NINDS) trial (0.6 vs. 6.4%), management of acute stroke remains challenging, considering the vast number of stroke patients without intravenous thrombolysis worldwide (18). Tan et al. (19) reported that 4.4% of ischemic stroke patients without thrombolysis developed sICH. But this is a single-center study with only 406 patients included. Another study based on a multicenter registry analyzed sICH in those who did not receive any antithrombotic therapy (20), while most patients with acute ischemic stroke would receive antithrombotic treatment after stroke, of which approximately one third underwent antiplatelet therapy (APT) prior to the onset of stroke in the real world (21, 22).
The purpose of this study is to investigate the risk factors and prognosis of sICH in patients with acute ischemic stroke that did not undergo thrombolytic therapy in a large multicenter, prospective cohort in China.
Methods
Study Design and Population
Data was obtained from the Chinese Acute Ischemic Stroke Treatment Outcome Registry (CASTOR), a multicenter, prospective, hospital-registry (n = 80) study conducted in 46 cities across China. The trial design and protocol were described elsewhere (23). The hospitals included in our study were required to have a neurology ward with over 100 stroke patients admitted every year. Consecutive patients from May 2015 to October 2017 were eligible for enrollment in the study if they met the following criteria: (1) age ≥18 years. (2) acute ischemic stroke diagnosed according to the Chinese Guideline for Diagnosis and Treatment of Ischemic Stroke (2014). (3) admitted within 1 week after onset of stroke. (4) consent to participation in this study. Patients with cerebral hemorrhage or an expected survival <3 months due to systemic diseases were excluded. Patients were assessed five times during the course of the study at admission, 7 ± 2 days after enrollment, discharge, and ~3- and 12- months post-stroke.
This study was registered with ClinicalTrials.gov (NCT02470624) and approved by the ethics committees of Peking University First Hospital (IRB approval number: 2015[922]) and all participating hospitals. This study was conducted in accordance with the International Conference on Harmonization Good Clinical Practice (ICH-GCP) guidelines and the Declaration of Helsinki. Written informed consent was obtained from all patients or an guardian (if the patient was unable to provide it) to participate.
Data Collection
Baseline information was collected predominantly by face-to-face interviews. The details of the diagnosis and treatment strategy during admission were obtained from the medical records and interviews with patients or their proxies. The information included: (1) demographic variables, including age and sex; (2) medical history, including hypertension (patients taking antihypertensive agents or with blood pressure >140/90 mmHg on repeated measurements), diabetes mellitus (patients taking antidiabetic agents, with fasting blood sugar level >126 mg/dL or HbA1c ≥6.5%, or with a casual plasma glucose level >200 mg/dL), hyperlipidemia (patients taking lipid-lowering agents or with an overnight fasting cholesterol level >240 mg/dL, triglyceride level >200 mg/dL, or low-density lipoprotein level >160 mg/dL), history of stroke (previous cerebral infarction and/or hemorrhage), atrial fibrillation(AF), coronary heart disease, history of tumors; (3) medication history within 3 months prior to onset of stroke (single antiplatelet agents, dual antiplatelet agents, lipid-lowering agents, antihypertensive agents, antidiabetic agents); (4) medication administered after onset of stroke (thrombolysis, antiplatelet agents, anticoagulation agents, lipid-lowering agents, antihypertensive agents, antidiabetic agents); (5) clinical features of the index stroke, including National Institutes of Health Stroke Scale score (NIHSS), Glasgow Coma Scale score (GCS) and systolic and diastolic blood pressure on admission.
Diagnosis of sICH
Repeated brain CT/MRI was suggested when neurological deterioration occurred in stroke patients. sICH was defined as the hemorrhage confirmed by CT/MRI scans during admission with clinical deterioration or an increase of four or more points in NIHSS score or adverse events indicating clinical worsening (e.g., drowsiness, increase of hemiparesis) recorded by the investigator, according to the definitions outlined in the European-Australasian Acute Stroke Study (ECASS-II) classification (24).
Outcome Assessment
The functional outcome measured using the modified Rankin scale (mRS) was collected via face-to-face or telephone interview at 3 and 12 months after the onset of symptoms. Poor outcome was defined as a mRS score of 3–6. All-cause death was defined as death from any cause and confirmed by a death certificate from the hospital or the local citizen registry. The outcomes in our study included the proportion of poor outcome and all-cause mortality at 3 and 12 months.
Statistical Analysis
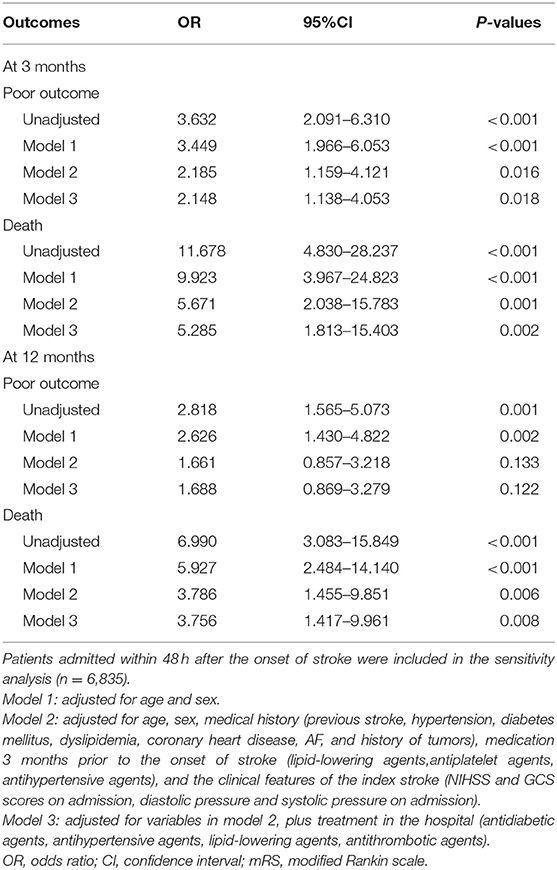
Data were expressed as median values, inter-quartile ranges (IQR) for continuous variables, and frequencies and percentages for discrete variables. The statistical significance of intergroup differences was assessed using Mann-Whitney U-test or χ2 tests as appropriate. Multivariate logistic regression analysis was subsequently used to identify the independent risk factors from those variables with p < 0.1 in the univariate analysis. Calculated odds ratios (ORs) were used to measure the association between sICH and risk factors. The relationship of sICH with poor outcome and all-cause mortality was assessed using several logistic regression models. Model 1 was adjusted for age and sex; Model 2 was further adjusted for medical history (previous stroke, hypertension, diabetes mellitus, dyslipidemia, coronary heart disease, AF, and history of tumors), medication 3 months prior to the onset of stroke (lipid-lowering agents antiplatelet agents, antihypertensive agents), and the clinical features of the index stroke (NIHSS and GCS scores on admission, diastolic pressure, and systolic pressure on admission) based on Model 1 and Model 3 was further adjusted for treatment in the hospital (antidiabetic agents, antihypertensive agents, lipid-lowering agents, antithrombotic agents) based on Model 2. A sensitivity analysis was performed that restricted the study population to those who were admitted within 48 h of stroke onset. All p-values were two-sided, with p < 0.05 considered statistically significant. All statistical analyses were performed using SPSS version 25.0 (SPSS Inc., Chicago, IL, USA).
Results
Patient Characteristics and Incidence of sICH
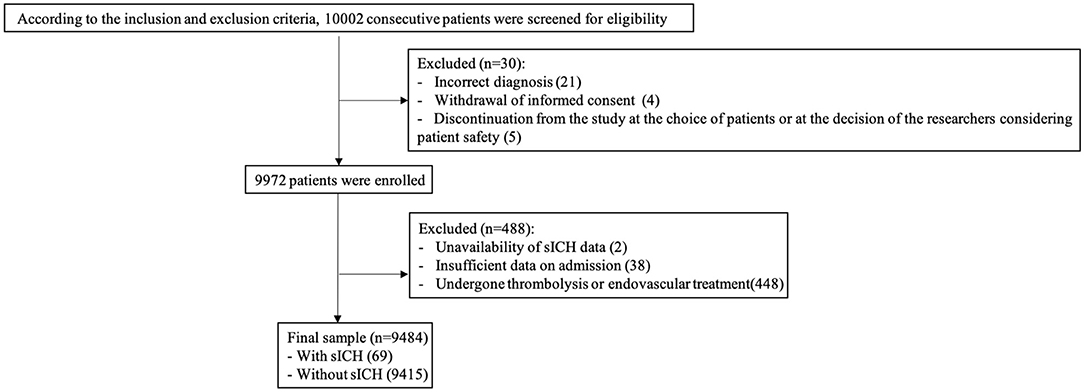
In total, 10,002 consecutive patients with ischemic stroke within 7 days of onset were enrolled in the CASTOR study. We excluded 518 patients for the following reasons: incorrect diagnosis (n = 21), withdrawal of informed consent (n = 4), discontinuation from the study at the choice of patients or at the decision of the researchers considering patient safety (n = 5), unavailability of sICH data (n = 2), insufficient data on admission (n = 38), and undergoing intravenous thrombolysis or endovascular treatment (n = 448). Finally, 9,484 patients (median age, 64.0 years; 65.6% males) were included in this the analysis (Figure 1). The median NIHSS score at admission was 4 (IQR 2–7). Among the 9,484 patients, 69 cases (0.73%; median age, 66.0 years; 66.7% males) had developed sICH during admission.
Predictors of sICH
The characteristics of patients with and without sICH are summarized in Table 1. Univariate analysis revealed the differences between patients with and without sICH were significant in the following features: age (p = 0.012), NIHSS score at admission (p < 0.001), GCS score at admission (p < 0.001), AF (p < 0.001), history of tumors (p = 0.012), antidiabetic agents during admission (p = 0.026), and antithrombotic agents during admission (p = 0.006). The multivariate logistic regression analysis showed that AF (OR, 3.682; 95% CI, 1.945–6.971; p < 0.001), history of tumors (OR, 2.956; 95% CI, 1.115–7.593; p = 0.024), and NIHSS score on admission ([6–15: OR, 2.344; 95% CI, 1.365–4.024; p = 0.002] [>15: OR, 4.731; 95% CI, 1.648–13.583; p = 0.004]) were the independent risk factors of sICH (Table 2).
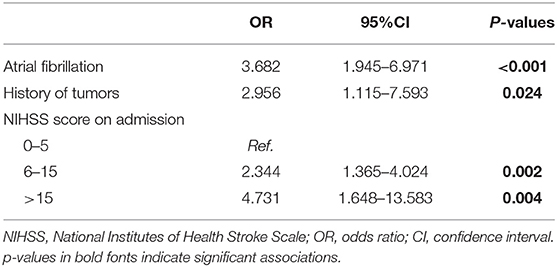
Table 2. Multivariate analysis to identify factors associated with sICH in patients with ischemic stroke without thrombolysis.
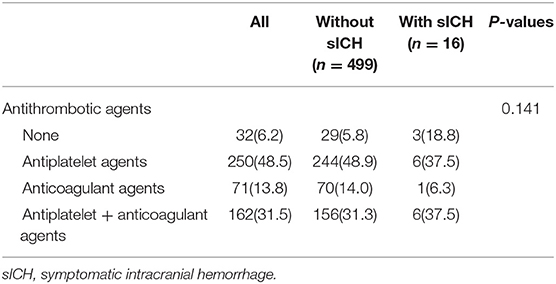
Antithrombotic Therapy and sICH in Patients With AF
In our study, 515 patients (5.4%) had a history of AF, of which 250 (48.5%) patients received antiplatelet therapy, 71 (13.8%) received anticoagulant therapy, 162 (31.5%) received both antiplatelet and anticoagulant therapy, and 32 (6.2%) did not receive any antithrombotic therapy during admission. In patients with AF, the development of sICH was not significantly associated with the antithrombotic regimen (Table 3).
Tumor Types and sICH
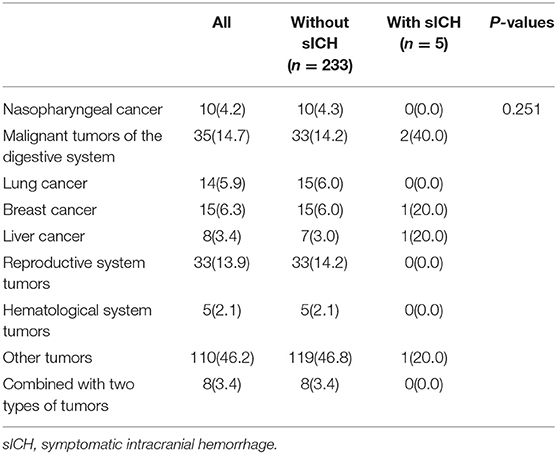
In our analysis, 238 (2.5%) patients had a history of tumors, of which 10 (42%) had nasopharyngeal cancer, 35 (14.7%) had malignant tumors of the digestive system, 14 (5.9%) had lung cancer, 15 (6.3%) had breast cancer, 8 (3.4%) had liver cancer, 33 (13.9%) had reproductive system tumors, 5 (2.1%) had tumors of the hematological system, 8 (3.4%) had a combination of two types of tumors, and 110 (46.2%) had other tumors. There was no significant association between tumor types and sICH (p = 0.251) (Table 4).
Prior APT and sICH
Among the 9,484 patients without thrombolysis, 843 (8.9%) received APT within 3 months prior to stroke onset, of which 115 received dual APT treatment with aspirin and clopidogrel, and 728 received single APT (663 patients with aspirin alone, 117 with clopidogrel alone, and 5 with cilostazol alone). There was no significant difference in the risk of sICH among the three groups (0.8% in the no APT group, 0.4% in the single APT group and 0.9% in the dual APT group, p = 0.575).
sICH and Poor Outcome
The mRS score at 3 months was collected in 8,890 (93.7%) patients, of which 1,734 (19.5%) had a mRS score of 3–5 and 95 (1.1%) patients had died (mRS = 6). At 12 months post stroke onset, the mRS score was collected in 8,332 (87.9%) patients, of which 1,209 (12.7%) had an mRS score 3–5 and 191(2.0%) patients had died. Compared to those without sICH, the patients with sICH had a higher risk of poor outcome (45.3 vs. 20.4%, p < 0.001; 33.3 vs. 16.7%, p = 0.001, respectively) and mortality (10.9 vs. 1.0%, p < 0.001; 13.3 vs. 2.2%, p < 0.001, respectively) at 3 and 12 months. After adjusting for the confounding variables, the differences in poor outcome (OR, 1.983; 95% CI, 1.117–3.521; p = 0.018) at 3 months and all-cause mortality at 3 (OR, 6.135; 95% CI, 2.328–16.169; p < 0.001) and 12 months (OR, 3.720; 95% CI, 1.513–9.148; p = 0.004) remained statistically significant (Table 5).
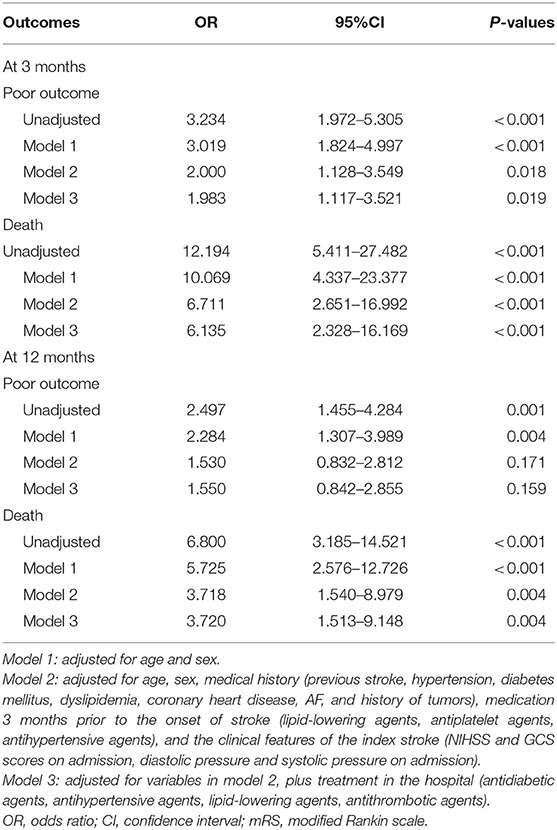
Table 5. Relationship of all-cause death and poor functional prognosis with sICH in patients without thrombolysis.
Sensitivity Analysis
When we restricted the study population to those who were admitted within 48 h of stroke onset, there were 6,835 patients were included in the sensitivity analysis, of which 55 (0.8%) patients developed sICH. AF (OR, 3.432; 95% CI, 1.723–6.835; p < 0.001), history of tumors (OR, 3.255; 95% CI, 1.128–9.391; p = 0.029), and NIHSS score at admission ([6–15: OR, 2.844; 95% CI, 1.524–5.307; p = 0.001] [>15: OR, 5.073; 95% CI, 1.601–16.072; p = 0.006]) were the independent predictors of sICH (Supplementary Tables 1–4). Further, sICH was significantly associated with the increased risk of poor outcome at 3 months and all-cause mortality at 3 and 12 months (Table 6).
Discussion
In this study, we found that sICH occurred in 0.73% of acute ischemic stroke patients without thrombolysis during admission and AF, history of tumors, and NIHSS score at admission were the independent risk factors of sICH. In these patients, sICH was associated with a higher risk of poor outcome at 3 months and an increased mortality at 3 and 12 months. No significant association between sICH and poor outcome at 12 months was observed.
Previous studies on acute ischemic stroke have focused on hemorrhagic transformation (HT), while the diagnosis of sICH required an imaging change and a deterioration in neurological function. In our study, the incidence of sICH in patients without thrombolysis was similar to that noted in NINDS trial (0.73 vs. 0.6%) (18). Several studies have reported a higher incidence of sICH in patients without thrombolysis. A systematic review showed that the incidence of sICH was 1.5% (4), which may be attributed to differences in patient selection criteria, the diagnostic criteria of sICH, the time interval between stroke onset and admission, and stroke treatment. Two studies from West China Hospital reported that the incidence of sICH was 1.3% in 2010–2011 and 4.4% in 2002–2005, respectively (15, 19). There were more patients with mild stroke in our cohort, which may explain this discrepancy. In addition, the sample sizes of these two studies were relatively small, and the patients were recruited from a single center.
We found AF was associated with an ~4-fold increase in the risk of sICH in the patients without thrombolysis. A similar result was reported by Tan et al. (19) In patients with thrombolysis, AF was also recognized as an independent risk factor of sICH with the OR ranging from 2.5 to 7 (25). Patients with cardiogenic stroke usually have rapid occlusion of arteries, less developed cerebral collateral circulation, small penumbra and large core infarction, which increases HT (26, 27). Most patients with AF may receive anticoagulant therapy, but previous trials have shown that anticoagulation could increase the risk of intracerebral bleeding in ischemic stroke patients (28). However, Lee et al. (29) reported that the incidence of sICH did not increase in patients with cardiogenic embolism who received early anticoagulation therapy within 1 week from stroke onset. Similarly, our analysis did not find a significant association between the antithrombotic regimen during admission and sICH in patients with AF, either. The risk in those with anticoagulation was not higher than that with antiplatelet medication. This finding was also consistent with those of several other studies (30–32), and this indicated that it was AF not the accompanying anticoagulation therapy which caused the increased the risk of sICH.
In this study, a higher NIHSS score at admission was associated with sICH in patients with acute ischemic stroke who did not undergo thrombolysis. Similar results were noted in those with ischemic stroke after thrombolysis (3, 25, 33, 34). In previous studies on patients without thrombolysis, although univariate analysis showed that patients with higher NIHSS score were more prone to HT, NIHSS score was not an independent risk factor for HT (2, 19, 20). This is perhaps explained by the smaller sample sizes of previous studies and the fact that previous studies only investigated the relationship between HT and NIHSS score at admission rather than the relationship between sICH and NIHSS score at admission. Severe ischemic stroke usually manifests as extensive brain tissue damage, including vascular damage, which is prone to bleeding.
Previous studies found that antithrombotic medications before acute ischemic stroke might increase the risk of sICH after intravenous thrombolysis (35, 36). In our study, although patients with prior APT were older and more likely to have vascular risk factors which may increase the risk of sICH, pre-stroke APT did not increased the risk of sICH, which suggests that prior use of APT did not increase the risk of sICH in stroke patients without thrombolysis. Similar results were reported in some other studies (2, 20). A study that included 12,415 patients without thrombolysis found no correlation between APT and HT, although reported a higher proportion of pre-stroke APT (17.54%) (20). The variations in the doses of pre-stroke antiplatelet therapy and the issue of patient compliance were not addressed in our study. Therefore, further research is needed.
Interestingly, we found an association between the history of tumors and sICH in acute ischemic stroke patients without thrombolysis, which had not been reported previously. Several studies investigated the relationship between a history of tumors and sICH after thrombolysis and their results varied greatly. The overall risk of hemorrhagic stroke occurrence in patients with cancer is significantly higher than that in the general population (37). Whether brain hemorrhage is directly or indirectly caused by cancer is not clear. In our study, the initial brain imaging before enrolment could exclude those patients with typical lesions of primary brain tumors or metastatic tumors. However, we could not rule out the possibility that some patients with atypical lesions of tumor had been included in the study and then had hemorrhagic transformation. Other studies indicated severe thrombocytopenia and coagulopathy may be a mechanism of cerebral hemorrhage related to tumor (37). Thus, further research is needed to investigate the mechanisms of sICH in acute ischemic stroke patients with history of tumors. Although in the latest acute ischemic stroke management guidelines, the American Heart Association and the American Stroke Association provided with some suggestions for patients with a history of tumors, such as intravenous thrombolysis is contraindicated in cases of intra-axial intracranial neoplasms and gastrointestinal malignancy, and recommended in cases of extra-axial intracranial neoplasms and systemic malignancy with reasonable (>6 months) life expectancy, the guidelines admitted that the efficacy and safety of the treatments in stroke patients with malignant disease are still unclear (38). With aging and the improvement in tumor therapy, there will be more tumor survivors who are also vulnerable to stroke. Our study showed a higher risk of sICH in stroke patients with a history of tumors, which indicated that there would be some different characteristics in these patients and warranted further research on the management of stroke in patients with history of tumors.
There was a relatively lower mortality (1.1% at 3 months and 2.0% at 12 months) in our study, which was similar to the results based on the Third China National Stroke Registry (1.3% at 3 months and 2.9% at 12 months) (39). Andrade et al. (40) reported a mortality of 6.9% at discharge and Paciaroni et al. (2) reported a mortality of 11.5% at 3 months in stroke patients. This difference may be due to the lower average age of patients and the lower median NIHSS score at admission in our cohort.
A prospective cohort study showed that post-thrombolytic sICH contributed to an increased risk of poor outcome and mortality. According to the different definitions of sICH used, the ranges of OR were 1.3–1.7 and 1.5–4.8, respectively (3). Another multicenter prospective cohort study showed that sICH could increase the risk of poor outcome by 3.57 times (7). Our findings suggested that patients with sICH had a higher risk of poor outcome at 3 months. Additionally, the patients with sICH still had a higher mortality rate at 3 and 12 months and it revealed sICH in stroke patients without thrombolysis was a serious problem in management of stroke since most patients did not receive thrombolysis in the real world. We speculated two possible reasons that would contribute to the worse prognosis. One was the direct influence of sICH, and the other reason was that the withdrawal of antithrombotic therapy at the early stage of treatment due to sICH increased the recurrent ischemic stroke. In our study, patients with sICH had a higher incidence of poor outcome at 12 months than those without sICH (33.3 vs. 16.7%, p = 0.001). However, the difference became insignificant after adjustment of the baseline characteristics and treatment during admission. Considering the mRS score was not collected in 1,152 patients at 12 months, we could not rule out the possibility of selective dropout.
The strengths of our study include a relatively large sample size and a multicenter design. However, our study has several limitations that need to be addressed. First, this study was an observational investigation, we adjusted for a series of identified confounding variables. However, due to the nature of observational studies, certain unmeasured or residual confounding effects were unavoidable. Therefore, we cannot conclude a causal relationship between sICH and poor outcome. Second, repeated brain imaging was often performed when the symptoms of stroke patients worsen in clinical practice, which may underestimate the risk of ICH, especially in the patients with asymptomatic or mild symptoms. However, our study focused on the patients with sICH, who had a deterioration in neurological function accompanying with ICH. So we think the risk of underestimation was limited in our study. Third, some information such as glucose level at admission, blood pressure during admission, details of brain imaging including radiographic classification of HT, and number of cerebral microbleeds were not collected in the database. So our study propably missed to elucidate the responsible mechanisms and potential protective measures of sICH. Fourth, the exact time of sICH was not recorded in our database. While sICH occurring at different timepoints may be due to varied pathological mechanisms. Fifth, although we found an association between history of tumors and sICH, the limited sample with tumor needed the further confirmation. Sixth, we did not include the patients who underwent endovascular treatment although thrombectomy treatment had been recommended in the current guidelines. Considering there were only 23 patients who received endovascular treatment in our study and the significant difference in the risk of bleeding, we focused on those who only received medication treatment. Seventh, the CASTOR study recruited the patients who were admitted within 7 days of onset from acute ischemic stroke, which indicates a possible heterogenous population, and those who had developed sICH at early stage would not be included in this registry, which may underestimate the incidence of sICH. However, in the sensitivity analysis which only included those admitted within 48 h, the results were similar to that of our main analysis. Finally, all the patients were recruited in China. The rate of receiving intravenous thrombolysis and endovascular treatment, as well as the mortality were relatively lower than other studies in the western countries (2, 41–44). Hence, caution is needed when generalizing our results to other populations.
Conclusions
In a large, multicenter cohort of acute ischemic stroke patients, sICH occurred in 0.73% of patients without thrombolysis and was associated with a worse prognosis at 3 and 12 months post stroke. AF, a history of tumors, and NIHSS score at admission were the independent risk factors of sICH.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by the ethics committee of Peking University First Hospital (IRB approval number: 2015[922]) and all participating hospitals. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
WeipS and YH: conceptualization, methodology, supervision, and writing—review and editing. ZS and YL: data curation. HJ, WeiS, RL, FL, JS, LT, GL, HC, GZ, LZ, XS, JQ, and YW: investigation. ZS: writing—original draft. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (Grant No. 81400944) and Peking University Medicine Seed Fund for Interdisciplinary Research (BMU2018MX020). The authors declare that this study received funding from Techpool Bio-Pharma Co., Ltd. The funder was not involved in the study design, collection, analysis, interpretation of data, the writing of this article or the decision to submit it for publication.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We thank all study participants and staffs in the CASTOR study.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2021.727304/full#supplementary-material
References
1. Vos T, Lim SS, Abbafati C, Abbas KM, Abbasi M, Abbasifard M, et al. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet. (2020) 396:1204–22. doi: 10.1016/S0140-6736(20)30925-9
2. Paciaroni M, Agnelli G, Corea F, Ageno W, Alberti A, Lanari A, et al. Early hemorrhagic transformation of brain infarction: rate, predictive factors, and influence on clinical outcome. Stroke. (2008) 39:2249–56. doi: 10.1161/STROKEAHA.107.510321
3. Strbian D, Sairanen T, Meretoja A, Pitkaniemi J, Putaala J, Salonen O, et al. Patient outcomes from symptomatic intracerebral hemorrhage after stroke thrombolysis. Neurology. (2011) 77:341–8. doi: 10.1212/WNL.0b013e3182267b8c
4. Lindley RI, Wardlaw JM, Sandercock PA, Rimdusid P, Lewis SC, Signorini DF, et al. Frequency and risk factors for spontaneous hemorrhagic transformation of cerebral infarction. J Stroke Cerebrovasc Dis. (2004) 13:235–46. doi: 10.1016/j.jstrokecerebrovasdis.2004.03.003
5. Terruso V, D'Amelio M, Di Benedetto N, Lupo I, Saia V, Famoso G, et al. Frequency and determinants for hemorrhagic transformation of cerebral infarction. Neuroepidemiology. (2009) 33:261–5. doi: 10.1159/000229781
6. Wang Y, Song Q, Cheng Y, Wei C, Ye C, Liu J, et al. Association between non-high-density lipoprotein cholesterol and haemorrhagic transformation in patients with acute ischaemic stroke. BMC Neurol. (2020) 20:47. doi: 10.1186/s12883-020-1615-9
7. Hong KS, Kang DW, Koo JS, Yu KH, Han MK, Cho YJ, et al. Impact of neurological and medical complications on 3-month outcomes in acute ischaemic stroke. Eur J Neurol. (2008) 15:1324–31. doi: 10.1111/j.1468-1331.2008.02310.x
8. Elsaid N, Mustafa W, Saied A. Radiological predictors of hemorrhagic transformation after acute ischemic stroke: an evidence-based analysis. Neuroradiol J. (2020) 33:118–33. doi: 10.1177/1971400919900275
9. Meretoja A, Putaala J, Tatlisumak T, Atula S, Artto V, Curtze S, et al. Off-label thrombolysis is not associated with poor outcome in patients with stroke. Stroke. (2010) 41:1450–8. doi: 10.1161/STROKEAHA.109.576140
10. Lee HJ, Lee JS, Choi JC, Cho YJ, Kim BJ, Bae HJ, et al. Simple estimates of symptomatic intracranial hemorrhage risk and outcome after intravenous thrombolysis using age and stroke severity. J Stroke. (2017) 19:229–31. doi: 10.5853/jos.2016.01109
11. Tong X, George MG, Yang Q, Gillespie C. Predictors of in-hospital death and symptomatic intracranial hemorrhage in patients with acute ischemic stroke treated with thrombolytic therapy: Paul Coverdell Acute Stroke Registry 2008-2012. Int J Stroke. (2014) 9:728–34. doi: 10.1111/ijs.12155
12. Seet RC, Rabinstein AA. Symptomatic intracranial hemorrhage following intravenous thrombolysis for acute ischemic stroke: a critical review of case definitions. Cerebrovasc Dis. (2012) 34:106–14. doi: 10.1159/000339675
13. Sung SF, Chen SC, Lin HJ, Chen YW, Tseng MC, Chen CH. Comparison of risk-scoring systems in predicting symptomatic intracerebral hemorrhage after intravenous thrombolysis. Stroke. (2013) 44:1561–6. doi: 10.1161/STROKEAHA.111.000651
14. Ginsberg MD, Hill MD. Symptomatic intracranial hemorrhage in the ALIAS Multicenter Trial: relationship to endovascular thrombolytic therapy. Int J Stroke. (2015) 10:494–500. doi: 10.1111/ijs.12476
15. Liu B, Wang D, Hao Z, Li D, Zhang J, Liu J, et al. Reduction in estimated glomerular filtration rate (eGFR) results in an increased risk of spontaneous hemorrhagic transformation in patients with large-artery atherosclerosis stroke. Curr Neurovasc Res. (2016) 13:75–81. doi: 10.2174/1567202612666151027151445
16. Hacke W, Kaste M, Bluhmki E, Brozman M, Dávalos A, Guidetti D, et al. Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. N Engl J Med. (2008) 359:1317–29. doi: 10.1056/NEJMoa0804656
17. Whiteley WN, Thompson D, Murray G, Cohen G, Lindley RI, Wardlaw J, et al. Targeting recombinant tissue-type plasminogen activator in acute ischemic stroke based on risk of intracranial hemorrhage or poor functional outcome. Stroke. (2014) 45:1000–6. doi: 10.1161/STROKEAHA.113.004362
18. Tissue plasminogen activator for acute ischemic stroke. N Engl J Med. (1995) 333:1581–7. doi: 10.1056/NEJM199512143332401
19. Tan S, Wang D, Liu M, Zhang S, Wu B, Liu B. Frequency and predictors of spontaneous hemorrhagic transformation in ischemic stroke and its association with prognosis. J Neurol. (2014) 261:905–12. doi: 10.1007/s00415-014-7297-8
20. Chen G, Wang A, Zhao X, Wang C, Liu L, Zheng H, et al. Frequency and risk factors of spontaneous hemorrhagic transformation following ischemic stroke on the initial brain CT or MRI: data from the China National Stroke Registry (CNSR). Neurol Res. (2016) 38:538–44. doi: 10.1080/01616412.2016.1187864
21. Wahlgren N, Ahmed N, Dávalos A, Ford GA, Grond M, Hacke W, et al. Thrombolysis with alteplase for acute ischaemic stroke in the Safe Implementation of Thrombolysis in Stroke-Monitoring Study (SITS-MOST): an observational study. Lancet. (2007) 369:275–82. doi: 10.1016/S0140-6736(07)60149-4
22. Malhotra K, Katsanos AH, Goyal N, Ahmed N, Strbian D, Palaiodimou L, et al. Safety and efficacy of dual antiplatelet pretreatment in patients with ischemic stroke treated with IV thrombolysis. Neurology. (2020) 94:e657–e66. doi: 10.1212/WNL.0000000000008961
23. Sun W, Ou Q, Zhang Z, Qu J, Huang Y. Chinese acute ischemic stroke treatment outcome registry (CASTOR): protocol for a prospective registry study on patterns of real-world treatment of acute ischemic stroke in China. BMC Complement Altern Med. (2017) 17:357. doi: 10.1186/s12906-017-1863-4
24. Hacke W, Kaste M, Fieschi C, von Kummer R, Davalos A, Meier D, et al. Randomised double-blind placebo-controlled trial of thrombolytic therapy with intravenous alteplase in acute ischaemic stroke (ECASS II). Lancet. (1998) 352:1245–51. doi: 10.1016/S0140-6736(98)08020-9
25. Liu M, Pan Y, Zhou L, Wang Y. Predictors of post-thrombolysis symptomatic intracranial hemorrhage in Chinese patients with acute ischemic stroke. PLoS ONE. (2017) 12:e0184646. doi: 10.1371/journal.pone.0184646
26. Tu HT, Campbell BC, Christensen S, Desmond PM, De Silva DA, Parsons MW, et al. Worse stroke outcome in atrial fibrillation is explained by more severe hypoperfusion, infarct growth, and hemorrhagic transformation. Int J Stroke. (2015) 10:534–40. doi: 10.1111/ijs.12007
27. Kim SJ, Seok JM, Bang OY, Kim GM, Kim KH, Jeon P, et al. MR mismatch profiles in patients with intracranial atherosclerotic stroke: a comprehensive approach comparing stroke subtypes. J Cereb Blood Flow Metab. (2009) 29:1138–45. doi: 10.1038/jcbfm.2009.38
28. Paciaroni M, Agnelli G, Micheli S, Caso V. Efficacy and safety of anticoagulant treatment in acute cardioembolic stroke. Stroke. (2007) 38:423–30. doi: 10.1161/01.STR.0000254600.92975.1f
29. Lee JH, Park KY, Shin JH, Cha JK, Kim HY, Kwon JH, et al. Symptomatic hemorrhagic transformation and its predictors in acute ischemic stroke with atrial fibrillation. Eur Neurol. (2010) 64:193–200. doi: 10.1159/000319048
30. Abdul-Rahim AH, Fulton RL, Frank B, Tatlisumak T, Paciaroni M, Caso V, et al. Association of improved outcome in acute ischaemic stroke patients with atrial fibrillation who receive early antithrombotic therapy: analysis from VISTA. Eur J Neurol. (2015) 22:1048–55. doi: 10.1111/ene.12577
31. Vannucchi V, Moroni F, Grifoni E, Marcucci R, Landini G, Prisco D, et al. Management of oral anticoagulation in very old patients with non valvular atrial fibrillation related acute ischemic stroke. J Thromb Thrombolysis. (2020) 49:86–93. doi: 10.1007/s11239-019-01972-0
32. Gioia LC, Kate M, Sivakumar L, Hussain D, Kalashyan H, Buck B, et al. Early rivaroxaban use after cardioembolic stroke may not result in hemorrhagic transformation: a prospective magnetic resonance imaging study. Stroke. (2016) 47:1917–9. doi: 10.1161/STROKEAHA.116.013491
33. Strbian D, Engelter S, Michel P, Meretoja A, Sekoranja L, Ahlhelm FJ, et al. Symptomatic intracranial hemorrhage after stroke thrombolysis: the SEDAN score. Ann Neurol. (2012) 71:634–41. doi: 10.1002/ana.23546
34. Emberson J, Lees KR, Lyden P, Blackwell L, Albers G, Bluhmki E, et al. Effect of treatment delay, age, and stroke severity on the effects of intravenous thrombolysis with alteplase for acute ischaemic stroke: a meta-analysis of individual patient data from randomised trials. Lancet. (2014) 384:1929–35. doi: 10.1016/S0140-6736(14)60584-5
35. Luo S, Zhuang M, Zeng W, Tao J. Intravenous thrombolysis for acute ischemic stroke in patients receiving antiplatelet therapy: a systematic review and meta-analysis of 19 studies. J Am Heart Assoc. (2016) 5:3242. doi: 10.1161/JAHA.116.003242
36. Larrue V, von Kummer RR, Müller A, Bluhmki E. Risk factors for severe hemorrhagic transformation in ischemic stroke patients treated with recombinant tissue plasminogen activator: a secondary analysis of the European-Australasian Acute Stroke Study (ECASS II). Stroke. (2001) 32:438–41. doi: 10.1161/01.STR.32.2.438
37. Huang G, Chen L, Qin C, Cheng D, Lu Q, Yu L, et al. Cerebral hemorrhage as the initial manifestation in patients with systemic cancer. Int J Neurosci. (2018) 128:48–54. doi: 10.1080/00207454.2017.1357553
38. Powers WJ, Rabinstein AA, Ackerson T, Adeoye OM, Bambakidis NC, Becker K, et al. Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 guidelines for the early management of acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. (2019) 50:e344–e418. doi: 10.1161/STR.0000000000000211
39. Wang A, Tian X, Gu H, Zuo Y, Meng X, Lv W, et al. CO2 combining power and outcomes in patients with acute ischaemic stroke or transient ischaemic attack. Stroke Vasc Neurol. (2021) 6:252–9. doi: 10.1136/svn-2020-000476
40. Andrade JBC, Mohr JP, Lima FO, de Carvalho JJF, Barros LCM, Nepomuceno CR, et al. The role of hemorrhagic transformation in acute ischemic stroke upon clinical complications and outcomes. J Stroke Cerebrovasc Dis. (2020) 29:104898. doi: 10.1016/j.jstrokecerebrovasdis.2020.104898
41. Xian Y, Xu H, Lytle B, Blevins J, Peterson ED, Hernandez AF, et al. Use of strategies to improve door-to-needle times with tissue-type plasminogen activator in acute ischemic stroke in clinical practice. Circulation. (2017) 10:3227. doi: 10.1161/CIRCOUTCOMES.116.003227
42. Meretoja A, Strbian D, Mustanoja S, Tatlisumak T, Lindsberg PJ, Kaste M. Reducing in-hospital delay to 20 minutes in stroke thrombolysis. Neurology. (2012) 79:306–13. doi: 10.1212/WNL.0b013e31825d6011
43. D'Amelio M, Terruso V, Famoso G, Di Benedetto N, Realmuto S, Valentino F, et al. Early and late mortality of spontaneous hemorrhagic transformation of ischemic stroke. J Stroke Cerebrovasc Dis. (2014) 23:649–54. doi: 10.1016/j.jstrokecerebrovasdis.2013.06.005
Keywords: ischemic stroke, symptomatic intracranial hemorrhage, risk factors, prognosis, atrial fibrillation, tumor
Citation: Shen Z, Jin H, Lu Y, Sun W, Liu R, Li F, Shu J, Tai L, Li G, Chen H, Zhang G, Zhang L, Sun X, Qiu J, Wei Y, Sun W and Huang Y (2021) Predictors and Prognosis of Symptomatic Intracranial Hemorrhage in Acute Ischemic Stroke Patients Without Thrombolysis: Analysis of Data From the Chinese Acute Ischemic Stroke Treatment Outcome Registry. Front. Neurol. 12:727304. doi: 10.3389/fneur.2021.727304
Received: 18 June 2021; Accepted: 27 August 2021;
Published: 28 September 2021.
Edited by:
Adriana Bastos Conforto, Universidade de São Paulo, BrazilReviewed by:
Yuishin Izumi, Tokushima University, JapanStefan Schwab, University Hospital Erlangen, Germany
Ashfaq Shuaib, University of Alberta, Canada
Copyright © 2021 Shen, Jin, Lu, Sun, Liu, Li, Shu, Tai, Li, Chen, Zhang, Zhang, Sun, Qiu, Wei, Sun and Huang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Weiping Sun, c3Vud2VpcGluZzE5NzkmI3gwMDA0MDsxNjMuY29t; Yining Huang, eW5odWFuZyYjeDAwMDQwO2JqbXUuZWR1LmNu
†These authors have contributed equally to this work and share last authorship
 Zhiyuan Shen
Zhiyuan Shen Haiqiang Jin1
Haiqiang Jin1 Ran Liu
Ran Liu Guozhong Li
Guozhong Li Huisheng Chen
Huisheng Chen Yining Huang
Yining Huang