- 1Department of Neurosurgery, Neurosurgery Research Institute, The First Affiliated Hospital, Fujian Medical University, Fuzhou, China
- 2Fujian Key Laboratory of Precision Medicine for Cancer, The First Affiliated Hospital, Fujian Medical University, Fuzhou, China
- 3Key Laboratory of Radiation Biology of Fujian Higher Education Institutions, The First Affiliated Hospital, Fujian Medical University, Fuzhou, China
Introduction: We explored whether higher preoperative serum levels of lactate dehydrogenase (LDH) predicted outcome 3 months after surgery in patients with aneurysmal subarachnoid hemorrhage (aSAH) treated using microsurgical clipping in our institution.
Methods: Patients with aSAH treated at our institution between 2010 and 2018 were enrolled. The following parameters were recorded: age, sex, smoking and drinking history, medical history, Hunt–Hess and Fisher grades, aneurysm location, aneurysm size, surgical treatment, delayed cerebral ischemia (DCI), intracranial infection, hydrocephalus, pneumonia, and preoperative serum LDH levels within 24 h of aSAH. We investigated whether preoperative serum LDH levels were associated with Hunt–Hess grade, Fisher grade, and functional neurological outcome.
Results: In total, 2,054 patients with aSAH were enrolled, 874 of whom were treated using microsurgical clipping. The average serum LDH level (U/L) was significantly lower in the good outcome group (180.096 ± 50.237) than in the poor outcome group (227.554 ± 83.002; p < 0.001). After propensity score matching, the average serum LDH level (U/L) was still lower in the good outcome group (205.356 ± 76.785) than in the poor outcome group (227.119 ± 86.469; p = 0.029). The area under the receiver operating characteristic (ROC) curve was 0.702 (95% confidence interval [CI]: 0.650–0.754; p < 0.001). Based on the ROC curve, the optimal cutoff value for serum LDH levels as a predictor of poor 3-month outcome (modified Rankin Scale score > 2) was 201.5 U/L. The results revealed that Hunt–Hess grade, Fisher grade, DCI, pneumonia, and serum LDH (>201.5 U/L) were significantly associated with poor outcome. After propensity score matching, serum LDH levels > 201.5 U/L were still considered an independent risk factor for poor outcome (odds ratio: 2.426, 95% CI = 1.378–4.271, p = 0.002). Serum LDH levels were associated with Hunt–Hess and Fisher grades and were correlated with functional neurological outcomes (p < 0.001).
Conclusions: Our findings showed that higher preoperative serum levels of LDH correlated with Hunt–Hess grade, Fisher grade, and neurological functional outcome, and predicted the outcome of aSAH treated by microsurgical clipping at 3 months, which was involved in the related mechanisms of early brain injury and showed its potential clinical significance in patients with aSAH.
Introduction
Lactate dehydrogenase (LDH) is a glycolytic enzyme that occurs in all important human organs, including the liver, heart, skeletal muscle, kidney, lung, and brain (1, 2). It catalyzes the dehydrogenation of lactic acid to pyruvic acid, promotes anaerobic glycolysis, and prevents lactic acid accumulation; the latter are associated with unfavorable clinical outcomes of traumatic brain injury (3). When cytolysis occurs or the cell membrane is destroyed, LDH is released into the blood, resulting in an increase in serum LDH (4). LDH activity can be detected in malignant tumor tissues and leukemic cells (5), and serum LDH levels are correlated with the prognosis of adult T-cell leukemia-lymphoma (6), prostate cancer (7), acute myeloid leukemia (8), melanoma (9), neuroblastoma (10), glioblastoma multiforme (11), acute encephalopathy (12), and Mycoplasma pneumoniae pneumonia (13). Serum LDH levels reflect the degree of brain tissue injury, and Yu et al. demonstrated that serum LDH activity is associated with middle cerebral artery occlusion in a dose-dependent manner (14). Several reports have shown that LDH quantification predicts neuronal injury (15, 16) and may predict poor prognosis of traumatic brain injury (17) and neonatal intracranial hemorrhage (18).
Several risk factors contribute to the poor prognosis in aneurysmal subarachnoid hemorrhage (aSAH), such as hypertension, poor Hunt–Hess grade, higher Fisher grade, hydrocephalus, pneumonia, and treatment modalities (19–21). However, few reports have explored the clinical significance of serum LDH levels in patients with aSAH, and the role of LDH in aSAH has not been fully established. At least two sources may contribute to higher serum LDH levels in patients with aSAH: (1) apoptotic/necrotic/damaged neurons or glial cells, (2) lytic red blood cells (RBCs) after release into the cerebrospinal fluid (CSF). Lu et al. reported that the number of apoptotic/necrotic/damaged cells was positively correlated with clinical condition in patients with aSAH, as well as with their Hunt–Hess grade (22). Similarly, the number of RBCs in the cerebra cisterna, sulcus, and/or ventricle was correlated with Fisher grade. Frontera found that early brain ischemia injury was associated with worse Hunt–Hess grade, which indicates poor acute neurological status and is correlated with worse functional outcomes after SAH (23). Claassen et al. showed that SAH completely filling the cistern or fissure, as well as intraventricular hemorrhage (IVH) on computed tomography (CT), were risk factors for delayed cerebral ischemia (DCI) (24), which is correlated with poor outcomes after SAH. However, few reports have explored the relationship between serum LDH levels and the extent of cerebral tissue injury in patients with aSAH. In the present study, we explored the clinical significance of serum LDH in patients with aSAH treated using microsurgical clipping in our institution. We hypothesized that higher preoperative serum levels of LDH, which may be correlated with Hunt–Hess grade and Fisher grade, predict 3-month outcome in patients with aSAH treated using microsurgical clipping.
Materials and Methods
Participants
Patients were enrolled in the study based on the following criteria: (1) diagnosis of SAH confirmed by CT; (2) presence of intracranial aneurysms confirmed using CT angiography (CTA) or digital subtraction angiography (DSA); (3) all aneurysms treated using microsurgical clipping; (4) CTA and/or DSA performed postoperatively. (5) patients were admitted 24 h after the onset of SAH. The exclusion criteria were as follows: (1) aSAH detected > 24 h after occurrence; (2) other cerebrovascular diseases (such as cerebral arteriovenous malformations, intracranial arteriovenous fistula, or moyamoya syndrome/disease) or intracranial tumors; (3) history of myocardial infarction, hepatitis, malignant tumor, pulmonary infarction, leukemia, hemolytic anemia, kidney disease, or progressive muscular atrophy. The data of patients with aSAH at our institution between 2010 and 2018 were collected. The following parameters were recorded: age, sex, smoking and drinking history, medical history (hypertension, diabetes, coronary heart disease, cerebral stroke), Hunt–Hess and Fisher grades, aneurysm location, aneurysm size, surgical treatment (conventional or decompressive craniotomy), delayed cerebral ischemia (DCI), intracranial infection, hydrocephalus, pneumonia, and preoperative serum LDH levels within 24 h of aSAH.
Treatment
After confirmation, ruptured intracranial aneurysms were treated using microsurgical clipping within 72 h of aSAH onset. After surgery, patients were treated according to current aSAH guidelines (25), including prevention of cerebral arterial narrowing, improvement of cerebral blood flow, neurotrophic treatment, stress ulcer prevention, and nutritional support.
Follow-Up Visit and Definition of Outcome
Postoperative complications were evaluated using CT scanning within 24 h of surgery. The neurological outcome was assessed at the 3-month follow-up and classified according to the modified Rankin Scale (mRS) score. Good clinical outcome was defined as an mRS score of 0–2, while poor outcome was assigned to patients with an mRS score of 3–6. Functional outcome was divided into four levels according to mRS: no symptoms (mRS = 0), no significant to slight disability (mRS = 1–2), moderate to serious disability (mRS = 3–4), and severe disability to death (mRS = 5–6). To define the relationship between serum LDH levels and clinical outcome in patients with aSAH, we investigated whether preoperative serum LDH levels were associated with Hunt–Hess grade, Fisher grade, or the upper four functional outcomes.
Statistical Analysis
All statistical analyses were performed using SPSS for Windows (version 25.0; IBM Corp., Armonk, NY, USA). The Kolmogorov–Smirnov test was used to check whether the data had a normal distribution. One-way analysis of variance or the Student's t-test were used to determine the significance of differences in continuous data. The χ2 test or Fisher's exact test was used to determine the significance of differences in qualitative data. Multivariable analysis was carried out using a backward stepwise logistic regression model that included all variables with a p-value of <0.10 in the univariate analysis. In the multivariable analysis model, age was divided into “ ≤ 60 years” and “> 60 years” (26), Hunt–Hess grade into “low grade” (I–III) and “high grade” (IV–V), Fisher grade into “low grade” (1–3) and “high grade” (4), and serum LDH levels into “ ≤ optimal cutoff value” and “> optimal cutoff value.” Statistical significance was set at a p-value of <0.05. The receiver operating characteristics (ROC) curve (MedCalc for Windows version 15.2.2; Mariakerke, Belgium) was generated to analyze the specificity and sensitivity of serum LDH levels for mRS. Propensity-score matching (PSM) was performed to remove imbalances in basic clinical characteristics between the good outcome and poor outcome groups, as well as between the pneumonia and non-pneumonia groups. Conditional probability was estimated using a logistic regression model. The good outcome and poor outcome groups were matched at a ratio of 1:1 using the nearest neighboring matching algorithm.
Results
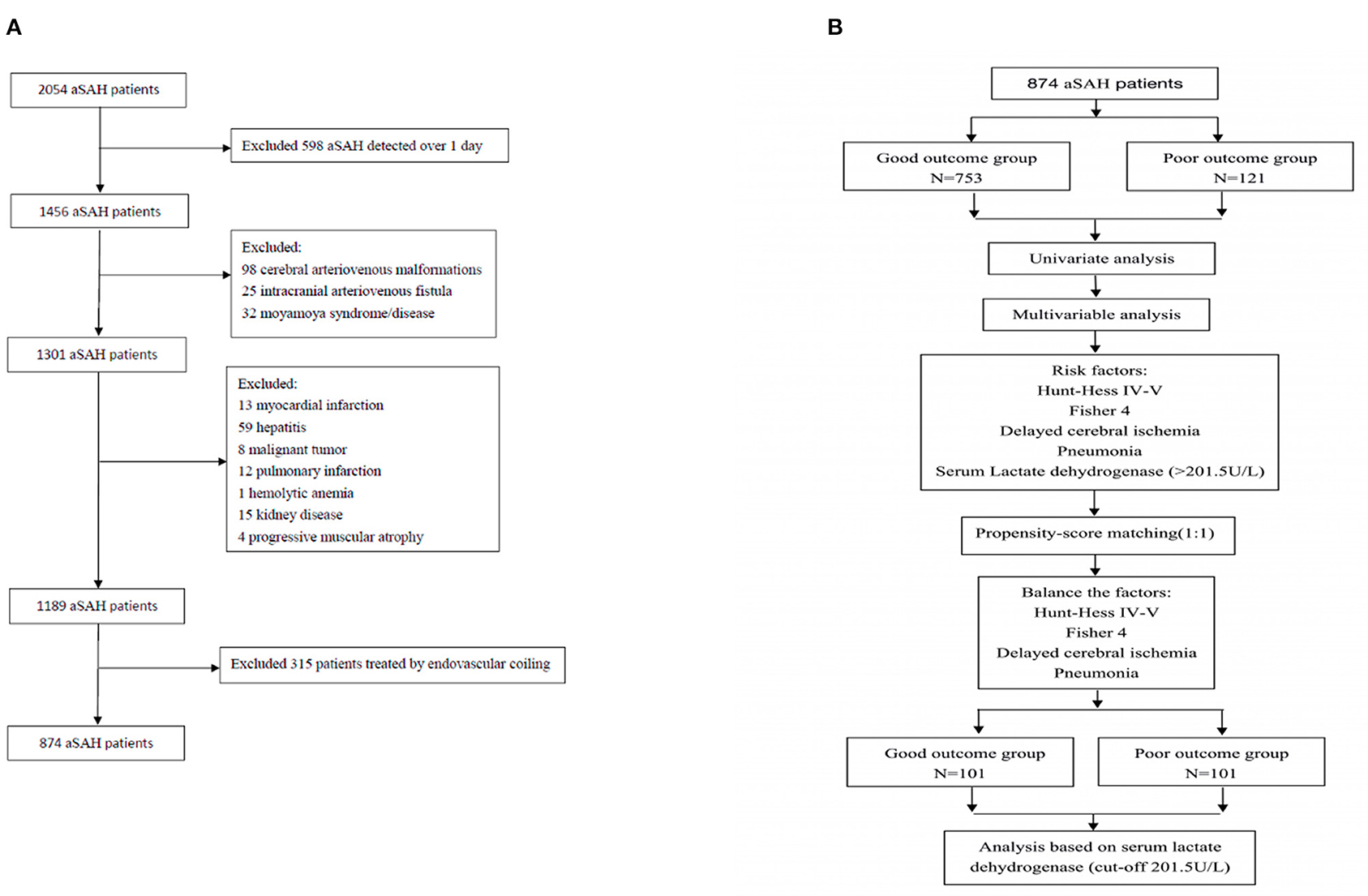
A total of 2,054 patients with aSAH were treated in our institution between 2010 and 2018, and 874 patients treated using microsurgical clipping were enrolled based on the above criteria (Figure 1). The incidence of poor outcomes following aSAH was 13.8% (121/874). The basic clinical characteristics of patients with aSAH are shown in Tables 1, 2. The average serum LDH level (U/L) was significantly lower in the good outcome group (180.096 ± 50.237) than in the poor outcome group (227.554 ± 83.002; p < 0.001). After PSM, the average serum LDH level (U/L) was still lower in the good outcome group (205.356 ± 76.785) than in the poor outcome group (227.119 ± 86.469; p = 0.029). The ROC curve of serum LDH levels for poor outcome in patients with aSAH at the 3-month follow-up is shown in Figure 2. The area under the ROC curve (AUC) was 0.702 (95% confidence interval [CI]: 0.650–0.754; p < 0.001). The optimal cutoff value for serum LDH levels as a predictor of poor 3-month outcome (mRS > 2) was determined to be 201.5 U/L based on the ROC curve. At this level, the sensitivity was 59.5% and the specificity 76.4%.
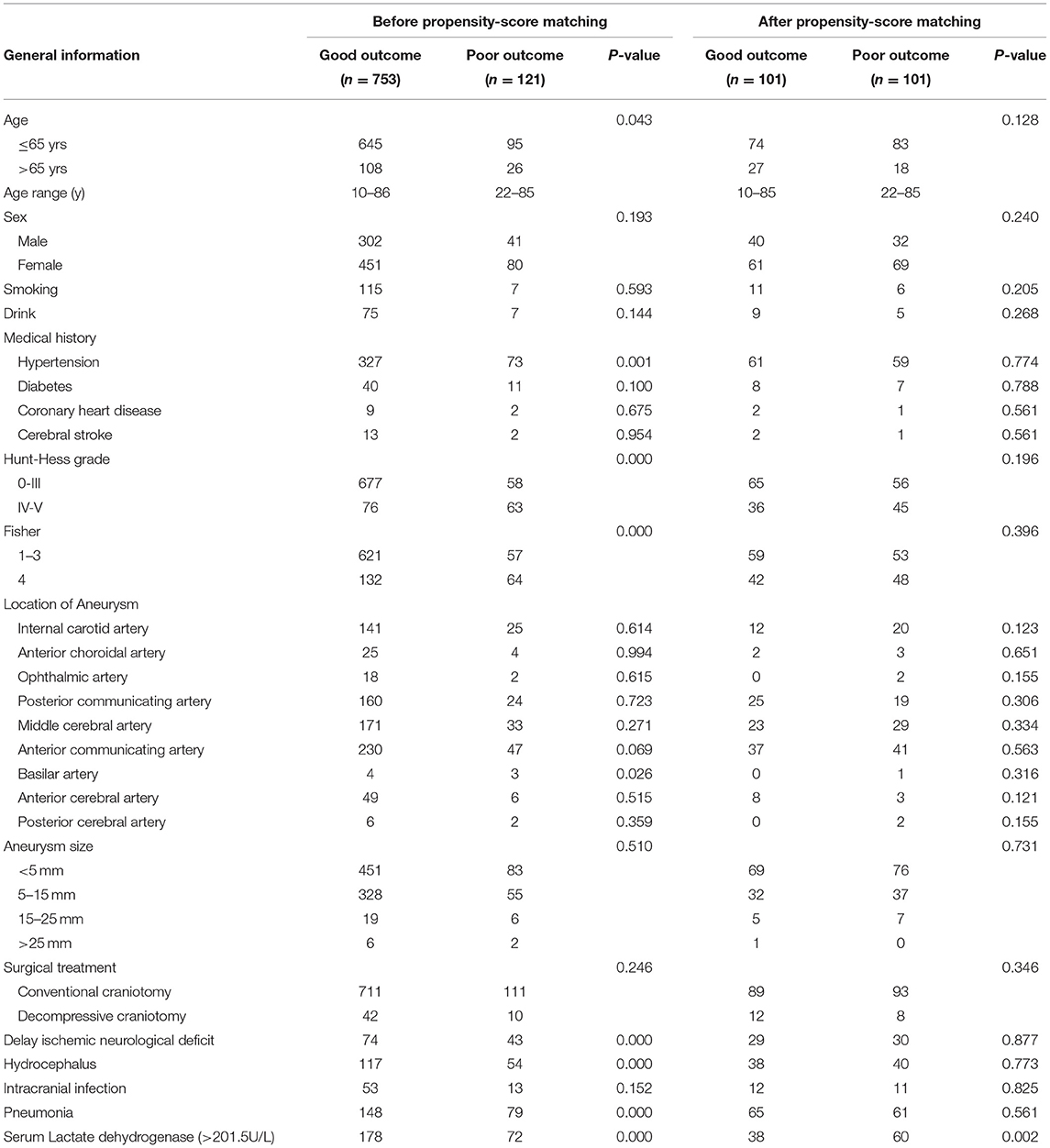
Table 1. Basic clinical characteristics of patients with aneurysmsal subarachnoid hemorrhage before and after propensity-score matching.
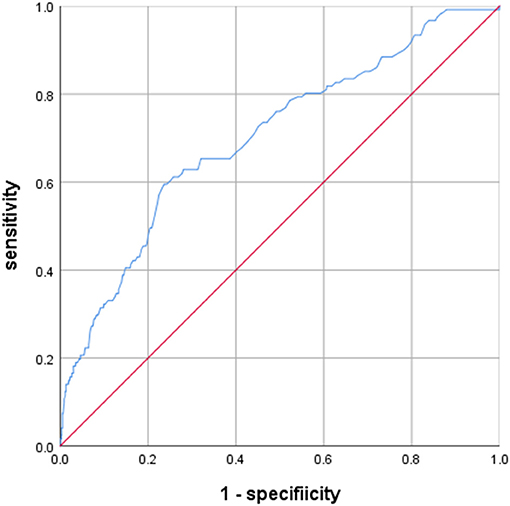
Figure 2. Predictive values of LDH for 3-month modified Rankin Scale (mRS) >2 area under curve 0.702 (95% confidence interval [CI], 0.650–0.754; p < 0.001).
To analyze the predictors of poor outcome in patients with aSAH, the following variables with a significance level of p < 0.10 were included in a univariate analysis: age, hypertension, diabetes, Hunt–Hess grade, Fisher grade, anterior communicating artery aneurysm, basilar artery aneurysm, DCI, hydrocephalus, pneumonia, and serum LDH levels of > 201.5 U/L. The results revealed that age, hypertension, Hunt–Hess grade, Fisher grade, DCI, hydrocephalus, pneumonia, and serum LDH levels > 201.5 U/L were associated with poor 3-month outcomes (Table 2; p < 0.05). In a multivariable analysis, Hunt–Hess grade, Fisher grade, DCI, pneumonia, and serum LDH levels >201.5 U/L were still significantly associated with outcome, whereas age, hypertension, diabetes, anterior communicating artery aneurysm, basilar artery aneurysm, and hydrocephalus were not. Patients with a Hunt–Hess grade of IV–V had a 1.6-fold increased risk of poor outcomes (odds ratio [OR]: 1.637; 95% CI: 1.266–2.118, p < 0.001). Those with a Fisher grade of 4 had a 1.5-fold increased risk of poor outcomes (OR: 1.517, 95% CI: 1.182–1.946, p = 0.001). DCI conferred a 4.2-fold increased risk of poor outcomes (OR: 4.234, 95% CI: 2.412–7.432, p < 0.001). Pneumonia was associated with a 3.8-fold increased risk of poor outcomes (OR: 3.848, 95% CI: 2.386–6.206, p < 0.001). Serum LDH levels >201.5 U/L showed a 2.7-fold increased risk of poor outcomes (OR: 2.702, 95% CI: 1.645–4.440, p < 0.001; Table 2). After PSM, there were no significant differences in Hunt–Hess grade, Fisher grade, DCI, or pneumonia between the good outcome and poor outcome groups (Tables 1, 2). In the logistic regression model (Table 2), serum LDH levels >201.5 U/L were still considered an independent risk factor for poor outcome (OR: 2.426, 95% CI: 1.378–4.271, p = 0.002).
Interestingly, serum LDH levels were associated with Hunt–Hess and Fisher grade, with levels of 163.880 ± 35.571 U/L in the Hunt–Hess grade I group, lower than those in the grade II (174.981 ± 49.616), III (188.306 ± 50.702), IV (225.609 ± 69.509), and V groups (252.851 ± 93.302). There were significant differences among the groups in this regard (p < 0.001), and there was a marked trend whereby serum LDH levels increased alongside Hunt–Hess grade (Figure 3). Serum LDH levels were 169.492 ± 41.621 in the Fisher grade 1 group, lower than in the grade 2 (177.097 ± 42.621), grade 3 (198.709 ± 72.553), and grade 4 groups (210.811 ± 68.962). There were statistically significant differences between grades 4 and 3, grades 4 and 2, grades 4 and 1, grades 3 and 2, grades 3 and 1 (p < 0.001 in all cases). There was a marked trend whereby serum LDH levels increased alongside Fisher grade (Figure 4).
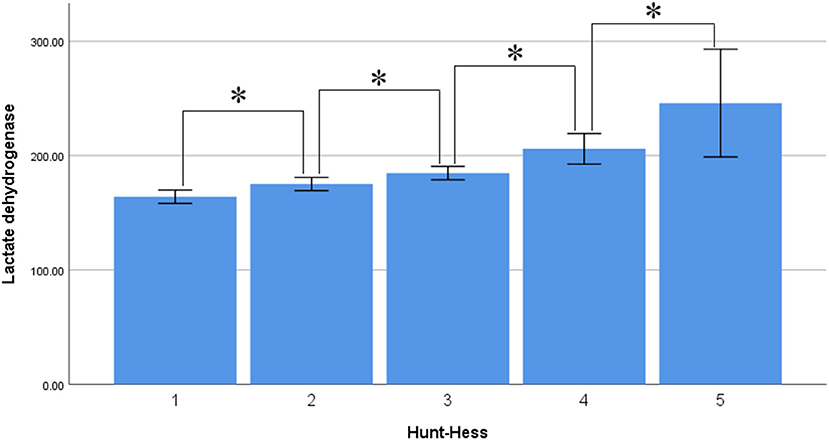
Figure 3. Average level of serum LDH in different Hunt-Hess grade (asterisk represent statistically significant differences).
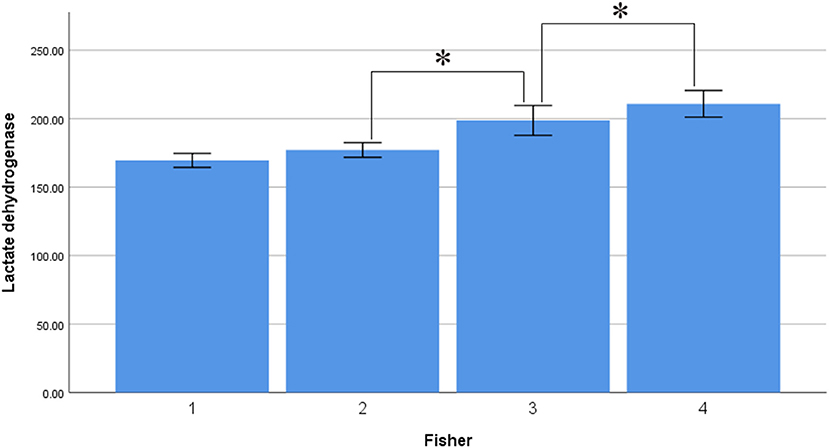
Figure 4. Average level of serum LDH in different fisher grade (asterisk represent statistically significant differences).
Serum LDH levels were also correlated with functional neurological outcome at the 3-month follow-up. The serum LDH levels were 179.247 ± 46.761 in the mRS 0 group, lower than in the no significant to slight disability (mRS 1–2; 193.977 ± 69.399), moderate to serious disability (mRS 3–4; 205.918 ± 59.203), and severe disability to death groups (mRS 5–6; 234.188 ± 108.336). There were significant differences among the groups in this regard (p < 0.001). There was a marked trend whereby serum LDH levels increased as neurological function deteriorated (Figure 5).
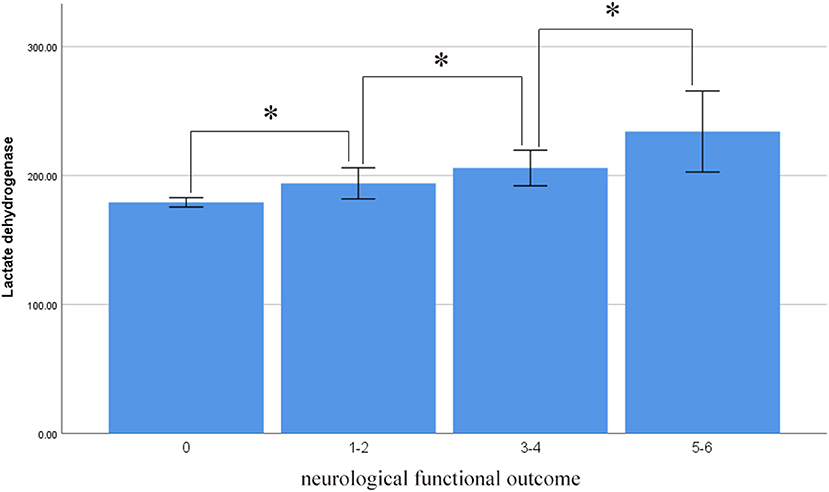
Figure 5. Average level of serum LDH in different neurological functional outcome (asterisk represent statistically significant differences).
Discussion
Our findings showed a marked trend whereby serum LDH levels increased alongside Hunt–Hess and Fisher grades. In addition, Hunt–Hess grade, Fisher grade, DCI, pneumonia, and higher serum LDH levels predicted and contributed to poor outcome in patients with aSAH at 3 months. The optimal cutoff value for serum LDH levels as a predictor of 3-month poor outcome (mRS > 2) was 201.5 U/L. Moreover, serum LDH levels were correlated with functional neurological outcomes. There was a marked trend whereby serum LDH levels increased as neurological function deteriorated. After PSM, serum LDH (>201.5 U/L) was still considered an independent risk factor of poor outcome.
Serum LDH levels are correlated with the prognosis of adult T-cell leukemia-lymphoma (6), prostate cancer (7), acute myeloid leukemia (8), melanoma (9), neuroblastoma (10), glioblastoma multiforme (11), acute encephalopathy (12), and Mycoplasma pneumoniae pneumonia (13). However, few studies have investigated the relationship between LDH and aSAH. Regional cerebral blood flow and arteriovenous difference of oxygen are reduced due to primary aSAH injury (27), and cerebral ischemia causes an anaerobic shift of metabolism, leading to lactic acidosis and upregulation of serum LDH levels (28). There is a significant correlation between serum LDH and lactic acid levels, and both reflect the degree of tissue damage (27, 29).
Serum LDH levels can also reflect the severity of brain tissue injury. Neuronal apoptosis and necrosis have been observed 24 h after SAH (30, 31) and can result in cytolysis and cell membrane destruction. Subsequently, LDH is released into the blood from the damaged or dead cells, resulting in increased serum LDH (4). In the study by Yu et al. (14), serum LDH activity was associated with infarct volume and degree of middle cerebral artery occlusion in a dose-dependent manner. Several studies have shown that LDH can be quantified to predict neuronal damage (15, 16), and inhibition of LDH release may reduce neuronal apoptosis (14). Rao et al. reported that a significant increase in serum LDH levels was a predictor of severe brain damage and poor prognosis of traumatic brain injury (17), while Engelke et al. indicated that LDH was significantly correlated with subsequent seizures, hydrocephalus, and adverse long-term outcomes of neonatal intracranial hemorrhage (18).
In the present study, serum LDH levels increased alongside Hunt–Hess grade. We deduced that serum LDH levels were correlated with Hunt–Hess grade, and that they reflected the degree of early brain injury and the clinical condition of patients with aSAH. Furthermore, there was a marked trend whereby serum LDH levels increased as neurological function at the 3-month follow-up deteriorated. Subarachnoid clots in sulci/fissures induce spreading depolarizations and acute cerebral infarction of the adjacent cortex after cerebral aneurysm rupture (30). This is a mechanism of early brain injury after SAH (32) and contributes to the clinical condition of patients with aSAH. In a study by Frontera et al. (23), early ischemic brain injury was related to worse Hunt–Hess grade, higher rates of death, and severe disability/death (mRS 4–6) at the 3-month follow-up. Increased ischemic lesion volume has been associated higher Hunt–Hess grade and 3-month mRS (33), corroborating our own findings.
Our findings also showed a marked trend whereby serum LDH levels increased alongside Fisher grade. Fisher grade is higher when there is more blood in the subarachnoid space, and higher Fisher grade correlates with poor aSAH outcome (34, 35). After cerebral aneurysm rupture, the blood brain barrier is destroyed and RBCs are released into the subarachnoid space from the artery. These RBCs break down in the cerebrospinal fluid (CSF) (35) and LDH from the lysed RBCs is absorbed into the blood after being released into the CSF (17), increasing serum LDH levels. Therefore, higher serum LDH levels are associated with higher Fisher grade, which is closely related to poor outcome in patients with aSAH (34, 36, 37).
However, our study had some limitations. Firstly, LDH occurs in all important human organs and lacks specificity to the central nervous system. LDH was not collected and measured in the CSF in our patients, and serum LDH levels do not directly reflect the true levels in brain tissue. Secondly, imaging data were not available to confirm the relationship between serum LDH levels and the degree of brain tissue damage. Thirdly, serum LDH levels were within the normal range in some patients with poor outcomes, so they do not fully explain these patients' prognosis; the detailed mechanism needs further exploration.Fourthly, LDH, like C-reactive protein, neutrophil to lymphocyte ratio and white blood cell count, are related to the prognosis of SAH. They are biomarkers of the prognosis of SAH. However, the specific mechanism of LDH is still unclear. And there is still no effective treatment to reduce the mortality and disability rate of aSAH patients.
Conclusions
Our findings showed that higher preoperative serum levels of LDH correlated with Hunt–Hess grade, Fisher grade, and functional neurological outcome, and that they predicted the 3-month outcome in patients with aSAH, which is associated with mechanisms of early brain injury and may have clinical significance in patients with aSAH.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by Ethics Committee of the First Affiliated Hospital of Fujian Medical University. Written informed consent from the participants' legal guardian/next of kin was not required to participate in this study in accordance with the national legislation and the institutional requirements.
Author Contributions
SZ, HW, GC, HS, and LY: acquisition of data and critical revision of manuscript for intellectual content. YL: study supervision. ZL, PY, and DK: study concept and design. PY and DK: analysis and interpretation of data and study supervision. All authors reviewed the manuscript.
Funding
Supported by key clinical specialty discipline construction program of Fujian, P.R.C, major project of Fujian provincial department of science and technology (No. 2014YZ0003 and No. 2014YZ01 to DK), the Young and Middle-aged Backbone Key Research Project of National Health and Family Planning Commission of Fujian Province (No. 2017-ZQN-46 to PY), Natural Science Funding of Fujian Province (No. 2018J01175 to PY and No. 2018J01176 to SZ) and Natural Science Funding of China (No. 81802492 to PY).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Podlasek SJ, McPherson RA, Threatte GA. Characterization of apparent lactate dehydrogenase isoenzyme 6: a lactate-independent dehydrogenase. Clin Chem. (1984) 30:266–70. doi: 10.1093/clinchem/30.2.266
2. Li W, Liu J, Chen JR, Zhu YM, Gao X, Ni Y, et al. Neuroprotective effects of DTIO, a novel analog of nec-1, in acute and chronic stages after ischemic stroke. Neurosci. (2018) 390:12–29. doi: 10.1016/j.neuroscience.2018.07.044
3. Engstrom M, Schott U, Nordstrom CH, Romner B, Reinstrup P. Increased lactate levels impair the coagulation system–a potential contributing factor to progressive hemorrhage after traumatic brain injury. J Neurosurg Anesth. (2006) 18:200–4. doi: 10.1097/01.ana.0000211002.63774.8a
4. Batt AM, Ferrari L. Manifestations of chemically induced liver damage. Clin Chem. (1995) 41:1882–7. doi: 10.1093/clinchem/41.12.1882
5. Fadeel B, Hassan Z, Hellstrom-Lindberg E, Henter JI, Orrenius S, Zhivotovsky B. Cleavage of bcl-2 is an early event in chemotherapy-induced apoptosis of human myeloid leukemia cells. Leukemia. (1999) 13:719–28. doi: 10.1038/sj.leu.2401411
6. Nakashima J, Imaizumi Y, Taniguchi H, Ando K, Iwanaga M, Itonaga H, et al. Clinical factors to predict outcome following mogamulizumab in adult t-cell leukemia-lymphoma. Int J Hematol. (2018) 108:516–23. doi: 10.1007/s12185-018-2509-0
7. Cotogno PM, Ranasinghe LK, Ledet EM, Lewis BE, Sartor O. Laboratory-based biomarkers and liver metastases in metastatic castration-resistant prostate cancer. Oncologist. (2018) 23:791–7. doi: 10.1634/theoncologist.2017-0564
8. Tachibana T, Andou T, Tanaka M, Ito S, Miyazaki T, Ishii Y, et al. Clinical significance of serum ferritin at diagnosis in patients with acute myeloid leukemia: a yacht multicenter retrospective study. Clin Lymphoma Myeloma Leuk. (2018) 18:415–21. doi: 10.1016/j.clml.2018.03.009
9. Nicholas MN, Khoja L, Atenafu EG, Hogg D, Quirt I, Butler M, et al. Prognostic factors for first-line therapy and overall survival of metastatic uveal melanoma: the princess margaret cancer centre experience. Melanoma Res. (2018) 28:571–7. doi: 10.1097/CMR.0000000000000468
10. Dorneburg C, Fischer M, Barth TFE, Mueller-Klieser W, Hero B, Gecht J, et al. LDHA in neuroblastoma is associated with poor outcome and its depletion decreases neuroblastoma growth independent of aerobic glycolysis. Clin Cancer Res. (2018) 24:5772–83. doi: 10.1158/1078-0432.CCR-17-2578
11. Daniele S, Giacomelli C, Zappelli E, Granchi C, Trincavelli ML, Minutolo F, et al. Lactate dehydrogenase-A inhibition induces human glioblastoma multiforme stem cell differentiation and death. Sci Rep. (2015) 5:15556. doi: 10.1038/srep15556
12. Oba C, Kashiwagi M, Tanabe T, Nomura S, Ogino M, Matsuda T, et al. Prognostic factors in the early phase of acute encephalopathy. Pediatr Int. (2018) 60:270–5. doi: 10.1111/ped.13492
13. Inamura N, Miyashita N, Hasegawa S, Kato A, Fukuda Y, Saitoh A, et al. Management of refractory mycoplasma pneumoniae pneumonia: utility of measuring serum lactate dehydrogenase level. J Infect Chemother. (2014) 20:270–3. doi: 10.1016/j.jiac.2014.01.001
14. Yu W, Wang Y, Zhou DX, Zhao LM, Li GR, Deng XL. Equol is neuroprotective during focal cerebral ischemia and reperfusion that involves p-Src and gp91(phox). Curr Neurovasc Res. (2014) 11:367–77. doi: 10.2174/1567202611666140908094517
15. Hu W, Dang XB, Wang G, Li S, Zhang YL. Peroxiredoxin-3 attenuates traumatic neuronal injury through preservation of mitochondrial function. Neurochem Int. (2018) 114:120–6. doi: 10.1016/j.neuint.2018.02.004
16. Zhang H, Zhang D, Li H, Yan H, Zhang Z, Zhou C, et al. Biphasic activation of nuclear factor-kb and expression of p65 and c-Rel following traumatic neuronal injury. Int J Mol Med. (2018) 41:3203–10. doi: 10.3892/ijmm.2018.3567
17. Rao CJ, Shukla PK, Mohanty S, Reddy YJ. Predictive value of serum lactate dehydrogenase in head injury. J Neurol Neurosurg Psychiatry. (1978) 41:948–53. doi: 10.1136/jnnp.41.10.948
18. Engelke S, Bridgers S, Saldanha RL, Trought WS. Cerebrospinal fluid lactate dehydrogenase in neonatal intracranial hemorrhage. Am J Med Sci. (1986) 291:391–5. doi: 10.1097/00000441-198606000-00004
19. Juvela S, Siironen J, Kuhmonen J. Hyperglycemia, excess weight, and history of hypertension as risk factors for poor outcome and cerebral infarction after aneurysmal subarachnoid hemorrhage. J Neurosurg. (2005) 102:998–1003. doi: 10.3171/jns.2005.102.6.0998
20. Zipfel GJ. Ultra-early surgery for aneurysmal subarachnoid hemorrhage. J Neurosurg. (2015) 122:381–2. doi: 10.3171/2014.8.JNS141613
21. Jeon SB, Choi HA, Badjatia N, Schmidt JM, Lantigua H, Claassen J, et al. Hyperoxia may be related to delayed cerebral ischemia and poor outcome after subarachnoid haemorrhage. J Neurol Neurosur Ps. (2014) 85:1301–7. doi: 10.1136/jnnp-2013-307314
22. Lu Y, Zhang XS, Zhang ZH, Zhou XM, Gao YY, Liu GJ, et al. Peroxiredoxin 2 activates microglia by interacting with toll-like receptor 4 after subarachnoid hemorrhage. J Neuroinflammation. (2018) 15:87. doi: 10.1186/s12974-018-1118-4
23. Frontera JA, Ahmed W, Zach V, Jovine M, Tanenbaum L, Sehba F, et al. Acute ischaemia after subarachnoid haemorrhage, relationship with early brain injury and impact on outcome: a prospective quantitative MRI study. J Neurol Neurosur Ps. (2015) 86:71–8. doi: 10.1136/jnnp-2013-307313
24. Claassen J, Bernardini GL, Kreiter K, Bates J, Du YE, Copeland D, et al. Effect of cisternal and ventricular blood on risk of delayed cerebral ischemia after subarachnoid hemorrhage: the fisher scale revisited. Stroke. (2001) 32:2012–20. doi: 10.1161/hs0901.095677
25. Connolly ES, Rabinstein AA, Carhuapoma JR, Derdeyn CP, Dion J, Higashida RT, et al. Guidelines for the management of aneurysmal subarachnoid hemorrhage: a guideline for healthcare professionals from the American heart association/American stroke association. Stroke. (2012) 43:1711–37. doi: 10.1161/STR.0b013e3182587839
26. Degos V, Gourraud PA, Tursis VT, Whelan R, Colonne C, Korinek AM, et al. Elderly age as a prognostic marker of 1-year poor outcome for subarachnoid hemorrhage patients through its interaction with admission hydrocephalus. Anesthesiology. (2012) 117:1289–99. doi: 10.1097/ALN.0b013e318267395b
27. Voldby B, Enevoldsen EM, Jensen FT. Regional CBF, intraventricular pressure, and cerebral metabolism in patients with ruptured intracranial aneurysms. J Neurosurg. (1985) 62:48–58. doi: 10.3171/jns.1985.62.1.0048
28. Yang S, Ning F, Li J, Guo D, Zhang L, Cui R, et al. Therapeutic effect analysis of sinomenine on rat cerebral ischemia-reperfusion injury. J Stroke Cerebrovasc Dis. (2016) 25:1263–9. doi: 10.1016/j.jstrokecerebrovasdis.2016.02.023
29. Zein JG, Lee GL, Tawk M, Dabaja M, Kinasewitz GT. Prognostic significance of elevated serum lactate dehydrogenase (LDH) in patients with severe sepsis. Chest. (2004) 126:873S. doi: 10.1378/chest.126.4_MeetingAbstracts.873S
30. Hartings JA, York J, Carroll CP, Hinzman JM, Mahoney E, Krueger B, et al. Subarachnoid blood acutely induces spreading depolarizations and early cortical infarction. Brain. (2017) 140:2673–90. doi: 10.1093/brain/awx214
31. Friedrich V, Flores R, Sehba FA. Cell death starts early after subarachnoid hemorrhage. Neurosci Lett. (2012) 512:6–11. doi: 10.1016/j.neulet.2012.01.036
32. Cahill J, Calvert JW, Zhang JH. Mechanisms of early brain injury after subarachnoid hemorrhage. J Cerebr Blood F Met. (2006) 26:1341–53. doi: 10.1038/sj.jcbfm.9600283
33. De Marchis GM, Filippi CG, Guo X, Pugin D, Gaffney CD, Dangayach NS, et al. Brain injury visible on early MRI after subarachnoid hemorrhage might predict neurological impairment and functional outcome. Neurocrit Care. (2015) 22:74–81. doi: 10.1007/s12028-014-0008-6
34. Dupont SA, Wijdicks EF, Manno EM, Lanzino G, Rabinstein AA. Prediction of angiographic vasospasm after aneurysmal subarachnoid hemorrhage: value of the Hijdra sum scoring system. Neurocrit Care. (2009) 11:172–6. doi: 10.1007/s12028-009-9247-3
35. Hugelshofer M, Sikorski CM, Seule M, Deuel J, Muroi CI, Seboek M, et al. Cell-free oxyhemoglobin in cerebrospinal fluid after aneurysmal subarachnoid hemorrhage: a biomarker and potential therapeutic target. World Neurosurg. (2018) 120:e660–6. doi: 10.1016/j.wneu.2018.08.141
36. Pereira AR, Sanchez-Pena P, Biondi A, Sourour N, Boch AL, Colonne C, et al. Predictors of 1-year outcome after coiling for poor-grade subarachnoid aneurysmal hemorrhage. Neurocrit Care. (2007) 7:18–26. doi: 10.1007/s12028-007-0053-5
Keywords: serum lactate dehydrogenase level, aneurysmal subarachnoid hemorrhage, risk factor, biomaker, outcome
Citation: Zheng S, Wang H, Chen G, Shangguan H, Yu L, Lin Z, Lin Y, Yao P and Kang D (2021) Higher Serum Levels of Lactate Dehydrogenase Before Microsurgery Predict Poor Outcome of Aneurysmal Subarachnoid Hemorrhage. Front. Neurol. 12:720574. doi: 10.3389/fneur.2021.720574
Received: 04 June 2021; Accepted: 19 July 2021;
Published: 12 August 2021.
Edited by:
Robert G. Kowalski, University of Colorado, United StatesReviewed by:
Yuanjian Fang, Zhejiang University, ChinaGang Wang, Southern Medical University, China
Copyright © 2021 Zheng, Wang, Chen, Shangguan, Yu, Lin, Lin, Yao and Kang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Peisen Yao, cGVpc2VuLnlhb0AxNjMuY29t; Dezhi Kang, a2R6OTk5ODhAdmlwLnNpbmEuY29t
†These authors have contributed equally to this work
 Shufa Zheng
Shufa Zheng Haojie Wang
Haojie Wang Guorong Chen1†
Guorong Chen1† Peisen Yao
Peisen Yao Dezhi Kang
Dezhi Kang