- 1Department of Neurology, Charité — Universitätsmedizin Berlin, Corporate Member of Freie Universität Berlin, Humboldt-Universität zu Berlin, Berlin, Germany
- 2NeuroCure Clinical Research Center, Charité — Universitätsmedizin Berlin, Corporate Member of Freie Universität Berlin, Humboldt-Universität zu Berlin, and Berlin Institute of Health, Berlin, Germany
- 3Center for Stroke Research Berlin, Charité — Universitätsmedizin Berlin, Corporate Member of Freie Universität Berlin, Humboldt-Universität zu Berlin, and Berlin Institute of Health, Berlin, Germany
- 4Department of Immunology, Charité — Universitätsmedizin Berlin, Berlin, Germany
- 5Department of Immunology, Labor Berlin, Charité Vivantes GmbH, Berlin, Germany
- 6German Myasthenia Gravis Society (Deutsche Myasthenie Gesellschaft, DMG), Bremen, Germany
- 7Department of Neurology, Jüdisches Krankenhaus Berlin, Berlin, Germany
Background: In chronic inflammatory demyelinating polyneuropathy (CIDP), there is an urgent need for biomarkers to monitor ongoing disease activity. Serum calprotectin (CLP) induces signaling pathways involved in inflammatory processes and has been shown to correlate with markers of disease activity in other autoimmune disorders. Thus, we wanted to study the potential value of CLP in comparison to serum neurofilament light chain (sNfl) to monitor disease activity.
Materials and Methods: Sera from 63 typical and atypical CIDP and 6 MMN patients with varying degrees of disease activity were analyzed in comparison with 40 healthy controls (HC) in a cross-sectional design. Association of CLP and sNfl levels with socio-demographics, disease duration, CIDP disease activity scale (CDAS), and impairment status [medical research council-sum score (MRC-SS), the inflammatory neuropathy cause and treatment disability score (INCAT-DS), grip strength, and maximum walking distance], patient-reported outcome (PRO) parameters [SF-36 questionnaire, Beck's depression index (BDI), and fatigue severity scale (FSS)], as well as treatment regime were investigated using uni- and multivariate analysis.
Results: CLP and sNfl levels were significantly higher in all CIDP patients compared to HC (p = 0.0009). Multivariate analysis adjusted for age and gender revealed that CLP acts as an independent predictor for CIDP and MMN. CLP was significantly associated with active disease course according to CDAS and correlated with MRC-SS, whereas sNfl correlated with parameters of disease impairment. There was no correlation with PRO, except for sNfl and the mental health composite score. Subgroup analysis revealed no differences between typical CIDP and atypical variants.
Conclusions: CLP was elevated in CIDP and variants and was associated with active disease course, whereas sNfl shows further potential as biomarker of axonal degeneration. Thus, CLP might be a suitable additive biomarker for measurement of ongoing inflammation, which is greatly needed to guide better patient care in CIDP.
Introduction
Chronic inflammatory demyelinating polyneuropathy (CIDP) is a rare, acquired immune-mediated demyelinating neuropathy, characterized by strong heterogeneity in terms of clinical presentation, prognosis, and treatment response (1). Diagnosis is based on progressive or relapsing course over 2 months, electrophysiological evidence of peripheral demyelination, and response to immune-modulating therapies (2–4). Different autoimmune targets are likely to be relevant, as both autoreactive T-cell responses against myelin antigens (5, 6) and auto antibodies (Abs) against paranodal (7) and ganglioside proteins (8) have been shown to be involved in the immunopathogenesis of CIDP. Thus, not only misdiagnosis is a common problem, as only a subgroup of patients presents with detectable auto-Abs, but also monitoring disease activity in order to guide treatment management, as nerve conduction studies and electromyography do not adequately reflect functional disability, especially in severe disease courses (9, 10).
Calprotectin (CLP), a calcium-binding protein of the S100 family, performs various biological functions via interaction with Toll-like receptor 4 (11) and is mainly released by activated leucocytes triggering signaling pathways involved in inflammatory processes (12). Thus, CLP has been investigated in other autoimmune diseases, mainly inflammatory bowel disease (IBD) (13, 14) and rheumatoid arthritis (RA) (15), where CLP strongly predicted disease activity and treatment response. CLP leads to the release of pro-inflammatory cytokines (16, 17) and induction of auto-reactive CD8+ T cells (17), which play a key role in the pathogenesis of CIDP (18, 19).
There is increasing evidence that serum neurofilament light chain (sNfl), which is released upon neuronal injury (20), is increased in patients with CIDP and correlates with markers of disease severity (21–24). Nevertheless, the major limitation of sNfl is the lack of age-specific cutoff values (25, 26). In addition, there is an unmet need for serum biomarkers to differentiate disease activity from disease progression.
Hence, we wanted to investigate the potential additive value of CLP as a disease activity biomarker using a cohort of CIDP patients with different variants compared to healthy controls (HC).
Materials and Methods
Standard Protocol Approvals, Registrations, and Patient Consent
The study was approved by the ethics committee of the Charité-Universitätsmedizin Berlin (EA1/025/11). All patients gave written informed consent in accordance with the Declaration of Helsinki in its currently applicable form.
Study Design
This is an exploratory, cross-sectional case–control study comparing CLP levels of CIDP and MMN patients as well as HC patients to assess the potential of CLP in comparison to sNfl for monitoring disease activity and impairment as measured by CIDP disease activity scale (CDAS) (27), clinical impairment status [medical research council-sum score (MRC-SS) (28), the inflammatory neuropathy cause and treatment disability score (INCAT-DS) (29), grip strength, and walking distance], PRO parameters [short form health survey (SF-36 questionnaire) (30), fatigue severity scale (FSS) (31), and Beck's depression inventory (BDI) (32)], disease duration, and treatment regime.
Patients and Controls
This study was performed at the outpatient center for inflammatory polyneuropathies of Charité-Universitätsmedizin Berlin, Germany. Patients over the age of 18 years who fulfilled the definite, probable, or possible EFNS/PNS criteria (3) were included independent of disease duration and severity. Moreover, all patients were tested for onconeural as well as paranodal and ganglioside antibodies (Abs) and were screened for the presence of other autoimmune disorders. Overall, 63 CIDP patients were consecutively screened at the outpatient clinic between April 2015 and February 2021 and were further categorized in subgroups according to clinical phenotype [typical, pure sensory, multifocal acquired demyelinating sensory, and motor polyneuropathy (MADSAM), and distal acquired demyelinating symmetric polyneuropathy (DADS)] (3, 33), aGAAbs-status, and treatment regime (therapy naïve; maintenance treatment with corticosteroids, intravenous immunoglobulins, immunosuppressive therapies; remission). Socio-demographics (age, sex), disease duration, patient-reported outcome (PRO) measures [SF-36 (30), FSS (31), BDI (32)], and comorbidities were collected in a database. Exclusion criteria were age <18, the presence of other autoimmune diseases due to potential influence in CLP levels, diagnosis of neurodegenerative diseases [atypical and typical parkinsonian disorders, frontotemporal dementia, Alzheimer's disease, and amyotrophic lateral sclerosis (34)], central nervous inflammatory diseases (multiple sclerosis and autoimmune encephalitis), and traumatic brain injury due to potential influence on sNFL levels. Healthy voluntary controls were enrolled as a HC group (n = 40).
Clinical Assessment
Patients were examined by the treating neurologist (JK, BH, and FS). Socio-demographics and current medication were documented. To measure the muscle strength on the level of impairment, the medical research council sum scale (MRC-SS) (28) was assessed. For the MRC-SS the following muscles had been tested on both sides: shoulder abduction, elbow flexion, wrist extension, index finger extension, hip flexion, knee extension, ankle dorsiflexion, and extension of the big toe. A sum score of 80 indicates a normal muscle strength. The adjusted inflammatory neuropathy cause and treatment disability score (INCAT-DS) (29) was used to assess the clinical disability in daily arm and leg mobility and has evolved as the most established primary outcome in clinical trials (35). Grip strength was measured by using the Martin Vigorimeter (36), and we assessed maximum walking distance. PRO was measured using the German version of the SF-36 questionnaire consisting of eight subscales and two summary scores—physical health composite score (PCS) and mental health composite score (MCS). Scores are ranging from 0 to 100, where higher scoring indicates a better quality of life. FSS was assessed to evaluate severity of fatigue (31) as well as BDI (32) to screen for depression.
In addition, we assessed treatment regime (corticosteroids, intravenous immunoglobulins, plasmapheresis, and immunosuppressive therapies) and treatment duration and further categorized according to CDAS (27) into active disease status and remission.
CLP and sNfl Measurements
Blood samples were collected from patients with CIDP and HC, immediately centrifuged, and stored at −80°C until being analyzed in May and February 2021. Serum levels of CLP were measured using the fCAL turbo method? on a COBAS 8000 semi-automated analyses (Bühlmann Laboratories AG, Schönenbuch, Switzerland) according to the manufacturer's protocol (37, 38). The fCAL turbo method? has been validated for accurate measurement of CLP levels in serum (37). sNfl concentrations were measured using SIMOA Nf-light kit® in SR-S immunoassay analyzer, SIMOA™ (Quanterix Corp., Boston, MA, USA) according to the manufacturer's protocol (39). SIMOA Nf-light kit® is an ultrasensitive paramagnetic bead-based enzyme-linked immunosorbent assay, is at least 125 times more sensitive than conventional immunoassays, and maintains a high analytical performance (40).
Statistical Analysis
All statistical analyses were performed using Prism (version 9.0.1, GraphPad Software, San Diego, CA, USA) and SPSS (version 25; IBM, Armonk, NY, USA). Continuous data are presented as means and standard deviation (SD) or median and quartiles as appropriate, and categorical variables are presented as absolute frequencies and percentages. CLP and sNfL levels between typical CIDP, atypical variants, and HC were compared using non-parametric tests given that CLP and sNfl levels are not normally distributed. Correlations between CLP, sNfL levels, and clinical assessments were analyzed by Spearman correlation. Mann–Whitney U and Kruskal–Wallis tests were used to compare medians of non-parametric variables between two or more groups, and Fisher's t-test was used to compare parametric variables. A two-tailed p < 0.05 was considered statistically significant. Multivariate logistic regression analysis was performed to assess dependency of CLP and sNfl levels on age and gender.
Primary Research Questions
Are CLP levels elevated in typical CIDP patients and atypical variants compared to HC and can elevated levels be used as a biomarker to monitor disease activity and PRO?
Results
Demographics and characteristics of CIDP and MMN patients
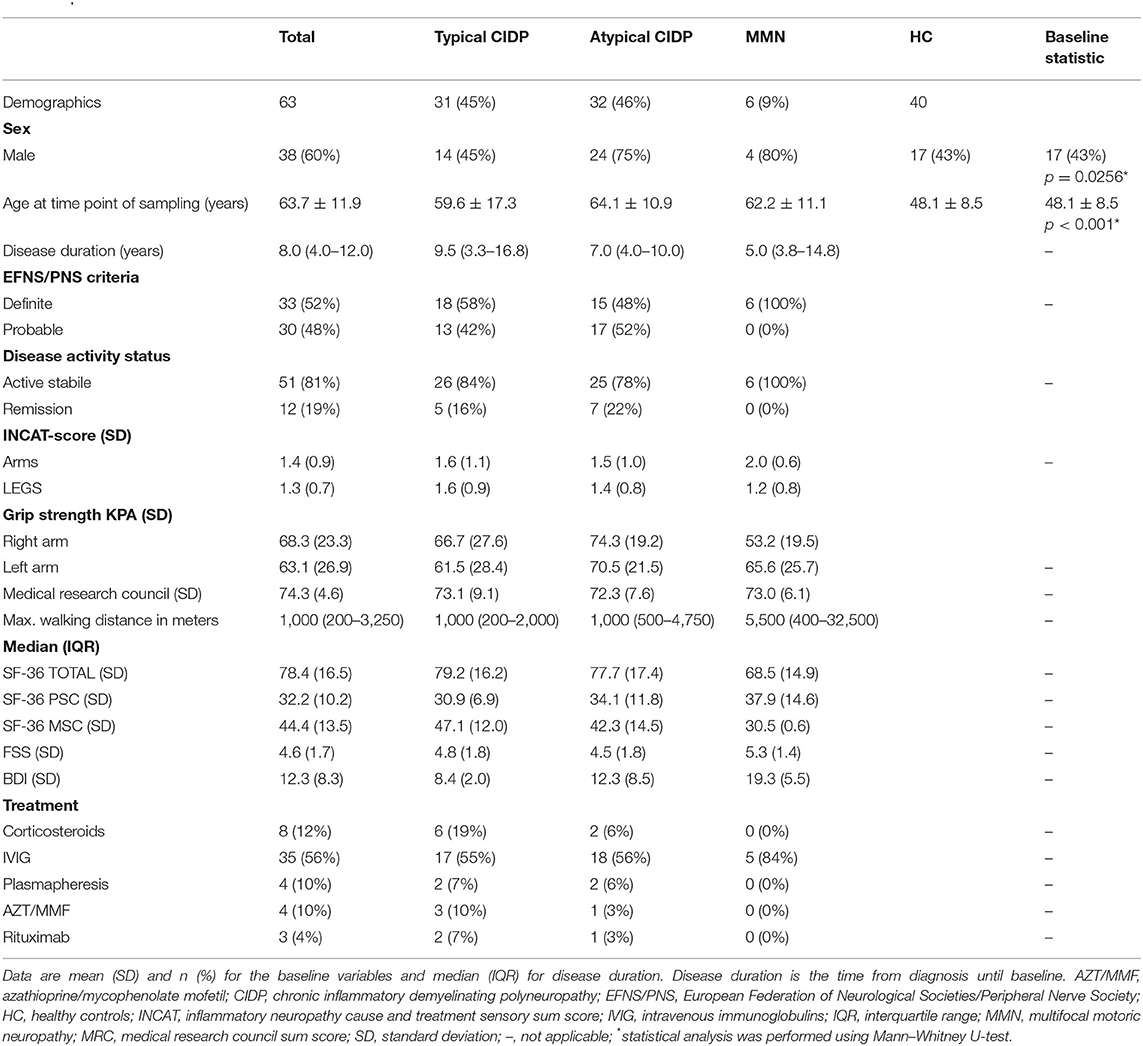
Altogether, we included 63 patients with CIDP and 40 HC (Table 1). Thirty-one patients suffered from typical CIDP (45%), and 32 (46%) suffered from an atypical variant. Of those with atypical variants, 9 patients suffered from pure sensory CIDP (14%), 14 (22%) from DADS, and 9 patients (14%) from MADSAM. Mean age was 63.7 years (SD 11.9 [IQR 55–73]); 60% (n = 38) were male. Median disease duration was 8.0 years (IQR 4.0–12.0). CIDP patients and HC did not differ in sex; however, atypical and MMN patients were more frequently male (75%, n = 24; 80%, n = 4). The majority of patients had an active disease activity status (81%, n = 51); 19% (n = 12) were in remission based on CDAS (27).
CLP and sNfl Is Independently Associated With CIDP and Variants
CLP levels were significantly higher in CIDP patients with a median of 2.6 μg/ml (IQR 1.8–4.1) compared to 1.4 μg/ml in HC (IQR 1.0–2.7; p = 0.0009, Mann–Whitney U-test; Figure 1). sNfl levels were significantly higher in CIDP patients with a median of 17.6 pg/ml (IQR 11.0–25.1) compared to 7.9 pg/ml in HC (IQR 6.5–9.5; p < 0.0001; Mann–Whitney U-test; Figure 2).
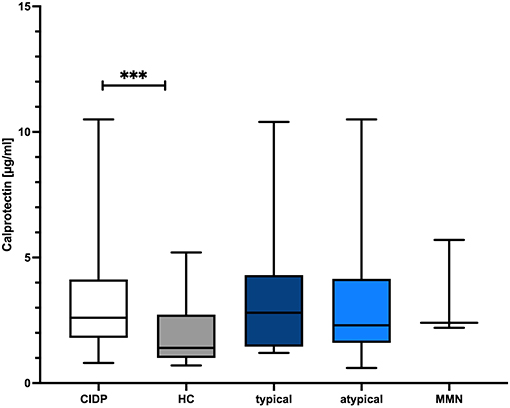
Figure 1. Comparison of CLP level in CIDP and HC. The boxplot bar represents median (min.–max.) of serum calprotectin (CLP) level in all CIDP patients (n = 63) and HC (n = 40). Median CLP levels were significantly increased in all CIDP patients regardless of subtype (p = 0.009) (typical: n = 31, atypical: n = 32, MMN n = 6). CIDP, chronic inflammatory demyelinating polyneuropathy; HC, healthy controls; min., minimum; max., maximum; MMN, multifocal motoric neuropathy; n, number of included study participants. ***p < 0.001, ****p < 0.0001.
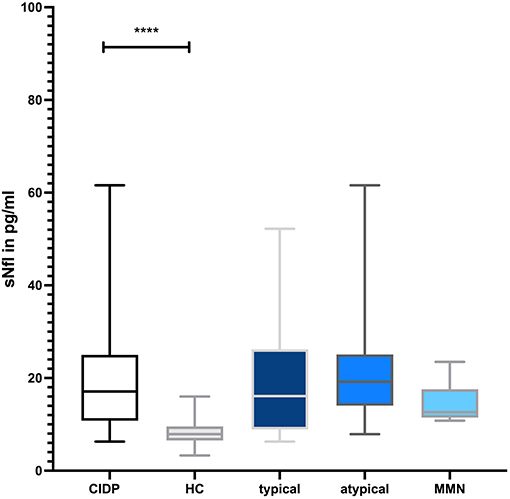
Figure 2. Comparison of sNfl level in CIDP and HC. The boxplot bar represents median (min.–max.) of serum neurofilament (sNfl) level in all CIDP patients (n = 63) and HC (n = 30). Median sNfl levels were significantly increased in all CIDP patients regardless of subtype (p < 0.001) (typical: n = 31, atypical: n = 32, MMN n = 6).
There was no significant difference in median CLP and sNfl levels between typical and atypical CIDP variants as well as MMN patients (p = 0.79 for CLP; p = 0.199 for sNfl, Kruskal–Wallis test). There was no correlation of CLP with age (r = 0.156, p = 0.136, Spearman correlation coefficient) and no association with gender (p = 0.299; Mann–Whitney U-test), whereas sNfl was highly associated with both age (r = 0.685, p < 0.001), and gender (p = 0.01; Mann–Whitney U-test).
Multivariate binary logistic regression models with backward stepwise inclusion to establish independence of age and gender were performed. In the model using sNFL to predict inflammatory neuropathies (p < 0.001), sNFL was the only remaining independent predictor, whereas the CLP model retained age and CLP (both p < 0.001) as independent predictor. Age was not distributed equally among the inflammatory neuropathies group and HC (Mann–Whitney U-test, p < 0.001).
CLP Levels Associated With CDAS and MRC-sum Score
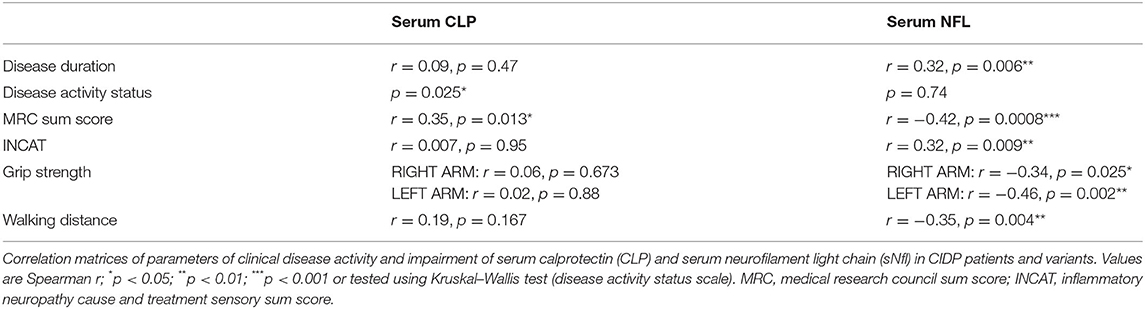
CLP was significantly associated with active disease course (unstable active disease and active disease) based on CDAS definition (27) (p = 0.025, Fisher's exact t-test) (Figure 3) and correlated moderately with MRC-sum score (r = 0.35, p = 0.015; Spearman correlation coefficient), whereas sNfl correlated with all parameters of disease impairment as well as with disease duration (r = 0.32, p = 0.006; Spearman correlation coefficient) (Table 2).
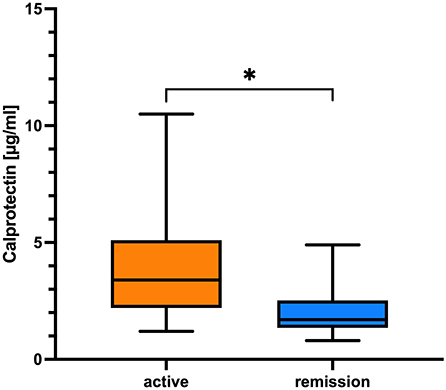
Figure 3. Comparison of CLP in regard to CDAS. The boxplot bar represents median (min.–max.) serum calprotectin level in regard to CIDP disease activity scale (CDAS) showing higher levels in patients with active disease (n = 51) in comparison to patients in remission (n = 12; *p = 0.025, Fisher's exact t-test).
CLP and sNfL Levels Are Not Associated With Patient-Reported Outcomes
Except for SF-MSC in correlation with sNfl (r = 0.32, p = 0.044; Spearman correlation coefficient), we found no association of CLP or sNfl in regard to PROs (FSS, BDI, and SF-PSC).
sNfL Levels and Treatment Regime
Neither CLP nor sNfl did significantly differ in regard to treatment regime (p = 0.643 and p = 0.5, respectively; Kruskal–Wallis test).
Discussion
This cross-sectional case–control study demonstrates for the first time that CLP is significantly increased in CIDP patients with different variants in comparison to HC. To emphasize, CLP was associated with active disease course as defined by CDAS and correlated with the MRC-SS. Thus, CLP might be of interest as an additional serum biomarker in the context of active CIDP. We also confirmed previous study results of sNfl being increased in CIDP patients correlating with parameters of clinical impairment. For emphasis and in order to reflect real-world-setting, we additionally examined the relationship of sNfl in regard to clinical characteristics and PRO parameters. The latter has not been investigated in other studies so far (21–24), although we found no significant relationship.
Despite recent advances in biomarker research for CIDP, nodal, paranodal, and anti-ganglioside antibodies (aGAAbs) can be detected only in a small proportion of patients (7, 8). For the larger CIDP population, diagnostic and prognostic blood biomarkers are still an unmet need. Even more important, there is a lack of objective, disease activity parameters to guide treatment response, especially in patients under maintenance therapy.
A recent report showed increased pathologic spontaneous activity to predict a more severe course of disease (9). Nevertheless, whether nerve conduction studies correlate with functional disability is still under discussion and requires external validation (10). Furthermore, electromyography is less readily available than a laboratory analysis, enabling repeated sampling and possibly allowing conclusions on the current disease activity.
CLP leads to induction of auto-reactive CD8+ T cells, IL-17 (41), and other pro-inflammatory cytokines like IL-1β. The imbalance of inflammatory cytokines and auto-reactive CD8+ T cells are crucially involved in the pathogenesis of CIDP and plays a central role. IL-17 was reported to be increased in peripheral blood mononuclear cell and cerebrospinal fluid of active CIDP in comparison with remitting CIDP or to other non-inflammatory neurological diseases (42). In addition, in a recent mouse model, auto-reactive CD8+ T cells were found in diseased nervous tissues, thus hinting further to a critical role in the pathogenesis and disease activity in CIDP (18). These results might explain the significant elevated CLP level in CIDP patients with disease activity according to CDAS, which we could not observe for sNfl. In addition, CLP correlated positively with MRC-SS, suggesting lower levels of axonal degeneration and persistent clinical impairment as in early phase of disease as well as in good treatment responders. This important finding relates to studies of CLP in IBD and RA, where CLP is routinely used as a disease activity marker (14, 43). CLP is a sensitive marker of inflammation mainly reflecting different degrees of disease activity and thus may guide treatment decisions, as proposed in other conditions like IBD (13) and RA (15, 44). In addition, CLP has the potential to serve as a possible diagnostic biomarker to differentiate inflammatory neuropathies from non-inflammatory neuropathies, as only a small percentage of CIDP patients present with nodal, paranodal, and aGAAbs. Future studies should therefore include non-inflammatory polyneuropathies as a diseased control group to further examine the potential diagnostic role of CLP in CIDP.
Our study confirms previous findings of elevated sNfl levels in patients with CIDP (21–24), although direct comparison of sNfl levels between studies should be done with caution, as methods of sNfl measurement vary. Hayashi et al. (23) found significantly elevated sNfl levels in 11 treatment naïve CIDP patients, which decreased after 1 month of IVIG therapy. Similar data were obtained by van Lieverloo et al., who found elevated sNlf levels in 29 patients starting induction therapy (21). However, in patients on maintenance therapy and in remission, increased sNfl levels were only infrequently found and did not differ from HC (21), which is in contrast to our results showing significant increased levels despite therapy. Mariotto et al. (22) and Fukami et al. (24) found a significant correlation of sNfl with disability using the overall neuropathy limitation in 12 CIDP patients and 58 CIDP patients, respectively. Latest study results are in line with our findings. Thus, the correlation of sNfl with disease impairment needs confirmation in future studies.
Neither CLP nor sNfl was significantly correlated with PRO, except for a weak correlation with the mental health composite score. Hence, CLP and sNfl objectively reflect ongoing clinical disease activity and impairment, although data on quality of life predictors in CIDP are still scarce. There is evidence that patients with more severe motor disability, short disease duration, and fatigue have a higher risk of worse quality of life (45). Thus, the relationship between PRO and blood biomarkers like CLP and sNfl should be investigated in larger, independent cohorts, since in other autoimmune diseases like IBD or multiple sclerosis, CLP and sNfl were shown to be a good predictor of health outcomes (46–48).
The major limitation of sNfl as a biomarker in neurological diseases is the lack of age-specific cutoff values, as sNfl levels in blood are age dependent increasing by an average of 2.2% per year in HC (25, 26). The reasons for this age-dependent increase might be mainly due to physiological age-dependent neuronal loss. In line with studies of sNfl in central neurodegenerative and neuroinflammatory diseases, establishing cutoff values over a wide range of ages for sNfl levels is critical to develop an individual, longitudinal biomarker, for assessment of disease severity in CIDP. In addition, sNfl correlated significantly with all parameters of disease impairment and disease duration hinting to its role to act as a biomarker of ongoing axonal degeneration.
In contrast, CLP is not an age-dependent biomarker (49), and we could not observe a correlation to age in our cohort. In addition, CLP is stable at room temperature and routinely measurable, which makes it an ideal candidate biomarker.
This study was conducted with a well-defined patient cohort with high diagnostic certainty. Nevertheless, there are several limitations to our pilot study. First, the cross-sectional cohort was rather small considering the subgroup analysis. In addition, the main proportion of included patients was not treatment naïve and heterogeneous in regard to treatment and disease duration. Nevertheless, this diversity reflects the typical makeup of a specialized clinic for inflammatory neuropathies. We have not included electrophysiological proxy parameters of axonal damage, mainly due to most included patients not having a nerve conduction study or myography within a short time of blood sample collection. We furthermore cannot rule out selection bias, as we recruited a convenience sample to perform this exploratory study. Our group of HC is significantly younger than the CIDP patients. We tried to match for age and gender as good as possible, which was often limited by confounding comorbidities increasingly prevalent with higher age. We accepted this convenience sample in light of evidence from many other studies and meta-analyses examining CLP in other autoimmune diseases like RA (15) or IBD (13, 14), that CLP is not influenced by age and tried to correct for its influence with multivariate analyses. Nevertheless, future confirmation studies will need to further control for this possible confounder with a much closer matched control group. In addition, in this pilot study, we have not included controls with non-inflammatory polyneuropathies as we first wanted to examine whether CLP is increased in CIDP patients and variants. Nevertheless, this study provides promise that CLP might also be of diagnostic value to discriminate inflammatory polyneuropathies from non-inflammatory polyneuropathies, which has to be examined in future studies.
In conclusion, this explorative cross-sectional study shows that CLP is significantly elevated in CIDP patients compared to HC and is associated with active disease course. In addition, this study confirms previous findings of elevated sNfl levels in CIDP, which correlate with disease impairment and disease duration, thus confirming sNFL as a potential marker of axonal degeneration. There is an unmet need of a validated, non-invasive biomarker in order to guide treatment decisions. CLP might potentially be a candidate additive biomarker for assessment of disease activity. Further multicentric, longitudinal investigations are strongly needed to determine the potential utility of CLP for a better patient care in CIDP.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by Charité-Universitätsmedizin Berlin (EA1/025/11). The patients/participants provided their written informed consent to participate in this study.
Author Contributions
FS: designed and conceptualized the study, had a major role in acquisition of data, analyzed and interpreted the data, drafted the manuscript for intellectual content, and takes full responsibility of the integrity of the data analyzed. BH: conceptualized the study, analyzed and interpreted the data, and revised the manuscript for intellectual content. SK: acquisition of data and revised the manuscript for intellectual content. CM: laboratory analysis and revised the manuscript for intellectual content. AM: interpreted the data and revised the manuscript for intellectual content. JK: designed and conceptualized the study, had a major role in acquisition of data, revised the manuscript for intellectual content, and revised the manuscript for intellectual content. All authors contributed to the article and approved the submitted version.
Conflict of Interest
BH reports compensation for the conduct of clinical trials by argenx BVBA. CM is employed by Labor Berlin, Charité Vivantes GmbH. AM received speaker honoraria from Alexion, GRIFOLS, and Hormosan. He received honoraria from Alexion, MorphoSys, and Vitaccess for consulting services and financial research support from Octapharma and Alexion. AM is chairman of the medical advisory board of the German Myasthenia Gravis Society. JK received speaker honoraria from Merck Serono, Bayer, Biogen, Roche, Sanofi Aventis, Grifols, and Octapharma. She received honoraria for consulting services from Biogen, Merck Serono, Roche as well as financial research support from Octapharma and Grifols.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We thank our co-workers at the NeuroCure Clinical Research Center Claudia Heibutzki and Dike Remstedt for patient management of the CIDP Outpatient Department, and M. Heinold, S. Märschenz, and S. Lischewski for administration support. The authors explicitly thank Arun Prakash Singh (NeuroHub) and Salomé Kiessling for their support in sample management.
References
1. Latov N. Diagnosis and treatment of chronic acquired demyelinating polyneuropathies. Nat Rev Neurol. (2014) 10:435–46. doi: 10.1038/nrneurol.2014.117
2. Mathey EK, Park SB, Hughes RA, Pollard JD, Armati PJ, Barnett MH, et al. Chronic inflammatory demyelinating polyradiculoneuropathy: from pathology to phenotype. J Neurol Neurosurg Psychiatry. (2015) 86:973–85. doi: 10.1136/jnnp-2014-309697
3. Van den Bergh PY, Hadden RD, Bouche P, Cornblath DR, Hahn A, Illa I, et al. European Federation of Neurological Societies/Peripheral Nerve Society guideline on management of chronic inflammatory demyelinating polyradiculoneuropathy: report of a joint task force of the European Federation of Neurological Societies and the Peripheral Nerve Society - first revision. Eur J Neurol. (2010) 17:356–63. doi: 10.1111/j.1468-1331.2009.02930.x
4. Lehmann HC, Burke D, Kuwabara S. Chronic inflammatory demyelinating polyneuropathy: update on diagnosis, immunopathogenesis and treatment. J Neurol Neurosurg Psychiatry. (2019) 90:981–7. doi: 10.1136/jnnp-2019-320314
5. Staudt M, Diederich JM, Meisel C, Meisel A, Klehmet J. Differences in peripheral myelin antigen-specific T cell responses and T memory subsets in atypical versus typical CIDP. BMC Neurol. (2017) 17:81. doi: 10.1186/s12883-017-0860-z
6. Diederich JM, Staudt M, Meisel C, Hahn K, Meinl E, Meisel A, et al. Neurofascin and compact myelin antigen-specific T cell response pattern in chronic inflammatory demyelinating polyneuropathy subtypes. Front Neurol. (2018) 9:171. doi: 10.3389/fneur.2018.00171
7. Cortese A, Lombardi R, Briani C, Callegari I, Benedetti L, Manganelli F, et al. Antibodies to neurofascin, contactin-1, and contactin-associated protein 1 in CIDP: clinical relevance of IgG isotype. Neurol Neuroimmunol Neuroinflamm. (2020) 7:e639. doi: 10.1212/NXI.0000000000000639
8. Klehmet J, Märschenz S, Ruprecht K, Wunderlich B, Büttner T, Hiemann R, et al. Analysis of anti-ganglioside antibodies by a line immunoassay in patients with chronic-inflammatory demyelinating polyneuropathies (CIDP). Clin Chem Lab Med. (2018) 56:919–26. doi: 10.1515/cclm-2017-0792
9. Grüter T, Motte J, Fisse AL, Bulut Y, Köse N, Athanasopoulos D, et al. Pathological spontaneous activity as a prognostic marker in chronic inflammatory demyelinating polyneuropathy. Eur J Neurol. (2020) 27:2595–603. doi: 10.1111/ene.14476
10. Kerasnoudis A, Pitarokoili K, Behrendt V, Gold R, Yoon MS. Correlation of nerve ultrasound, electrophysiological and clinical findings in chronic inflammatory demyelinating polyneuropathy. J Neuroimaging. (2015) 25:207–16. doi: 10.1111/jon.12079
11. Ehrchen JM, Sunderkötter C, Foell D, Vogl T, Roth J. The endogenous Toll-like receptor 4 agonist S100A8/S100A9 (calprotectin) as innate amplifier of infection, autoimmunity, and cancer. J Leukoc Biol. (2009) 86:557–66. doi: 10.1189/jlb.1008647
12. Shabani F, Farasat A, Mahdavi M, Gheibi N. Calprotectin (S100A8/S100A9): a key protein between inflammation and cancer. Inflammat Res. (2018) 67:801–12. doi: 10.1007/s00011-018-1173-4
13. Suárez Ferrer C, Abadía Barno M, Martín Arranz E, Jochems A, García Ramírez L, Poza Cordón J, et al. The use of serum calprotectin as a biomarker for inflammatory activity in inflammatory bowel disease. Rev Esp Enferm Dig. (2019) 111:744–9. doi: 10.17235/reed.2019.5797/2018
14. Kalla R, Kennedy NA, Ventham NT, Boyapati RK, Adams AT, Nimmo ER, et al. Serum calprotectin: a novel diagnostic and prognostic marker in inflammatory bowel diseases. Am J Gastroenterol. (2016) 111:1796–805. doi: 10.1038/ajg.2016.342
15. Abildtrup M, Kingsley GH, Scott DL. Calprotectin as a biomarker for rheumatoid arthritis: a systematic review. J Rheumatol. (2015) 42:760–70. doi: 10.3899/jrheum.140628
16. Sunahori K, Yamamura M, Yamana J, Takasugi K, Kawashima M, Yamamoto H, et al. The S100A8/A9 heterodimer amplifies proinflammatory cytokine production by macrophages via activation of nuclear factor kappa B and p38 mitogen-activated protein kinase in rheumatoid arthritis. Arthritis Res Ther. (2006) 8:R69. doi: 10.1186/ar1939
17. Loser K, Vogl T, Voskort M, Lueken A, Kupas V, Nacken W, et al. The Toll-like receptor 4 ligands Mrp8 and Mrp14 are crucial in the development of autoreactive CD8+ T cells. Nature medicine. (2010) 16:713–7. doi: 10.1038/nm.2150
18. Yang M, Peyret C, Shi XQ, Siron N, Jang JH, Wu S, et al. Evidence from human and animal studies: pathological roles of CD8(+) T cells in autoimmune peripheral neuropathies. Front Immunol. (2015) 6:532. doi: 10.3389/fimmu.2015.00532
19. Klehmet J, Staudt M, Ulm L, Unterwalder N, Meisel A, Meisel C. Circulating lymphocyte and T memory subsets in glucocorticosteroid versus IVIG treated patients with CIDP. J Neuroimmunol. (2015) 283:17–22. doi: 10.1016/j.jneuroim.2015.03.023
20. Khalil M, Teunissen CE, Otto M, Piehl F, Sormani MP, Gattringer T, et al. Neurofilaments as biomarkers in neurological disorders. Nat Rev Neurol. (2018) 14:577–89. doi: 10.1038/s41582-018-0058-z
21. van Lieverloo GGA, Wieske L, Verhamme C, Vrancken AFJ, van Doorn PA, Michalak Z, et al. Serum neurofilament light chain in chronic inflammatory demyelinating polyneuropathy. J Peripher Nerv Syst. (2019) 24:187–94. doi: 10.1111/jns.12319
22. Mariotto S, Farinazzo A, Magliozzi R, Alberti D, Monaco S, Ferrari S. Serum and cerebrospinal neurofilament light chain levels in patients with acquired peripheral neuropathies. J Peripher Nerv Syst. (2018) 23:174–7. doi: 10.1111/jns.12279
23. Hayashi T, Nukui T, Piao JL, Sugimoto T, Anada R, Matsuda N, et al. Serum neurofilament light chain in chronic inflammatory demyelinating polyneuropathy. Brain Behav. (2021) 11:e02084. doi: 10.1002/brb3.2084
24. Fukami Y, Iijima M, Koike H, Yamada S, Hashizume A, Katsuno M. Association of serum neurofilament light chain levels with clinicopathology of chronic inflammatory demyelinating polyneuropathy, including NF155 reactive patients. J Neurol. (2021). doi: 10.1007/s00415-021-10537-2. [Epub ahead of print].
25. Bridel C, van Wieringen WN, Zetterberg H, Tijms BM, Teunissen CE, Alvarez-Cermeño JC, et al. Diagnostic value of cerebrospinal fluid neurofilament light protein in neurology: a systematic review and meta-analysis. JAMA Neurol. (2019) 76:1035–48. doi: 10.1001/jamaneurol.2019.1534
26. Khalil M, Pirpamer L, Hofer E, Voortman MM, Barro C, Leppert D, et al. Serum neurofilament light levels in normal aging and their association with morphologic brain changes. Nat Commun. (2020) 11:812. doi: 10.1038/s41467-020-14612-6
27. Gorson KC, van Schaik IN, Merkies IS, Lewis RA, Barohn RJ, Koski CL, et al. Chronic inflammatory demyelinating polyneuropathy disease activity status: recommendations for clinical research standards and use in clinical practice. J Peripher Nerv Syst. (2010) 15:326–33. doi: 10.1111/j.1529-8027.2010.00284.x
28. Kleyweg RP, van der Meché FG, Schmitz PI. Interobserver agreement in the assessment of muscle strength and functional abilities in Guillain-Barré syndrome. Muscle Nerve. (1991) 14:1103–9. doi: 10.1002/mus.880141111
29. Merkies IS, Schmitz PI, Van Der Meché FG, Samijn JP, Van Doorn PA. Psychometric evaluation of a new handicap scale in immune-mediated polyneuropathies. Muscle Nerve. (2002) 25:370–7. doi: 10.1002/mus.10045
30. Brazier JE, Harper R, Jones NM, O'Cathain A, Thomas KJ, Usherwood T, et al. Validating the SF-36 health survey questionnaire: new outcome measure for primary care. BMJ. (1992) 305:160–4. doi: 10.1136/bmj.305.6846.160
31. Krupp LB, LaRocca NG, Muir-Nash J, Steinberg AD. The fatigue severity scale. Application to patients with multiple sclerosis and systemic lupus erythematosus. Archiv Neurol. (1989) 46:1121–3. doi: 10.1001/archneur.1989.00520460115022
32. Beck AT, Steer RA, Carbin MG. Psychometric properties of the Beck Depression Inventory: twenty-five years of evaluation. Clin Psychol Rev. (1988) 8:77–100. doi: 10.1016/0272-7358(88)90050-5
33. Doneddu PE, Cocito D, Manganelli F, Fazio R, Briani C, Filosto M, et al. Atypical CIDP: diagnostic criteria, progression and treatment response. Data from the Italian CIDP database. J Neurol Neurosurg Psychiatry. (2019) 90:125–32. doi: 10.1136/jnnp-2018-318714
34. Alshekhlee A, Basiri K, Miles JD, Ahmad SA, Katirji B. Chronic inflammatory demyelinating polyneuropathy associated with tumor necrosis factor-alpha antagonists. Muscle Nerve. (2010) 41:723–7. doi: 10.1002/mus.21584
35. Hughes R, Bensa S, Willison H, Van den Bergh P, Comi G, Illa I, et al. Randomized controlled trial of intravenous immunoglobulin versus oral prednisolone in chronic inflammatory demyelinating polyradiculoneuropathy. Ann Neurol. (2001) 50:195–201. doi: 10.1002/ana.1088
36. Vanhoutte EK, Latov N, Deng C, Hanna K, Hughes RA, Bril V, et al. Vigorimeter grip strength in CIDP: a responsive tool that rapidly measures the effect of IVIG–the ICE study. Eur J Neurol. (2013) 20:748–55. doi: 10.1111/j.1468-1331.2012.03851.x
37. Åsberg A, Løfblad L, Felic A, Hov GG. Measuring calprotectin in plasma and blood with a fully automated turbidimetric assay. Scand J Clin Lab Investig. (2019) 79:50–7. doi: 10.1080/00365513.2018.1550810
38. Šumová B, Cerezo LA, Szczuková L, Nekvindová L, Uher M, Hulejová H, et al. Circulating S100 proteins effectively discriminate SLE patients from healthy controls: a cross-sectional study. Rheumatol Int. (2019) 39:469–78. doi: 10.1007/s00296-018-4190-2
39. Rissin DM, Kan CW, Campbell TG, Howes SC, Fournier DR, Song L, et al. Single-molecule enzyme-linked immunosorbent assay detects serum proteins at subfemtomolar concentrations. Nat Biotechnol. (2010) 28:595–9. doi: 10.1038/nbt.1641
40. Kuhle J, Barro C, Andreasson U, Derfuss T, Lindberg R, Sandelius Å, et al. Comparison of three analytical platforms for quantification of the neurofilament light chain in blood samples: ELISA, electrochemiluminescence immunoassay and Simoa. Clin Chem Lab Med. (2016) 54:1655–61. doi: 10.1515/cclm-2015-1195
41. Schaffert H, Pelz A, Saxena A, Losen M, Meisel A, Thiel A, et al. IL-17-producing CD4(+) T cells contribute to the loss of B-cell tolerance in experimental autoimmune myasthenia gravis. Eur J Immunol. (2015) 45:1339–47. doi: 10.1002/eji.201445064
42. Chi LJ, Xu WH, Zhang ZW, Huang HT, Zhang LM, Zhou J. Distribution of Th17 cells and Th1 cells in peripheral blood and cerebrospinal fluid in chronic inflammatory demyelinating polyradiculoneuropathy. J Peripher Nerv Syst. (2010) 15:345–56. doi: 10.1111/j.1529-8027.2010.00294.x
43. Nordal HH, Brokstad KA, Solheim M, Halse AK, Kvien TK, Hammer HB. Calprotectin (S100A8/A9) has the strongest association with ultrasound-detected synovitis and predicts response to biologic treatment: results from a longitudinal study of patients with established rheumatoid arthritis. Arthritis Res Ther. (2017) 19:3. doi: 10.1186/s13075-016-1201-0
44. Choi IY, Gerlag DM, Herenius MJ, Thurlings RM, Wijbrandts CA, Foell D, et al. MRP8/14 serum levels as a strong predictor of response to biological treatments in patients with rheumatoid arthritis. Ann Rheum Dis. (2015) 74:499. doi: 10.1136/annrheumdis-2013-203923
45. Bozovic I, Kacar A, Peric S, Nikolic A, Bjelica B, Cobeljic M, et al. Quality of life predictors in patients with chronic inflammatory demyelinating polyradiculoneuropathy. J Neurol. (2017) 264:2481–6. doi: 10.1007/s00415-017-8658-x
46. Galetta K, Deshpande C, Healy BC, Glanz B, Ziehn M, Saxena S, et al. Serum neurofilament levels and patient-reported outcomes in multiple sclerosis. Ann Clin Transl Neurol. (2021) 8:631–8. doi: 10.1002/acn3.51305
47. Thebault S, D RT, Lee H, Bowman M, Bar-Or A, Arnold DL, et al. High serum neurofilament light chain normalizes after hematopoietic stem cell transplantation for MS. Neurol Neuroimmunol Neuroinflammation. (2019) 6:e598. doi: 10.1212/NXI.0000000000000598
48. Morris MW, Stewart SA, Heisler C, Sandborn WJ, Loftus EV, Zello GA, et al. Biomarker-based models outperform patient-reported scores in predicting endoscopic inflammatory disease activity. Inflamm Bowel Dis. (2018) 24:277–85. doi: 10.1093/ibd/izx018
Keywords: calprotectin (S100A8/S100A9), serum neurofilament light chain, chronic inflammatory demyelinating polyneuropathy, CIDP variants, biomarker
Citation: Stascheit F, Hotter B, Klose S, Meisel C, Meisel A and Klehmet J (2021) Calprotectin in Chronic Inflammatory Demyelinating Polyneuropathy and Variants—A Potential Novel Biomarker of Disease Activity. Front. Neurol. 12:723009. doi: 10.3389/fneur.2021.723009
Received: 09 June 2021; Accepted: 12 August 2021;
Published: 13 September 2021.
Edited by:
Elisabeth Chroni, University of Patras, GreeceReviewed by:
Fiore Manganelli, University of Naples Federico II, ItalyLuis Querol, Hospital de la Santa Creu i Sant Pau, Spain
Copyright © 2021 Stascheit, Hotter, Klose, Meisel, Meisel and Klehmet. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Frauke Stascheit, ZnJhdWtlLnN0YXNjaGVpdEBjaGFyaXRlLmRl
†These authors have contributed equally to this work
 Frauke Stascheit
Frauke Stascheit Benjamin Hotter
Benjamin Hotter Sarah Klose
Sarah Klose Christian Meisel
Christian Meisel Andreas Meisel
Andreas Meisel Juliane Klehmet
Juliane Klehmet