
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Neurol. , 02 December 2021
Sec. Stroke
Volume 12 - 2021 | https://doi.org/10.3389/fneur.2021.716778
This article is part of the Research Topic Cerebral Venous Thrombosis View all 6 articles
Background and Purpose: The mechanism of action of Batroxobin included the decomposition of the fibrinogen to fibrin degradation products (FDPs) and D-dimer and mobilization of endothelial cells to release endogenous nt-PA and to promote thrombolysis. This review aims to summarize current study findings about batroxobin on correcting cerebral arterial, venous, and peripheral vascular diseases, to explore the mechanism of batroxobin on anti-thrombosis process.
Methods: A thorough literature search was conducted utilizing the PubMed Central (PMC) and EMBASE databases to identify studies up to June 2021. Data from clinical studies and animal experiments about batroxobin were extracted, integrated and analyzed based on Cochrane handbook for systematic reviews of interventions approach and the Preferred Reporting Items for Systematic Review and Meta-Analysis Protocols (PRISMA-P), including the condition of subjects, the usage and dosage, research observation index and main findings.
Results: A total of 62 studies were enrolled in this systematic review, including 26 clinical studies and 36 animal experiments. The 26 clinical studies involved 873 patients with arterial ischemic events, 92 cases with cerebral venous thrombosis, 13 cases with cerebral cortical vein thrombosis, and 1,049 cases with peripheral vascular diseases. These patients included 452 males and 392 females aged 65.6 ± 5.53 years. The results revealed that batroxobin had broad effects, including improving clinical prognosis (n = 12), preventing thrombosis (n = 7), promoting thrombolysis (n = 6), and improving vascular cognitive dysfunction (n = 1). The effects of batroxobin on reducing neuronal apoptosis (n = 8),relieving cellular edema (n = 4), improving spatial memory (n = 3), and promoting thrombolysis (n = 13) were concluded in animal experiments. The predominant mechanisms explored in animal experiments involved promoting depolymerization of fibrinogen polymers (n = 6), regulating the expression of related molecules (n = 9); such as intercellular adhesion molecule, heat shock proteins, tumor necrosis factor), reducing oxidative stress (n = 5), and reducing inflammation response (n = 4).
Conclusion: Batroxobin can correct both arterial and venous ischemic diseases by promoting depolymerization of fibrinogen polymers, regulating the expression of related molecules, reducing oxidative stress, and reducing the inflammation response.
Batroxobin, isolated from Bothrops atrox moojeni venom, is widely used in clinical such as postoperative hemostasis of surgery because of its hemostatic effect (1–4). Batroxobin has also been investigated for the treatment of deep vein thrombosis and cerebral infarction as it promotes thrombolysis, prevents recurrence of thrombus, and provides neuroprotection (5–8). In recent years, the role of Batroxobin in cerebral venous thrombotic diseases has attracted more attention with two clinical articles proposing to study the clinical value of Batroxobin in cerebral venous thrombosis (CVT) and cerebral venous sinus thrombosis (CVST), respectively (9, 10). Batroxobin may promote venous sinus recanalization thrombosis recanalization, and is a potentially safe and effective adjunct therapeutic agent in patients with a high level of fibrinogen. Another small clinical study investigated the efficacy of Batroxobin in cerebral cortical vein thrombosis (CCVT). Batroxobin significantly improved the prognosis of patients with CCVT (11). All these studies prove that Batroxobin has a wide range of clinical applications. The mechanism of action of Batroxobin included the decomposition of the fibrinogen to fibrin degradation products (FDPs) and D-dimer (12, 13) and mobilization of endothelial cells to release endogenous nt-PA and to promote thrombolysis (14, 15). However, there is a lack of literature review that summarizes the clinical effects and related mechanisms of Batroxobin. Since there is a growing interest in studying Batroxobin as a treatment strategy in cerebral venous system diseases, our study aims to summarize the previous findings to provide a theoretical basis for the use of Batroxobin in cerebral venous system diseases and facilitate future research.
In this study, we review previous studies investigating Batroxobin in both clinical and experimental settings and summarize the most recent findings to provide a deep understanding of Batroxobin in treating thrombotic diseases. We also discuss the potential use of Batroxobin in the treatment of cerebral venous thrombotic diseases.
A systematic review of the literature has been performed on PubMed Central (PMC) and EMBASE databases using the keywords “Batroxobin,” “animal study,” or “clinical study.” Our review includes studies published till June 2021 that investigated Batroxobin. Cochrane handbook for systematic reviews of interventions approach and the Preferred Reporting Items for Systematic Review and Meta-Analysis Protocols (PRISMA-P) was followed accordingly (Supplementary Table 1).
Clinical (prospective and retrospective) and experimental studies that evaluated the efficacy of Batroxobin were included. Studies not related to vascular system diseases and their complications were excluded. Conference abstracts, reviews, case reports, and letters were also not included in the analysis. If two or more studies had duplicate or overlapping data, then the study with the larger sample size and more detailed data was selected. Two reviewers (D-L and SY-S) independently performed the study selection and any disagreements were resolved by discussion (Figure 1).
Two authors (D-L and SY-S) extracted data from the selected studies, which was evaluated by another author (BL-J). The data were further extracted and summarized as follows: the name of the first author, year of publication, country, study characteristics (sample size and research type), subject characteristics (population and animal status, comorbid status and animal model type), detailed information of Batroxobin use, primary outcome and other main findings. All disagreements were resolved by consensus.
The main outcomes of the clinical trials in this review were coagulation indicators, improvement of neurological function, and thrombus recanalization and recurrence. The main outcomes of animal experiments were histopathological indexes and blood factor indexes.
Sixty-two studies, including 26 clinical studies and 36 animal experiments, were selected for the systematic review. The specific screening process is shown in Figure 1 and detailed information about the selected studies is listed in Tables 1, 2.
Two clinical studies, including 31 and 61 subjects, evaluated the efficacy of the combination of Batroxobin and anticoagulation in cerebral venous thrombosis (CVT) and cerebral venous sinus thrombosis (CVST), respectively (9, 10). Higher recanalization rates were found in both Batroxobin groups (adjusted OR [95% CI] of 2.5 [1.1–5.0]; adjusted OR [95%CI] of 8.10 [1.61–40.7], respectively) compared with the control groups, especially in patients with high levels of fibrinogen (adjusted OR [95% CI] of 4.7 [1.4–16.7]). The results of the two studies were inconsistent in concluding whether Batroxobin improved neurological deficits. National Institute of Health Stroke Scale (NIHSS) scores significantly improved at discharge in the Batroxobin group [0(0, 4.25)−5(2, 11), p = 0.036] compared with the baseline in only one study (9). A clinical study with 13 patients evaluated the effectiveness of Batroxobin in acute cerebral cortical vein thrombosis (CCVT) (11). Compared with the non-Batroxobin group, the Batroxobin group achieved a significantly improved prognosis, evaluated by the global impression of change (PGIC) (p = 0.030) in patients.
Ten studies investigated the efficacy of Batroxobin in patients with acute ischemic stroke (AIS). Six studies reported significant improvement of nerve function evaluated by NIHSS (n = 1), Neurological deficit scale (NDS) (n = 2), European stroke scale (ESS) (n = 2) (16–19, 21, 22). Two studies reported a positive association between Batroxobin and prevention of recurrence of stroke (7, 16). Three studies concluded that Batroxobin significantly decreases the level of fibrinogen and increases the level of D-dimer (18, 20, 23).
One study investigated the effect of Batroxobin in improving vascular cognitive dysfunction (24). Significant differences were observed in Mini-mental state examination (MMSE) and activities of daily living (ADL) scores compared with baseline.
The application of Batroxobin was also tested in peripheral vascular disease, deep venous thrombosis (DVT) (n = 5) (5, 25–27, 64), peripheral arterial thrombosis (PAT) (n = 5) (4, 28–30, 32), trial fibrillation (AF) (n = 1) (34) and healthy subjects (n = 1) (35). In all five DVT studies, Batroxobin promoted the recanalization of thrombosis and decreased the occurrence of restenosis of PAT. Batroxobin promoted favorable clinical outcomes in patients with peripheral arteriovenous thrombosis, evaluated by ankle-brachial index (ABI). Coagulation tests with Batroxobin showed a significant decrease in FIB (5, 27, 30) and prolongation of thrombin time (TT) (35) in these studies. Batroxobin also affected other clotting indicators such as prothrombin time (PT) and activated partial thromboplastin time (APTT), but the exact role is controversial (30, 35).
In animal experiments, the main objective was to understand the central vascular damage model that is involved in acute cerebral ischemia (ACI) [n = 8; rat(n = 6) and gerbil (n = 2)], cerebral ischemia-reperfusion (IR) [n = 6; rat(n = 3) and gerbil(n = 3)], intracerebral hemorrhage (ICH) (n = 2; rat), and spinal cord injury (SCI) (n = 2; rat). Four studies also assessed the effect of Batroxobin in the rat models of anoxic damage, nigrostriatal pathway injury, demyelinating disease, and experimental autoimmune encephalomyelitis. Twelve studies showed that Batroxobin reduces neuronal apoptosis (n = 8) (8, 36–39, 41, 43, 48) and relieves cellular edema (n = 4) (14, 15, 42, 65) by promoting the expression of growth-associated protein-43 (GAP-43) (38), increasing the level of adenosine triphosphate (ATP) (8), decreasing the hydroxyl radical production (41, 44, 65), down-regulating the heat shock proteins (HSP) (49), and down-regulating complement expression (15). Three experiments concluded that Batroxobin significantly improved the spatial memory and cognitive function in rats by regulating the expression of HSP32, HSP70 and neural cell adhesion molecule (NCAM) (40, 45, 66).
The peripheral vascular model included three bleeding models; the rest were all ischemic models including acute myocardial ischemia (AMI) (n = 3; dog), disseminated intravascular coagulation (DIC) (n = 2; rat), peripheral artery thrombosis/ischemic injury [n = 4; dog(n = 2) and rat (n = 2)], and atherosclerosis (n = 1; rabbit). Four experiments confirmed that Batroxobin decreased fibrinogen levels (47, 54, 60, 61). Further, Batroxobin decreased blood counts, platelet counts, and hematocrit level (60, 61). Two experiments showed that Batroxobin also promoted coagulation (57, 67). Other reports showed that Batroxobin also participated in stabilizing the atherosclerotic plaque, inhibiting human vascular smooth muscle cell migration, accelerating tissue repair, and expediting vascular regeneration (59, 62, 63).
Our review for the first time summarizes the clinical applications and possible mechanisms of Batroxobin by systemically reviewing current clinical and experimental studies (Figure 2).
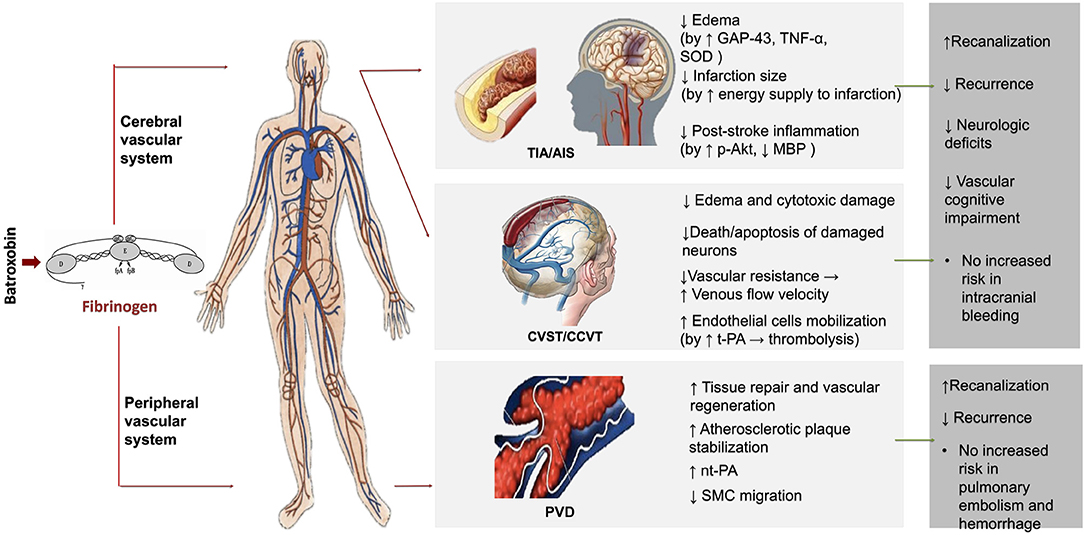
Figure 2. The possible mechanisms and clinical application of batroxobin. TIA, Transient ischemic attack; AIS, Acute ischemic stroke; CVST, Cerebral venous sinus thrombosis; CCVT, Cerebral cortical vein thrombosis; PVD, Peripheral vascular disease; GAP-43, Growth-associated protein-43; TNF-α, Tumor necrosis factor alpha; SOD, Superoxide dismutase; nt-PA, Native tissue type plasminogen activator; MBP, Maltose-binding protein; p-Akt, Phospho-Akt (Ser473); SMC, Smooth muscle cell.
The effectiveness of Batroxobin in promoting recanalization (9, 10) and preventing recurrence (7, 16) of thrombus in all patients with ischemic disease, including cerebral venous sinus thrombosis (CVST) or acute ischemic stroke (AIS), were supported by several studies. In addition to its benefit for recanalization and secondary stroke prevention, treatment with Batroxobin also improved the neurologic deficits which secondary to CVST or AIS (1, 8, 14–16, 23, 67). A case-control study showed that Batroxobin in combination with aspirin improved vascular cognitive impairment (VCI) (24). Batroxobin did not increase the relative risk of any adverse events, including intracranial bleeding (9), compared with the control group.
In animal models of cerebral ischemia or ischemia-reperfusion, Batroxobin reduced the number of apoptotic neurons (8, 14, 36, 39, 41, 43), the degree of edema (14, 42) and the size of infarction (37–39, 42, 46) and the occurrence of micro-thrombosis (14). Batroxobin may produce these effects through a variety of pathophysiological mechanisms, including promotion of the expression of growth-associated protein-43 (GAP-43) (38), inhibition of the excessive increase of Tumor necrosis factor-alpha (TNF-α) (14), increase of the Superoxide dismutase (SOD) activities (44), reduction of oxygen-free damage (41, 44) and increase of the energy supply to the infarct area (8). Batroxobin increases the expression of neural cell adhesion molecule (NCAM) and downregulates the generations o9f heat shock proteins (HSP), such as HSP32 and HSP70, and c-jun, thereby, improving spatial memory disorder (40, 45, 66). In the models of intracerebral hemorrhage (ICH), Batroxobin effectively attenuated brain edema formation and decreased bleeding, possibly by decreasing the concentration of malondialdehyde (MDA) and free Ca2+, increasing the SOD activities and down-regulating the expression of Intercellular Adhesion Molecule 1 (ICAM-1) and complements, such as C3d and C9 (15, 65). Batroxobin was also effective in other animal models of central disease, including nigrostriatal pathway injury, widespread anoxic damage, demyelinating disease and spinal cord injury (SCI) (47–52). Batroxobin also attenuates the scar formation (48), display a direct neuroprotective effect on anoxic neuron (49) and delay the onset and the course of demyelinating disease; (50, 51) possible mechanisms include relieving inflammation (48, 51), decreasing the deposition of fibrin, down-regulating the expression of phospho-Akt (p-Akt), and up-regulating the expression of myelin basic protein (MBP) (51).
Batroxobin treatment alone or in combination with other anticoagulant drugs could promote complete recanalization and prevent the incidence of postoperative deep venous thrombosis (DVT) without adverse events such as pulmonary embolism (PE) and hemorrhage (5, 25, 26, 64). Also, injection of Batroxobin with long-term micropump may get a better efficacy for DVT (27). Batroxobin in combination with aspirin also prevented restenosis after arterial angioplasty which may be mediated by decreased regional inflammation (4, 28–30, 32). In patients with atrial fibrillation (AF), Batroxobin improved blood rheology, decreased blood cell aggregation, and prevented left atrial thrombus formation (34).
In peripheral vascular-related animal models, Batroxobin improved hemostasis (56, 57, 67), and prevented thrombosis (54, 58), accelerating tissue repair and vascular regeneration and stabilizing the atherosclerotic plaque (59, 62). The effect of Batroxobin on fibrinogen metabolism played an important role in ameliorating the formation of disseminated intravascular coagulation (DIC) (60, 61). As an adjunct, Batroxobin enhanced the thrombolytic effects of native tissue-type plasminogen activator (nt-PA) (55). The role of Batroxobin in inhibiting human vascular smooth muscle cell (SMC) migration may also play a clinical value in the future (63).
Timely diagnosis and treatment are essential for faster and more complete recanalization and better outcomes in patients with cerebral venous sinus thrombosis (68–70). However, the primary treatment of CVST is long-term oral anticoagulation. For acute and severe CVST, endovascular therapy is always used first (71). Whereas, venous recanalization is time consuming and there remains a risk of hemorrhagic transformation after anticoagulation. Further complications of endovascular interventions make these interventions a dilemma for most physicians. Therefore, exploration of optimized treatment strategies in CVST is necessary.
Hyperfibrinogenemia, decreased blood flow velocity, and increased viscosity of hyperfibrinogenemia are the three major factors that promote venous thrombosis (72). Batroxobin is a serine protease extracted from the venom of the snake Bothrops atrox moojeni, and it exerts defibrinogenating effects (13). Batroxobin reduces the concentration of fibrinogen in blood by degrading fibrinogen to fibrin degradation products (FDPs) and D-dimer (12, 13). The defibrinogenating effect of batroxobin improves microcirculation by reducing vascular resistance and increasing blood flow velocity (30). Batroxobin can also mobilize endothelial cells to release endogenous t-PA, which indirectly promotes thrombolysis (12, 13). Therefore, Batroxobin can play both preventative and therapeutic roles in pat without increasing the risk of bleeding events in patients with a high risk of CVST.
Despite the controversial effect of Batroxobin on coagulation status, the significant reduction of the amount of bleeding and the effect on hemostasis by Batroxobin was well studied. Batroxobin combined with anticoagulation can significantly promote the recanalization of CVST and cortical venous thrombosis (CCVT) without increasing the risk of bleeding (10, 11). Venous stasis and the embolism from the venous sinus, especially the superior sagittal sinus, were the main risks CCVT in CVT patients (73–75). CCVT is often secondary to venous infarct and hemorrhagic transformation. A previous study reported that Batroxobin reduced the death/apoptosis of damaged neurons, the size of the ischemic infarct, and the risk of bleeding conversion (36). Therefore, CCVT patients are likely to benefit from Batroxobin treatment. CVST or venous infarct-induced cerebral edema resulted in a series of clinical symptoms of intracranial hypertension, which is often a predictor of poor prognosis (75, 76). Previous studies showed that CVST patients benefit from decompressive craniotomy (77). However, decompressive craniotomy might be better suited for severe cerebral edema caused by large venous infarcts. For CVST patients with mild intracranial hypertension caused by edema, Batroxobin may be a better choice since it reduces tissue edema and inhibits cytotoxic damage, as demonstrated in previous studies (14, 15, 42, 65).
CVST patients always showed good neurological and cognitive long-term outcomes (78). However, some patients also presented with significant neurological impairment or neuropsychological deficits due to the disruption of functional areas or conduction tracts when the cerebral cortex is infarcted because of CVST or thrombosis in the deep cerebral venous sinus (75, 79). Cognitive dysfunction is an important factor affecting patients' quality of life and aggravating family burden. Therefore, in the acute stage of CVST or venous infarcts, intervention measures are needed to protect nerve cells in the damaged area to avoid or mitigate cognitive impairment as much as possible. Batroxobin improves free radical scavenging leading to neuroprotective function. A previous study reported that Batroxobin was effective in improving vascular cognitive impairment (VCI) caused by ischemic cerebrovascular disease after long-term treatment (24). Future studies are needed to investigate whether the cognitive dysfunction associated with CVST can benefit from the use of Batroxobin.
In summary, Batroxobin had broad clinical applications in both arterial and venous thrombosis, including promotion of thrombolysis, prevention of thrombotic formation, reduction of edema in infarcted areas, improvement of vascular cognitive dysfunction, and neuroprotection. The potential mechanisms include promotion of depolymerization of fibrinogen polymers, increase in the capacity of free radical scavenging, reduction of inflammation, and regulation of endogenous plasminogen activator expression. Batroxobin can also be therapeutic in CVST and their secondary diseases. However, the application of Batroxobin was still limited to clinical studies with small sample size. Future multi-centered studies with randomized design and larger sample size would provide more evidence on the potential effect of Batroxobin in cerebral vascular diseases.
Batroxobin could treat both arterial and venous ischemic diseases by promoting depolymerization of fibrinogen polymers, regulating the expression of related molecules, reducing oxidative stress, and reducing the inflammation response. However, current evidence of the beneficial effect of Batroxobin in cerebral vascular diseases was mostly from clinical and experimental studies with small sample size and high heterogeneity. Multi-centered clinical trials with randomized design and larger sample size would be needed in the future.
DL and SYS: manuscript drafting and revision, study concept and design, collection, assembly, and interpretation of the data. BLJ: collection, assembly, and interpretation of the data. RM, YHL, and SYS: manuscript drafting and revision, study concept and design, deeply edited the revised version and contributed critical revision, and final approval of the manuscript.
This work was supported by the National Key R&D Program of China under Grant (2017YFC1308400), the National Natural Science Foundation under Grant (81371289), and the Beijing Natural Science Foundation (7212047).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The reviewer PW declared a shared affiliation, with no collaboration, with the authors to the handling editor at the time of the review.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
We would like to thank all participators in this study for their cooperation.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2021.716778/full#supplementary-material
Supplementary Table 1. PRISMA 2020 checklist.
1. Swamy DF, Barretto ES, Rodrigues JSL. Effectiveness of topical haemocoagulase as a haemostatic agent in children undergoing extraction of primary teeth: a split-mouth, randomised, double-blind, clinical trial. Eur Arch Paediatr Dent. (2019) 20:311–7. doi: 10.1007/s40368-018-0406-0
2. Zeng Z, Xiao P, Chen J, Wei Y. Are batroxobin agents effective for perioperative hemorrhage in thoracic surgery? A systematic review of randomized controlled trials. Blood Coagul Fibrinolysis. (2009) 20:101–7. doi: 10.1097/MBC.0b013e3283254532
3. Kjaergard HK, Trumbull HR. Vivostat system autologous fibrin sealant: preliminary study in elective coronary bypass grafting. Ann Thoracic Surg. (1998) 66:482–6. doi: 10.1016/S0003-4975(98)00470-6
4. Ding G, Li S, Pan Z, Gao C, Ma H. Effects of batroxobin on perioperative blood loss and coagulation in patients with low molecular weight heparin when undergoing the total hip replacement. Zhonghua Liu Xing Bing Xue Za Zhi. (2014) 35:737–40.
5. Zhang L, Lu SH, Li L, Tao YG, Wan YL, Senga H, et al. Batroxobin mobilizes circulating endothelial progenitor cells in patients with deep vein thrombosis. Clin Appl Thromb Hemost. (2011) 17:75–9. doi: 10.1177/1076029609347903
6. Qin J, Xu Z, Shi D, Chen D, Dai J, Teng H, et al. Deep vein thrombosis after total hip arthroplasty and total knee arthroplasty in patients with previous ischemic stroke. Int J Lower Extrem Wounds. (2013) 12:316–9. doi: 10.1177/1534734613493291
7. Xu G, Liu X, Zhu W, Yin Q, Zhang R, Fan X. Feasibility of treating hyperfibrinogenemia with intermittently administered batroxobin in patients with ischemic stroke/transient ischemic attack for secondary prevention. Blood Coagul Fibrinolysis. (2007) 18:193–7. doi: 10.1097/MBC.0b013e328040c0f2
8. Chen Q, Zeng YM, Xu PC, Fan JW. Influence of batroxobin on cerebral ischemia-reperfusion injury in gerbils. Acta Pharmacol Sin. (2000) 21:161–4.
9. Ding J, Zhou D, Hu Y, Elmadhoun O, Pan L, Ya J, et al. The efficacy and safety of Batroxobin in combination with anticoagulation on cerebral venous sinus thrombosis. J Thromb Thrombolysis. (2018) 46:371–8. doi: 10.1007/s11239-018-1718-y
10. Ding JY, Pan LQ, Hu YY, Rajah GB, Zhou D, Bai CB, et al. Batroxobin in combination with anticoagulation may promote venous sinus recanalization in cerebral venous thrombosis: a real-world experience. CNS Neurosci Therapeutics. (2019) 25:638–46. doi: 10.1111/cns.13093
11. Song SY, Dornbos III D, Lan D, Jiao BL, Wan SL, Guo YB, et al. High-Resolution magnetic resonance black blood thrombus imaging and serum d-dimer in the confirmation of acute cortical vein thrombosis. Front Neurol. (2021) 12:680040. doi: 10.3389/fneur.2021.680040
12. Hioki M, Iedokoro Y, Yamagishi S, Yamashita Y, Orii K, Hirano S, et al. Prevention of postoperative pericardial adhesions with a defibrinogenating agent. Int Surg. (1998) 83:11–4.
13. Fukutake K, Fujimaki M, Nagasawa H, Kato M. [Clinico-pharmacological observations of batroxobin (Defibrase) administered to normal human adults (author's transl)]. Nihon Ketsueki Gakkai Zasshi. (1981) 44:1178–94.
14. Kang Z, Cao H, Mei B. Neuroprotective role of Batroxobin in cardiopulmonary resuscitation rabbits. Res Lett. (2007) 2:254–6. doi: 10.1016/S1673-5374(07)60057-7
15. Wu G, Huang FP. Effects of venom defibrase on brain edema after intracerebral hemorrhage in rats. Acta Neurochir Suppl. (2005) 95:381–7. doi: 10.1007/3-211-32318-X_78
16. He Y, Ma K, Tang B, Fu X, Zhan Y, Cai Z, et al. Effects of batroxobin with continuous transcranial doppler monitoring in patients with acute cerebral stroke: a randomized controlled trial. Echocardiography. (2014) 31:1283–92. doi: 10.1111/echo.12559
17. Wu FP, Cai ZL. Observation of curative effects of Edaravone combining Batroxobin on treating acute cerebral infarction. J Clin Neurol. (2010) 23:227–9.
18. Ren NY, Zhao KR, Zhang WF. Curative effffects of Edaravone combined Batroxobin on treating progressive cerebral infarction. J Clin Neurol. (2009) 22:148–50.
19. Hao Q, Zhang ZB, Yang YF, Liu CH. Assessment of batroxobin combined with local mild hypothermia in the treatment of cerebral infarction. Chin J Cerebrovasc Dis. (2008) 5:121–4.
20. Wang XQ, Yin JJ, Meng FW. Curative effects of Edaravone combined Batroxobin on treating acute progressive cerebral infarction. J Clin Neurol. (2008) 21:111–2.
21. Gusev EI, Skvortsova VI, Suslina ZA, Avakian GN, Martynov MI, Temirbaeva SL, et al. Batroxobin in patients with ischemic stroke in the carotid system (the multicenter study). Zhurnal nevrologii i psikhiatrii imeni S.S. Korsakova/Ministerstvo zdravookhraneniia i meditsinskoi promyshlennosti Rossiiskoi Federatsii, Vserossiiskoe obshchestvo nevrologov [i] Vserossiiskoe obshchestvo psikhiatrov. (2006) 106:31–4.
22. Yu B, Xin M, Shuyao Z. The clinical study on therapy of acute brain infaction by Tobish Batroxobin (DF-521). J Xi'an Med Univer. Chinese Edition. (1997) 18:488–91.
23. Tanahashi N, Fukuuchi Y, Tomita M, Kobari M, Takeda H, Yokoyama M, et al. Effect of single intravenous administration of batroxobin on erythrocyte aggregability in patients with acute-stage cerebral infarction. Clin Hemorheol. (1995) 15:89–96. doi: 10.3233/CH-1995-15111
24. Zhai QJ, Yue XY, Hong Z, Xu GL, Liu XF. Efficacy observation of batroxobin for treatment of vascular cognitive impairment. Chin J Cerebrovasc Dis. (2010) 7:73–6. doi: 10.3969/j.issn.1672-5921.2010.02.005
25. Chen D, Li Q, Rong Z, Yao Y, Xu Z, Shi D, et al. Incidence and risk factors of deep venous thrombosis following arthroscopic posterior cruciate ligament reconstruction. Medicine (United States). (2017) 96:e7074. doi: 10.1097/MD.0000000000007074
26. Ye S, Dongyang C, Zhihong X, Dongquan S, Jin D, Jianghui Q, et al. The incidence of deep venous thrombosis after arthroscopically assisted anterior cruciate ligament reconstruction. Arthroscopy. (2013) 29:742–7. doi: 10.1016/j.arthro.2013.01.017
27. Wang HT, Jiang WL, Zhang YN, Sun ZF, Sun QF, Ma J. Multicentre clinical observation of anticoagulation and thrombolysis for the deep venous thrombosis. Zhonghua yi xue za zhi. (2009) 89:3181–5.
28. Xue B, Zhang PL, Wang J, Li MH, Zhao JG, Zhu YQ, et al. Evaluation of batroxobin in preventing vascular restenosis in diabetic patients after infrapopliteal arterial angioplasty: a randomized comparative study. J Interv Radiol. (2011) 20:202–6.
29. Wang J, Zhu YQ, Liu F, Li MH, Zhao JG, Tan HQ, et al. Batroxobin for prevention of restenosis in diabetic patients after infrapopliteal arterial angioplasty: a small randomized pilot trial. Ann Vasc Surg. (2010) 24:876–84. doi: 10.1016/j.avsg.2010.03.030
30. Yasunaga K, Kumada K, Matsuda K. Coagulation studies on the patients treated with defibrase, a snake venom batroxobin. Jpn Arch Intern Med. (1979) 26:465–74.
31. Li J, Wang J. Zhu YQ, Zhang PL., Application of batroxobin plus aspirin in preventing post - Intervention re - Stenosis in patients with diabetic lower-limb ischemia: Analysis of therapeutic effects. J Interv Radiol (China). (2014) 23:865–9. doi: 10.3969/j.issn.1008-794X.2014.10.007
32. Wang J, Zhu YQ, Li MH, Zhao JG, Tan HQ, Wang JB, et al. Batroxobin plus aspirin reduces restenosis after angioplasty for arterial occlusive disease in diabetic patients with lower-limb ischemia. J Vasc Interv Radiol. (2011) 22:987–94. doi: 10.1016/j.jvir.2011.03.015
33. Xiao G, Cao Y, Zhang X, Zhang C, Liu C. Can batroxobin depress the inflammatory reaction due to mechanical injury of CAS? Eur J Neurol. (2010). 17:407. doi: 10.1111/j.1468-1331.2010.03233.x
34. Sakamoto S, Mizushige K, Takagi Y, Ueda T, Ohmori K, Matsuo H. Effect of batroxobin on spontaneous echo contrast and hemorheology in left atrial appendage in atrial fibrillation assessed by transesophageal echocardiograpy. Am J Cardiol. (1999) 84:816–9. doi: 10.1016/S0002-9149(99)00443-9
35. Choi SK, Kim CW, Kim JT, Seomun Y, Park MS, Kim CO. Coagulant effect and tolerability of yeast-produced recombinant batroxobin in healthy adult subjects. Clin Drug Investig. (2018) 38:829–35. doi: 10.1007/s40261-018-0673-x
36. Li J, Ding XS, Gao ZQ, Feng MJ, Yin WB, Gong J. Effect of batroxobin and edaravone cooperation on apoptosis after transient forebrain ischemia in gerbils. Pharm Biotechnol. (2007) 14:432–5.
37. Hu XS, Zhou D, Hu XY, Zahng YZ, Tian LY, Huang J. Effectiveness of urokinase used in combination with batroxobin (DF-521) in rat model of focal cerebral ischemia-reperfusion. J Sichuan Univer. (2004) 35:395–7.
38. Wu W, Guan X, Zhang X, Kuang P. GAP-43 expression and pathological changes of temporal infarction in rats and effects of batroxobin. J Tradit Chin Med. (2002) 22:42–6.
39. Wu W, Kuang P, Li Z. Effect of batroxobin on neuronal apoptosis during focal cerebral ischemia and reperfusion in rats. J Tradit Chin Med. (2001) 21:136–40.
40. Wu W, Kuang P, Jiang S, Yang J, Sui N, Chen A, et al. Effect of batroxobin on expression of c-Jun in left temporal ischemic rats with spatial learning and memory disorder. J Tradit Chin Med. (2000) 20:147–51.
41. Qun C, Yin-Ming Z, Shi-Lei W, Peng-Cheng X, Jian-Wei F. Effects of batroxobin on delayed neuronal death in hippocampus CA1 following cerebral ischemia in gerbils. Chin Pharmacol Bull. (1998) 14:522–24.
42. Namikata S, Sato S, Yoshida M, Nishikawa T, Takenaga K, Senga Y. Effects of snake venom batroxobin (Defibrase®) on cerebral infarction in a rat model. Jpn Pharmacol Therapeut. (1992) 20:41–53.
43. Xu LB, Yin WB, Ding XS. Influence of applied times of Batroxobin on neuroprotective effects in cerebral ischemic-reperfusion injury gerbils. J Clin Neurol. (2008) 21:123–5.
44. Zhang L, Zhang P, Zeng Y, Chen Q. Batroxobin plus hypothermia for protection of cerebral ischemia/reperfusion injury models in gerbils. (2006) 1:405–7.
45. Wu W, Guan X, Kuang P, Jiang S, Yang J, Sui N, et al. Effect of batroxobin on expression of neural cell adhesion molecule in temporal infarction rats and spatial learning and memory disorder. J Tradit Chin Med. (2001) 21:294–98.
46. Yi T, Peigen K, Jing S. The in vivo changes of extracellular fluid adenosine on brain ischemia/reperfusion and the effects of batroxobin on these changes in rats. Chin J Neurol. (1997) 30:165–8.
47. Fan H, Liu X, Tang HB, Xiao P, Wang YZ, Ju G. Protective effects of Batroxobin on spinal cord injury in rats. Neurosci Bull. (2013) 29:501–8. doi: 10.1007/s12264-013-1354-7
48. Li D, Tong L, Kawano H, Liu N, Liu L, Li HP. Protective effects of batroxobin on a nigrostriatal pathway injury in mice. Brain Res Bull. (2016) 127:195–201. doi: 10.1016/j.brainresbull.2016.09.014
49. Liu J, Kuang P, Wu W, Wang F, Ding A. Batroxobin against anoxic damage of rat hippocampal neurons in culture: morphological changes and Hsp70 expression. J Tradit Chin Med. (2001) 21:215–9.
50. Inoue A, Koh CS, Yamazaki M, Yanagisawa N, Ishihara Y, Kim BS. Fibrin deposition in the central nervous system correlates with the degree of Theiler's murine encephalomyelitis virus-induced demyelinating disease. J Neuroimmunol. (1997) 77:185–94. doi: 10.1016/S0165-5728(97)00072-6
51. Yang Y, Tian SJ, Wu L, Huang DH, Wu WP. Fibrinogen depleting agent batroxobin has a beneficial effect on experimental autoimmune encephalomyelitis. Cell Mol Neurobiol. (2011) 31:437–48. doi: 10.1007/s10571-010-9637-2
52. Yu H, Lin B, He Y, Zhang W, Xu Y. Batroxobin protects against spinal cord injury in rats by promoting the expression of vascular endothelial growth factor to reduce apoptosis. Exp Ther Med. (2015) 9:1631–8. doi: 10.3892/etm.2015.2368
53. Jiang ZS, Xia CF, Tian QP, Fu MG, Wang XH, Pang YZ, et al. Effect of batroxobin against dog heart ischemia/reperfusion injury. Acta Pharmacol Sinica, (2000) 21:70–4.
54. Tomaru T, Nakamura F, Aoki N, Sakamoto Y, Omata M, Uchida Y. Local treatment with are antithrombotic drug reduces thrombus size in corollary and peripheral thrombosed arteries. Heart Vessels. (1996) 11:133–44. doi: 10.1007/BF01745171
55. Tomaru T, Uchida Y, Nakamura F, Sonoki H, Tsukamoto M, Sugimoto T. Enhancement of arterial thrombolysis with native tissue type plasminogen activator by pretreatment with heparin or batroxobin: an angioscopic study. Am Heart J. (1989). 117:275–81. doi: 10.1016/0002-8703(89)90769-2
56. Seon GM, Lee MH, Kwon BJ, Kim MS, Koo MA, Kim D, et al. Functional improvement of hemostatic dressing by addition of recombinant batroxobin. Acta Biomater. (2017) 48:175–85. doi: 10.1016/j.actbio.2016.10.024
57. You KE, Koo MA, Lee DH, Kwon BJ, Lee MH, Hyon SH, et al. The effective control of a bleeding injury using a medical adhesive containing batroxobin. Biomed Mater. (2014) 9:025002. doi: 10.1088/1748-6041/9/2/025002
58. Tomaru T, Nakamura F, Fujimori Y, Omata M, Kawai S, Okada R, et al. Local treatment with antithrombotic drugs can prevent thrombus formation: an angioscopic and angiographic study. J Am Coll Cardiol. (1995) 26:1325–32. doi: 10.1016/0735-1097(95)00324-X
59. Masuda H, Sato A, Shizuno T, Yokoyama K, Suzuki Y, Tokunaga M, et al. Batroxobin accelerated tissue repair via neutrophil extracellular trap regulation and defibrinogenation in a murine ischemic hindlimb model. PLoS ONE. (2019) 14:e0220898. doi: 10.1371/journal.pone.0220898
60. Yoshikawa T, Murakami M, Furukawa Y. The effects of defibrinogenation with batroxobin on endotoxin-induced disseminated intravascular coagulation in rats. Thromb Res. (1983) 31:729–35. doi: 10.1016/0049-3848(83)90103-2
61. Markwardt F, Nowak G. The influence of drugs on disseminated intravascular coagulation (DIC) IV. Effects of the thrombin-like enzyme batroxobin on thrombin-induced DIC in rats. Thromb Res. (1980) 17:103–11. doi: 10.1016/0049-3848(80)90298-4
62. Huang J, Zhou D, Tian L, Wu H, Zhang J, Zhang S, et al. The effect of batroxobin on atherosclerosis. Sheng Wu Yi Xue Gong Cheng Xue Za Zhi. (2003) 20:197–201.
63. Wang DS, Hanamoto M, Fang F, Ohba M, Ishii M, Kimura F, et al. Defibrinogenating effect of batroxobin (Defibrase®) in rats and inhibition of migration of human vascular smooth muscle cells by the plasma of batroxobin-treated rats in vitro. Atherosclerosis. (2001) 156:73–80. doi: 10.1016/S0021-9150(00)00628-6
64. Hu YC, Wan SY, Qin DD, Zhan YQ, Ding Y. The topically hemostatic effects of batroxobin on the carotid arteries adventitia removal rabbit. Natl Med J China. (2013) 93:3152–4.
65. Qi L, Dong Z, Ma J. Neuroprotective effect of batroxobin on experimental intracerebral hemorrhage in rats. Yaoxue Xuebao. (2009) 44:338–43.
66. Wu W, Kuang P, Jiang S, Zhang X, Yang J, Sui N, et al. Effects of batroxobin on spatial learning and memory disorder of rats with temporal ischemia and the expression of HSP32 and HSP70. J Tradit Chin Med. (2000) 20:297–301.
67. Seon GM, Lee MH, Kwon BJ, Kim MS, Koo MA, Seomun Y, et al. Recombinant batroxobin-coated nonwoven chitosan as hemostatic dressing for initial hemorrhage control. Int J Biol Macromol. (2018) 113:757–63. doi: 10.1016/j.ijbiomac.2018.03.017
68. Krajíčková D, Klzo L, Krajina A, Vyšata O, Herzig R, Vališ M. Cerebral venous sinus thrombosis: clinical characteristics and factors influencing clinical outcome. Clin Appl Thromb Hemost. (2016) 22:665–72. doi: 10.1177/1076029615576739
69. Gazioglu S, Eyuboglu I, Yildirim A, Aydin CO, Alioglu Z. Cerebral venous sinus thrombosis: clinical features, long-term outcome and recanalization. J Clin Neurosci. (2017) 45:248–51. doi: 10.1016/j.jocn.2017.07.028
70. Stolz E, Rahimi A, Gerriets T, Kraus J, Kaps M. Cerebral venous thrombosis: an all or nothing disease? Prognostic factors and long-term outcome. Clin Neurol Neurosurg. (2005) 107:99–107. doi: 10.1016/j.clineuro.2004.06.002
71. Olaf M, Cooney R. Deep venous thrombosis. Emerg Med Clin North Am. (2017) 35:743–70. doi: 10.1016/j.emc.2017.06.003
72. Meng R, Li ZY, Ji X, Ding Y, Meng S, Wang X. Antithrombin III associated with fibrinogen predicts the risk of cerebral ischemic stroke. Clin Neurol Neurosurg. (2011) 113:380–6. doi: 10.1016/j.clineuro.2010.12.016
73. Sato T, Terasawa Y, Mitsumura H, Komatsu T, Sakuta K, Sakai K, et al. Venous stasis and cerebrovascular complications in cerebral venous sinus thrombosis. Eur Neurol. (2017) 78:154–60. doi: 10.1159/000478980
74. Gunes HN, Cokal BG, Guler SK, Yoldas TK, Malkan UY, Demircan CS, et al. Clinical associations, biological risk factors and outcomes of cerebral venous sinus thrombosis. J Int Med Res. (2016) 44:1454–61. doi: 10.1177/0300060516664807
75. Itrat A, Shoukat S, Kamal AK. Pathophysiology of cerebral venous thrombosis–an overview. J Pak Med Assoc. (2006) 56:506–8.
76. Salottolo K, Wagner J, Frei DF, Loy D, Bellon RJ, McCarthy K, et al. Epidemiology, endovascular treatment, and prognosis of cerebral venous thrombosis: US Center study of 152 patients. J Am Heart Assoc. (2017) 6:e005480. doi: 10.1161/JAHA.117.005480
77. Aaron S, Alexander M, Moorthy RK, Mani S, Mathew V, Patil AK, et al. Decompressive craniectomy in cerebral venous thrombosis: a single centre experience. J Neurol Neurosurg Psychiatry. (2013) 84:995–1000. doi: 10.1136/jnnp-2012-303356
78. Buccino G, Scoditti U, Patteri I, Bertolino C, Mancia D. Neurological and cognitive long-term outcome in patients with cerebral venous sinus thrombosis. Acta Neurol Scand. (2003) 107:330–5. doi: 10.1034/j.1600-0404.2003.00031.x
Keywords: batroxobin, vascular disease, ischemic, effects, mechanism
Citation: Lan D, Song SY, Liu YH, Jiao BL and Meng R (2021) Use of Batroxobin in Central and Peripheral Ischemic Vascular Diseases: A Systematic Review. Front. Neurol. 12:716778. doi: 10.3389/fneur.2021.716778
Received: 29 May 2021; Accepted: 03 November 2021;
Published: 02 December 2021.
Edited by:
Mirjam R. Heldner, University Hospital Bern, SwitzerlandReviewed by:
Jiayue Ding, Tianjin Medical University General Hospital, ChinaCopyright © 2021 Lan, Song, Liu, Jiao and Meng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ran Meng, cmFubWVuZzIwMTFAcGt1Lm9yZy5jbg==
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.