
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Neurol. , 22 October 2021
Sec. Stroke
Volume 12 - 2021 | https://doi.org/10.3389/fneur.2021.727171
Background and Purpose: Prediction models for functional outcomes after ischemic stroke are useful for statistical analyses in clinical trials and guiding patient expectations. While there are models predicting dichotomous functional outcomes after ischemic stroke, there are no models that predict ordinal mRS outcomes. We aimed to create a model that predicts, at the time of hospital discharge, a patient's modified Rankin Scale (mRS) score on day 90 after ischemic stroke.
Methods: We used data from three multi-center prospective studies: CRISP, DEFUSE 2, and DEFUSE 3 to derive and validate an ordinal logistic regression model that predicts the 90-day mRS score based on variables available during the stroke hospitalization. Forward selection was used to retain independent significant variables in the multivariable model.
Results: The prediction model was derived using data on 297 stroke patients from the CRISP and DEFUSE 2 studies. National Institutes of Health Stroke Scale (NIHSS) at discharge and age were retained as significant (p < 0.001) independent predictors of the 90-day mRS score. When applied to the external validation set (DEFUSE 3, n = 160), the model accurately predicted the 90-day mRS score within one point for 78% of the patients in the validation cohort.
Conclusions: A simple model using age and NIHSS score at time of discharge can predict 90-day mRS scores in patients with ischemic stroke. This model can be useful for prognostication in routine clinical care and to impute missing data in clinical trials.
Prediction models of functional outcome after ischemic stroke can aid clinical decision making for providers, patients, and families by guiding rehabilitation goals, discharge planning, and patient expectations (1–3). They can also be useful for imputing missing data in clinical trials. These models stroke have generally focused on predicting a dichotomization of the modified Rankin Scale (mRS) such as functional independence (mRS 0–2) vs. functional dependency or death (mRS 3–6), or alive (mRS 0–5) vs. dead (mRS 6) (4–6). While these dichotomizations are meaningful, a model that could predict outcome across the entire spectrum of the mRS would be more informative. For example, for patients who have less severe strokes, a model predicting mortality may be less useful than a model that predicts the exact score on the mRS (7). Such a model could also be used to impute missing data in clinical trials when patients are lost to follow-up or when outcome data is not yet available.
To address this need, we aimed to develop an ordinal logistic regression model that predicts the 90-day mRS score based on variables available at the time of hospital discharge.
The data that support the findings of this study are available from the corresponding author upon reasonable request.
This study used de-identified patient data from three prior studies: CRISP, DEFUSE 2, and DEFUSE 3 (8–10). CRISP and DEFUSE 2 were multi-center prospective cohort studies and DEFUSE 3 was a prospective randomized open-label multicenter trial of endovascular therapy. Patients were older than 18, and only DEFUSE3 had an upper age limit of 90 years. Other inclusion criteria included diagnosis of ischemic stroke, eligibility for endovascular therapy, baseline NIHSS ≥ 5 (CRISP, DEFUSE 2) or ≥6 (DEFUSE 3). All studies received approval from local institutional review boards, and patients or their proxies provided written informed consent.
The dependent variable was the mRS score obtained 90 days after the index event. For patients with missing 90-day mRS scores, 30-day mRS scores were carried forward. Patients missing both outcome measures were excluded from the study. Patients who died during the initial hospitalization were also excluded.
Predictor variables included baseline characteristics [sex, age, history of atrial fibrillation, history of diabetes, history of hypertension, history of stroke or transient ischemic stroke (TIA)], imaging measures (24-h follow-up infarct volume, hemorrhagic transformation per the European Cooperative Acute Stroke Study criteria), and clinical measures (NIHSS score at discharge or day 5 of hospitalization, whichever occurred earlier).
The CRISP and DEFUSE 2 datasets were used as the derivation set. The ordinal outcome measure was the mRS score at 90 days. We used a proportional odds model, which estimates intercepts for each level, but assumes a common coefficient across ordered response categories. Validity of the proportional odds assumption was verified by trending univariate odds ratios for each cutoff and plotting partial residuals. The derivation dataset was “upsampled” to account for the relatively small number of participants with an mRS score of five at day 90. The upsampling method augments the minority class by sampling random observations from this class with replacement (i.e., bootstrapping). We implemented upsampling using the upsample function in the package R-splitters. After upsampling, variables that were associated with outcome at a p < 0.2 in univariate analysis were entered in a multivariable model and were retained if they reduced the AIC by seven or more points.
The model was internally and externally validated to assess model performance on unseen data, thereby mitigating possible overfitting of the model. The model was internally validated within the derivation set using five-fold cross-validation. Univariate screening and forward selection were repeated to derive a model for each fold. The model was externally validated using the DEFUSE 3 dataset.
Measures used to evaluate for model performance included R2 (coefficient of determination), mean absolute error (MAE), and the percentage of predicted outcomes that fell within one point of the observed outcomes. As an additional measure of model performance, we ran the primary efficacy analysis of the DEFUSE 3 trial based on imputed 90-day mRS scores and compared it to the same analysis using observed 90-day mRS scores.
Proportions were compared using Fisher's exact test, and distributions of continuous and ordinal variables were compared using the t-test or Wilcoxon rank-sum test. We report two-sided results and used a p < 0.05 as a threshold for statistical significance.
All statistical analyses were performed using R software (version 3.6.2) and SAS software (version 9.4).
There were 201 patients enrolled in the CRISP study, 130 in DEFUSE 2, and 182 in DEFUSE 3. In the derivation set (CRISP and DEFUSE 2), we excluded two patients with missing 30 and 90-day mRS outcome data, and 32 patients who died during their initial hospitalization. In the validation set (DEFUSE 3), we excluded 22 patients who died during their initial hospitalization. (Supplementary Figure I). The patient characteristics are presented in Table 1. Age (65.6 vs. 68.9, p = 0.02), prevalence of hypertension (66.7 vs. 78.8%, p = 0.01), hemorrhagic transformation score (p = 0.01), NIHSS score at discharge (6 vs. 8, p = 0.03), and infarct volume at early follow up (26.2 vs. 37.7 ml, p < 0.001) were different between the derivation and validation groups.
In the derivation set, predictors that were associated (p < 0.2) with the 90-day mRS score in univariate analyses, included age (p < 0.001), hypertension (p < 0.001), diabetes mellitus (p < 0.001), history of stroke (p = 0.12), hemorrhagic transformation (p < 0.001), NIHSS score at discharge (p < 0.001), infarct volume at early follow-up (p < 0.001). After forward selection, age and the NIHSS score at discharge remained as independent predictors of the 90-day mRS score in the multivariable ordinal regression model (p < 0.001 for each, Table 2).
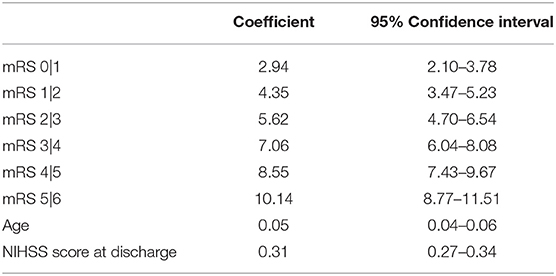
Table 2. Ordinal logistic regression predicting 90-day mRS derived from the full derivation set (n = 297).
With five-fold internal cross-validation, age and NIHSS were retained in each model and no other variable was retained in a majority of models. The mean R2 of the five validation models was 0.60, the average mean absolute error was 0.88 (95% CI 0.82–0.94), and a mean of 80% of predictions were within one of the observed value. Additional results of the five-fold internal cross-validation are shown in Table 3 and Supplementary Figure II. In external validation, using the DEFUSE 3 dataset, the model had an R2 of 0.60, and a mean absolute error of 0.94 (95% CI 0.80–1.07). The model predicted 34% of the 90-day mRS scores correctly, 78% of the scores within one point of the observed value, 96% within two points of the observed value, and 99% within three points of the observed value. Additional performance metrics of the model in external validation are presented in Table 3 and illustrated in Figure 1. There was no significant difference in the model's performance when validated on the subset of DEFUSE 3 patients who were treated with endovascular therapy and those who were treated with medical management alone (MAE 0.85 vs. 1.04, p = 0.15, Supplementary Figure III). We also compared the model in the subset of DEFUSE 3 patients with more severe disability at discharge or day 5 (75% quantile: NIHSS ≥ 16), and there was no significant difference in the models' performance (MAE 0.90 vs. 0.95, p = 0.75, Supplementary Figure IV).
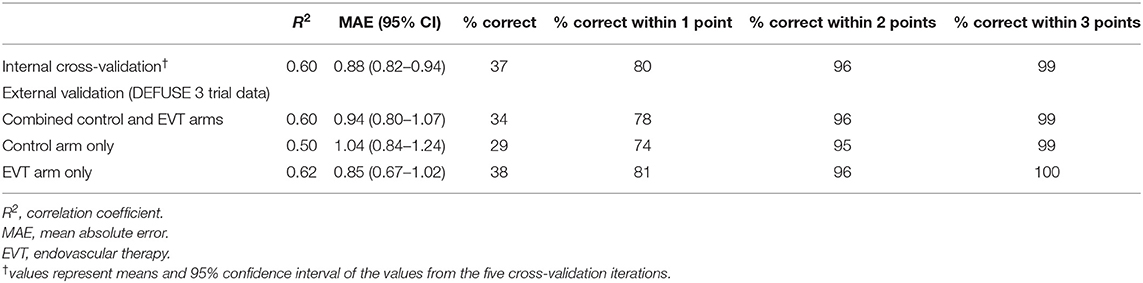
Table 3. Performance metrics of the 90-day mRS prediction model in internal and external validation.
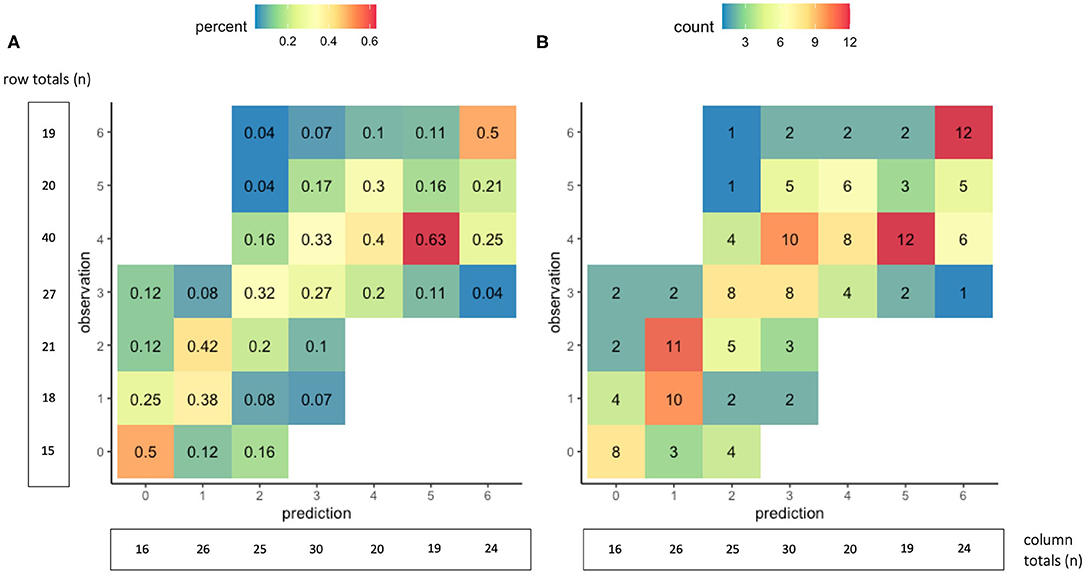
Figure 1. Accuracy of the 90-day mRS prediction model in external validation. The graphs show cross-tabulations between the observed and predicted values of the 90-day mRS for the 160 patients in the external validation group. Values in graph (A) show proportions, where the columns represent the distribution of observed outcomes for a given predicted outcome. For example, of the 16 patients with a predicted mRS score of 0, the observed 90-day mRS score was 0 in 50%, one in 25%, two in 12.5%, and three in 12.5%. Values in graph (B) show counts of the observed and predicted mRS scores at 90 days.
The primary analysis of the DEFUSE 3 trial, a comparison of the distribution of 90-day mRS scores between patients in the endovascular treatment and control groups, was rerun with 90-day mRS scores predicted by the final model. The odds ratio for benefit from endovascular therapy using predicted mRS scores was 5.01 (95% CI 2.77–9.05), which is similar to the results of the same analysis performed using observed mRS scores (OR 3.45, 95% 1.94–6.13).
A prediction model based on two easily obtainable clinical variables, age and NIHSS score assessed at the time of hospital discharge, had moderately high accuracy for predicting a patient's 90-day modified Rankin Scale score. It accurately predicted the 90-day mRS within one point in ~ 80% of patients. The model's independent variables — age and NIHSS score — are corroborated by prior studies that have demonstrated that these factors are correlated with functional outcome after ischemic stroke (11–14). This model can be used for prognostication in clinical practice and for imputation of 90-day outcome data in clinical trials.
Several prior studies have focused on predicting survival or other dichotomized functional outcomes after ischemic stroke (5, 6). A recent meta-analysis by Fahey et al. identified over 60 models for outcomes following ischemic stroke (4). Variables such as sex, age, disease characteristics, and comorbidities were the best predictors for mortality and functional outcomes after ischemic stroke. The meta-analysis noted that a limitation of current models is that few models are externally validated (4). In addition, because the current models were derived with dichotomous outcome data, none predict a patient's exact score on the mRS at long-term follow-up. In contrast, our externally validated ordinal logistic regression model predicts a patient's 90-day mRS score with moderately high accuracy.
One of the relative advantages of an ordinal regression is that there is less information loss as compared to dichotomous outcome models. Multiple studies have shown that ordinal analyses increase statistical power and efficiency, and suggest that further clinical research could benefit from increased utilization of ordinal analyses where relevant (15–17). Because of this, most recent acute stroke studies use an ordinal model for their primary outcome analysis. Unlike dichotomous prediction models, our model could be used to impute missing outcomes if 30- and 90-day mRS data are missing. Moreover, an ordinal prognostic model could help optimize future randomized trials of stroke rehabilitation interventions, by excluding patients who are likely to be non-responders because they have either a high chance of spontaneous recovery or a high chance of mortality (18). In addition, our prediction model could be used in clinical practice as a tool to assist with the assessment of a patient's prognosis. This could improve stroke rehabilitation by personalizing rehabilitation plans, reducing variation in therapy, and increasing equity of services. A recent review of prediction tools for stroke rehabilitation found that models were most helpful if they were available at the time of rehabilitation or discharge planning and predicted functional status beyond binary outcomes. The authors were concerned that a general prognosis of good or poor is not sufficiently detailed to be useful (6). Thus, 90-day ordinal predictions could be a helpful reference in discussions with the patient and family and provide additional context when discussing rehabilitation and future residence destination (19). Even though our model is only able to predict well within one point, we believe this presents an advantage over dichotomous models which predict a range without identifying the most likely outcome within that range.
Some researchers have cited concern about the use of ordinal regression models and the need to test for proportionality of the odds. To address this, we confirmed proportionality by visual inspection of the univariate odds ratios and partial residuals. In addition, we empirically demonstrated the model's performance by validating its accuracy in an external dataset.
There are limitations to our study. First, our dataset is limited to patients from three endovascular stroke therapy studies and is therefore not representative of all patients with ischemic strokes. Specifically, the model may not apply to patients with mild strokes or strokes in the posterior circulation who were not eligible for the trials. The model may also not apply well to patients with pre-existing severe disability who were excluded from the studies. The model did perform similarly in patients who underwent endovascular therapy and those who did not, likely because the effect of endovascular therapy is captured in the NIHSS score at discharge, which is one of the prediction variables in the model (20, 21) Future studies validating this model in a larger cohort could provide additional information about the generalizability of this model. Second, while the simplicity of our model, which only includes two predictor variables, is a relative strength, a model derived from an even larger dataset could identify additional independent variables that might further improve the model's performance.
In summary, our internally and externally validated model predicts the ordinal mRS score at 90 days after ischemic stroke with moderately good accuracy and could be used for prognostication in clinical practice and to impute missing data in clinical trials.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by the Stanford IRB (DEFUSE 2 and CRISP) and the University of Cincinnati IRB serving as the Central IRB for StrokeNet (DEFUSE 3). The patients/participants provided their written informed consent to participate in this study.
ML and MM organized the database. MZ wrote the first draft of the manuscript. All authors contributed to conception and design of the study, statistical analysis, manuscript revision, read, and approved the submitted version.
The study was funded by grants from the National Institute for Neurological Disorders and Stroke (principal investigators, ML and GA; 1TR01ActNS075209Project01A1Year, 5TR01ActNS075209Project02Year, 5TR01ActNS075209Project03Year, 5TR01ActNS075209Project04Year, 5TR01ActNS075209Project05Year, 2TR01ActNS075209Project06Year, 5TR01ActNS075209Project07Year, 5TR01ActNS075209Project08Year, 1TK23ActNS051372Project01A1Year, 5TK23ActNS051372Project02Year, 5TK23ActNS051372Project03Year, 5TK23ActNS051372Project04Year, 3TK23ActNS051372Project04S1Year, 5TK23ActNS051372Project05Year, 1TU10ActNS086487Project01Year, 5TU10ActNS086487Project02Year, 5TU10ActNS086487Project03Year, 4TU10ActNS086487Project04Year, 5TU10ActNS086487Project05Year, 1TU01ActNS092076Project01A1Year, 5TU01ActNS092076Project02Year) and the Stanford Medical Scholars Research Program.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2021.727171/full#supplementary-material
1. Weimar C, Ziegler A, König IR, Diener HC. Predicting functional outcome and survival after acute ischemic stroke. J Neurol. (2002) 249:888–95. doi: 10.1007/s00415-002-0755-8
2. Wang Y, Lim LLY, Heller RF, Fisher J, Levi CR. A prediction model of 1-year mortality for acute ischemic stroke patients. Arch Phys Med Rehabil. (2003) 84:1006–11. doi: 10.1016/S0003-9993(03)00032-7
3. Kwakkel G, Wagenaar RC, Kollen BJ, Lankhorst GJ. Predicting disability in stroke - a critical review of the literature. Age Ageing. (1996) 25:479–89. doi: 10.1093/ageing/25.6.479
4. Fahey M, Crayton E, Wolfe C, Douiri A. Clinical prediction models for mortality and functional outcome following ischemic stroke: a systematic review and meta-analysis. PLoS ONE. (2018) 13:1–13. doi: 10.1371/journal.pone.0185402
5. Veerbeek JM, Kwakkel G, van Wegen EEH, Ket JCF, Heymans MW. Early prediction of outcome of activities of daily living after stroke: a systematic review. Stroke. (2011) 42:1482–8. doi: 10.1161/STROKEAHA.110.604090
6. Stinear CM, Smith MC, Byblow WD. Prediction tools for stroke rehabilitation. Stroke. (2019) 50:3314–22. doi: 10.1161/STROKEAHA.119.025696
7. Risselada R, Lingsma HF, Molyneux AJ, Kerr RSC, Yarnold J, Sneade M, et al. Prediction of two month modified Rankin scale with an ordinal prediction model in patients with aneurysmal subarachnoid haemorrhage. BMC Med Res Methodol. (2010) 10:86. doi: 10.1186/1471-2288-10-86
8. Lansberg MG, Christensen S, Kemp S, Mlynash M, Mishra N, Federau C, et al. Computed tomographic perfusion to predict response to recanalization in ischemic stroke. Ann Neurol. (2017) 81:849–56. doi: 10.1002/ana.24953
9. Lansberg MG, Straka M, Kemp S, Mlynash M, Wechsler LR, Jovin TG, et al. Magnetic resonance imaging profile and response to endovascular reperfusion: results of the DEFUSE 2 prospective cohort study. Lancet Neurol. (2012) 11:860–7. doi: 10.1016/S1474-4422(12)70203-X
10. Albers GW, Marks MP, Kemp S, Christensen S, Tsai JP, Ortega-Gutierrez S, et al. Thrombectomy for stroke at 6 to 16 hours with selection by perfusion imaging. N Engl J Med. (2018) 378:708–18. doi: 10.1056/NEJMoa1713973
11. Wahlgren NG, Ranasinha KW, Rosolacci T, Franke CL, van Erven PMM, Ashwood T, et al. Clomethiazole acute stroke study (CLASS): results of a randomized, controlled trial of clomethiazole versus placebo in 1360 acute stroke patients. Stroke. (1999) 30:21–8. doi: 10.1161/01.STR.30.1.21
12. Adams HP, Davis PH, Leira EC, Chang K-C, Bendixen BH, Clarke WR, et al. Baseline NIH stroke scale score strongly predicts outcome after stroke: a report of the trial of org 10172 in acute stroke treatment (TOAST). Neurology. (1999) 53:126. doi: 10.1212/WNL.53.1.126
13. Johnston KC, Connors AF, Wagner DP, Knaus WA, Wang X-Q, Haley EC, et al. Predictive risk model for outcomes of ischemic stroke. Stroke. (2000) 31:448–55. doi: 10.1161/01.STR.31.2.448
14. Wardlaw JM, Dorman PJ, Candelise L, Signorini DF. The influence of baseline prognostic variables on outcome after thrombolysis. J Neurol. (1999) 246:1059–62. doi: 10.1007/s004150050512
15. Chen L. Overview of clinical prediction models. Ann Transl Med. (2020) 8:71. doi: 10.21037/atm.2019.11.121
16. Roozenbeek B, Lingsma HF, Perel P, Edwards P, Roberts I, Murray GD, et al. The added value of ordinal analysisin clinical trials: an example in traumatic brain injury. Crit Care. (2011) 15:R127. doi: 10.1186/cc10240
17. McHugh GS, Butcher I, Steyerberg EW, Marmarou A, Lu J, Lingsma HF, et al. Simulation study evaluating approaches to the analysis of ordinal outcome data in randomized controlled trials in traumatic brain injury: results from the IMPACT project. Clin Trials. (2010) 7:44–57. doi: 10.1177/1740774509356580
18. König IR, Ziegler A, Bluhmki E, Hacke W, Bath PMW, Sacco RL, et al. Predicting long-term outcome after acute ischemic stroke: a simple index works in patients from controlled clinical trials. Stroke. (2008) 39:1821–6. doi: 10.1161/STROKEAHA.107.505867
19. de Ridder IR, Dijkland SA, Scheele M, den Hertog HM, Dirks M, Westendorp WF, et al. Development and validation of the Dutch stroke score for predicting disability and functional outcome after ischemic stroke: a tool to support efficient discharge planning. Eur Stroke J. (2018) 3:165–73. doi: 10.1177/2396987318754591
20. Kissela B, Lindsell CJ, Kleindorfer D, Alwell K, Moomaw CJ, Woo D, et al. Clinical prediction of functional outcome after ischemic stroke. Stroke. (2009) 40:530–6. doi: 10.1161/STROKEAHA.108.521906
Keywords: modified rankin scale, neurology, ischemic stroke, outcome, ordinal regression
Citation: Zhang MY, Mlynash M, Sainani KL, Albers GW and Lansberg MG (2021) Ordinal Prediction Model of 90-Day Modified Rankin Scale in Ischemic Stroke. Front. Neurol. 12:727171. doi: 10.3389/fneur.2021.727171
Received: 18 June 2021; Accepted: 21 September 2021;
Published: 22 October 2021.
Edited by:
Vasileios-Arsenios Lioutas, Beth Israel Deaconess Medical Center and Harvard Medical School, United StatesReviewed by:
Apostolos Safouris, Metropolitan Hospital, GreeceCopyright © 2021 Zhang, Mlynash, Sainani, Albers and Lansberg. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Maarten G. Lansberg, bGFuc2JlcmdAc3RhbmZvcmQuZWR1
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.