
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Neurol. , 14 January 2022
Sec. Dementia and Neurodegenerative Diseases
Volume 12 - 2021 | https://doi.org/10.3389/fneur.2021.721427
Some studies show that low serum vitamin D levels are associated with white matter hyperintensity (WMH), while other studies report no association. This meta-analysis aimed to investigate the presence of an association between serum 25-hydroxy vitamin D [25(OH)D] levels and WMH. PubMed, Embase, the Cochrane Library, CNKI, WANFANG, and VIP were searched for available papers published up to December 2020. The outcomes were the odds ratios (ORs) with 95% confidence intervals (CIs) for the association between different vitamin D statuses and WMH. All meta-analyses were performed using a random-effects model. Five studies (4393 patients) were included. Compared with sufficient 25(OH)D levels, 25(OH)D deficiency was not associated with WMH (OR = 1.67, 95%CI: 0.92–3.04; I2 = 70.2%, Pheterogeneity = 0.009), nor was 25(OH)D insufficiency (OR = 1.21, 95%CI: 0.89–1.65; I2 = 48.1%, Pheterogeneity = 0.103). A decrease of 25 nmol/L in 25(OH)D levels was associated with WMH (OR = 1.83, 95%CI: 1.34-2.49; I2 = 0%, Pheterogeneity= 0.512). The sensitivity analyses showed that the results were robust. 25(OH)D deficiency and insufficiency are not associated with WMH. A decrease of 25 nmol/L in 25(OH)D levels was associated with WMH, but this result will have to be confirmed. Prospective trials, both cross-sectional and longitudinal, are necessary to examine the association between 25(OH)D levels and WMH.
Neurodegenerative diseases are characterized by protein aggregation and inclusion body formation. Most neurodegenerative diseases appear in older people (1, 2). Traditionally, these diseases had to be diagnosed by post-mortem examinations, but recent advances in neuroimaging now allow us to infer the diagnosis of neurodegenerative diseases early in the course of the disease (3). These new techniques involve magnetic resonance imaging (MRI), single-photon emission computed tomography (SPECT), positron emission tomography (PET), protein-specific imaging, and scintigraphy (3). Nevertheless, neuroimaging can also reveal non-specific lesions and signals, and the increased use of these imaging methods led to an increase in the incidence of such lesions (3, 4). Indeed, the aging brain is characterized by a non-specific increase in white matter hyperintensity (WMH), called leukoaraiosis (5–11). WMHlesions are easily visualized on MRI either in the deep white matter (D-WMH) or in contact with lateral cerebral ventricles (periventricularWMH, P-WMH) (5–11). The clinical relevance of both D-WMH and P-WMH is related to the disruption of cortico-subcortical white matter tracts that connect important cognitive regions of the brain (12), making them risk factors associated with cognitive decline (5–7).
Recent prospective studies have found that low serum vitamin D levels are associated with WMH (13–17). It was hypothesized that the age-related decrease in vitamin D level could explain, at least in part, the WMH observed in older adults (15, 18–20). Hypovitaminosis D is a common finding in the elderly (21, 22). Epidemiological studies have reported that older adults with hypovitaminosis D have more frequent and more severe cognitive disturbances than those with appropriate blood levels of vitamin D (23–25). Epidemiological and clinical studies suggest that low 25-hydroxy vitamin D [25(OH)D] levels are associated with higher risks of large-vessel diseases such as myocardial infarction, ischemic stroke, and carotid atherosclerosis (26–31). Consistently, vitamin D is active in the brain and can reverse some age-related brain changes (32). Several actions are described, including participation in neurophysiology through the genetic regulation of neurotransmitters, neurotrophins, and dendritic growth (33–35) and neuroprotective effects based on antioxidant and anti-inflammatory properties (32–34, 36).
On the other hand, some studies suggested that vitamin D levels are not associated with WMH (37, 38). Many of the available studies are characterized by relatively small numbers of individuals, limiting the statistical power for observing differences. In addition, these studies were limited to specific populations, limiting the generalizability of the results. Some studies also included patients with WMH concomitant with other neurological diseases that could influence the associations, such as Alzheimer's disease (39), stroke (17), and dementia (13). In this situation, in view of the lack of large-scale population studies, the meta-analysis methodology can be used to obtain general associations that might help determine a general relationship between 25(OH)D levels and WMH. No previous meta-analysis examined the association between 25(OH)D levels and the presence of WMH. Indeed, 25(OH)D is viewed as a superior biomarker for assessing the vitamin D status (28, 40, 41).
Therefore, this systematic review and meta-analysis aimed to investigate the presence of an association between serum 25(OH)D levels and WMH. The results could help define the role of 25(OH)D as a possible biomarker for WMH.
This systematic review and meta-analysis was performed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (42) and the PICO principle (43). PubMed, Embase, the Cochrane Library, CNKI, WANFANG, and VIP were searched for available papers published up to December 2020 using the MeSH terms of “Vitamin D”, “White matter”, “Cerebral disease”, “Leukoaraiosis”, as well as relevant key words, followed by screening based on the eligibility criteria.
The eligibility criteria were (1) exposure: 25(OH)D levels, (2) outcome: cerebral disease (WMH by MRI), (3) language: English or Chinese, and (4) outcome: the results were presented as odds ratios (ORs) with 95% confidence intervals (CIs).
Study characteristics (authors, year of publication, country, study design, sex, sample size, diagnosis, and cut-off value to define deficiency, insufficient, and sufficient serum 25(OH)D levels) and outcomes (ORs with 95%CIs at different vitamin D statuses; if several models were reported, the data from the most adjusted models were extracted; using sufficient vitamin D as the reference, the ORs of insufficient vitamin D were extracted) were extracted by two different investigators (Yilei Zhao and Jingfeng Xu) according to a pre-specified protocol. Discrepancies in the assessments were reappraised by the third investigator.
The level of evidence of all articles was assessed independently by two authors (Zhan Feng and Jincheng Wang) according to the Newcastle-Ottawa Scale (NOS) criteria for quality assessment of cohort and case-control studies (44) and the AHRQ criteria for quality assessment of cross-sectional studies (45). Discrepancies in the assessment were resolved by discussion until a consensus was reached.
For studies that categorized the vitamin levels into four groups, the second and third percentile were synthesized and defined as insufficient vitamin levels in the present meta-analysis. All analyses were performed using STATA SE 14.0 (StataCorp, College Station, TX, USA). Statistical heterogeneity among studies was calculated by Cochran's Q-test and the I2 index. An I2 > 50% and Q-test P < 0.10 indicated high heterogeneity). All meta-analyses were performed using a random-effects model. P-values < 0.05 were considered statistically different. The possible publication bias was not assessed using funnel plots and Egger's test because the number of studies included in each quantitative analysis was <10, in which case the funnel plots and Egger's test could yield misleading results (46, 47).
Figure 1 presents the study selection process. The initial search yielded 501 records; 103 were removed because they were duplicates, 398 records were screened based on the titles, and 222 were excluded (reviews, n = 104; conference abstracts, n = 59; meta-analysis, n = 1; editorial, n = 5; note/report, n = 8; survey, n = 1; letter, n = 7; others, n = 14; animal, n = 13; not accessible, n = 10). Then, 176 full-text articles or abstracts were assessed for eligibility, and 171 were excluded (study aim/design, n = 104; population, n = 12; outcomes, n = 22; exposure, n = 31; language, n = 2).
Table 1 presents the five included studies (4,393 patients) (16, 17, 37, 38, 48). Two studies were from the United States of America (37, 38), one from Korea (16), and two from China (17, 48). The mean/median age was 62 to 73.6±4.5 years. The percentage of males was 30.8–59.2%. According to the NOS, the case-control study (48) scored 6 points (Supplementary Table 1a), two cohort studies (17, 38) scored 7 points, and one cohort study (37) scored 9 points (Supplementary Table 1b). According to AHRQ criteria, the cross-sectional study (16) scored 7 points (Supplementary Table 1c).
All five studies (16, 17, 37, 38, 48) examined the association between 25(OH)D deficiency/insufficiency and WMH. Compared with the patients with sufficient 25(OH)D levels, those with 25(OH)D deficiency did not show a higher frequency of WMH (OR = 1.67, 95%CI: 0.92–3.04; I2 = 70.2%, Pheterogeneity = 0.009) (Figure 2). The same was observed for the patients with 25(OH)D insufficiency (OR = 1.21, 95%CI: 0.89–1.65; I2 = 48.1%, Pheterogeneity = 0.103) (Figure 3). Using regression analyses, two studies (16, 48) showed that a decrease of 25 nmol/L in the absolute levels of 25(OH)D was associated with WMH lesions (OR = 1.83, 95%CI: 1.34–2.49; I2 = 0%, Pheterogeneity= 0.512) (Figure 4).
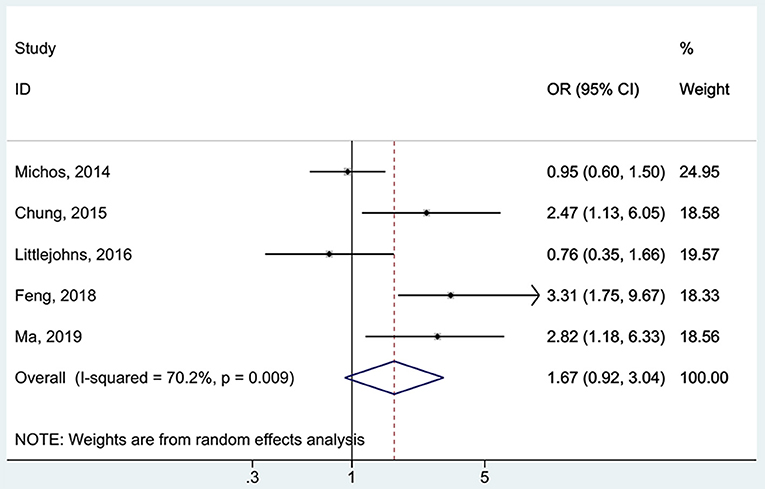
Figure 2. Forest plot of deficient vs. sufficient vitamin D level. % Weight represents the contribution of the sample size of each study to the total number of patients included in the analysis.
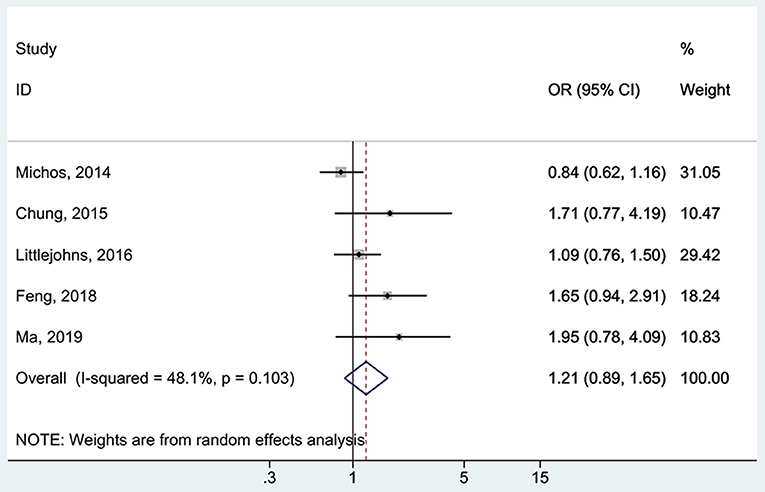
Figure 3. Forest plot of insufficient vs. sufficient vitamin D level. % Weight represents the contribution of the sample size of each study to the total number of patients included in the meta-analysis.
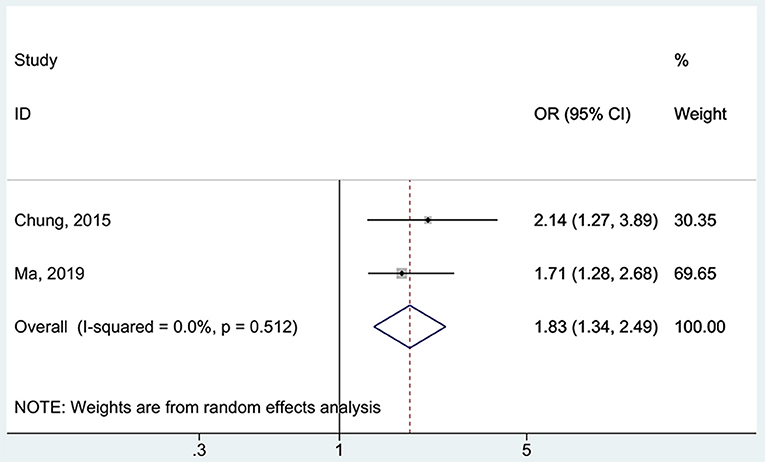
Figure 4. Forest plot of vitamin D decreased by 25 nmol/L. % Weight represents the contribution of the sample size of each study to the total number of patients included in the meta-analysis.
Figures 5, 6 indicate that the analyses were robust. The sequential exclusion of each study did not influence the results significantly.
Evidence suggests that low serum vitamin D levels are associated with WMH (13–20), but conflicting results are reported among studies (37, 38). Therefore, this study aimed to investigate the presence of an association between serum 25-hydroxy vitamin D [25(OH)D] levels and WMH. The results showed that 25(OH)D deficiency and insufficiency are not associated with WMH, but a decrease of 25 nmol/L in 25(OH)D levels were associated with WMH.
WMH has retained attention because of its association with cognitive decline in older adults (5–7). Vitamin D levels are associated with neurological conditions involving lower cognitive abilities levels (23–25). Thus, some authors suggested that there might be an association between vitamin D levels and WMH (15, 18–20). This association is mechanistically possible since WMH on MRI represents brain areas where the cortico-subcortical white matter tracts are disrupted (12) and because vitamin D is known to have several neuroprotective effects (32–36). The association between low vitamin D levels and WMH has been suggested by several studies (13–20), but not all of them could be included in the present meta-analysis because of the selection criteria and because some of them did not measure 25(OH)D, which is considered the most reliable marker for vitamin D metabolism (28, 40, 41). Still, they support the neuroprotective effects of vitamin D. On the other hand, other studies do not support this association (37, 38). The present meta-analysis also supports that 25(OH)D deficiency and insufficiency are not associated with WMH. This observation might be because vitamin D is only one of many factors that affect WMH development. Still, only five studies could be included, and the highly negative results of Michos et al. (37) and Littlejohns et al. (38) might drive the lack of association. In addition, because there were <10 studies included, publication bias could not be analyzed (47). Future studies should examine these other factors as well.
Significant heterogeneity was observed in this meta-analysis and could be explained by the characteristics of the included studies. In the study by Feng et al. (17), 234 patients with first-ever minor ischemic stroke or transient ischemic attacks (TIA) and who underwent MRI were included. 25(OH)D levels were measured using an immunoassay. They concluded that 25(OH)D levels were associated with WMH in patients with minor brain ischemia. That study, of course, suffers from a selection bias. Chung et al. (16) examined the 25(OH)D levels (by immunoassay) of 759 patients who underwent MRI for ischemic stroke or TIA. They showed that 25(OH)D levels were inversely associated with WMH. Ma et al. (48) examined the association between 25(OH)D levels and WMH in 163 patients with lacunar infarction and 158 healthy volunteers. They showed that the 25(OH)D levels were associated with lacunar infarction and with the presence of WMH. In the longitudinal study by Michos et al. (37), participants without neurological conditions at baseline underwent MRI (n = 1622) and a second MRI 10 years later (n = 888). That study was based on the Atherosclerosis Risk in Communities (ARIC) Brain MRI study. The 25(OH)D levels were measured only at baseline, and this cross-sectional measurement was not associated with WMH development. Littlejohns et al. (38) also performed a longitudinal study, based on the US-based Cardiovascular Health Study, in which 1,658 participants aged >65 years and free of any cognitive-related neurological condition at baseline (in 1992–1993) were followed using MRI for a mean follow-up of 5.0 years. Hence, using a longitudinal design, they showed that 25(OH)D levels were not associated with WMH development in elderly patients. The measurement of 25(OH)D levels by mass spectrometry and liquid chromatography-tandem mass spectrometry, as in the two longitudinal studies (37, 38), is far more sensitive and precise than immunoassays but more expensive and requires more equipment and technical expertise (49, 50). Unfortunately, in the two longitudinal studies, only baseline 25(OH)D levels were measured, explaining the lack of association with WMH. Future longitudinal studies should perform multiple measurements of 25(OH)D in time. On the other hand, three studies (16, 17, 48) showed associations between 25(OH)D levels and WMH were performed in patients who underwent MRI because of stroke or TIA, resulting in selection bias and limiting the generalizability of the results.
Differences in vitamin D levels among countries and ethnicities could indeed influence the associations observed among the various studies and contribute to heterogeneity. In the USA population, Parva et al. (51) reported that low levels of vitamin D were observed in Hispanics and African-Americans. Wei et al. (52) reported that the prevalence of vitamin D deficiency was high in China and in ethnic minorities in the USA. The prevalence of vitamin D deficiency is higher among individuals of African origin and living at high latitudes (53). There are also large differences in vitamin D deficiency prevalence among European and Middle East countries (54).
This meta-analysis has limitations. Because of the strict eligibility criteria, the number of studies that could be included was small. All five studies included together 4,393 patients. Considering that WMH must be diagnosed by MRI, it is a relatively large number of patients. On the other hand, epidemiologically speaking, this number is small, and only three countries are represented. Additional studies are necessary to determine the epidemiological characteristics of WMH in relation to 25(OH)D levels. The present meta-analysis highlights this need. Furthermore, the quality of a meta-analysis is determined by the quality of the studies that match the eligibility criteria, and the quality of the included studies was relatively low. The definitions for vitamin D sufficiency, vitamin D insufficiency, and vitamin D deficiency varied among the studies and the methods for measuring the 25(OH)D levels. In addition, the selection of the patients, as well as the indications for MRI, varied among studies. These factors contributed to the heterogeneity observed among the studies. Finally, many causative relationships can be responsible for the association between WMH progression and cognitive impairment, including 25(OH)D levels and aging, 25(OH)D and impairment of cognitive functions, and white matter change and aging, among others. This meta-analysis was not designed to determine the causal relationships, and several covariates affecting cognitive impairment could not be considered. Future studies will have to examine such relationships more closely.
In conclusion, 25(OH)D deficiency and insufficiency are not associated with WMH. A decrease of 25 nmol/L in 25(OH)D levels was associated with WMH, but this result will have to be confirmed. Prospective trials, both cross-sectional and longitudinal, are necessary to examine the association between 25(OH)D levels and WMH, as well as the causal relationships.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
All authors designed the systematic review protocol. JX and ZF searched the literature for potentially eligible studies. ZF and JW carried the selection of studies. YZ extracted the information. YZ and JX evaluated the methodology quality. ZF conducted the meta-analysis. YZ wrote this paper.
This study was funded by the Zhejiang Natural Science Foundation, Association of Mathematical and Physical Medicine (No. LSY19H180014); Zhejiang Basic Public Welfare Research Project (No. LGF19H220003).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
We acknowledge the help of Dr. Kang Wang, Department of Neurology, the First Affiliated Hospital, School of Medicine, Zhejiang University.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2021.721427/full#supplementary-material
1. Hou Y, Dan X, Babbar M, Wei Y, Hasselbalch SG, Croteau DL, et al. Ageing as a risk factor for neurodegenerative disease. Nat Rev Neurol. (2019) 15:565–81. doi: 10.1038/s41582-019-0244-7
2. Wyss-Coray T. Ageing, neurodegeneration and brain rejuvenation. Nature. (2016) 539:180–6. doi: 10.1038/nature20411
3. Shimizu S, Hirose D, Hatanaka H, Takenoshita N, Kaneko Y, Ogawa Y, et al. Role of neuroimaging as a biomarker for neurodegenerative diseases. Front Neurol. (2018) 9:265. doi: 10.3389/fneur.2018.00265
4. Sivakolundu DK, West KL, Zuppichini MD, Wilson A, Moog TM, Blinn AP, et al. BOLD signal within and around white matter lesions distinguishes multiple sclerosis and non-specific white matter disease: a three-dimensional approach. J Neurol. (2020) 267:2888–96. doi: 10.1007/s00415-020-09923-z
5. Marek M, Horyniecki M, Fraczek M, Kluczewska E. Leukoaraiosis - new concepts and modern imaging. Pol J Radiol. (2018) 83:e76–81. doi: 10.5114/pjr.2018.74344
6. Schmidt R, Petrovic K, Ropele S, Enzinger C, Fazekas F. Progression of leukoaraiosis and cognition. Stroke. (2007) 38:2619–25. doi: 10.1161/STROKEAHA.107.489112
8. Ben-Assayag E, Mijajlovic M, Shenhar-Tsarfaty S, Bova I, Shopin L, Bornstein NM. Leukoaraiosis is a chronic atherosclerotic disease. ScientificWorldJournal. (2012) 2012:532141. doi: 10.1100/2012/532141
9. Fazekas F, Schmidt R, Scheltens P. Pathophysiologic mechanisms in the development of age-related white matter changes of the brain. Dement Geriatr Cogn Disord. (1998) 9:2–5. doi: 10.1159/000051182
10. Pantoni L, Garcia JH. Pathogenesis of leukoaraiosis: a review. Stroke. (1997) 28:652–9. doi: 10.1161/01.STR.28.3.652
11. Gass A, Oster M, Cohen S, Daffertshofer M, Schwartz A, Hennerici MG. Assessment of T2- and T1-weighted MRI brain lesion load in patients with subcortical vascular encephalopathy. Neuroradiology. (1998) 40:503–6. doi: 10.1007/s002340050633
12. Annweiler C, Montero-Odasso M. Vascular burden as a substrate for higher-level gait disorders in older adults. A review of brain mapping literature. Panminerva Med. (2012) 54:189–204.
13. Buell JS, Dawson-Hughes B, Scott TM, Weiner DE, Dallal GE, Qui WQ, et al. 25-Hydroxyvitamin D, dementia, and cerebrovascular pathology in elders receiving home services. Neurology. (2010) 74:18–26. doi: 10.1212/WNL.0b013e3181beecb7
14. Annweiler C, Annweiler T, Bartha R, Herrmann FR, Camicioli R, Beauchet O. Vitamin D and white matter abnormalities in older adults: a cross-sectional neuroimaging study. Eur J Neurol. (2014) 21:1436–e95. doi: 10.1111/ene.12511
15. Prager JM, Thomas C, Ankenbrandt WJ, Meyer JR, Gao Y, Ragin A, et al. Association of white matter hyperintensities with low serum 25-hydroxyvitamin D levels. AJNR Am J Neuroradiol. (2014) 35:1145–9. doi: 10.3174/ajnr.A3840
16. Chung PW, Park KY, Kim JM, Shin DW, Park MS, Chung YJ, et al. 25-hydroxyvitamin D status is associated with chronic cerebral small vessel disease. Stroke. (2015) 46:248–51. doi: 10.1161/STROKEAHA.114.007706
17. Feng C, Tang N, Huang H, Zhang G, Qi X, Shi F. 25-Hydroxy vitamin D level is associated with total MRI burden of cerebral small vessel disease in ischemic stroke patients. Int J Neurosci. (2019) 129:49–54. doi: 10.1080/00207454.2018.1503182
18. Wang L, Zhao XM, Yuan XZ, Wang FY, Shen J, Wang Y. Association between Serum 25-Hydroxyvitamin D level and cognitive impairment in patients with white matter lesions: a cross-sectional study. Med Princ Pract. (2020) 29:451–7. doi: 10.1159/000506864
19. Annweiler C, Bartha R, Karras SN, Gautier J, Roche F, Beauchet O. Vitamin D and white matter abnormalities in older adults: a quantitative volumetric analysis of brain MRI. Exp Gerontol. (2015) 63:41–7. doi: 10.1016/j.exger.2015.01.049
20. Pieters B, Staals J, Knottnerus I, Rouhl R, Menheere P, Kessels A, et al. Periventricular white matter lucencies relate to low vitamin B12 levels in patients with small vessel stroke. Stroke. (2009) 40:1623–6. doi: 10.1161/STROKEAHA.108.523431
21. Nowson CA, McGrath JJ, Ebeling PR, Haikerwal A, Daly RM, Sanders KM, et al. Vitamin D and health in adults in Australia and New Zealand: a position statement. Med J Aust. (2012) 196:686–7. doi: 10.5694/mja11.10301
22. Holick MF. The vitamin D deficiency pandemic: Approaches for diagnosis, treatment and prevention. Rev Endocr Metab Disord. (2017) 18:153–65. doi: 10.1007/s11154-017-9424-1
23. Panwar B, Judd SE, Howard VJ, Jenny NS, Wadley VG, Gutierrez OM. Vitamin D, fibroblast growth factor 23 and incident cognitive impairment: findings from the REGARDS study. PLoS ONE. (2016) 11:e0165671. doi: 10.1371/journal.pone.0165671
24. Balion C, Griffith LE, Strifler L, Henderson M, Patterson C, Heckman G, et al. Vitamin D, cognition, and dementia: a systematic review and meta-analysis. Neurology. (2012) 79:1397–405. doi: 10.1212/WNL.0b013e31826c197f
25. Llewellyn DJ, Lang IA, Langa KM, Muniz-Terrera G, Phillips CL, Cherubini A, et al. Vitamin D and risk of cognitive decline in elderly persons. Arch Intern Med. (2010) 170:1135–41. doi: 10.1001/archinternmed.2010.173
26. Carlsson M, Wanby P, Brudin L, Lexne E, Mathold K, Nobin R, et al. Older swedish adults with high self-perceived health show optimal 25-hydroxyvitamin d levels whereas vitamin d status is low in patients with high disease burden. Nutrients. (2016) 8:717. doi: 10.3390/nu8110717
27. Brondum-Jacobsen P, Nordestgaard BG, Schnohr P, Benn M. 25-hydroxyvitamin D and symptomatic ischemic stroke: an original study and meta-analysis. Ann Neurol. (2013) 73:38–47. doi: 10.1002/ana.23738
28. Sizar O, Khare S, Goyal A, Bansal P, Givler A. Vitamin D Deficiency. In: StatPearls. Treasure Island (FL) (2021)
29. Berghout BP, Fani L, Heshmatollah A, Koudstaal PJ, Ikram MA, Zillikens MC, et al. Vitamin D status and risk of stroke: the Rotterdam study. Stroke. (2019) 50:2293–8. doi: 10.1161/STROKEAHA.119.025449
30. Makariou SE, Michel P, Tzoufi MS, Challa A, Milionis HJ. Vitamin D and stroke: promise for prevention and better outcome. Curr Vasc Pharmacol. (2014) 12:117–24. doi: 10.2174/15701611113119990119
31. Judd SE, Morgan CJ, Panwar B, Howard VJ, Wadley VG, Jenny NS, et al. Vitamin D deficiency and incident stroke risk in community-living black and white adults. Int J Stroke. (2016) 11:93–102. doi: 10.1177/1747493015607515
32. Briones TL, Darwish H. Vitamin D mitigates age-related cognitive decline through the modulation of pro-inflammatory state and decrease in amyloid burden. J Neuroinflammation. (2012) 9:244. doi: 10.1186/1742-2094-9-244
33. Kalueff AV, Tuohimaa P. Neurosteroid hormone vitamin D and its utility in clinical nutrition. Curr Opin Clin Nutr Metab Care. (2007) 10:12–9. doi: 10.1097/MCO.0b013e328010ca18
34. Annweiler C, Schott AM, Berrut G, Chauvire V, Le Gall D, Inzitari M, et al. Vitamin D and ageing: neurological issues. Neuropsychobiology. (2010) 62:139–50. doi: 10.1159/000318570
35. Brown J, Bianco JI, McGrath JJ, Eyles DW. 1,25-dihydroxyvitamin D3 induces nerve growth factor, promotes neurite outgrowth and inhibits mitosis in embryonic rat hippocampal neurons. Neurosci Lett. (2003) 343:139–43. doi: 10.1016/S0304-3940(03)00303-3
36. Ibi M, Sawada H, Nakanishi M, Kume T, Katsuki H, Kaneko S, et al. Protective effects of 1 alpha,25-(OH)(2)D(3) against the neurotoxicity of glutamate and reactive oxygen species in mesencephalic culture. Neuropharmacology. (2001) 40:761–71. doi: 10.1016/S0028-3908(01)00009-0
37. Michos ED, Carson KA, Schneider AL, Lutsey PL, Xing L, Sharrett AR, et al. Vitamin D and subclinical cerebrovascular disease: the Atherosclerosis Risk in Communities brain magnetic resonance imaging study. JAMA Neurol. (2014) 71:863–71. doi: 10.1001/jamaneurol.2014.755
38. Littlejohns TJ, Kos K, Henley WE, Lang IA, Annweiler C, Beauchet O, et al. Vitamin D and risk of neuroimaging abnormalities. PLoS ONE. (2016) 11:e0154896. doi: 10.1371/journal.pone.0154896
39. Shih EJ, Lee WJ, Hsu JL, Wang SJ, Fuh JL. Effect of vitamin D on cognitive function and white matter hyperintensity in patients with mild Alzheimer's disease. Geriatr Gerontol Int. (2020) 20:52–8. doi: 10.1111/ggi.13821
40. Hypponen E, Turner S, Cumberland P, Power C, Gibb I. Serum 25-hydroxyvitamin D measurement in a large population survey with statistical harmonization of assay variation to an international standard. J Clin Endocrinol Metab. (2007) 92:4615–22. doi: 10.1210/jc.2007-1279
41. Kennel KA, Drake MT, Hurley DL. Vitamin D deficiency in adults: when to test and how to treat. Mayo Clin Proc. (2010) 85:752–7. doi: 10.4065/mcp.2010.0138
42. Selcuk AA. A Guide for systematic reviews: PRISMA. Turk Arch Otorhinolaryngol. (2019) 57:57–8. doi: 10.5152/tao.2019.4058
43. Aslam S, Emmanuel P. Formulating a researchable question: a critical step for facilitating good clinical research. Indian J Sex Transm Dis AIDS. (2010) 31:47–50. doi: 10.4103/0253-7184.69003
44. Lo CK, Mertz D, Loeb M. Newcastle-Ottawa Scale: comparing reviewers' to authors' assessments. BMC Med Res Methodol. (2014) 14:45. doi: 10.1186/1471-2288-14-45
45. Agency for Healthcare Research and Quality: Agency for Healthcare Research and Quality (AHRQ) methodology checklist for cross-sectional studies. Available online at: https://effectivehealthcare.ahrq.gov/sites/default/files/assessing-the-risk-of-bias_draft-report.pdf. Agency for Healthcare Research and Quality, Rockville (2011)
46. Higgins JP, Altman DG, Gotzsche PC, Juni P, Moher D, Oxman AD, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. (2011) 343:d5928. doi: 10.1136/bmj.d5928
47. Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al. Cochrane Handbook for Systematic Reviews of Interventions version 6, 1. Cochrane Collaboration, London (2020). doi: 10.1002/9781119536604
48. Ma N, Bian O, Yan SM, Ding L, Sin DM, Wang H, et al. Correlation between serum 25-hydroxyvitamin D level and small vessel disease in elderly patients. Clin J Med Offic. (2019) 47:345–7. doi: 10.1101/2020.09.25.20201756
49. Farrell CJ, Martin S, McWhinney B, Straub I, Williams P, Herrmann M. State-of-the-art vitamin D assays: a comparison of automated immunoassays with liquid chromatography-tandem mass spectrometry methods. Clin Chem. (2012) 58:531–42. doi: 10.1373/clinchem.2011.172155
50. Malik A, Chakraborty B, Patel V, Khan A. Comparison of the analytical and diagnostic performance of an automated immunoassay and liquid chromatography tandem mass spectrometry for 25-hydroxy vitamin D. Am J Clin Pathol. (2016) 146:86. doi: 10.1093/ajcp/aqw163.011
51. Parva NR, Tadepalli S, Singh P, Qian A, Joshi R, Kandala H, et al. Prevalence of Vitamin D deficiency and associated risk factors in the US population (2011-2012). Cureus. (2018) 10:e2741. doi: 10.7759/cureus.2741
52. Wei J, Zhu A, Ji JS. A comparison study of vitamin D deficiency among older adults in China and the United States. Sci Rep. (2019) 9:19713. doi: 10.1038/s41598-019-56297-y
53. Ames BN, Grant WB, Willett WC. Does the high prevalence of vitamin D deficiency in african americans contribute to health disparities? Nutrients. (2021) 13:499. doi: 10.3390/nu13020499
54. Lips P, Cashman KD, Lamberg-Allardt C, Bischoff-Ferrari HA, Obermayer-Pietsch B, Bianchi ML, et al. Current vitamin D status in European and Middle East countries and strategies to prevent vitamin D deficiency: a position statement of the European Calcified Tissue Society. Eur J Endocrinol. (2019) 180:23–P54. doi: 10.1530/EJE-18-0736
Keywords: white matter abnormality, magnetic resonance imaging, vitamin D deficiency, odds ratios, systematic review, meta-analysis
Citation: Zhao Y, Xu J, Feng Z and Wang J (2022) Impact of 25-Hydroxy Vitamin D on White Matter Hyperintensity in Elderly Patients: A Systematic Review and Meta-Analysis. Front. Neurol. 12:721427. doi: 10.3389/fneur.2021.721427
Received: 07 June 2021; Accepted: 13 December 2021;
Published: 14 January 2022.
Edited by:
Rufus Olusola Akinyemi, University of Ibadan, NigeriaReviewed by:
Lan Zhang, First Affiliated Hospital of Henan University of Traditional Chinese Medicine, ChinaCopyright © 2022 Zhao, Xu, Feng and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yilei Zhao, MTMwOTAzNUB6anUuZWR1LmNu
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.