
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
MINI REVIEW article
Front. Neurol., 22 September 2021
Sec. Stroke
Volume 12 - 2021 | https://doi.org/10.3389/fneur.2021.719329
This article is part of the Research TopicEmerging Areas in Extracranial Carotid Stenosis Evaluation and ManagementView all 11 articles
 Joseph Kamtchum-Tatuene1*
Joseph Kamtchum-Tatuene1* Ali Z. Nomani2
Ali Z. Nomani2 Sarina Falcione2
Sarina Falcione2 Danielle Munsterman2
Danielle Munsterman2 Gina Sykes2
Gina Sykes2 Twinkle Joy2
Twinkle Joy2 Elena Spronk2
Elena Spronk2 Maria Isabel Vargas3
Maria Isabel Vargas3 Glen C. Jickling2
Glen C. Jickling2Embolic stroke of unknown source (ESUS) represents one in five ischemic strokes. Ipsilateral non-stenotic carotid plaques are identified in 40% of all ESUS. In this narrative review, we summarize the evidence supporting the potential causal relationship between ESUS and non-stenotic carotid plaques; discuss the remaining challenges in establishing the causal link between non-stenotic plaques and ESUS and describe biomarkers of potential interest for future research. In support of the causal relationship between ESUS and non-stenotic carotid plaques, studies have shown that plaques with high-risk features are five times more prevalent in the ipsilateral vs. the contralateral carotid and there is a lower incidence of atrial fibrillation during follow-up in patients with ipsilateral non-stenotic carotid plaques. However, non-stenotic carotid plaques with or without high-risk features often coexist with other potential etiologies of stroke, notably atrial fibrillation (8.5%), intracranial atherosclerosis (8.4%), patent foramen ovale (5–9%), and atrial cardiopathy (2.4%). Such puzzling clinical associations make it challenging to confirm the causal link between non-stenotic plaques and ESUS. There are several ongoing studies exploring whether select protein and RNA biomarkers of plaque progression or vulnerability could facilitate the reclassification of some ESUS as large vessel strokes or help to optimize secondary prevention strategies.
Ischemic stroke is considered cryptogenic when no definite cause is identified during the baseline etiological workup (1). According to the Cryptogenic Stroke/Embolic Stroke of Undetermined Source International Working Group, the baseline etiological workup should include brain imaging with computed tomography (CT) or magnetic resonance imaging (MRI), assessment of the heart rhythm with 12-lead ECG and continuous cardiac monitoring for at least 24 h with automated rhythm detection, transthoracic cardiac ultrasound, and imaging of cervical and intracranial vessels supplying the infarcted brain region (using CT, MRI, conventional angiography, or ultrasonography) (2).
Cryptogenic strokes represent ~30% of all ischemic strokes. They could be further classified into three subgroups: stroke with no cause despite complete baseline workup, stroke with multiple possible underlying causes, and stroke with incomplete baseline workup (3). In the subgroup of cryptogenic strokes with complete workup, embolic stroke of unknown source (ESUS) is a clinical construct referring to non-lacunar ischemic strokes (size >1.5 cm on CT or >2.0 cm on diffusion MRI) of presumable embolic origin (superficial/cortical brain lesion) despite the absence of any obvious sources of cardiac or arterial embolism (e.g., atrial fibrillation, carotid, or intracranial stenosis > 50%) (Figure 1) (2). ESUS represent ~17% of all ischemic strokes with a recurrent stroke rate of 4.5% per year despite antithrombotic therapy (4–6).
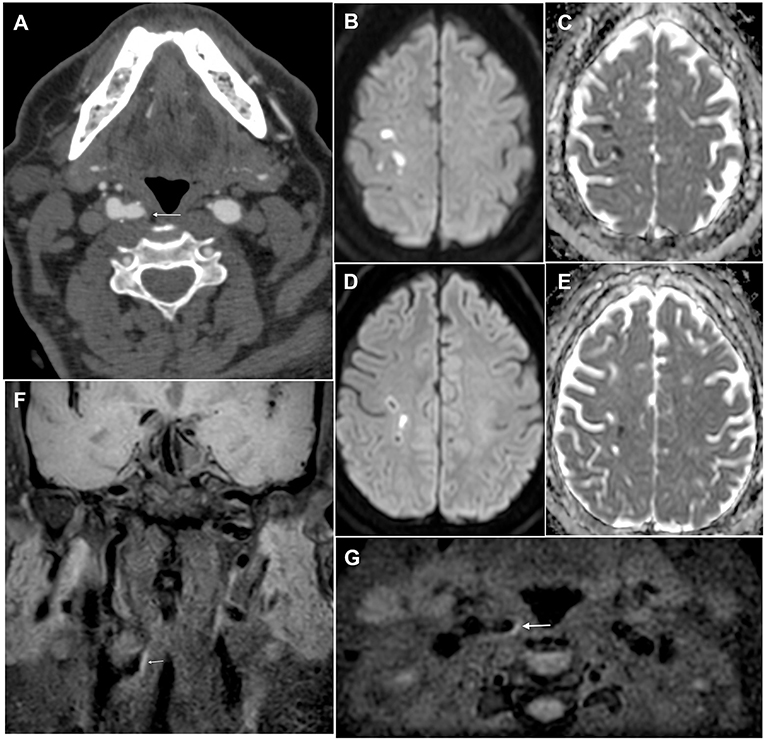
Figure 1. Brain and plaque imaging findings in a 64-year-old man with ESUS. (A) Axial angio-CT scan slice showing a hypodense non-stenotic carotid plaque in the right internal carotid artery (white arrow). (B–E) Axial diffusion-weighted imaging slices (with corresponding ADC maps) showing multiple embolic strokes in the right pre-and post-central area. (F,G) Coronal and axial T1-weighted black blood sequence showing hyperintensity of the non-stenotic plaque in the right internal carotid artery (white arrow), thus confirming the presence of intraplaque hemorrhage.
The definition of ESUS was based on the assumptions that cryptogenic strokes may be related to covert atrial fibrillation and that a relationship between non-stenotic atherosclerotic plaques (causing <50% stenosis) and stroke was unlikely. However, there is now evidence to suggest that ESUS represents a heterogeneous group including patients with various other potential causes of stroke besides atrial fibrillation (7–9). Such causes include atrial cardiopathy (10), patent foramen ovale (PFO) (11), cancer (12), and non-stenotic plaques affecting the aortic arch or carotid, vertebral, or intracranial arteries (7, 13, 14). Atrial cardiopathy is a concept referring to a dysfunction of the left atrium that is thought to favor and precede the onset of atrial fibrillation and its eventual detection by electrocardiographic devices. The diagnosis is based on the identification of imaging markers (e.g., left atrial enlargement, spontaneous echocontrast in the left atrium or the left atrial appendage, atrial fibrosis with delayed gadolinium enhancement on MRI), electrocardiographic markers (e.g., paroxysmal supraventricular tachycardia, increased P-wave terminal force in V1, interatrial block, prolonged PR), and blood biomarkers (e.g., N-terminal pro-brain natriuretic peptide, highly sensitive cardiac troponin T) (10).
Non-stenotic carotid plaques are found in 40% of patients with ESUS and 10–15% of patients with ESUS have mild stenosis (20–49%) (2, 15–17). Here we review the evidence supporting the relationship between non-stenotic carotid plaques with high-risk features and stroke in patients with ESUS. We present the remaining challenges in the process of formally establishing the causal link between non-stenotic plaques and ESUS, notably those related to the identification of blood biomarkers of vulnerable plaque. Finally, we discuss the management of non-stenotic carotid plaques in patients with ESUS and highlight areas for future research.
The relationship between non-stenotic carotid plaques and ESUS is supported by a set of three clinical observations.
First, in patients with ESUS, carotid plaques are more prevalent on the side of the stroke than on the contralateral side. In a cross-sectional study of 85 patients with ESUS, non-stenotic carotid plaques thicker than 3 mm were present in 35% of ipsilateral carotid arteries vs. 15% of the contralateral carotid arteries (18). A similar finding was observed in a review of 138 ESUS cases from the prospective multicenter INTERRSeCT study (The Predicting Early Recanalization and Reperfusion With IV Alteplase and Other Treatments Using Serial CT Angiography). The investigators found a non-stenotic carotid plaque ipsilateral to the stroke in 29.2% of patients and contralateral to the stroke in 18.7% (17).
Second, in patients with ESUS, there is a lower incidence of atrial fibrillation detected during follow-up in patients with ipsilateral non-stenotic carotid plaques than in those without, thus suggesting that non-stenotic carotid plaques may be related to the stroke. In 777 participants of the New Approach Rivaroxaban Inhibition of Factor Xa in a Global Trial vs. ASA to Prevent Embolism in Embolic Stroke of Undetermined Source (NAVIGATE-ESUS) trial who were followed up for a median of 2 years, the incidence of atrial fibrillation was 2.9 per 100 person-years in patients with ipsilateral non-stenotic carotid plaques vs. 5.0 per 100 person-years in those without (overall rate: 8.5 vs. 19.0%; adjusted hazard ratio: 0.57, 95% CI 0.37–0.84) (15).
Third, plaques with high-risk features are more prevalent on the side of the stroke in patients with ESUS. In a meta-analysis of 8 studies enrolling 323 patients with ESUS, plaques with high-risk features were present in 32.5% of the ipsilateral carotid arteries vs. 4.6% of the contralateral carotid arteries. More specifically, the odds of finding a non-stenotic carotid plaque with a ruptured fibrous cap in the ipsilateral vs. the contralateral carotid artery was 17.5, reinforcing the idea that non-stenotic carotid plaques should not be considered as benign coincidental findings in patients with ESUS (13).
High-risk plaques have features on brain or vascular imaging that are associated with a higher risk of stroke in patients with either symptomatic or asymptomatic carotid atherosclerosis, independent of the grade of stenosis (19–24). The most common high-risk plaque features are echolucency, impaired cerebrovascular reserve, intraplaque hemorrhage (Figure 1), silent brain infarcts, lipid-rich necrotic core, large juxtaluminal black hypoechoic area, large plaque volume, plaque thickness, microembolic signals, mural thrombus, neovascularization, plaque irregularity, plaque inflammation or hypermetabolism, thin or ruptured fibrous cap, and ulceration (19, 21, 25–31). The American Heart Association combines some of these features to derive a classification of atherosclerotic plaques into 6 types reflecting increasing instability and risk of cardiovascular events (Table 1) (32–37). On average, high-risk plaque features are three times more prevalent in patients with symptomatic vs. asymptomatic carotid stenosis (OR = 3.4, 95% CI: 2.5–4.6) (19). They are detected using various vascular imaging modalities (Table 2). To date, there are no data on the risk of recurrent stroke associated with each of the high-risk features in patients with ESUS. Analysis of secondary outcome data from the Carotid Plaque Imaging in Acute Stroke study (CAPIAS; NCT01284933) might help to address this knowledge gap (35, 39).
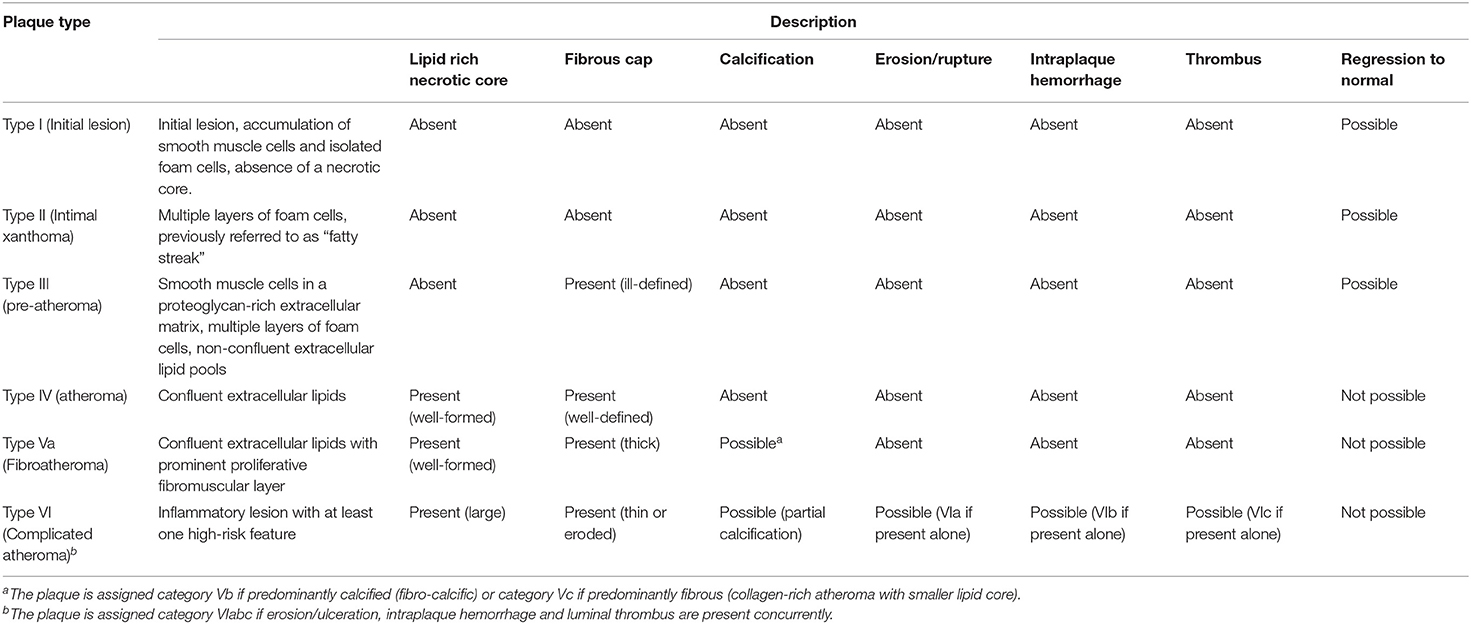
Table 1. American Heart Association comprehensive morphological classification scheme for atherosclerotic lesions (32–34).
Although studies of high-risk features have provided evidence of an association between non-stenotic carotid plaques and brain infarction in patients with ESUS, establishing causality remains challenging in most cases. The dilemma rests on four clinical observations. First, high-risk features are often found in plaques in the absence of related clinical symptoms (19, 40). In a meta-analysis of eight studies enrolling 323 patients with ESUS, a non-stenotic carotid plaque with high-risk features was identified in the contralateral carotid artery in 4.6% of cases (95% CI: 0.1–13.1) (13). Likewise, in a meta-analysis of 64 studies enrolling 20,571 patients with asymptomatic carotid stenosis of various grades, 26.5% of patients were found to have at least one high-risk plaque feature (95% CI: 22.9–30.3). The highest prevalence was observed for neovascularization (43.4%, 95% CI: 31.4–55.8) and the lowest for mural thrombus (7.3%, 95% CI: 2.5–19.4). On average, intraplaque hemorrhage was found in 1 out of 5 patients (19). Second, high-risk plaque features are not specific for symptomatic carotid plaques. In a meta-analysis of data from 20 prospective studies enrolling 1,652 patients with symptomatic carotid stenosis, high-risk plaque features were identified in <1 in 2 patients (43.3%, 95% CI: 33.6–53.2) (19). Third, in patients with stroke, there is an association between the presence of high-risk plaque features and atrial fibrillation. In a study of 68 patients with embolic stroke, including 45 ESUS, the presence of high-risk plaque features on carotid ultrasound (ulceration, thickness ≥ 3 mm, and echolucency) was independently associated with detection of atrial fibrillation on admission or during follow-up (OR = 4.5, 95% CI: 1.0–19.6) (41). Fourth, in some patients with ESUS diagnosed using the current clinical definition, non-stenotic carotid plaques often coexist with other potential causes of stroke, including atrial fibrillation (8.5%) (15), intracranial atherosclerosis (8.4%) (42), PFO (5–9%) (43, 44), and atrial cardiopathy (2.4%) (45).
The identification of an ipsilateral non-stenotic carotid plaque with or without high-risk features is not sufficient to reclassify ESUS as stroke due to large vessel disease. Further research is, therefore, needed to determine whether combination of vascular imaging findings, clinical data, and candidate biomarkers of plaque progression/instability or atheroembolism (46–82) into multiparameter scores could improve the ability to (1) establish a causal link between ESUS and a non-stenotic carotid plaque, (2) predict plaque progression or stroke recurrence, and (3) select patients who might benefit from adjuvant anti-inflammatory and lipid-lowering therapies as briefly discussed in the next section. Some biomarkers of plaque progression and instability that warrant further investigation specifically in patients with ESUS are presented in Table 3. There are several ongoing projects exploring biomarkers in patients with ESUS or cryptogenic stroke, notably the Searching for Explanations for Cryptogenic Stroke in the Young: Revealing the Etiology, Triggers, and Outcome study (SECRETO, NCT01934725) (95), the Carotid Plaque Imaging in Acute Stroke study (CAPIAS, NCT01284933) (35), and the Biomarkers of Acute Stroke Etiology study (BASE, NCT02014896) (96). Efforts to establish a causal relationship between non-stenotic carotid stenosis and ESUS using biomarkers and multimodal vascular imaging in well-phenotyped prospective cohorts will also benefit from research aiming to identify alternative causes of stroke in patients with ESUS (14, 68, 97–104).
As a result of the challenges to determine the root cause of an ESUS, the optimal treatment strategy for patients with ESUS remains unclear, and a tailored approach would likely be the most appropriate (9). In this section, we briefly describe the strategies that have been explored so far and discuss possible future directions.
Following the results of the Platelet-Oriented Inhibition in New TIA and Minor Ischemic Stroke (POINT) (105) and the Clopidogrel in High-Risk Patients with Acute Non-disabling Cerebrovascular Events (CHANCE) (106) trials, patients with ESUS are treated with Aspirin-based dual antiplatelet therapy for 21 days provided that their baseline NIHSS is low. After 3 weeks, patients ideally return to single antiplatelet therapy and switching from Aspirin to Clopidogrel is considered in patients who had an ESUS while on Aspirin (107). A meta-analysis of data from CHANCE and POINT showed that extending the treatment beyond 3 weeks might increase the bleeding risk without additional benefit for secondary stroke prevention (108). Whether the presence of ipsilateral non-stenotic carotid plaque with or without high-risk features would modify the magnitude (absolute risk reduction) and duration (beyond 21 days) of the benefits derived from dual antiplatelet therapy in patients with ESUS remains unknown. In patients allergic to Clopidogrel and in carriers of a CYP2C19 loss of function allele, Ticagrelor might be an alternative according to findings of the Acute Stroke or Transient Ischemic Attack Treated with Ticagrelor and ASA [acetylsalicylic acid] for Prevention of Stroke and Death (THALES) trial (109–112). The ongoing Clopidogrel with Aspirin in High-risk patients with Acute Non-disabling Cerebrovascular Events II (CHANCE-2, NCT04078737) trial is evaluating the superiority of the Ticagrelor-Aspirin combination over Clopidogrel-Aspirin therapy in CYP2C19 loss of function carriers with minor stroke or transient ischemic attack (TIA) (113). There is currently no evidence supporting the use of dual antiplatelet therapies not containing Aspirin or triple antiplatelet therapies (with or without Aspirin) for secondary stroke prevention in patients with acute stroke or TIA (114).
The New Approach Rivaroxaban Inhibition of Factor Xa in a Global Trial vs. ASA [Acetylsalicylic Acid] to Prevent Embolism in Embolic Stroke of Undetermined Source (NAVIGATE-ESUS) and the Randomized Double-Blind Evaluation in Secondary Stroke Prevention Comparing The Efficacy Of Oral Thrombin Inhibitor Dabigatran Etexilate for Secondary Stroke Prevention in Patients With Embolic Stroke of Undetermined Source (RE-SPECT-ESUS) trials have shown that universal full-dose oral anticoagulation is not an effective strategy to reduce the risk of stroke recurrence in patients with ESUS (5, 6). These results are likely explained by the heterogeneity of stroke mechanisms in patients with ESUS as discussed earlier, with atrial fibrillation being diagnosed in only 24.8% of cases at 24 months using insertable cardiac monitors (115). Moreover, there is no evidence that patients with ESUS and ipsilateral non-stenotic carotid plaques should be treated differently than those without plaques. In a subgroup analysis of data from 2,905 patients with non-stenotic carotid plaques enrolled in the NAVIGATE-ESUS trial, there was no difference between Rivaroxaban and Aspirin with respect to the prevention of ipsilateral ischemic stroke [Hazard ratio [HR] = 0.6, 95% CI: 0.2–1.9]. Major bleeding complications were significantly more frequent in patients taking anticoagulation (HR = 3.7, 95% CI: 1.6–8.7) (16).
In the Cardiovascular Outcomes for People Using Anticoagulation Strategies (COMPASS) trial, the combination Rivaroxaban-Aspirin (2.5 mg twice daily plus Aspirin 100 mg once per day) was superior to Aspirin alone (100 mg once daily) for the prevention of cardioembolic strokes (HR = 0.4, 95% CI: 0.2–0.8) and ESUS (HR = 0.3, 95% CI: 0.1–0.7) but there was no effect on the incidence of stroke due to moderate-to-severe carotid stenosis (HR = 0.9, 95% CI: 0.5–1.6) (116). Although these results suggest that the combination of Aspirin and low-dose Rivaroxaban could be an effective secondary stroke prevention strategy, they are not directly applicable to patients with ESUS since all patients with acute stroke (<1 month) were excluded from the trial due to the perceived higher risk of major intracranial bleeding (117). Furthermore, the baseline proportion of patients with non-stenotic carotid plaque, with or without high-risk features, was not reported. The prevalence of ipsilateral non-stenotic carotid plaque in participants diagnosed with ESUS during follow-up was also not reported.
According to currently available data, patients with ESUS and features of atrial cardiopathy, notably atrial enlargement, constitute the only subgroup that may benefit from anticoagulation (118). However, since these results are derived from a post-hoc analysis of the NAVIGATE-ESUS trial, they might not be used to justify universal prescription of anticoagulation until confirmation is obtained in dedicated trials. The ongoing Atrial Cardiopathy and Antithrombotic Drugs in Prevention After Cryptogenic Stroke (ARCADIA, NCT03192215) (101), Apixaban for Treatment of Embolic Stroke of Undetermined Source (ATTICUS, NCT02427126), and A Study on BMS-986177 (oral factor XIa inhibitor) for the Prevention of a Stroke in Patients Receiving Aspirin and Clopidogrel (AXIOMATIC-SSP, NCT03766581) trials will, hopefully, provide conclusive results to guide patient care. Likewise, in the Oxford Vascular Study, a large patent foramen ovale is present in 36% of patients with a cryptogenic stroke aged >60 years (119) and associated with a 2.5 times higher risk of recurrent ischemic stroke (120), thus suggesting it might be worth trialing PFO closure or anticoagulation in elderly patients with a large PFO. However, the causal relationship between the PFO and the recurrent stroke was not formally established and the prevalence of ipsilateral non-stenotic carotid plaque not reported. Because PFO closure or anticoagulation are not expected to prevent strokes due to large vessel atherosclerosis, trials of PFO closure or anticoagulation in elderly patients with a large PFO should carefully plan subgroup analyses according to the presence of alternative candidate causes of the recurrent stroke, notably an atrial cardiopathy or an ipsilateral non-stenotic carotid plaque that may coexist with PFO (43, 44, 121).
Currently, patients with ESUS receive intensive lipid-lowering therapy (e.g., statins, ezetimibe) to achieve a level of LDL cholesterol <70 mg/dL (1.8 mmol/L) as early as possible after stroke (122–124). The treatment is maintained long-term if well-tolerated, even in older adults (125–128). Specific targets of LDL cholesterol have not been assessed in patients with ESUS and it is unknown if the presence of an ipsilateral non-stenotic carotid plaque would modify the effect of lipid-lowering drugs as suggested by findings of the Stroke Prevention by Aggressive Reduction in Cholesterol Levels (SPARCL) (129). Furthermore, the potential role of newer classes of lipid-lowering drugs for plaque stabilization and secondary stroke prevention is yet to be defined. Such drugs include proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors (small interfering RNA—inclisiran or monoclonal antibodies—evolocumab or alirocumab) and Apo(a) antisense oligonucleotides that reduce plasma levels of both LDL cholesterol and lipoprotein(a) [Lp(a)]; as well as anti- angiopoietin-like 3 monoclonal antibodies that do not affect Lp(a) levels and bempedoic acid (92, 130–135). Like ezetimibe (93, 136), the new lipid-lowering drugs may be useful as add-on or statin-sparing agents in cases of allergy or intolerance to statins, familial hypercholesterolemia, refractory hypercholesterolemia, or in patients with high Lp(a) levels at the time of stroke since statins increase plasma levels of Lp(a) (90, 137). There are reports of an association between high Lp(a) levels and cryptogenic stroke (138, 139) suggesting that Lp(a) could represent a biomarker to guide optimization of lipid-lowering therapy in patients with ESUS as is the case in other cardiovascular diseases.
Systemic inflammation, a hallmark of atherosclerosis, modulates the risk of stroke and the effect of lipid-lowering agents (140–142). This explains the benefit of various anti-inflammatory drugs (e.g., canakinumab, colchicine) for the prevention of atherosclerotic cardiovascular diseases (86, 87, 143). In patients with ESUS and ipsilateral non-stenotic carotid plaque, the effect of anti-inflammatory agents is worth exploring, especially in those with high-risk plaque features since they would not be offered revascularization procedures as first-line treatment according to current guidelines (144–146). Data from the ongoing Colchicine for Prevention of Vascular Inflammation in Non-Cardioembolic Stroke (CONVINCE, NCT02898610) might answer the question of whether patients with ESUS with or without ipsilateral non-stenotic carotid plaques would benefit from the addition of low-dose colchicine to best medical therapy for secondary stroke prevention (147). The relevance of serial vascular imaging to monitor carotid plaque progression and stability is another aspect of the management that remains unexplored.
Besides pharmacological treatments, there is a variety of lifestyle interventions that are beneficial for cardiovascular risk reduction and are recommended by the American Heart Association for secondary stroke prevention no matter the suspected underlying etiology. Such interventions include smoking cessation, regular physical activity, weight loss, improved sleep hygiene, avoidance of noise and air pollution, reduction of salt and sugar intake, higher consumption of fish, fruits, and vegetables (148–155).
ESUS is a common subtype of stroke that is frequently associated with an ipsilateral non-stenotic carotid plaque. Evidence suggests that advanced multimodal vascular imaging and biomarkers might help reclassify some ESUS as large vessel strokes. However, the precise algorithm for this reclassification remains to be designed. Despite significant research efforts since the term ESUS was coined in 2014, the optimal management strategy for patients with ESUS remains unclear. There are several ongoing trials investigating various interventions. While waiting for more evidence to support the design of tailored therapeutic guidelines for the various well-phenotyped subgroups of patients with ESUS, clinicians should continue to fully implement all previously validated stroke prevention strategies, whether an ipsilateral non-stenotic carotid plaque is present or not. Such strategies include short-term dual antiplatelet therapy if appropriate, long-term intensive lipid lowering therapy, control of modifiable cardiovascular risk factors (e.g., hypertension, diabetes, smoking, obesity), and lifestyle changes.
JK-T did the literature search and wrote the manuscript. MV and JK-T prepared the figure. AN, SF, DM, GS, TJ, ES, MV, and GJ critically revised the manuscript. All authors approved the final version.
GJ received research grant support from Canadian Institutes of Health Research (CIHR), Heart and Stroke Foundation, University Hospital Foundation, Canada Foundation for Innovation (CFI), and National Institutes of Health (NIH). JK-T was supported by the Faculty of Medicine and Dentistry Motyl Graduate Studentship in Cardiac Sciences, an Alberta Innovates Graduate Student Scholarship, the Ballermann Translational Research Fellowship, the Izaak Walton Killam Memorial Scholarship, and the Andrew Stewart Memorial Graduate Prize.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Adams HP Jr, Bendixen BH, Kappelle LJ, Biller J, Love BB, Gordon DL, et al. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke. (1993) 24:35–41. doi: 10.1161/01.STR.24.1.35
2. Hart RG, Diener HC, Coutts SB, Easton JD, Granger CB, O'Donnell MJ, et al. Embolic strokes of undetermined source: the case for a new clinical construct. Lancet Neurol. (2014) 13:429–38. doi: 10.1016/S1474-4422(13)70310-7
3. Tsivgoulis G, Katsanos AH, Kohrmann M, Caso V, Lemmens R, Tsioufis K, et al. Embolic strokes of undetermined source: theoretical construct or useful clinical tool? Ther Adv Neurol Disord. (2019) 12:1756286419851381. doi: 10.1177/1756286419851381
4. Hart RG, Catanese L, Perera KS, Ntaios G, Connolly SJ. Embolic stroke of undetermined source: a systematic review and clinical update. Stroke. (2017) 48:867–72. doi: 10.1161/STROKEAHA.116.016414
5. Hart RG, Sharma M, Mundl H, Kasner SE, Bangdiwala SI, Berkowitz SD, et al. Rivaroxaban for stroke prevention after embolic stroke of undetermined source. N Engl J Med. (2018) 378:2191–201. doi: 10.1056/NEJMoa1802686
6. Diener HC, Sacco RL, Easton JD, Granger CB, Bernstein RA, Uchiyama S, et al. Dabigatran for prevention of stroke after embolic stroke of undetermined source. N Engl J Med. (2019) 380:1906–17. doi: 10.1056/NEJMoa1813959
7. Ntaios G. Embolic stroke of undetermined source: JACC review topic of the week. J Am Coll Cardiol. (2020) 75:333–40. doi: 10.1016/j.jacc.2019.11.024
8. Li L, Yiin GS, Geraghty OC, Schulz UG, Kuker W, Mehta Z, et al. Incidence, outcome, risk factors, and long-term prognosis of cryptogenic transient ischaemic attack and ischaemic stroke: a population-based study. Lancet Neurol. (2015) 14:903–13. doi: 10.1016/S1474-4422(15)00132-5
9. Kamel H, Merkler AE, Iadecola C, Gupta A, Navi BB. Tailoring the approach to embolic stroke of undetermined source: a review. JAMA Neurol. (2019) 76:855–61. doi: 10.1001/jamaneurol.2019.0591
10. Yaghi S, Kamel H, Elkind MSV. Atrial cardiopathy: a mechanism of cryptogenic stroke. Expert Rev Cardiovasc Ther. (2017) 15:591–9. doi: 10.1080/14779072.2017.1355238
11. Kasner SE, Swaminathan B, Lavados P, Sharma M, Muir K, Veltkamp R, et al. Rivaroxaban or aspirin for patent foramen ovale and embolic stroke of undetermined source: a prespecified subgroup analysis from the NAVIGATE ESUS trial. Lancet Neurol. (2018) 17:1053–60. doi: 10.1016/S1474-4422(18)30319-3
12. Navi BB, Kasner SE, Elkind MSV, Cushman M, Bang OY, DeAngelis LM. Cancer and embolic stroke of undetermined source. Stroke. (2021) 52:1121–30. doi: 10.1161/STROKEAHA.120.032002
13. Kamtchum-Tatuene J, Wilman A, Saqqur M, Shuaib A, Jickling GC. carotid plaque with high-risk features in embolic stroke of undetermined source: systematic review and meta-analysis. Stroke. (2020) 51:311–4. doi: 10.1161/STROKEAHA.119.027272
14. Tao L, Li XQ, Hou XW, Yang BQ, Xia C, Ntaios G, et al. Intracranial atherosclerotic plaque as a potential cause of embolic stroke of undetermined source. J Am Coll Cardiol. (2021) 77:680–91. doi: 10.1016/j.jacc.2020.12.015
15. Ntaios G, Perlepe K, Sirimarco G, Strambo D, Eskandari A, Karagkiozi E, et al. Carotid plaques and detection of atrial fibrillation in embolic stroke of undetermined source. Neurology. (2019) 92:e2644–52. doi: 10.1212/WNL.0000000000007611
16. Ntaios G, Swaminathan B, Berkowitz SD, Gagliardi RJ, Lang W, Siegler JE, et al. Efficacy and safety of rivaroxaban versus aspirin in embolic stroke of undetermined source and carotid atherosclerosis. Stroke. (2019) 50:2477–85. doi: 10.1161/STROKEAHA.119.025168
17. Ospel JM, Singh N, Marko M, Almekhlafi M, Dowlatshahi D, Puig J, et al. Prevalence of ipsilateral nonstenotic carotid plaques on computed tomography angiography in embolic stroke of undetermined source. Stroke. (2020) 51:1743–9. doi: 10.1161/STROKEAHA.120.029404
18. Coutinho JM, Derkatch S, Potvin AR, Tomlinson G, Kiehl TR, Silver FL, et al. Nonstenotic carotid plaque on CT angiography in patients with cryptogenic stroke. Neurology. (2016) 87:665–72. doi: 10.1212/WNL.0000000000002978
19. Kamtchum-Tatuene J, Noubiap JJ, Wilman AH, Saqqur M, Shuaib A, Jickling GC. Prevalence of high-risk plaques and risk of stroke in patients with asymptomatic carotid stenosis: a meta-analysis. JAMA Neurol. (2020) 77:1018–27. doi: 10.1001/jamaneurol.2020.2658
20. Schindler A, Schinner R, Altaf N, Hosseini AA, Simpson RJ, Esposito-Bauer L, et al. Prediction of stroke risk by detection of hemorrhage in carotid plaques: meta-analysis of individual patient data. JACC Cardiovasc Imaging. (2019) 13(2 Pt 1):395–406. doi: 10.1016/j.jcmg.2019.03.028
21. Saba L, Saam T, Jager HR, Yuan C, Hatsukami TS, Saloner D, et al. Imaging biomarkers of vulnerable carotid plaques for stroke risk prediction and their potential clinical implications. Lancet Neurol. (2019) 18:559–72. doi: 10.1016/S1474-4422(19)30035-3
22. Bos D, Arshi B, van den Bouwhuijsen QJA, Ikram MK, Selwaness M, Vernooij MW, et al. Atherosclerotic carotid plaque composition and incident stroke and coronary events. J Am Coll Cardiol. (2021) 77:1426–35. doi: 10.1016/j.jacc.2021.01.038
23. Kelly PJ, Camps-Renom P, Giannotti N, Marti-Fabregas J, McNulty JP, Baron JC, et al. A risk score including carotid plaque inflammation and stenosis severity improves identification of recurrent stroke. Stroke. (2020) 51:838–45. doi: 10.1161/STROKEAHA.119.027268
24. Baradaran H, Gupta A. Extracranial vascular disease: carotid stenosis and plaque imaging. Neuroimaging Clin N Am. (2021) 31:157–66. doi: 10.1016/j.nic.2021.02.002
25. Bayer-Karpinska A, Schindler A, Saam T. Detection of vulnerable plaque in patients with cryptogenic stroke. Neuroimaging Clin N Am. (2016) 26:97–110. doi: 10.1016/j.nic.2015.09.008
26. Fabiani I, Palombo C, Caramella D, Nilsson J, De Caterina R. Imaging of the vulnerable carotid plaque: role of imaging techniques and a research agenda. Neurology. (2020) 94:922–32. doi: 10.1212/WNL.0000000000009480
27. Paraskevas KI, Veith FJ, Spence JD. How to identify which patients with asymptomatic carotid stenosis could benefit from endarterectomy or stenting. Stroke Vasc Neurol. (2018) 3:92–100. doi: 10.1136/svn-2017-000129
28. Ringelstein EB, Droste DW, Babikian VL, Evans DH, Grosset DG, Kaps M, et al. Consensus on microembolus detection by TCD. International Consensus Group on Microembolus Detection. Stroke. (1998) 29:725–9. doi: 10.1161/01.STR.29.3.725
29. Saam T, Ferguson MS, Yarnykh VL, Takaya N, Xu D, Polissar NL, et al. Quantitative evaluation of carotid plaque composition by in vivo MRI. Arterioscler Thromb Vasc Biol. (2005) 25:234–9. doi: 10.1161/01.ATV.0000149867.61851.31
30. Markus HS, Harrison MJ. Estimation of cerebrovascular reactivity using transcranial Doppler, including the use of breath-holding as the vasodilatory stimulus. Stroke. (1992) 23:668–73. doi: 10.1161/01.STR.23.5.668
31. Rafailidis V, Li X, Sidhu PS, Partovi S, Staub D. Contrast imaging ultrasound for the detection and characterization of carotid vulnerable plaque. Cardiovasc Diagn Ther. (2020) 10:965–81. doi: 10.21037/cdt.2020.01.08
32. Stary HC. Natural history and histological classification of atherosclerotic lesions: an update. Arterioscler Thromb Vasc Biol. (2000) 20:1177–8. doi: 10.1161/01.ATV.20.5.1177
33. Virmani R, Kolodgie FD, Burke AP, Farb A, Schwartz SM. Lessons from sudden coronary death: a comprehensive morphological classification scheme for atherosclerotic lesions. Arterioscler Thromb Vasc Biol. (2000) 20:1262–75. doi: 10.1161/01.ATV.20.5.1262
34. Saba L, Brinjikji W, Spence JD, Wintermark M, Castillo M, Borst GJD, et al. Roadmap consensus on carotid artery plaque imaging and impact on therapy strategies and guidelines: an international, multispecialty, expert review and position statement. AJNR Am J Neuroradiol. (2021). doi: 10.3174/ajnr.A7223. [Epub ahead of print].
35. Bayer-Karpinska A, Schwarz F, Wollenweber FA, Poppert H, Boeckh-Behrens T, Becker A, et al. The carotid plaque imaging in acute stroke (CAPIAS) study: protocol and initial baseline data. BMC Neurol. (2013) 13:201. doi: 10.1186/1471-2377-13-201
36. Freilinger TM, Schindler A, Schmidt C, Grimm J, Cyran C, Schwarz F, et al. Prevalence of nonstenosing, complicated atherosclerotic plaques in cryptogenic stroke. JACC Cardiovasc Imaging. (2012) 5:397–405. doi: 10.1016/j.jcmg.2012.01.012
37. Hyafil F, Schindler A, Sepp D, Obenhuber T, Bayer-Karpinska A, Boeckh-Behrens T, et al. High-risk plaque features can be detected in non-stenotic carotid plaques of patients with ischaemic stroke classified as cryptogenic using combined (18)F-FDG PET/MR imaging. Eur J Nucl Med Mol Imaging. (2016) 43:270–9. doi: 10.1007/s00259-015-3201-8
38. Buon R, Guidolin B, Jaffre A, Lafuma M, Barbieux M, Nasr N, et al. Carotid ultrasound for assessment of nonobstructive carotid atherosclerosis in young adults with cryptogenic stroke. J Stroke Cerebrovasc Dis. (2018) 27:1212–6. doi: 10.1016/j.jstrokecerebrovasdis.2017.11.043
39. Kopczak A, Schindler A, Bayer-Karpinska A, Koch ML, Sepp D, Zeller J, et al. Complicated carotid artery plaques as a cause of cryptogenic stroke. J Am Coll Cardiol. (2020) 76:2212–22. doi: 10.1016/j.jacc.2020.09.532
40. Sun J, Underhill HR, Hippe DS, Xue Y, Yuan C, Hatsukami TS. Sustained acceleration in carotid atherosclerotic plaque progression with intraplaque hemorrhage: a long-term time course study. JACC Cardiovasc Imaging. (2012) 5:798–804. doi: 10.1016/j.jcmg.2012.03.014
41. Grosse GM, Sieweke JT, Biber S, Ziegler NL, Gabriel MM, Schuppner R, et al. Nonstenotic carotid plaque in embolic stroke of undetermined source: interplay of arterial and atrial disease. Stroke. (2020) 51:3737–41. doi: 10.1161/STROKEAHA.120.030537
42. Ameriso SF, Amarenco P, Pearce LA, Perera KS, Ntaios G, Lang W, et al. Intracranial and systemic atherosclerosis in the NAVIGATE ESUS trial: recurrent stroke risk and response to antithrombotic therapy. J Stroke Cerebrovasc Dis. (2020) 29:104936. doi: 10.1016/j.jstrokecerebrovasdis.2020.104936
43. Ntaios G, Sagris D, Strambo D, Perlepe K, Sirimarco G, Georgiopoulos G, et al. Carotid atherosclerosis and patent foramen ovale in embolic stroke of undetermined source. J Stroke Cerebrovasc Dis. (2021) 30:105409. doi: 10.1016/j.jstrokecerebrovasdis.2020.105409
44. Jaffre A, Guidolin B, Ruidavets JB, Nasr N, Larrue V. Non-obstructive carotid atherosclerosis and patent foramen ovale in young adults with cryptogenic stroke. Eur J Neurol. (2017) 24:663–6. doi: 10.1111/ene.13275
45. Kamel H, Pearce LA, Ntaios G, Gladstone DJ, Perera K, Roine RO, et al. Atrial cardiopathy and nonstenosing large artery plaque in patients with embolic stroke of undetermined source. Stroke. (2020) 51:938–43. doi: 10.1161/STROKEAHA.119.028154
46. Barreto J, Karathanasis SK, Remaley A, Sposito AC. Role of LOX-1 (Lectin-like oxidized low-density lipoprotein receptor 1) as a cardiovascular risk predictor: mechanistic insight and potential clinical use. Arterioscler Thromb Vasc Biol. (2021) 41:153–66. doi: 10.1161/ATVBAHA.120.315421
47. Hofmann A, Brunssen C, Wolk S, Reeps C, Morawietz H. Soluble LOX-1: a novel biomarker in patients with coronary artery disease, stroke, and acute aortic dissection? J Am Heart Assoc. (2020) 9:e013803. doi: 10.1161/JAHA.119.013803
48. Markstad H, Edsfeldt A, Yao Mattison I, Bengtsson E, Singh P, Cavalera M, et al. High levels of soluble lectinlike oxidized low-density lipoprotein receptor-1 are associated with carotid plaque inflammation and increased risk of ischemic stroke. J Am Heart Assoc. (2019) 8:e009874. doi: 10.1161/JAHA.118.009874
49. Yokota C, Sawamura T, Watanabe M, Kokubo Y, Fujita Y, Kakino A, et al. High levels of soluble lectin-like oxidized low-density lipoprotein receptor-1 in acute stroke: an age- and sex-matched cross-sectional study. J Atheroscler Thromb. (2016) 23:1222–6. doi: 10.5551/jat.32466
50. Li XM, Jin PP, Xue J, Chen J, Chen QF, Luan XQ, et al. Role of sLOX-1 in intracranial artery stenosis and in predicting long-term prognosis of acute ischemic stroke. Brain Behav. (2018) 8:e00879. doi: 10.1002/brb3.879
51. Wu J, Zhang J, Wang A, Chen S, Wu S, Zhao X. Association between non-high-density lipoprotein cholesterol levels and asymptomatic vulnerable carotid atherosclerotic plaques. Eur J Neurol. (2019) 26:1433–8. doi: 10.1111/ene.13973
52. Katan M, Moon YP, Paik MC, Wolfert RL, Sacco RL, Elkind MS. Lipoprotein-associated phospholipase A2 is associated with atherosclerotic stroke risk: the Northern Manhattan Study. PLoS ONE. (2014) 9:e83393. doi: 10.1371/journal.pone.0083393
53. Yang M, Wang A, Li J, Zhao X, Liu L, Meng X, et al. Lp-PLA2 and dual antiplatelet agents in intracranial arterial stenosis. Neurology. (2019) 94:e181–9. doi: 10.1212/WNL.0000000000008733
54. Kamtchum-Tatuene J, Jickling GC. Blood biomarkers for stroke diagnosis and management. Neuromolecular Med. (2019) 21:344–68. doi: 10.1007/s12017-019-08530-0
55. Koenig W, Khuseyinova N. Biomarkers of atherosclerotic plaque instability and rupture. Arterioscler Thromb Vasc Biol. (2007) 27:15–26. doi: 10.1161/01.ATV.0000251503.35795.4f
56. Wang Y, Li B, Jiang Y, Zhang R, Meng X, Zhao X, et al. YKL-40 is associated with ultrasound-determined carotid atherosclerotic plaque instability. Front Neurol. (2021) 12:622869. doi: 10.3389/fneur.2021.622869
57. Skjelland M, Michelsen AE, Krohg-Sorensen K, Tennoe B, Dahl A, Bakke S, et al. Plasma levels of granzyme B are increased in patients with lipid-rich carotid plaques as determined by echogenicity. Atherosclerosis. (2007) 195:e142–6. doi: 10.1016/j.atherosclerosis.2007.05.001
58. Nasr N, Ruidavets JB, Arnal JF, Sie P, Larrue V. Association of neutrophil count with microembolization in patients with symptomatic carotid artery stenosis. Atherosclerosis. (2009) 207:519–23. doi: 10.1016/j.atherosclerosis.2009.05.003
59. Jiao Y, Qin Y, Zhang Z, Zhang H, Liu H, Li C. Early identification of carotid vulnerable plaque in asymptomatic patients. BMC Cardiovasc Disord. (2020) 20:429. doi: 10.1186/s12872-020-01709-5
60. Handberg A, Skjelland M, Michelsen AE, Sagen EL, Krohg-Sorensen K, Russell D, et al. Soluble CD36 in plasma is increased in patients with symptomatic atherosclerotic carotid plaques and is related to plaque instability. Stroke. (2008) 39:3092–5. doi: 10.1161/STROKEAHA.108.517128
61. Georgakis MK, van der Laan SW, Asare Y, Mekke JM, Haitjema S, Schoneveld AH, et al. Monocyte-chemoattractant protein-1 levels in human atherosclerotic lesions associate with plaque vulnerability. Arterioscler Thromb Vasc Biol. (2021) 1:2038–48. doi: 10.1161/ATVBAHA.121.316091
62. Dolz S, Gorriz D, Tembl JI, Sanchez D, Fortea G, Parkhutik V, et al. Circulating microRNAs as novel biomarkers of stenosis progression in asymptomatic carotid stenosis. Stroke. (2017) 48:10–6. doi: 10.1161/STROKEAHA.116.013650
63. Basic J, Stojkovic S, Assadian A, Rauscher S, Duschek N, Kaun C, et al. The relevance of vascular endothelial growth factor, hypoxia inducible factor-1 alpha, and clusterin in carotid plaque instability. J Stroke Cerebrovasc Dis. (2019) 28:1540–5. doi: 10.1016/j.jstrokecerebrovasdis.2019.03.009
64. Ammirati E, Moroni F, Norata GD, Magnoni M, Camici PG. Markers of inflammation associated with plaque progression and instability in patients with carotid atherosclerosis. Mediators Inflamm. (2015) 2015:718329. doi: 10.1155/2015/718329
65. Xiao J, Chen L, Melander O, Orho-Melander M, Nilsson J, Borne Y, et al. Circulating vimentin is associated with future incidence of stroke in a population-based cohort study. Stroke. (2021) 52:937–44. doi: 10.1161/STROKEAHA.120.032111
66. Alhazmi H, Bani-Sadr A, Bochaton T, Paccalet A, Da Silva CC, Buisson M, et al. Large vessel cardioembolic stroke and embolic stroke of undetermined source share a common profile of matrix metalloproteinase-9 level and susceptibility vessel sign length. Eur J Neurol. (2021) 28:1977–83. doi: 10.1111/ene.14806
67. Jickling GC, Xu H, Stamova B, Ander BP, Zhan X, Tian Y, et al. Signatures of cardioembolic and large-vessel ischemic stroke. Ann Neurol. (2010) 68:681–92. doi: 10.1002/ana.22187
68. Choi KH, Kim JH, Kim JM, Kang KW, Lee C, Kim JT, et al. d-dimer level as a predictor of recurrent stroke in patients with embolic stroke of undetermined source. Stroke. (2021) 52:2292–301. doi: 10.1161/STROKEAHA.120.033217
69. Xu T, Zuo P, Cao L, Gao Z, Ke K. Omentin-1 is associated with carotid plaque instability among ischemic stroke patients. J Atheroscler Thromb. (2018) 25:505–11. doi: 10.5551/jat.42135
70. Yanofsky R, Sancho C, Gasbarrino K, Zheng H, Doonan RJ, Jaunet F, et al. Expression of Resistin, Chemerin, and Chemerin's receptor in the unstable carotid atherosclerotic plaque. Stroke. (2021) 52:2537–46. doi: 10.1161/STROKEAHA.120.030228
71. Eltoft A, Arntzen KA, Wilsgaard T, Mathiesen EB, Johnsen SH. Interleukin-6 is an independent predictor of progressive atherosclerosis in the carotid artery: the Tromso Study. Atherosclerosis. (2018) 271:1–8. doi: 10.1016/j.atherosclerosis.2018.02.005
72. Ridker PM. From RESCUE to ZEUS: will interleukin-6 inhibition with ziltivekimab prove effective for cardiovascular event reduction? Cardiovasc Res. (2021). doi: 10.1093/cvr/cvab231. [Epub ahead of print].
73. Ridker PM, Devalaraja M, Baeres FMM, Engelmann MDM, Hovingh GK, Ivkovic M, et al. IL-6 inhibition with ziltivekimab in patients at high atherosclerotic risk (RESCUE): a double-blind, randomised, placebo-controlled, phase 2 trial. Lancet. (2021) 397:2060–9. doi: 10.1016/S0140-6736(21)00520-1
74. Ridker PM, Rane M. Interleukin-6 signaling and anti-interleukin-6 therapeutics in cardiovascular disease. Circ Res. (2021) 128:1728–46. doi: 10.1161/CIRCRESAHA.121.319077
75. Pothineni NVK, Karathanasis SK, Ding Z, Arulandu A, Varughese KI, Mehta JL. LOX-1 in atherosclerosis and myocardial ischemia: biology, genetics, and modulation. J Am Coll Cardiol. (2017) 69:2759–68. doi: 10.1016/j.jacc.2017.04.010
76. Si W, He P, Wang Y, Fu Y, Li X, Lin X, et al. Complement complex C5b-9 levels are associated with the clinical outcomes of acute ischemic stroke and carotid plaque stability. Transl Stroke Res. (2018) 10:279–86. doi: 10.1007/s12975-018-0658-3
77. Shi X, Xie WL, Kong WW, Chen D, Qu P. Expression of the NLRP3 inflammasome in carotid atherosclerosis. J Stroke Cerebrovasc Dis. (2015) 24:2455–66. doi: 10.1016/j.jstrokecerebrovasdis.2015.03.024
78. Arthurs ZM, Andersen C, Starnes BW, Sohn VY, Mullenix PS, Perry J. A prospective evaluation of C-reactive protein in the progression of carotid artery stenosis. J Vasc Surg. (2008) 47:744–50; discussion 751. doi: 10.1016/j.jvs.2007.11.066
79. Klein JH, Hegele RA, Hackam DG, Koschinsky ML, Huff MW, Spence JD. Lipoprotein(a) is associated differentially with carotid stenosis, occlusion, and total plaque area. Arterioscler Thromb Vasc Biol. (2008) 28:1851–6. doi: 10.1161/ATVBAHA.108.169292
80. Muramatsu Y, Minami Y, Kato A, Katsura A, Sato T, Kakizaki R, et al. Lipoprotein (a) level is associated with plaque vulnerability in patients with coronary artery disease: an optical coherence tomography study. Int J Cardiol Heart Vasc. (2019) 24:100382. doi: 10.1016/j.ijcha.2019.100382
81. Rehberger Likozar A, Zavrtanik M, Sebestjen M. Lipoprotein(a) in atherosclerosis: from pathophysiology to clinical relevance and treatment options. Ann Med. (2020) 52:162–77. doi: 10.1080/07853890.2020.1775287
82. Ganji M, Nardi V, Prasad M, Jordan KL, Bois MC, Franchi F, et al. carotid plaques from symptomatic patients are characterized by local increase in xanthine oxidase expression. Stroke. (2021) 52:1636–42. doi: 10.1161/STROKEAHA.120.032964
83. Stability Investigators, White HD, Held C, Stewart R, Tarka E, Brown R, et al. Darapladib for preventing ischemic events in stable coronary heart disease. N Engl J Med. (2014) 370:1702–11. doi: 10.1056/NEJMoa1315878
84. Pires N, Gota V, Gulia A, Hingorani L, Agarwal M, Puri A. Safety and pharmacokinetics of Withaferin-A in advanced stage high grade osteosarcoma: a phase I trial. J Ayurveda Integr Med. (2020) 11:68–72. doi: 10.1016/j.jaim.2018.12.008
85. Thurman JM. New anti-complement drugs: not so far away. Blood. (2014) 123:1975–6. doi: 10.1182/blood-2014-02-555805
86. Ridker PM. Anticytokine agents: targeting interleukin signaling pathways for the treatment of atherothrombosis. Circ Res. (2019) 124:437–50. doi: 10.1161/CIRCRESAHA.118.313129
87. Ridker PM. From CANTOS to CIRT to COLCOT to clinic: will all atherosclerosis patients soon be treated with combination lipid-lowering and inflammation-inhibiting agents? Circulation. (2020) 141:787–9. doi: 10.1161/CIRCULATIONAHA.119.045256
88. Ridker PM, Everett BM, Thuren T, MacFadyen JG, Chang WH, Ballantyne C, et al. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N Engl J Med. (2017) 377:1119–31. doi: 10.1056/NEJMoa1707914
89. Ridker PM. From C-reactive protein to interleukin-6 to interleukin-1: moving upstream to identify novel targets for atheroprotection. Circ Res. (2016) 118:145–56. doi: 10.1161/CIRCRESAHA.115.306656
90. Kamtchum-Tatuene J, Jickling GC. Letter by Kamtchum-Tatuene and Jickling Regarding Article, “Elevated Lp(a) (Lipoprotein[a]) Levels Increase Risk of 30-Day Major Adverse Cardiovascular Events in Patients Following Carotid Endarterectomy”. Stroke. (2021) 52:e64–5. doi: 10.1161/STROKEAHA.120.032698
91. Tsimikas S, Karwatowska-Prokopczuk E, Gouni-Berthold I, Tardif JC, Baum SJ, Steinhagen-Thiessen E, et al. Lipoprotein(a) reduction in persons with cardiovascular disease. N Engl J Med. (2020) 382:244–55. doi: 10.1056/NEJMoa1905239
92. Hegele RA, Tsimikas S. Lipid-lowering agents. Circ Res. (2019) 124:386–404. doi: 10.1161/CIRCRESAHA.118.313171
93. Michos ED, McEvoy JW, Blumenthal RS. Lipid management for the prevention of atherosclerotic cardiovascular disease. N Engl J Med. (2019) 381:1557–67. doi: 10.1056/NEJMra1806939
94. Magenta A, Sileno S, D'Agostino M, Persiani F, Beji S, Paolini A, et al. Atherosclerotic plaque instability in carotid arteries: miR-200c as a promising biomarker. Clin Sci. (2018) 132:2423–36. doi: 10.1042/CS20180684
95. Putaala J, Martinez-Majander N, Saeed S, Yesilot N, Jakala P, Nerg O, et al. Searching for explanations for cryptogenic stroke in the young: revealing the triggers, causes, and outcome (SECRETO): rationale and design. Eur Stroke J. (2017) 2:116–25. doi: 10.1177/2396987317703210
96. Jauch EC, Barreto AD, Broderick JP, Char DM, Cucchiara BL, Devlin TG, et al. Biomarkers of Acute Stroke Etiology (BASE) study methodology. Transl Stroke Res. (2017) 8:424–8. doi: 10.1007/s12975-017-0537-3
97. Chang AD, Ignacio GC, Akiki R, Grory BM, Cutting SS, Burton T, et al. increased left atrial appendage density on computerized tomography is associated with cardioembolic stroke. J Stroke Cerebrovasc Dis. (2020) 29:104604. doi: 10.1016/j.jstrokecerebrovasdis.2019.104604
98. Ricci B, Chang AD, Hemendinger M, Dakay K, Cutting S, Burton T, et al. A simple score that predicts paroxysmal atrial fibrillation on outpatient cardiac monitoring after embolic stroke of unknown source. J Stroke Cerebrovasc Dis. (2018) 27:1692–6. doi: 10.1016/j.jstrokecerebrovasdis.2018.01.028
99. Ntaios G, Perlepe K, Lambrou D, Sirimarco G, Strambo D, Eskandari A, et al. External performance of the HAVOC score for the prediction of new incident atrial fibrillation. Stroke. (2020) 51:457–61. doi: 10.1161/STROKEAHA.119.027990
100. Ntaios G, Perlepe K, Lambrou D, Sirimarco G, Strambo D, Eskandari A, et al. Identification of patients with embolic stroke of undetermined source and low risk of new incident atrial fibrillation: the AF-ESUS score. Int J Stroke. (2021) 16:29–38. doi: 10.1177/1747493020925281
101. Kamel H, Longstreth WT Jr, Tirschwell DL, Kronmal RA, Broderick JP, Palesch YY, et al. The AtRial cardiopathy and antithrombotic drugs in prevention after cryptogenic stroke randomized trial: rationale and methods. Int J Stroke. (2018) 14:207–14. doi: 10.1177/1747493018799981
102. Zhang K, Kamtchum-Tatuene J, Li M, Jickling GC. Cardiac natriuretic peptides for diagnosis of covert atrial fibrillation after acute ischaemic stroke: a meta-analysis of diagnostic accuracy studies. Stroke Vasc Neurol. (2020) 6:128–32. doi: 10.1136/svn-2020-000440
103. Goyal M, Singh N, Marko M, Hill MD, Menon BK, Demchuk A, et al. Embolic stroke of undetermined source and symptomatic nonstenotic carotid disease. Stroke. (2020) 51:1321–5. doi: 10.1161/STROKEAHA.119.028853
104. Strambo D, Sirimarco G, Nannoni S, Perlepe K, Ntaios G, Vemmos K, et al. Embolic stroke of undetermined source and patent foramen ovale: risk of paradoxical embolism score validation and atrial fibrillation prediction. Stroke. (2021) 52:1643–52. doi: 10.1161/STROKEAHA.120.032453
105. Johnston SC, Easton JD, Farrant M, Barsan W, Conwit RA, Elm JJ, et al. Clopidogrel and aspirin in acute ischemic stroke and high-risk TIA. N Engl J Med. (2018) 379:215–25. doi: 10.1056/NEJMoa1800410
106. Wang Y, Wang Y, Zhao X, Liu L, Wang D, Wang C, et al. Clopidogrel with aspirin in acute minor stroke or transient ischemic attack. N Engl J Med. (2013) 369:11–9. doi: 10.1056/NEJMoa1215340
107. Lee M, Saver JL, Hong KS, Rao NM, Wu YL, Ovbiagele B. antiplatelet regimen for patients with breakthrough strokes while on aspirin: a systematic review and meta-analysis. Stroke. (2017) 48:2610–3. doi: 10.1161/STROKEAHA.117.017895
108. Pan Y, Elm JJ, Li H, Easton JD, Wang Y, Farrant M, et al. Outcomes associated with clopidogrel-aspirin use in minor stroke or transient ischemic attack: a pooled analysis of clopidogrel in high-risk patients with acute non-disabling cerebrovascular events (CHANCE) and platelet-oriented inhibition in new TIA and minor ischemic stroke (POINT) trials. JAMA Neurol. (2019) 76:1466–73. doi: 10.1001/jamaneurol.2019.2531
109. Damman P, Woudstra P, Kuijt WJ, de Winter RJ, James SK. P2Y12 platelet inhibition in clinical practice. J Thromb Thrombolysis. (2012) 33:143–53. doi: 10.1007/s11239-011-0667-5
110. Pan Y, Chen W, Xu Y, Yi X, Han Y, Yang Q, et al. Genetic polymorphisms and clopidogrel efficacy for acute ischemic stroke or transient ischemic attack: a systematic review and meta-analysis. Circulation. (2017) 135:21–33. doi: 10.1161/CIRCULATIONAHA.116.024913
111. Johnston SC, Amarenco P, Denison H, Evans SR, Himmelmann A, James S, et al. Ticagrelor and aspirin or aspirin alone in acute ischemic stroke or TIA. N Engl J Med. (2020) 383:207–17. doi: 10.1056/NEJMoa1916870
112. Li ZX, Xiong Y, Gu HQ, Fisher M, Xian Y, Johnston SC, Wang YJ. P2Y12 inhibitors plus aspirin versus aspirin alone in patients with minor stroke or high-risk transient ischemic attack. Stroke. (2021) 52:2250–7. doi: 10.1161/STROKEAHA.120.033040
113. Wang Y, Johnston C, Bath PM, Meng X, Jing J, Xie X, et al. Clopidogrel with aspirin in high-risk patients with acute non-disabling cerebrovascular events II (CHANCE-2): rationale and design of a multicentre randomised trial. Stroke Vasc Neurol. (2021) 6:280–5. doi: 10.1136/svn-2020-000791
114. Xiong Y, Bath PM. Antiplatelet therapy for transient ischemic attack and minor stroke. Stroke. (2020) 51:3472–4. doi: 10.1161/STROKEAHA.120.031763
115. Noubiap JJ, Agbaedeng TA, Kamtchum-Tatuene J, Fitzgerald JL, Middeldorp ME, Kleinig T, et al. Rhythm monitoring strategies for atrial fibrillation detection in patients with cryptogenic stroke: a systematic review and meta-analysis. Int J Cardiol Heart Vasc. (2021) 34:100780. doi: 10.1016/j.ijcha.2021.100780
116. Perera KS, Ng KKH, Nayar S, Catanese L, Dyal L, Sharma M, et al. Association between low-dose rivaroxaban with or without aspirin and ischemic stroke subtypes: a secondary analysis of the COMPASS trial. JAMA Neurol. (2020) 77:43–8. doi: 10.1001/jamaneurol.2019.2984
117. Sharma M, Hart RG, Connolly SJ, Bosch J, Shestakovska O, Ng KKH, et al. Stroke outcomes in the COMPASS trial. Circulation. (2019) 139:1134–45. doi: 10.1161/CIRCULATIONAHA.118.035864
118. Healey JS, Gladstone DJ, Swaminathan B, Eckstein J, Mundl H, Epstein AE, et al. Recurrent stroke with rivaroxaban compared with aspirin according to predictors of atrial fibrillation: secondary analysis of the NAVIGATE esus randomized clinical trial. JAMA Neurol. (2019) 76:764–73. doi: 10.1001/jamaneurol.2019.0617
119. Mazzucco S, Li L, Binney L, Rothwell PM Oxford vascular study phenotyped C. prevalence of patent foramen ovale in cryptogenic transient ischaemic attack and non-disabling stroke at older ages: a population-based study, systematic review, and meta-analysis. Lancet Neurol. (2018) 17:609–17. doi: 10.1016/S1474-4422(18)30167-4
120. Mazzucco S, Li L, Rothwell PM. Prognosis of cryptogenic stroke with patent foramen ovale at older ages and implications for trials: a population-based study and systematic review. JAMA Neurol. (2020) 77:1279–87. doi: 10.1001/jamaneurol.2020.1948
121. Yaghi S, Boehme AK, Hazan R, Hod EA, Canaan A, Andrews HF, et al. Atrial cardiopathy and cryptogenic stroke: a cross-sectional pilot study. J Stroke Cerebrovasc Dis. (2016) 25:110–4. doi: 10.1016/j.jstrokecerebrovasdis.2015.09.001
122. Powers WJ, Rabinstein AA, Ackerson T, Adeoye OM, Bambakidis NC, Becker K, et al. 2018 Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. (2018) 49:e46–110. doi: 10.1161/STR.0000000000000158
123. Amarenco P, Kim JS, Labreuche J, Charles H, Abtan J, Bejot Y, et al. A comparison of two LDL cholesterol targets after ischemic stroke. N Engl J Med. (2020) 382:9. doi: 10.1056/NEJMoa1910355
124. Turan TN, Voeks JH, Chimowitz MI, Roldan A, LeMatty T, Haley W, et al. Rationale, design, and implementation of intensive risk factor treatment in the CREST2 trial. Stroke. (2020) 51:2960–71. doi: 10.1161/STROKEAHA.120.030730
126. Raal FJ, Mohamed F. Never too old to benefit from lipid-lowering treatment. Lancet. (2020) 396:1608–9. doi: 10.1016/S0140-6736(20)32333-3
127. Cheung BMY, Lam KSL. Never too old for statin treatment? Lancet. (2019) 393:379–80. doi: 10.1016/S0140-6736(18)32263-3
128. Dearborn-Tomazos JL, Hu X, Bravata DM, Phadke MA, Baye FM, Myers LJ, et al. Deintensification or no statin treatment is associated with higher mortality in patients with ischemic stroke or transient ischemic attack. Stroke. (2021) 52:2521–9. doi: 10.1161/STROKEAHA.120.030089
129. Sillesen H, Amarenco P, Hennerici MG, Callahan A, Goldstein LB, Zivin J, et al. Atorvastatin reduces the risk of cardiovascular events in patients with carotid atherosclerosis: a secondary analysis of the Stroke Prevention by Aggressive Reduction in Cholesterol Levels (SPARCL) trial. Stroke. (2008) 39:3297–302. doi: 10.1161/STROKEAHA.108.516450
130. Sabatine MS, Giugliano RP, Wiviott SD, Raal FJ, Blom DJ, Robinson J, et al. Efficacy and safety of evolocumab in reducing lipids and cardiovascular events. N Engl J Med. (2015) 372:1500–9. doi: 10.1056/NEJMoa1500858
131. Schwartz GG, Steg PG, Szarek M, Bhatt DL, Bittner VA, Diaz R, et al. Alirocumab and cardiovascular outcomes after acute coronary syndrome. N Engl J Med. (2018) 379:2097–107. doi: 10.1056/NEJMoa1801174
132. Julius U, Tselmin S, Schatz U, Fischer S, Bornstein SR. Lipoprotein(a) and proprotein convertase subtilisin/kexin type 9 inhibitors. Clin Res Cardiol Suppl. (2019) 14:45–50. doi: 10.1007/s11789-019-00099-z
133. Rosenson RS, Burgess LJ, Ebenbichler CF, Baum SJ, Stroes ESG, Ali S, et al. Evinacumab in patients with refractory hypercholesterolemia. N Engl J Med. (2020) 383:2307–19. doi: 10.1056/NEJMoa2031049
134. Ruscica M, Zimetti F, Adorni MP, Sirtori CR, Lupo MG, Ferri N. Pharmacological aspects of ANGPTL3 and ANGPTL4 inhibitors: new therapeutic approaches for the treatment of atherogenic dyslipidemia. Pharmacol Res. (2020) 153:104653. doi: 10.1016/j.phrs.2020.104653
135. Di Minno A, Lupoli R, Calcaterra I, Poggio P, Forte F, Spadarella G, et al. Efficacy and safety of Bempedoic acid in patients with hypercholesterolemia: systematic review and meta-analysis of randomized controlled trials. J Am Heart Assoc. (2020) 9:e016262. doi: 10.1161/JAHA.119.016262
136. Awad K, Mikhailidis DP, Katsiki N, Muntner P, Banach M, Lipid, Blood Pressure Meta-Analysis Collaboration Group. Effect of ezetimibe monotherapy on plasma Lipoprotein(a) concentrations in patients with primary hypercholesterolemia: a systematic review and meta-analysis of randomized controlled trials. Drugs. (2018) 78:453–62. doi: 10.1007/s40265-018-0870-1
137. Tsimikas S, Gordts P, Nora C, Yeang C, Witztum JL. Statin therapy increases lipoprotein(a) levels. Eur Heart J. (2020) 41:2275–84. doi: 10.1093/eurheartj/ehz310
138. Beheshtian A, Shitole SG, Segal AZ, Leifer D, Tracy RP, Rader DJ, et al. Lipoprotein (a) level, apolipoprotein (a) size, and risk of unexplained ischemic stroke in young and middle-aged adults. Atherosclerosis. (2016) 253:47–53. doi: 10.1016/j.atherosclerosis.2016.08.013
139. Lin WV, Vickers A, Prospero Ponce CM, Lee AG. Elevated lipoprotein(a) levels as the cause of cryptogenic stroke in a young Ashkenazi Jewish female. Can J Ophthalmol. (2019) 54:e126–8. doi: 10.1016/j.jcjo.2018.07.011
140. Esenwa CC, Elkind MS. Inflammatory risk factors, biomarkers and associated therapy in ischaemic stroke. Nat Rev Neurol. (2016) 12:594–604. doi: 10.1038/nrneurol.2016.125
141. Libby P, Buring JE, Badimon L, Hansson GK, Deanfield J, Bittencourt MS, et al. Atherosclerosis. Nat Rev Dis Primers. (2019) 5:56. doi: 10.1038/s41572-019-0106-z
142. Puri R, Nissen SE, Arsenault BJ, St John J, Riesmeyer JS, Ruotolo G, et al. Effect of C-reactive protein on Lipoprotein(a)-associated cardiovascular risk in optimally treated patients with high-risk vascular disease: a prespecified secondary analysis of the ACCELERATE trial. JAMA Cardiol. (2020) 5:1136–43. doi: 10.1001/jamacardio.2020.2413
143. Lawler PR, Bhatt DL, Godoy LC, Luscher TF, Bonow RO, Verma S, et al. Targeting cardiovascular inflammation: next steps in clinical translation. Eur Heart J. (2021) 42:113–31. doi: 10.1093/eurheartj/ehaa099
144. Brott TG, Halperin JL, Abbara S, Bacharach JM, Barr JD, Bush RL, et al. 2011 ASA/ACCF/AHA/AANN/AANS/ACR/ASNR/CNS/SAIP/SCAI/SIR/SNIS/SVM/SVS guideline on the management of patients with extracranial carotid and vertebral artery disease: executive summary: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, and the American Stroke Association, American Association of Neuroscience Nurses, American Association of Neurological Surgeons, American College of Radiology, American Society of Neuroradiology, Congress of Neurological Surgeons, Society of Atherosclerosis Imaging and Prevention, Society for Cardiovascular Angiography and Interventions, Society of Interventional Radiology, Society of NeuroInterventional Surgery, Society for Vascular Medicine, and Society for Vascular Surgery. J Am Coll Cardiol. (2011) 57:1002–44. doi: 10.1016/j.jacc.2010.11.005
145. Naylor AR, Ricco JB, de Borst GJ, Debus S, de Haro J, Halliday A, et al. Editor's Choice - management of atherosclerotic carotid and vertebral artery disease: 2017 clinical practice guidelines of the European Society for Vascular Surgery (ESVS). Eur J Vasc Endovasc Surg. (2018) 55:3–81. doi: 10.1016/j.ejvs.2017.06.021
146. Bonati LH, Kakkos S, Berkefeld J, de Borst GJ, Bulbulia R, Halliday A, et al. European Stroke Organisation guideline on endarterectomy and stenting for carotid artery stenosis. Eur Stroke J. (2021) 6:I–XLVII. doi: 10.1177/23969873211026990
147. Katsanos AH, Palaiodimou L, Price C, Giannopoulos S, Lemmens R, Kosmidou M, et al. Colchicine for stroke prevention in patients with coronary artery disease: a systematic review and meta-analysis. Eur J Neurol. (2020) 27:1035–8. doi: 10.1111/ene.14198
148. Kleindorfer DO, Towfighi A, Chaturvedi S, Cockroft KM, Gutierrez J, Lombardi-Hill D, et al. 2021 Guideline for the prevention of stroke in patients with stroke and transient ischemic attack: a guideline From the American Heart Association/American Stroke Association. Stroke. (2021) 52:e364–467. doi: 10.1161/STR.0000000000000375
149. Rippe JM. Lifestyle strategies for risk factor reduction, prevention, and treatment of cardiovascular disease. Am J Lifestyle Med. (2019) 13:204–12. doi: 10.1177/1559827618812395
150. Munzel T, Sorensen M, Daiber A. Transportation noise pollution and cardiovascular disease. Nat Rev Cardiol. (2021) 18:619–36. doi: 10.1038/s41569-021-00532-5
151. Munzel T, Sorensen M, Gori T, Schmidt FP, Rao X, Brook FR, et al. Environmental stressors and cardio-metabolic disease: part II-mechanistic insights. Eur Heart J. (2017) 38:557–64. doi: 10.1093/eurheartj/ehw294
152. Munzel T, Sorensen M, Gori T, Schmidt FP, Rao X, Brook J, et al. Environmental stressors and cardio-metabolic disease: part I-epidemiologic evidence supporting a role for noise and air pollution and effects of mitigation strategies. Eur Heart J. (2017) 38:550–6. doi: 10.1093/eurheartj/ehw269
153. McAlpine CS, Kiss MG, Rattik S, He S, Vassalli A, Valet C, et al. Sleep modulates haematopoiesis and protects against atherosclerosis. Nature. (2019) 566:383–7. doi: 10.1038/s41586-019-0948-2
154. Leng Y, Cappuccio FP, Wainwright NW, Surtees PG, Luben R, Brayne C, et al. Sleep duration and risk of fatal and nonfatal stroke: a prospective study and meta-analysis. Neurology. (2015) 84:1072–9. doi: 10.1212/WNL.0000000000001371
Keywords: stroke, carotid stenosis, carotid plaque, biomarkers, atherosclerosis
Citation: Kamtchum-Tatuene J, Nomani AZ, Falcione S, Munsterman D, Sykes G, Joy T, Spronk E, Vargas MI and Jickling GC (2021) Non-stenotic Carotid Plaques in Embolic Stroke of Unknown Source. Front. Neurol. 12:719329. doi: 10.3389/fneur.2021.719329
Received: 02 June 2021; Accepted: 30 August 2021;
Published: 22 September 2021.
Edited by:
Christopher Bladin, Monash University, AustraliaReviewed by:
Klearchos Psychogios, Metropolitan Hospital, GreeceCopyright © 2021 Kamtchum-Tatuene, Nomani, Falcione, Munsterman, Sykes, Joy, Spronk, Vargas and Jickling. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Joseph Kamtchum-Tatuene, a2FtdGNodW1AdWFsYmVydGEuY2E=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.