
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Neurol. , 26 July 2021
Sec. Stroke
Volume 12 - 2021 | https://doi.org/10.3389/fneur.2021.713738
This article is part of the Research Topic Ischemic Stroke Management: From Symptom Onset to Successful Reperfusion and Beyond View all 60 articles
 Aravind Ganesh1
Aravind Ganesh1 Johanna Maria Ospel2
Johanna Maria Ospel2 Martha Marko1,3
Martha Marko1,3 Wim H. van Zwam4
Wim H. van Zwam4 Yvo B. W. E. M. Roos5
Yvo B. W. E. M. Roos5 Charles B. L. M. Majoie6
Charles B. L. M. Majoie6 Mayank Goyal1,7,8*
Mayank Goyal1,7,8*Background and Purpose: During the months and years post-stroke, treatment benefits from endovascular therapy (EVT) may be magnified by disability-related differences in morbidity/mortality or may be eroded by recurrent strokes and non-stroke-related disability/mortality. Understanding the extent to which EVT benefits may be sustained at 5 years, and the factors influencing this outcome, may help us better promote the sustenance of EVT benefits until 5 years post-stroke and beyond.
Methods: In this review, undertaken 5 years after EVT became the standard of care, we searched PubMed and EMBASE to examine the current state of the literature on 5-year post-stroke outcomes, with particular attention to modifiable factors that influence outcomes between 3 months and 5 years post-EVT.
Results: Prospective cohorts and follow-up data from EVT trials indicate that 3-month EVT benefits will likely translate into lower 5-year disability, mortality, institutionalization, and care costs and higher quality of life. However, these group-level data by no means guarantee maintenance of 3-month benefits for individual patients. We identify factors and associated “action items” for stroke teams/systems at three specific levels (medical care, individual psychosocioeconomic, and larger societal/environmental levels) that influence the long-term EVT outcome of a patient. Medical action items include optimizing stroke rehabilitation, clinical follow-up, secondary stroke prevention, infection prevention/control, and post-stroke depression care. Psychosocioeconomic aspects include addressing access to primary care, specialist clinics, and rehabilitation; affordability of healthy lifestyle choices and preventative therapies; and optimization of family/social support and return-to-work options. High-level societal efforts include improving accessibility of public/private spaces and transportation, empowering/engaging persons with disability in society, and investing in treatments/technologies to mitigate consequences of post-stroke disability.
Conclusions: In the longtime horizon from 3 months to 5 years, several factors in the medical and societal spheres could negate EVT benefits. However, many factors can be leveraged to preserve or magnify treatment benefits, with opportunities to share responsibility with widening circles of care around the patient.
Endovascular therapy (EVT) is a highly efficacious treatment for acute ischemic stroke with large vessel occlusion (LVO), promoting post-stroke functional independence (1). Through successful reperfusion of brain tissue, EVT results in lower post-treatment infarct volumes when performed rapidly (2–4). However, fast and successful EVT alone does not guarantee a good outcome. Several critical factors operate in the post-stroke period that can influence the 3-month recovery of the patient. Some are unmodifiable, like advanced age and comorbidities like cardiovascular disease or cancer (Figure 1A) (5). Others are modifiable through attention to the quality of post-acute care, such as the occurrence of post-stroke complications like pneumonia or deep vein thrombosis (5, 6).
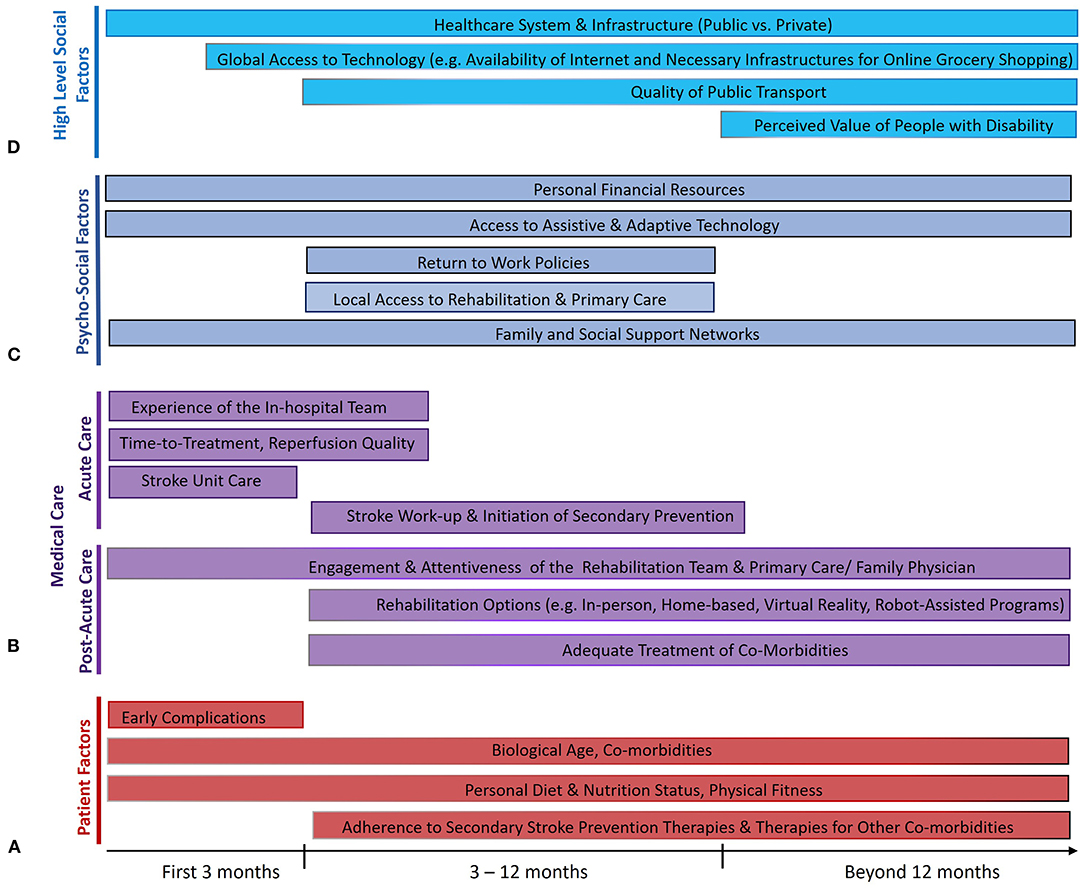
Figure 1. Factors that influence the maintenance of treatment benefits of endovascular therapy (EVT) from 3 months to 5 years after ischemic stroke from large vessel occlusion. Several factors relate to the individual patients themselves (A), some of which are modifiable (like risk of early complications) and rely on effective collaboration between patients and physicians, while several others directly relate to the quality of acute and post-acute medical care (B). Many psychosocioeconomic factors in the life of the patient also play a crucial role (C), as do higher-level societal factors (D) that influence the ability of the patient to reintegrate post-stroke and live a fulfilling life. The length of the bars reflects the portion of the post-stroke time period where each factor is thought to play an important role.
Notwithstanding the various potential pitfalls in stroke recovery from EVT to 3 months, the longer time horizon from 3 months to 5 years is fraught with even greater uncertainty. Some patients may experience further recovery from disability beyond 3 months, while some others successfully maintain their independence, with magnification of treatment-related differences in terms of long-term disability and mortality (7). On the other hand, EVT-related benefits may be eroded by recurrent strokes, accrual of non-stroke-related disability, or by non-stroke-related deaths, especially since stroke occurs more often in elderly people with progressive comorbidities. This raises the question of how we may maximally sustain the initial gains from EVT.
In this review, 5 years after EVT became the standard of care for acute ischemic stroke with LVO, we examine the current state of the literature on 5-year post-stroke outcomes, with particular attention to the modifiable factors that influence the evolution of outcome of the patients between 3 months and 5 years post-stroke. This knowledge may help us better ensure that the therapeutic benefits of EVT are sustained to the greatest possible extent until 5 years post-stroke and beyond.
We searched the literature for studies that (1) involved patients with ischemic stroke and (2) examined a post-stroke outcome of interest beyond the 3-month period (search strategy in the Supplementary Material). Although we were most interested in studies that examined long-term outcomes after EVT or LVO-associated stroke, there continues to be a paucity of high-quality studies examining longer-term outcomes in this specific population. Therefore, we took a more inclusive approach and considered studies in the general ischemic stroke population as well, since most aspects of post-acute care are not unique to the LVO population. We limited the search to studies of humans published in English. The literature search is up-to-date as of April 30, 2021. We excluded case reports, case series, and opinions or editorials.
The sustenance of EVT benefits between 3 months and 5 years post-stroke is predicated on maximizing 3-month treatment effects in the first place. Therefore, it is worth briefly reviewing key factors of acute stroke care that can optimize 3-month EVT benefits (Figure 1B).
Tremendous gains have been made in EVT technology, techniques, and workflow. Improvements in thrombectomy devices (specifically stent retrievers) were crucial to the dawn of successful EVT for stroke (8, 9), and the continued evolution of these devices—with better size choices and longer, more radio-opaque designs—and of EVT training programs holds promise for further enhancing EVT benefits (10–12). Speed is also critical (13); indeed, shortened treatment times from IMS-III (Interventional Management of Stroke trial-III) to the major EVT trials in 2015 helped drive efficacy (14, 15). Further refinement of EVT techniques like CAPTIVE (continuous aspiration prior to intracranial vascular embolectomy) (16) or BADDASS (BAlloon guiDe with large bore DISTAL ACCESS catheter with dual aspiration with Stent retriever as Standard approach) (17) is also crucial to continue improving reperfusion rates. The population benefitting from EVT continues to expand, such as “late-window” patients with salvageable penumbra (18, 19) and potentially patients with more extensive early ischemic changes for whom trials are ongoing (20, 21). Three-month outcomes may be further improved by neuroprotective therapies (22); a promising treatment is nerinetide, which may improve outcomes in patients not receiving alteplase (23). Artificial intelligence and machine learning approaches may further improve outcomes through decision support for stroke identification/triage, imaging interpretation, and patient selection for treatment (24, 25).
Following EVT, attention to post-acute care, ideally on organized stroke units, is essential to prevent or mitigate complications like aspiration pneumonia or deep vein thrombosis, which can rapidly negate EVT benefits (5, 6). Larger, systems-level factors also matter, such as whether care occurs in the context of integrated systems of stroke care. Such systems involve concerted efforts across the continuum from prehospital care all the way to post-stroke rehabilitation and secondary prevention (26) and may promote lower 30-day mortality (27). Even in regions with more fragmented systems, the adoption of certain concerted approaches to stroke workflow, such as prehospital notification of incoming “code strokes” and rapid patient triage, stroke team activation, and neuroimaging completion, can improve onset-to-groin-puncture times and thus improve 3-month outcomes (28, 29). The organization of stroke systems in the field to ensure efficient transport of patients with LVO for EVT remains a work in progress (30). Several scales have been developed for prehospital identification of LVO, with attendant limitations (31, 32), and geographical modeling of optimal transport options has emerged as an important technology to guide routing decisions (33, 34).
The enduring burden of long-term disability in ischemic stroke has been reported in many cohorts, with about 31–36% of patients being functionally disabled patients 5 years post-stroke (35–38). Three-month modified Rankin Scale (mRS) scores strongly predicted 5-year disability in the population-based Oxford Vascular Study (OXVASC), implying that treatments like EVT that reduce 3-month disability likely promote long-term functional independence (39).
As for mortality, at 1 year post-stroke and beyond, the most frequent causes are respiratory infections and cardiovascular disease (40). Functional dependency, with attendant issues of immobility and incontinence, is associated with complications like infections and pressure sores (41). Observational studies have shown that early post-stroke disability predicts long-term mortality (key studies shown in Table 1). These data suggest that early disability reductions from EVT will likely translate into lower long-term mortality, without much erosion by non-stroke-related deaths. Cognitive impairment is a well-recognized post-stroke complication, progressing to dementia in up to one-third of patients (48, 49), with dementia incidence being nearly 50 times higher 1 year post-stroke compared with the general population (50). Post-stroke dementia contributes to dependency (51, 52), institutionalization, and mortality (53). In OXVASC, each 3-month mRS increment was associated with higher 5-year risk of dementia (54).
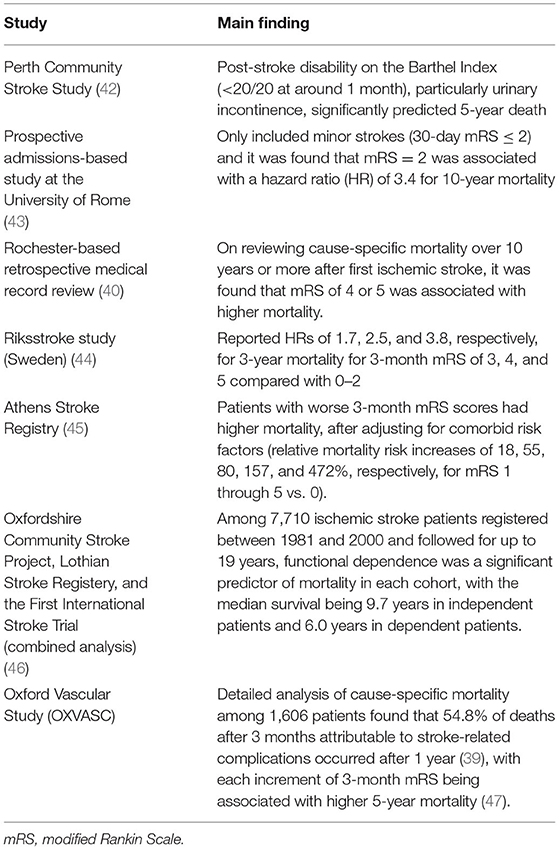
Table 1. Key observational studies examining the relationship of short-term post-stroke disability or functional outcome to 5-year (or longer-term) mortality.
Healthcare costs also reflect long-term treatment effects. Three-month functional outcome again predicts long-term post-stroke costs. A systematic review of costing studies between 2004 and 2015 found that costs consistently increased with increasing mRS (55). In OXVASC, each increment of worsening 3-month mRS was associated with higher 5-year healthcare/social care costs (47), regardless of premorbid disability (56). Analyses of the North-East Melbourne Stroke Incidence Study (NEMESIS) have shown that 5-year outcomes provide a robust estimate of lifetime post-stroke costs (57). Long-term costs are closely tied to institutionalization, i.e., admission to residential care or nursing homes, affecting 9–15% of patients by 5 years post-stroke (36, 37, 58–60) and over 40% of initially hospitalized patients with severe strokes (61–63). Unsurprisingly, early disability predicts 5-year institutionalization. The Erlangen Stroke Project found that urinary incontinence on the Barthel Index at 7 days conferred a four-fold higher risk of 12-month institutionalization (64). In OXVASC, 1-month/3-month mRS predicted 5-year institutionalization (>35% with mRS of 3–5 vs. <10% with mRS 0–2) (36, 54).
Higher post-stroke disability is also associated with poorer quality of life. Indeed, the 3-year Australian POISE (Psychosocial Outcomes In Stroke) study found that functional independence at 28 days was the strongest predictor of return-to-work within 1 year post-stroke (65). In OXVASC, each 3-month mRS increment was associated with worse quality-of-life ratings and 5-year quality-adjusted life expectancy (QALE) (54, 66). The VISTA (Virtual International Stroke Trials Archive) collaborators found that 3-month mRS scores accounted for 65–71% of variation in health utilities generated using EQ-5D data for different countries (67).
From these observational data, we may extrapolate that 3-month EVT benefits will likely translate into lower 5-year disability, mortality, institutionalization, and care costs and higher quality of life/QALE. This suggests that the 3-month benefits are probably preserved and potentially magnified at 5 years, but a caveat is that many/most of the patients in these cohorts did not have LVOs (although OXVASC reported sensitivity analyses in potentially “treatable” strokes) (39). Preliminary real-world data showing these long-term benefits have come from the analyses of the MR CLEAN (Multicenter Randomized Clinical trial of Endovascular treatment for Acute ischemic stroke in the Netherlands) and REVASCAT (a randomized trial of revascularization with SOLITAIRE FR® device vs. best medical therapy in the treatment of acute stroke due to anterior circulation LVO presenting within 8 h of symptom onset) trials. An analysis of 2-year mRS data from 391 of 500 patients enrolled in MR CLEAN (78.2%) showed an adjusted OR of 1.68 (95% CI 1.15–2.45) for a shift of mRS in favor of EVT (68). One-year mRS data from REVASCAT, available for 205 of 206 patients (99%), showed that 89% of the positive treatment effect was already observed at 90 days (69). In REVASCAT, EVT was also associated with better cognitive performance at 3 months and 1 year on the trail-making—test part B, especially among patients with mRS 0–2 (70).
Interestingly, a recent OXVASC analysis that applied prognostic weights derived for each level of the 3-month mRS to EVT trial data estimated very similar long-term treatment effects as the actual MR CLEAN and REVASCAT analyses. For example, OXVASC estimated a 2.5% lower mortality (95% CI −7.1 to 12.0%) and 0.06 additional QALY (0.003–0.13) in the REVASCAT EVT arm at 1 year, similar to the non-significant 1% mortality difference and 0.12 (0.03–0.22) utility difference reported in the 1-year REVASCAT analysis (69). Similarly, OXVASC estimated a 5.5% lower mortality (−0.5 to 11.4%) and 0.14 additional QALY (0.06–0.23) in the MR CLEAN EVT arm at 2 years, which was close to the 5% mortality and 0.10 (0.03–0.16) utility differences reported in the 2-year MR CLEAN analysis (68). Buoyed by these robust estimates, the 5-year benefits of EVT were extrapolated using weighted ordinal analyses of pooled 3-month mRS results of all major EVT trials. Endovascular therapy was predicted to confer an 11% lower risk (95% CI 9–14%) of death/dementia/institutionalization, a $10,193 (7,405–12,981) reduction in healthcare/social care costs, and an additional 0.55 (0.43–0.66) QALYs over 5 years vs. control treatments. A subsequent analysis from the HERMES collaboration estimated that every 10 min of earlier EVT results in an average gain of 39 days of disability-free life and increases net monetary benefit by $10,519 for healthcare costs and $10,915 for societal costs over the lifetime of a patient, indicating the long-term benefits of faster EVT (71).
Importantly, the strong group-level observational and clinical trial data for the extrapolation of 3-month benefits of EVT to 5 years and beyond by no means guarantee the maintenance of 3-month benefits for individual patients. At the individual level, there are numerous factors occurring as part of the medical care of the patient (both physician- and patient-dependent) that likely influence how the long-term EVT outcome of the patient will play out (Figure 1B).
Firstly, the 3-month disability need not guarantee 5-year disability. Whereas, post-stroke recovery was conventionally thought to occur mostly in the first 3 months post-stroke (72), rehabilitation strategies like constraint-induced movement therapy (CIMT) have been shown to be effective in the 3- to 9-month window (73, 74), indicating that patients may demonstrate late functional improvement (75). In OXVASC (76) and in an analysis of three randomized multicenter trials of acute ischemic stroke (2,555 patients), such improvement (by ≥1 mRS grades) was observed in about one in four patients with ischemic stroke between 3 and 12 months post-stroke (77). Whereas, analyses of 11 rehabilitation pilot studies demonstrated a gradient of recovery fading to asymptotic levels after about 18 months post-stroke (78), functional improvement was also seen in about 1 in 10 patients in OXVASC between 1 and 5 years post-stroke (76). Although such late improvements, particular between 3 and 12 months, seem more common among those with lacunar strokes (76), patients who demonstrated late improvement in OXVASC, regardless of subtype, had lower 5-year mortality, institutionalization, and healthcare/social care costs (79). These findings should motivate clinicians and patients to maximize late recovery in practice. Robot-assisted rehabilitation holds promise for promoting intensive, interactive, and individualized practice, but methodologically limited studies to date have only shown small effects on motor control and medium effects on strength (80). Augmentation of rehabilitation interventions with virtual reality, particularly involving a gaming component, improves treatment gains by over 10% compared with conventional approaches (81). These approaches may help further promote late recovery in the future.
In addition, attention to secondary stroke prevention and care for post-stroke complications is critical. It is essential to address and control all modifiable cardiovascular risk factors to prevent recurrent stroke. Anticoagulation for atrial fibrillation is one important example, given the high stroke recurrence in the absence of anticoagulation. Some observational studies suggest that early initiation of direct oral anticoagulants post-stroke may be associated with an acceptably low risk of ICH (82, 83); randomized controlled trials are currently investigating the optimal time point to start anticoagulation (e.g., ELAN—NCT03148457, OPTIMAS—NCT03759938, TIMING—NCT02961348) (84). Organized clinical follow-up is associated with lower hospitalization rates several months post-stroke (85–88). There is a wide variation in the availability of secondary prevention services and medical follow-up (89, 90). In a recent American study, 59.3% of patients had primary care follow-up within 1 month post-stroke and only 24% had neurology/stroke service follow-up (87). Similar challenges have been noted in other countries; in Sweden, only 75% of patients in the Riksstroke registry had 90-day follow-up (91). The added benefits of predefined care models and specialized stroke prevention clinics are being systematically studied in clinical trials (92, 93), which may facilitate their wider adoption.
Moreover, patient compliance and lifestyle modification are critical to maintain functional independence. Beyond prescriptions, patients need appropriately tailored information and education to mitigate risk and promote timely recognition of recurrent strokes (94). The quality of patient/caregiver educational strategies is quite variable, with some approaches showing limited effect on long-term outcomes (95, 96). Patients also benefit from organizational and behavioral interventions to meet secondary prevention goals like blood pressure or low-density lipoprotein targets (97, 98). Underscoring the importance of follow-up, patients without 90-day follow-up have lower medication compliance (91). Only around 65% of patients adhere to statins (99, 100), while 60% adhere to anticoagulation (101–103). Barriers to adherence include challenges with self-care, limited knowledge about stroke and its dangers, frequent medication changes, and high treatment burden and complexity (104). Lifestyle modification, especially smoking cessation, is key for secondary prevention. Yet in a recent analysis of the National Health and Nutrition Examination Survey and the Behavioral Risk Factor Surveillance System survey, active smoking had not become less prevalent among stroke survivors over the past 20 years in the United States, in contrast to the general population (105).
Aside from recurrent cardiovascular events, infections are an important cause for readmission post-stroke (106). Such infections, including aspiration pneumonia (39), are associated with increased mortality (107). Particular vigilance is required for patients with dysphagia, associated with pneumonia and increased morbidity/mortality (108). Importantly, swallowing therapy improves long-term survival (109), emphasizing the importance of multidisciplinary care, in this case including speech and language pathologists.
Furthermore, depression affects one-third of patients after stroke and adversely affects long-term outcomes. Optimal treatment options and benefit of antidepressants for daily activities remain uncertain, but early recognition with a combination of pharmacological and non-pharmacological approaches is prudent (110). Recent trials of fluoxetine in the early post-stroke period have shown benefits for mood and emotional control (111, 112) with reduced incidence of new post-stroke depression (112), but no benefits for functional outcomes.
Based on these insights, we can identify a set of “action items” for stroke teams to address between 3 months and 5 years, ideally in tandem with primary care and multidisciplinary teams, to help maximize long-term EVT benefits (Table 2).
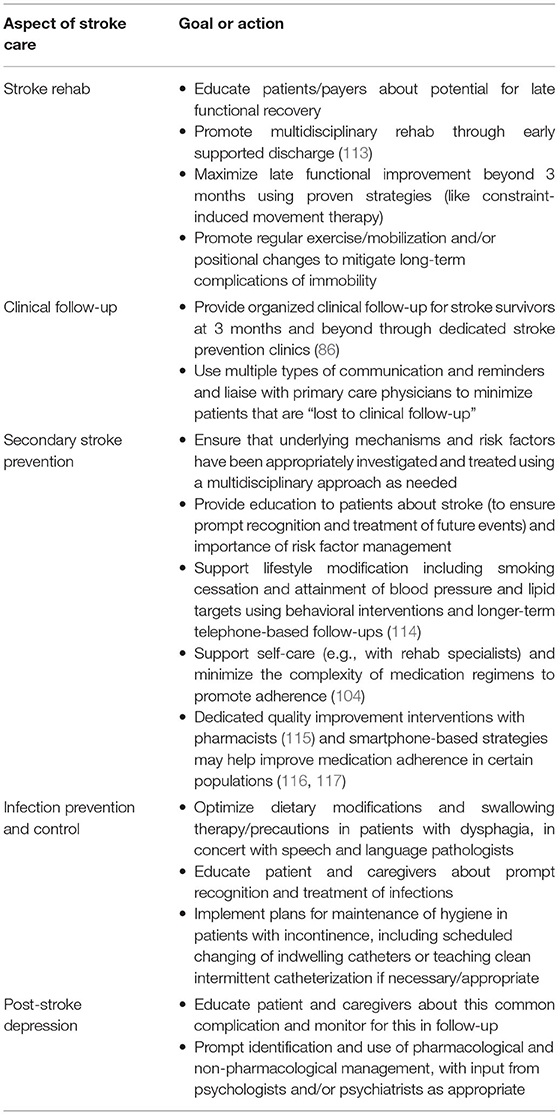
Table 2. Medical action items for stroke teams to address between 3 months and 5 years post-stroke to ensure maintenance of EVT benefits.
Aside from psychological effects of the stroke itself, there are several relevant psycho-social factors operating in the immediate environment of the patient that play a major role in their long-term recovery and, thus, their long-term EVT benefit (Figure 1C).
There is a consistent association of lower socioeconomic status and lower education with higher long-term morbidity/mortality post-stroke (118–120). The growing wealth and income disparity worldwide can be expected to contribute to greater disparities in stroke prevention and outcomes (121, 122). Whereas, socioeconomic or insurance status has been studied mostly in relation to acute/in-hospital care in the United States (123, 124), important data on longer-term care metrics have recently come from European studies. Socioeconomic deprivation was associated with lower survival and greater enduring functional impairment on the Barthel Index at 7 years post-stroke in England (125, 126). Higher education was associated with better motor and functional recovery during rehabilitation in Europe (127) and lower 1-year stroke-related mortality in Finland (128), whereas low income was associated with lower 6-month motor improvement post-stroke in Europe (127) and higher 1-year stroke-related mortality in Finland (128). Some of these differences relate to disparities in accessing good-quality care. For example, patients with socioeconomic deprivation were less likely to receive appropriate post-stroke care during 5 years of follow-up in London, including swallowing assessments, medications for atrial fibrillation, and in Black patients, physiotherapy and occupational therapy (129).
In addition, once patients are discharged from the hospital, their access to rehabilitation programs is highly variable. Insurance policies in countries like the United States often restrict stroke patients from accessing rehabilitation after discharge (75). Even in countries with universal healthcare insurance like the United Kingdom and Canada, patients struggle to access rehabilitation services beyond the first few months post-stroke (130). The aforementioned benefits of late post-stroke recovery should incentivize payers to expand coverage for proven late therapies like CIMT (73) beyond 3 months post-stroke, as such investment can pay off with sustained independence and lower healthcare/social care costs.
However, even with excellent post-acute stroke care, patients may suffer from suboptimal management of non-stroke-related comorbidities due to poor access to primary care or allied health professionals. Timely involvement of primary care physicians is enshrined in guidelines for post-acute care (131), yet options may be limited for patients living in remote/rural areas. Financial barriers also hamper secondary prevention efforts in more subtle ways. Besides making healthy eating habits unaffordable, they create competing priorities for patients trying to attend appointments; for example, patients may struggle with the double hit of lost income on the day of an appointment and transport/parking costs (130).
Besides, there is a substantial need for family support post-stroke to optimize physical recovery and outcomes (132–134). The experience of a patient of residual disability post-EVT can be dramatically different depending on how invested their families are in helping them thrive at home. Closely tied to this is the social support network of the patient; besides having a more enriching quality of life, patients with more open and vibrant social networks extending beyond their family are also more likely to be brought in for timely medical attention with future emergencies like stroke (135, 136). Social support also influences more intimate aspects of daily life; a poor relationship with the person feeding them (strangers vs. family/friends) can, for example, worsen meal-skipping, malnutrition, frailty, and isolation among stroke survivors (137). Compounded by changing family and social dynamics, social isolation is a major public health issue (138) and results in worse post-stroke outcomes (139, 140).
Access to assistive or adaptive technology is another huge determinant of whether post-stroke impairments cause functional disability. Robots and other technologies designed to compensate for impaired skills may help patients retain functional independence (141). Technological options also influence post-stroke return-to-work, a major component of self-perceived autonomy (142). Only two-thirds of “working-age” patients achieve return-to-work within 4 years of stroke (143, 144), with contributory factors falling into personal (impairment, adaptation, motivation), rehabilitative (availability, appropriateness), and workplace (demands, support, disability management) domains (145). Relatively simple professional supports may help facilitate return-to-work, like practice typing for office jobs (146). Unsurprisingly, socioeconomic disparities again play a role, with patients who worked in higher management positions more likely to return than blue-collar workers or farmers (147).
These psychosocioeconomic factors extend beyond the typically demarcated circle of care of stroke teams, but there are still important action items to consider (Table 3). Rather than relying on medical expertise, addressing these challenges requires stroke teams to build collaborations with the family of the patient, social networks, and allied-health community partners and to be effective advocates for patients. One powerful way for stroke teams to help attain these goals is by advocating for and joining integrated stroke systems that empower concerted efforts across the continuum of stroke care (26).
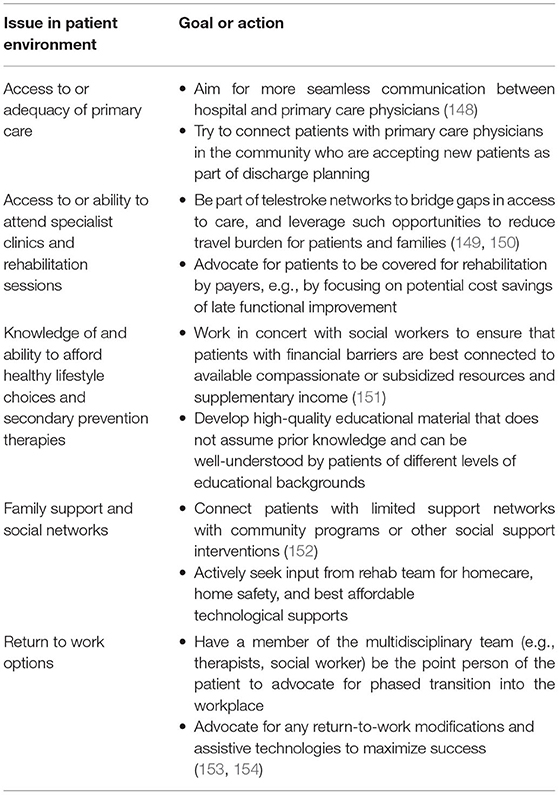
Table 3. Psychosocioeconomic action items for stroke teams to address or advocate for between 3 months and 5 years post-stroke to help maintain EVT benefits.
The long-term post-stroke trajectory of a patient is also influenced by much higher-level, upstream societal environmental factors (155). The relevance of such factors is apparent when considering macrogeographical disparities in stroke outcome, evidenced by higher stroke mortality in lower/middle-income countries (LMICs) (120), and microgeographical disparities, evidenced by higher 1-year mortality in disadvantaged parts of a given city (156). Whereas, the general organization of a healthcare system may dictate the access of a patient to care as discussed above, it is worth noting the factors outside the healthcare sphere that influence patient re-engagement post-stroke and, thus, their long-term outcomes (Figure 1D).
How a society organizes its public and private spaces can greatly affect the ability of a patient to have a fulfilling life post-stroke. Physical barriers like inaccessible entryways, bathrooms, and door thresholds can lock even mildly disabled patients out of economic and leisurely pursuits (157). In societies where having a car becomes essential, patients who are unable to drive and rely on specialized transport services have a worse quality of life (158). The availability of accessible and affordable public transport may help mitigate these challenges.
In addition, how a society values people with disability in the workplace and the public sphere may determine successful re-engagement post-stroke. Is there a supportive niche for stroke survivors or are they discriminated against? These attitudes also influence the self-perceptions and ability of the patient to thrive post-stroke. For example, negative attitudes of employers and colleagues (reflecting prevailing societal attitudes) hamper return-to-work post-stroke (159). The experiences of patients of negative public attitudes toward their need for assistance or accommodations can be especially detrimental to their progress (157).
These high-level factors are clearly beyond the control of an individual physician or stroke team. However, the potential impact of addressing such factors through collaborative efforts (Table 4) between policymakers, governments, or private/public partnerships is substantial. In a world of competing demands on resources, this calls for stroke systems to identify and promote highly motivated and visionary health professionals to leadership positions in public and political spheres where they may champion these areas of reform.
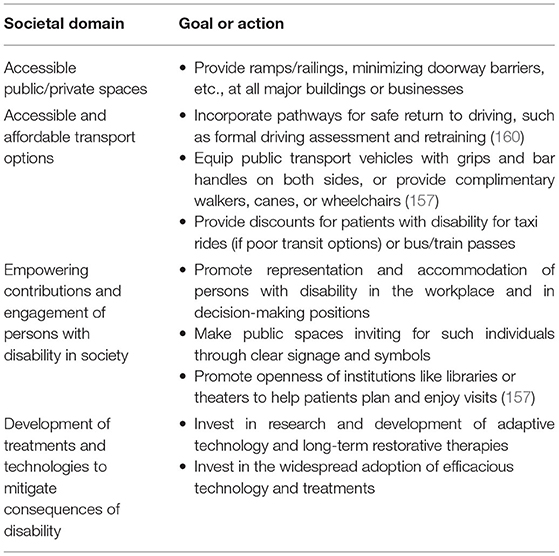
Table 4. Action items for societies to address to help maintain EVT benefits in stroke survivors at 5 years and beyond.
Endovascular therapy is one of the most efficacious therapies in modern medicine. Current evidence from 2-year follow-up of EVT trials and 5-year follow-up from longitudinal studies of ischemic stroke indicates that the 3-month group-level benefits of EVT will likely be sustained at 5 years, further supporting its long-term cost-effectiveness. In this paper, we have examined the various factors that can potentially modify the long-term outcomes of patients after ischemic stroke, drawing on the best available evidence in the literature. The adoption of regular audits and feedback as quality improvement strategies could help healthcare systems optimize these various aspects of patient care and follow-up across the continuum of stroke care in the months and years after stroke.
Our review has some important limitations. Many of the factors discussed here—such as secondary prevention, rehabilitation, and social reintegration strategies—have not been systematically examined in the EVT or LVO population. In the absence of better data, it is reasonable to extrapolate relevant insights from the general ischemic stroke population to help us optimize longer-term post-EVT care and outcomes in our current practice. Nevertheless, there remains a need for high-quality evidence from prospective cohort studies and longer-term follow-up of EVT trials or LVO cohorts to further validate the benefits of the various action items suggested in our paper. In addition, many of the insights about post-stroke care discussed in this paper have come from observational studies and are yet to be validated in randomized controlled trials. That being said, it is neither practical nor advisable to randomize patients into control arms for several non-pharmacological aspects like physician follow-up or societal accommodations for disability, so it is likely that we will have to continue relying on best-available observational data in many such cases. It is also important to note that various aspects of post-stroke care may not be generalizable to different healthcare systems owing to differences in care delivery and available resources.
When treating individual patients, stroke teams may perceive a loss of control over the long-term outcome of the patients as more time elapses post-stroke. Indeed, in the longtime horizon from 3 months to 5 years, several factors at the medical, psychosocioeconomic, and larger societal–environmental levels could erode EVT benefits. However, several factors at each level can also be leveraged to preserve or magnify treatment benefits, with opportunities to share responsibility with widening circles of care around the patient, involving primary care physicians, family/social supports, and policymakers. The race from stroke onset to EVT is a sprint, but the maintenance of EVT benefits from 3 months to 5 years post-stroke is a marathon.
AG co-conceived the paper, performed literature review, interpreted results, and wrote the first draft of the manuscript. JO and MM co-conceived the paper, performed literature review, interpreted results, and revised the manuscript. WZ, YR, and CM interpreted results and critically revised the manuscript. MG co-conceived the paper, provided supervision, and critically revised the manuscript. All authors contributed to the article and approved the submitted version.
AG reports membership in editorial boards of Neurology, Stroke, and Neurology Clinical Practice, and Stroke; speaker honoraria from NHS Health Education England; consulting fees from MD Analytics, MyMedicalPanel, Adkins Research Group, and Genome BC; research support from The Rhodes Trust, Wellcome Trust, the University of Calgary, Alberta Innovates, the Canadian Cardiovascular Society, and the Canadian Institutes of Health Research; stock/stock options from SnapDx, TheRounds.com, and Advanced Health Analytics (AHA Health Ltd.); and has a provisional patent application (US 63/024,239) for a system to deliver remote ischemic conditioning or other cuff-based therapies. JO is supported by the Julia Bangerter Rhyner Foundation, University of Basel Research Foundation, and Freiwillige Akademische Gesellschaft Basel. MG reports consulting fees from Medtronic, Stryker, Microvention, and Mentice and has a patent for Systems of stroke diagnosis licensed to GE Healthcare.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2021.713738/full#supplementary-material
EVT, endovascular treatment; LVO, large vessel occlusion; mRS, modified Rankin Scale; QALY, quality-adjusted life year.
1. Goyal M, Menon BK, van Zwam WH, Dippel DWJ, Mitchell PJ, Demchuk AM, et al. Endovascular thrombectomy after large-vessel ischaemic stroke: a meta-analysis of individual patient data from five randomised trials. Lancet. (2016) 387:1723–31. doi: 10.1016/S0140-6736(16)00163-X
2. Al-Ajlan FS, Al Sultan AS, Minhas P, Assis Z, de Miquel MA, Millan M, et al. Posttreatment infarct volumes when compared with 24-hour and 90-day clinical outcomes: insights from the REVASCAT randomized controlled trial. Am J Neuroradiol. (2018) 39:107–10. doi: 10.3174/ajnr.A5463
3. Al-Ajlan FS, Goyal M, Demchuk AM, Minhas P, Sabiq F, Assis Z, et al. Intra-arterial therapy and post-treatment infarct volumes: insights from the ESCAPE randomized controlled trial. Stroke. (2016) 47:777–81. doi: 10.1161/STROKEAHA.115.012424
4. Boers AMM, Jansen IGH, Beenen LFM, Devlin TG, San Roman L, Heo JH, et al. Association of follow-up infarct volume with functional outcome in acute ischemic stroke: a pooled analysis of seven randomized trials. J Neurointerv Surg. (2018) 10:1137–42. doi: 10.1136/neurintsurg-2017-013724
5. Ganesh A, Menon BK, Assis ZA, Demchuk AM, Al-Ajlan FS, Almekhlafi MA, et al. Discrepancy between post-treatment infarct volume and 90-day outcome in the ESCAPE randomized controlled trial. Int J Stroke. (2020) 2020:1747493020929943. doi: 10.1177/1747493020929943
6. Middleton S, McElduff P, Ward J, Grimshaw JM, Dale S, D'Este C, et al. Implementation of evidence-based treatment protocols to manage fever, hyperglycaemia, and swallowing dysfunction in acute stroke (QASC): a cluster randomised controlled trial. Lancet. (2011) 378:1699–706. doi: 10.1016/S0140-6736(11)61485-2
7. group ISTc. Effect of thrombolysis with alteplase within 6 h of acute ischaemic stroke on long-term outcomes (the third International Stroke Trial [IST-3]): 18-month follow-up of a randomised controlled trial. Lancet Neurol. (2013) 12:768–76. doi: 10.1016/S1474-4422(13)70130-3
8. Saver JL, Jahan R, Levy EI, Jovin TG, Baxter B, Nogueira RG, et al. Solitaire flow restoration device versus the Merci Retriever in patients with acute ischaemic stroke (SWIFT): a randomised, parallel-group, non-inferiority trial. Lancet. (2012) 380:1241–9. doi: 10.1016/S0140-6736(12)61384-1
9. Nogueira RG, Lutsep HL, Gupta R, Jovin TG, Albers GW, Walker GA, et al. Trevo versus Merci retrievers for thrombectomy revascularisation of large vessel occlusions in acute ischaemic stroke (TREVO 2): a randomised trial. Lancet. (2012) 380:1231–40. doi: 10.1016/S0140-6736(12)61299-9
10. Zhu Y, Zhang H, Zhang Y, Wu H, Wei L, Zhou G, et al. Endovascular metal devices for the treatment of cerebrovascular diseases. Adv Mater. (2019) 31:e1805452. doi: 10.1002/adma.201970058
11. Wallace AN, Kansagra AP, McEachern J, Moran CJ, Cross DT III, Derdeyn CP. Evolution of endovascular stroke therapies and devices. Expert Rev Med Devices. (2016) 13:263–70. doi: 10.1586/17434440.2016.1143772
12. Ganesh A, Goyal M. Thrombectomy for acute ischemic stroke: recent insights and future directions. Curr Neurol Neurosci Rep. (2018) 18:59. doi: 10.1007/s11910-018-0869-8
13. Saver JL, Goyal M, van der Lugt A, Menon BK, Majoie CB, Dippel DW, et al. Time to treatment with endovascular thrombectomy and outcomes from ischemic stroke: a meta-analysis. JAMA. (2016) 316:1279–88. doi: 10.1001/jama.2016.13647
14. Goyal M, Almekhlafi MA, Fan L, Menon BK, Demchuk AM, Yeatts SD, et al. Evaluation of interval times from onset to reperfusion in patients undergoing endovascular therapy in the Interventional Management of Stroke III trial. Circulation. (2014) 130:265–72. doi: 10.1161/CIRCULATIONAHA.113.007826
15. Goyal M, Jadhav AP, Bonafe A, Diener H, Mendes Pereira V, Levy E, et al. Analysis of workflow and time to treatment and the effects on outcome in endovascular treatment of acute ischemic stroke: results from the SWIFT PRIME randomized controlled trial. Radiology. (2016) 279:888–97. doi: 10.1148/radiol.2016160204
16. McTaggart RA, Tung EL, Yaghi S, Cutting SM, Hemendinger M, Gale HI, et al. Continuous aspiration prior to intracranial vascular embolectomy (CAPTIVE): a technique which improves outcomes. J Neurointerv Surg. (2017) 9:1154–9. doi: 10.1136/neurintsurg-2016-012838
17. Ospel JM, Volny O, Jayaraman M, McTaggart R, Goyal M. Optimizing fast first pass complete reperfusion in acute ischemic stroke - the BADDASS approach (BAlloon guiDe with large bore DISTAL ACCESS catheter with dual aspiration with Stent-retriever as Standard approach). Expert Rev Med Devices. (2019) 16:955–63. doi: 10.1080/17434440.2019.1684263
18. Albers GW, Marks MP, Kemp S, Christensen S, Tsai JP, Ortega-Gutierrez S, et al. Thrombectomy for stroke at 6 to 16 hours with selection by perfusion imaging. N Engl J Med. (2018) 378:708–18. doi: 10.1056/NEJMoa1713973
19. Nogueira RG, Jadhav AP, Haussen DC, Bonafe A, Budzik RF, Bhuva P, et al. Thrombectomy 6 to 24 hours after stroke with a mismatch between deficit and infarct. N Engl J Med. (2018) 378:11–21. doi: 10.1056/NEJMoa1706442
20. Roman LS, Menon BK, Blasco J, Hernandez-Perez M, Davalos A, Majoie C, et al. Imaging features and safety and efficacy of endovascular stroke treatment: a meta-analysis of individual patient-level data. Lancet Neurol. (2018) 17:895–904. doi: 10.1016/S1474-4422(18)30242-4
21. Kaesmacher J, Chaloulos-Iakovidis P, Panos L, Mordasini P, Michel P, Hajdu SD, et al. Mechanical thrombectomy in ischemic stroke patients with alberta stroke program early computed tomography score 0-5. Stroke. (2019) 50:880–8. doi: 10.1161/STROKEAHA.118.023465
22. Neuhaus AA, Couch Y, Hadley G, Buchan AM. Neuroprotection in stroke: the importance of collaboration and reproducibility. Brain. (2017) 140:2079–92. doi: 10.1093/brain/awx126
23. Hill MD, Goyal M, Menon BK, Nogueira RG, McTaggart RA, Demchuk AM, et al. Efficacy and safety of nerinetide for the treatment of acute ischaemic stroke (ESCAPE-NA1): a multicentre, double-blind, randomised controlled trial. Lancet. (2020) 395:878–87. doi: 10.1016/S0140-6736(20)30258-0
24. Abedi V, Khan A, Chaudhary D, Misra D, Avula V, Mathrawala D, et al. Using artificial intelligence for improving stroke diagnosis in emergency departments: a practical framework. Ther Adv Neurol Disord. (2020) 13:1756286420938962. doi: 10.1177/1756286420938962
25. Bivard A, Churilov L, Parsons M. Artificial intelligence for decision support in acute stroke - current roles and potential. Nat Rev Neurol. (2020) 16:575–85. doi: 10.1038/s41582-020-0390-y
26. Adeoye O, Nystrom KV, Yavagal DR, Luciano J, Nogueira RG, Zorowitz RD, et al. Recommendations for the establishment of stroke systems of care: a 2019 update. Stroke. (2019) 50:e187–210. doi: 10.1161/STR.0000000000000173
27. Ganesh A, Lindsay P, Fang J, Kapral MK, Cote R, Joiner I, et al. Integrated systems of stroke care and reduction in 30-day mortality: a retrospective analysis. Neurology. (2016) 86:898–904. doi: 10.1212/WNL.0000000000002443
28. Aghaebrahim A, Streib C, Rangaraju S, Kenmuir CL, Giurgiutiu DV, Horev A, et al. Streamlining door to recanalization processes in endovascular stroke therapy. J Neurointerv Surg. (2017) 9:340–5. doi: 10.1136/neurintsurg-2016-012324
29. Menon BK, Sajobi TT, Zhang Y, Rempel JL, Shuaib A, Thornton J, et al. Analysis of workflow and time to treatment on thrombectomy outcome in the Endovascular Treatment for Small Core and Proximal Occlusion Ischemic Stroke (ESCAPE) randomized controlled trial. Circulation. (2016) 133:2279–86. doi: 10.1161/CIRCULATIONAHA.116.024877
30. Almekhlafi MA, Holodinsky JK, Hill MD, Kamal N, Goyal M. Organizing stroke systems in the field for patients with suspected large vessel occlusion acute stroke. Expert Rev Cardiovasc Ther. (2019) 17:3–9. doi: 10.1080/14779072.2019.1550717
31. Lima FO. Mont'Alverne FJA, Bandeira D, Nogueira RG. Pre-hospital assessment of large vessel occlusion strokes: implications for modeling and planning stroke systems of care. Front Neurol. (2019) 10:955. doi: 10.3389/fneur.2019.00955
32. Schlemm L, Schlemm E. Clinical benefit of improved prehospital stroke scales to detect stroke patients with large vessel occlusions: results from a conditional probabilistic model. BMC Neurol. (2018) 18:16. doi: 10.1186/s12883-018-1021-8
33. Milne MS, Holodinsky JK, Hill MD, Nygren A, Qiu C, Goyal M, et al. Drip 'n ship versus mothership for endovascular treatment: modeling the best transportation options for optimal outcomes. Stroke. (2017) 48:791–4. doi: 10.1161/STROKEAHA.116.015321
34. Holodinsky JK, Williamson TS, Demchuk AM, Zhao H, Zhu L, Francis MJ, et al. Modeling stroke patient transport for all patients with suspected large-vessel occlusion. JAMA Neurol. (2018) 75:1477–86. doi: 10.1001/jamaneurol.2018.2424
35. Bamford J, Sandercock P, Dennis M, Burn J, Warlow C. Classification and natural history of clinically identifiable subtypes of cerebral infarction. Lancet. (1991) 337:1521–6. doi: 10.1016/0140-6736(91)93206-O
36. Luengo-Fernandez R, Paul NL, Gray AM, Pendlebury ST, Bull LM, Welch SJ, et al. Population-based study of disability and institutionalization after transient ischemic attack and stroke: 10-year results of the Oxford Vascular Study. Stroke. (2013) 44:2854–61. doi: 10.1161/STROKEAHA.113.001584
37. Feigin VL, Barker-Collo S, Parag V, Senior H, Lawes CM, Ratnasabapathy Y, et al. Auckland stroke outcomes study. Part 1: gender, stroke types, ethnicity, and functional outcomes 5 years poststroke. Neurology. (2010) 75:1597–607. doi: 10.1212/WNL.0b013e3181fb44b3
38. Hankey GJ, Jamrozik K, Broadhurst RJ, Forbes S, Anderson CS. Long-term disability after first-ever stroke and related prognostic factors in the Perth Community Stroke Study, 1989–1990. Stroke. (2002) 33:1034–40. doi: 10.1161/01.STR.0000012515.66889.24
39. Ganesh A, Luengo-Fernandez R, Wharton RM, Gutnikov SA, Silver LE, Mehta Z, et al. Time course of evolution of disability and cause-specific mortality after ischemic stroke: implications for trial design. J Am Heart Assoc. (2017) 6:e005788. doi: 10.1161/JAHA.117.005788
40. Vernino S, Brown RD Jr, Sejvar JJ, Sicks JD, Petty GW, O'Fallon WM. Cause-specific mortality after first cerebral infarction: a population-based study. Stroke. (2003) 34:1828–32. doi: 10.1161/01.STR.0000080534.98416.A0
41. Langhorne P, Stott DJ, Robertson L, MacDonald J, Jones L, McAlpine C, et al. Medical complications after stroke: a multicenter study. Stroke. (2000) 31:1223–9. doi: 10.1161/01.STR.31.6.1223
42. Hankey GJ, Jamrozik K, Broadhurst RJ, Forbes S, Burvill PW, Anderson CS, et al. Five-year survival after first-ever stroke and related prognostic factors in the Perth Community Stroke Study. Stroke. (2000) 31:2080–6. doi: 10.1161/01.STR.31.9.2080
43. Prencipe M, Culasso F, Rasura M, Anzini A, Beccia M, Cao M, et al. Long-term prognosis after a minor stroke: 10-year mortality and major stroke recurrence rates in a hospital-based cohort. Stroke. (1998) 29:126–32. doi: 10.1161/01.STR.29.1.126
44. Eriksson M, Norrving B, Terent A, Stegmayr B. Functional outcome 3 months after stroke predicts long-term survival. Cerebrovasc Dis. (2008) 25:423–9. doi: 10.1159/000121343
45. Huybrechts KF, Caro JJ, Xenakis JJ, Vemmos KN. The prognostic value of the modified Rankin Scale score for long-term survival after first-ever stroke. Results from the Athens Stroke Registry Cerebrovasc Dis. (2008) 26:381–7. doi: 10.1159/000151678
46. Slot KB, Berge E, Dorman P, Lewis S, Dennis M, Sandercock P, et al. Impact of functional status at six months on long term survival in patients with ischaemic stroke: prospective cohort studies. BMJ. (2008) 336:376–9. doi: 10.1136/bmj.39456.688333.BE
47. Ganesh A, Luengo-Fernandez R, Wharton RM, Rothwell PM, Oxford Vascular S. Ordinal vs dichotomous analyses of modified Rankin Scale, 5-year outcome, and cost of stroke. Neurology. (2018) 91:e1951–60. doi: 10.1212/WNL.0000000000006554
48. Sachdev PS, Chen X, Brodaty H, Thompson C, Altendorf A, Wen W. The determinants and longitudinal course of post-stroke mild cognitive impairment. J Int Neuropsychol Soc. (2009) 15:915–23. doi: 10.1017/S1355617709990579
49. Kjork E, Blomstrand C, Carlsson G, Lundgren-Nilsson A, Gustavsson C. Daily life consequences, cognitive impairment, and fatigue after transient ischemic attack. Acta Neurol Scand. (2016) 133:103–10. doi: 10.1111/ane.12435
50. Pendlebury ST, Rothwell PM, Oxford Vascular S. Incidence and prevalence of dementia associated with transient ischaemic attack and stroke: analysis of the population-based Oxford Vascular Study. Lancet Neurol. (2019) 18:248–58. doi: 10.1016/S1474-4422(18)30442-3
51. Barba R, Martinez-Espinosa S, Rodriguez-Garcia E, Pondal M, Vivancos J, Del Ser T. Poststroke dementia: clinical features and risk factors. Stroke. (2000) 31:1494–501. doi: 10.1161/01.STR.31.7.1494
52. Leys D, Henon H, Mackowiak-Cordoliani MA, Pasquier F. Poststroke dementia. Lancet Neurol. (2005) 4:752–9. doi: 10.1016/S1474-4422(05)70221-0
53. Desmond DW, Moroney JT, Sano M, Stern Y. Mortality in patients with dementia after ischemic stroke. Neurology. (2002) 59:537–43. doi: 10.1212/WNL.59.4.537
54. Ganesh A, Luengo-Fernandez R, Pendlebury ST, Rothwell PM, on behalf of the Oxford Vascular Study. Weights for ordinal analyses of the modified Rankin Scale in stroke trials: a population-based cohort study. E Clin Med. (2020) 23:100415. doi: 10.1016/j.eclinm.2020.100415
55. Wilson A, Bath PMW, Berge E, Cadilhac DA, Cuche M, Ford GA, et al. Understanding the relationship between costs and the modified Rankin Scale: a systematic review, multidisciplinary consensus and recommendations for future studies. Eur Stroke J. (2017) 2:3–12. doi: 10.1177/2396987316684705
56. Ganesh A, Luengo-Fernandez R, Pendlebury ST, Rothwell PM. Long-term consequences of worsened poststroke status in patients with premorbid disability. Stroke. (2018) 49:2430–6. doi: 10.1161/STROKEAHA.118.022416
57. Gloede TD, Halbach SM, Thrift AG, Dewey HM, Pfaff H, Cadilhac DA. Long-term costs of stroke using 10-year longitudinal data from the North East Melbourne Stroke Incidence Study. Stroke. (2014) 45:3389–94. doi: 10.1161/STROKEAHA.114.006200
58. Hardie K, Hankey GJ, Jamrozik K, Broadhurst RJ, Anderson C. Ten-year risk of first recurrent stroke and disability after first-ever stroke in the Perth Community Stroke Study. Stroke. (2004) 35:731–5. doi: 10.1161/01.STR.0000116183.50167.D9
59. Liman TG, Heuschmann PU, Endres M, Floel A, Schwab S, Kolominsky-Rabas PL. Impact of low mini-mental status on health outcome up to 5 years after stroke: the Erlangen Stroke Project. J Neurol. (2012) 259:1125–30. doi: 10.1007/s00415-011-6312-6
60. Ayerbe L, Ayis S, Rudd AG, Heuschmann PU, Wolfe CD. Natural history, predictors, and associations of depression 5 years after stroke: the South London Stroke Register. Stroke. (2011) 42:1907–11. doi: 10.1161/STROKEAHA.110.605808
61. Walsh T, Donnelly T, Carew S. O' Connor C, O' Riordan R, Lyons D. Stroke unit care: recurrence, mortality and institutionalisation rates-a four year follow-up study. Ir J Med Sci. (2008) 177:135–9. doi: 10.1007/s11845-007-0110-2
62. Portelli R, Lowe D, Irwin P, Pearson M, Rudd AG, Intercollegiate Stroke Working Party. Institutionalization after stroke. Clin Rehabil. (2005) 19:97–108. doi: 10.1191/0269215505cr822oa
63. Brodaty H, Altendorf A, Withall A, Sachdev PS. Mortality and institutionalization in early survivors of stroke: the effects of cognition, vascular mild cognitive impairment, and vascular dementia. J Stroke Cerebrovasc Dis. (2010) 19:485–93. doi: 10.1016/j.jstrokecerebrovasdis.2009.09.006
64. Kolominsky-Rabas PL, Hilz MJ, Neundoerfer B, Heuschmann PU. Impact of urinary incontinence after stroke: results from a prospective population-based stroke register. Neurourol Urodyn. (2003) 22:322–7. doi: 10.1002/nau.10114
65. Hackett ML, Glozier N, Jan S, Lindley R. Returning to paid employment after stroke: the Psychosocial Outcomes In StrokE (POISE) cohort study. PLoS ONE. (2012) 7:e41795. doi: 10.1371/journal.pone.0041795
66. Luengo-Fernandez R, Gray AM, Bull L, Welch S, Cuthbertson F, Rothwell PM, et al. Quality of life after TIA and stroke: ten-year results of the Oxford Vascular Study. Neurology. (2013) 81:1588–95. doi: 10.1212/WNL.0b013e3182a9f45f
67. Ali M, MacIsaac R, Quinn TJ, Bath PM, Veenstra DL, Xu Y, et al. Dependency and health utilities in stroke: data to inform cost-effectiveness analyses. Eur Stroke J. (2017) 2:70–6. doi: 10.1177/2396987316683780
68. van den Berg LA, Dijkgraaf MG, Berkhemer OA, Fransen PS, Beumer D, Lingsma HF, et al. Two-year outcome after endovascular treatment for acute ischemic stroke. N Engl J Med. (2017) 376:1341–9. doi: 10.1056/NEJMoa1612136
69. Davalos A, Cobo E, Molina CA, Chamorro A, de Miquel MA, Roman LS, et al. Safety and efficacy of thrombectomy in acute ischaemic stroke (REVASCAT): 1-year follow-up of a randomised open-label trial. Lancet Neurol. (2017) 16:369–76. doi: 10.1016/S1474-4422(17)30047-9
70. Lopez-Cancio E, Jovin TG, Cobo E, Cerda N, Jimenez M, Gomis M, et al. Endovascular treatment improves cognition after stroke: a secondary analysis of REVASCAT trial. Neurology. (2017) 88:245–51. doi: 10.1212/WNL.0000000000003517
71. Kunz WG, Hunink MG, Almekhlafi MA, Menon BK, Saver JL, Dippel DWJ, et al. Public health and cost consequences of time delays to thrombectomy for acute ischemic stroke. Neurology. (2020). doi: 10.1212/WNL.0000000000010867
72. Jorgensen HS, Nakayama H, Raaschou HO, Vive-Larsen J, Stoier M, Olsen TS. Outcome and time course of recovery in stroke. Part II: time course of recovery The Copenhagen Stroke Study. Arch Phys Med Rehabil. (1995) 76:406–12. doi: 10.1016/S0003-9993(95)80568-0
73. Wolf SL, Winstein CJ, Miller JP, Taub E, Uswatte G, Morris D, et al. Effect of constraint-induced movement therapy on upper extremity function 3 to 9 months after stroke: the EXCITE randomized clinical trial. JAMA. (2006) 296:2095–104. doi: 10.1001/jama.296.17.2095
74. Page SJ, Levine P, Leonard A, Szaflarski JP, Kissela BM. Modified constraint-induced therapy in chronic stroke: results of a single-blinded randomized controlled trial. Phys Ther. (2008) 88:333–40. doi: 10.2522/ptj.20060029
75. Belagaje SR. Stroke rehabilitation. Continuum (Minneap Minn). (2017) 23(1, Cerebrovascular Disease):238–53. doi: 10.1212/CON.0000000000000423
76. Ganesh A, Gutnikov SA, Rothwell PM, Oxford Vascular S. Late functional improvement after lacunar stroke: a population-based study. J Neurol Neurosurg Psychiatry. (2018) 89:1301–7. doi: 10.1136/jnnp-2018-318434
77. Qureshi A, Afghan S, Malik A, Saeed O, Janjua N. Abstract 22: late functional improvement in acute ischemic stroke patients: pooled analysis of three multicenter clinical trials. Stroke. (2018) 49:A22. doi: 10.1161/str.49.suppl_1.22
78. Ballester BR, Maier M, Duff A, Cameirao M, Bermudez S, Duarte E, et al. A critical time window for recovery extends beyond one-year post-stroke. J Neurophysiol. (2019) 122:350–7. doi: 10.1152/jn.00762.2018
79. Ganesh A, Luengo-Fernandez R, Rothwell PM. Late functional improvement and 5-year poststroke outcomes: a population-based cohort study. J Neurol Neurosurg Psychiatry. (2020) 91:831–9. doi: 10.1136/jnnp-2019-322365
80. Ferreira F, Chaves MEA, Oliveira VC, Van Petten A, Vimieiro CBS. Effectiveness of robot therapy on body function and structure in people with limited upper limb function: a systematic review and meta-analysis. PLoS ONE. (2018) 13:e0200330. doi: 10.1371/journal.pone.0200330
81. Karamians R, Proffitt R, Kline D, Gauthier LV. Effectiveness of virtual reality- and gaming-based interventions for upper extremity rehabilitation poststroke: a meta-analysis. Arch Phys Med Rehabil. (2020) 101:885–96. doi: 10.1016/j.apmr.2019.10.195
82. Seiffge DJ, Paciaroni M, Wilson D, Koga M, Macha K, Cappellari M, et al. Direct oral anticoagulants versus vitamin K antagonists after recent ischemic stroke in patients with atrial fibrillation. Ann Neurol. (2019) 85:823–34. doi: 10.1002/ana.25489
83. Mizoguchi T, Tanaka K, Toyoda K, Yoshimura S, Itabashi R, Takagi M, et al. Early initiation of direct oral anticoagulants after onset of stroke and short- and long-term outcomes of patients with nonvalvular atrial fibrillation. Stroke. (2020) 51:883–91. doi: 10.1161/STROKEAHA.119.028118
84. Asberg S, Hijazi Z, Norrving B, Terent A, Ohagen P, Oldgren J. Timing of oral anticoagulant therapy in acute ischemic stroke with atrial fibrillation: study protocol for a registry-based randomised controlled trial. Trials. (2017) 18:581. doi: 10.1186/s13063-017-2313-9
85. Terman SW, Reeves MJ, Skolarus LE, Burke JF. Association between early outpatient visits and readmissions after ischemic stroke. Circ Cardiovasc Qual Outcomes. (2018) 11:e004024. doi: 10.1161/CIRCOUTCOMES.117.004024
86. Webster F, Saposnik G, Kapral MK, Fang J, O'Callaghan C, Hachinski V. Organized outpatient care: stroke prevention clinic referrals are associated with reduced mortality after transient ischemic attack and ischemic stroke. Stroke. (2011) 42:3176–82. doi: 10.1161/STROKEAHA.111.621524
87. Leppert MH, Sillau S, Lindrooth RC, Poisson SN, Campbell JD, Simpson JR. Relationship between early follow-up and readmission within 30 and 90 days after ischemic stroke. Neurology. (2020) 94:e1249–e58. doi: 10.1212/WNL.0000000000009135
88. Condon C, Lycan S, Duncan P, Bushnell C. Reducing readmissions after stroke with a structured nurse practitioner/registered nurse transitional stroke program. Stroke. (2016) 47:1599–604. doi: 10.1161/STROKEAHA.115.012524
89. Webb A, Heldner MR, Aguiar de. Sousa D, Sandset EC, Randall G, Bejot Y, et al. Availability of secondary prevention services after stroke in Europe: an ESO/SAFE survey of national scientific societies and stroke experts. Eur Stroke J. (2019) 4:110–8. doi: 10.1177/2396987318816136
90. van Schaik SM, de Vries BS, Weinstein HC, Visser MC, Van den Berg-Vos RM. Practice variation in long-term secondary stroke prevention in The Netherlands. J Stroke Cerebrovasc Dis. (2015) 24:566–72. doi: 10.1016/j.jstrokecerebrovasdis.2014.09.031
91. Ullberg T, Glader EL, Zia E, Petersson J, Eriksson M, Norrving B. Associations between ischemic stroke follow-up, socioeconomic status, and adherence to secondary preventive drugs in southern sweden: observations from the Swedish Stroke Register (Riksstroke). Neuroepidemiology. (2017) 48:32–8. doi: 10.1159/000456618
92. Duncan PW, Bushnell CD, Rosamond WD, Jones Berkeley SB, Gesell SB, D'Agostino RB Jr, et al. The Comprehensive Post-Acute Stroke Services (COMPASS) study: design and methods for a cluster-randomized pragmatic trial. BMC Neurol. (2017) 17:133. doi: 10.1186/s12883-017-0907-1
93. Sharrief AZ, Hinojosa E, Cooksey G, Okpala MN, Avritscher EB, Pedroza C, et al. Does care in a specialised stroke prevention clinic improve poststroke blood pressure control: a protocol for a randomised comparative effectiveness study. BMJ Open. (2019) 9:e024695. doi: 10.1136/bmjopen-2018-024695
94. Hafsteinsdóttir TB, Vergunst M, Lindeman E, Schuurmans M. Educational needs of patients with a stroke and their caregivers: a systematic review of the literature. Patient Educ Couns. (2011) 85:14–25. doi: 10.1016/j.pec.2010.07.046
95. Eames S, Hoffmann T, Worrall L, Read S, Wong A. Randomised controlled trial of an education and support package for stroke patients and their carers. BMJ Open. (2013) 3:5. doi: 10.1136/bmjopen-2012-002538
96. Maasland L, Brouwer-Goossensen D, den Hertog HM, Koudstaal PJ, Dippel DW. Health education in patients with a recent stroke or transient ischaemic attack: a comprehensive review. Int J Stroke. (2011) 6:67–74. doi: 10.1111/j.1747-4949.2010.00541.x
97. Bridgwood B, Lager KE, Mistri AK, Khunti K, Wilson AD, Modi P. Interventions for improving modifiable risk factor control in the secondary prevention of stroke. Cochrane Database Syst Rev. (2018) 5:Cd009103. doi: 10.1002/14651858.CD009103.pub3
98. Lawrence M, Pringle J, Kerr S, Booth J, Govan L, Roberts NJ. Multimodal secondary prevention behavioral interventions for TIA and stroke: a systematic review and meta-analysis. PLoS ONE. (2015) 10:e0120902. doi: 10.1371/journal.pone.0120902
99. Chung PW, Yoon BW, Lee YB, Shin BS, Kim HY, Park JH, et al. Medication adherence of statin users after acute ischemic stroke. Eur Neurol. (2018) 80:106–14. doi: 10.1159/000493530
100. D NC, C NC, Akijian L, Callaly EL, Hannon N, Kelly L, et al. Suboptimal lipid management before and after ischaemic stroke and TIA-the North Dublin Population Stroke Study. Ir J Med Sci. (2018) 187:739–46. doi: 10.1007/s11845-018-1739-8
101. Gumbinger C, Holstein T, Stock C, Rizos T, Horstmann S, Veltkamp R. Reasons underlying non-adherence to and discontinuation of anticoagulation in secondary stroke prevention among patients with atrial fibrillation. Eur Neurol. (2015) 73:184–91. doi: 10.1159/000371574
102. Sauer R, Sauer EM, Bobinger T, Blinzler C, Huttner HB, Schwab S, et al. Adherence to oral anticoagulation in secondary stroke prevention–the first year of direct oral anticoagulants. J Stroke Cerebrovasc Dis. (2015) 24:78–82. doi: 10.1016/j.jstrokecerebrovasdis.2014.07.032
103. Shah R, Li S, Stamplecoski M, Kapral MK. Low use of oral anticoagulant prescribing for secondary stroke prevention: results from the ontario stroke registry. Med Care. (2016) 54:907–12. doi: 10.1097/MLR.0000000000000589
104. Jamison J, Graffy J, Mullis R, Mant J, Sutton S. Barriers to medication adherence for the secondary prevention of stroke: a qualitative interview study in primary care. Br J Gen Pract. (2016) 66:e568–76. doi: 10.3399/bjgp16X685609
105. Parikh NS, Chatterjee A, Diaz I, Merkler AE, Murthy SB, Iadecola C, et al. Trends in active cigarette smoking among stroke survivors in the United States, 1999 to 2018. Stroke. (2020) 51:1656–61. doi: 10.1161/STROKEAHA.120.029084
106. Bjerkreim AT, Thomassen L, Brøgger J, Waje-Andreassen U, Næss H. Causes and predictors for hospital readmission after ischemic stroke. J Stroke Cerebrovasc Dis. (2015) 24:2095–101. doi: 10.1016/j.jstrokecerebrovasdis.2015.05.019
107. Heikinheimo T, Broman J, Haapaniemi E, Kaste M, Tatlisumak T, Putaala J. Preceding and poststroke infections in young adults with first-ever ischemic stroke: effect on short-term and long-term outcomes. Stroke. (2013) 44:3331–7. doi: 10.1161/STROKEAHA.113.002108
108. Martino R, Foley N, Bhogal S, Diamant N, Speechley M, Teasell R. Dysphagia after stroke: incidence, diagnosis, and pulmonary complications. Stroke. (2005) 36:2756–63. doi: 10.1161/01.STR.0000190056.76543.eb
109. Lo YK, Fu TC, Chen CP, Yuan SS, Hsu CC. Involvement of swallowing therapy is associated with improved long-term survival in patients with post-stroke dysphagia. Eur J Phys Rehabil Med. (2019) 55:728–34. doi: 10.23736/S1973-9087.19.05893-3
110. Villa RF, Ferrari F, Moretti A. Post-stroke depression: mechanisms and pharmacological treatment. Pharmacol Ther. (2018) 184:131–44. doi: 10.1016/j.pharmthera.2017.11.005
111. AFFINITY Trail Collaboration. Safety and efficacy of fluoxetine on functional outcome after acute stroke (AFFINITY): a randomised, double-blind, placebo-controlled trial. Lancet Neurol. (2020) 19:651–60. doi: 10.1016/S1474-4422(20)30207-6
112. EFFECTS Trial Collaboration. Safety and efficacy of fluoxetine on functional recovery after acute stroke (EFFECTS): a randomised, double-blind, placebo-controlled trial. Lancet Neurol. (2020) 19:661–9. doi: 10.1016/S1474-4422(20)30219-2
113. Langhorne P, Baylan S. Early supported discharge services for people with acute stroke. Cochrane Database Syst Rev. (2017) 7:CD000443. doi: 10.1002/14651858.CD000443.pub4
114. Ögren J, Irewall AL, Söderström L, Mooe T. Long-term, telephone-based follow-up after stroke and TIA improves risk factors: 36-month results from the randomized controlled NAILED stroke risk factor trial. BMC Neurol. (2018) 18:153. doi: 10.1186/s12883-018-1158-5
115. Hedegaard U, Kjeldsen LJ, Pottegård A, Henriksen JE, Lambrechtsen J, Hangaard J, et al. Improving medication adherence in patients with hypertension: a randomized trial. Am J Med. (2015) 128:1351–61. doi: 10.1016/j.amjmed.2015.08.011
116. Machline-Carrion MJ, Santucci EV, Damiani LP, Bahit C, Málaga G, Pontes-Neto OM, et al. An international cluster-randomized quality improvement trial to increase the adherence to evidence-based therapies for acute ischemic stroke and transient ischemic attack patients: rationale and design of the BRIDGE STROKE Trial. Am Heart J. (2019) 207:49–57. doi: 10.1016/j.ahj.2018.09.009
117. Zhang Y, Fan D, Ji H, Qiao S, Li X. Treatment adherence and secondary prevention of ischemic stroke among discharged patients using mobile phone- and WeChat-based improvement services: cohort study. JMIR Mhealth Uhealth. (2020) 8:e16496. doi: 10.2196/16496
118. Belleudi V, Sciattella P, Agabiti N, Di Martino M, Di Domenicantonio R, Davoli M, et al. Socioeconomic differences in one-year survival after ischemic stroke: the effect of acute and post-acute care-pathways in a cohort study. BMC Public Health. (2016) 16:408. doi: 10.1186/s12889-016-3019-8
119. Vivanco-Hidalgo RM, Ribera A, Abilleira S. Association of socioeconomic status with ischemic stroke survival. Stroke. (2019) 50:3400–7. doi: 10.1161/STROKEAHA.119.026607
120. Marshall IJ, Wang Y, Crichton S, McKevitt C, Rudd AG, Wolfe CD. The effects of socioeconomic status on stroke risk and outcomes. Lancet Neurol. (2015) 14:1206–18. doi: 10.1016/S1474-4422(15)00200-8
121. Song T, Pan Y, Chen R, Li H, Zhao X, Liu L, et al. Is there a correlation between socioeconomic disparity and functional outcome after acute ischemic stroke? PLoS ONE. (2017) 12:e0181196. doi: 10.1371/journal.pone.0181196
122. Abdalla SM, Yu S, Galea S. Trends in cardiovascular disease prevalence by income level in the United States. JAMA Netw Open. (2020) 3:e2018150. doi: 10.1001/jamanetworkopen.2020.18150
123. Lai PM, Dasenbrock H, Lin N, Du R. The impact of insurance status on the outcomes after aneurysmal subarachnoid hemorrhage. PLoS ONE. (2013) 8:e78047. doi: 10.1371/journal.pone.0078047
124. Shen JJ, Washington EL. Disparities in outcomes among patients with stroke associated with insurance status. Stroke. (2007) 38:1010–6. doi: 10.1161/01.STR.0000257312.12989.af
125. Chen R, McKevitt C, Rudd AG, Wolfe CD. Socioeconomic deprivation and survival after stroke: findings from the prospective South London Stroke Register of 1995 to 2011. Stroke. (2014) 45:217–23. doi: 10.1161/STROKEAHA.113.003266
126. Chen R, Crichton S, McKevitt C, Rudd AG, Sheldenkar A, Wolfe CD. Association between socioeconomic deprivation and functional impairment after stroke: the South London Stroke Register. Stroke. (2015) 46:800–5. doi: 10.1161/STROKEAHA.114.007569
127. Putman K, De Wit L, Schoonacker M, Baert I, Beyens H, Brinkmann N, et al. Effect of socioeconomic status on functional and motor recovery after stroke: a European multicentre study. J Neurol Neurosurg Psychiatry. (2007) 78:593–9. doi: 10.1136/jnnp.2006.094607
128. Jakovljevic D, Sarti C, Sivenius J, Torppa J, Mahonen M, Immonen-Raiha P, et al. Socioeconomic status and ischemic stroke: the FINMONICA Stroke Register. Stroke. (2001) 32:1492–8. doi: 10.1161/01.STR.32.7.1492
129. Chen R, McKevitt C, Crichton SL, Rudd AG, Wolfe CD. Socioeconomic deprivation and provision of acute and long-term care after stroke: the South London Stroke Register cohort study. J Neurol Neurosurg Psychiatry. (2014) 85:1294–300. doi: 10.1136/jnnp-2013-306413
130. Ganesh A, King-Shier K, Manns BJ, Hill MD, Campbell DJ. Money is brain: financial barriers and consequences for canadian stroke patients. Can J Neurol Sci. (2017) 44:146–51. doi: 10.1017/cjn.2016.411
131. Cameron JI, O'Connell C, Foley N, Salter K, Booth R, Boyle R, et al. Canadian stroke best practice recommendations: managing transitions of care following stroke, guidelines update 2016. Int J Stroke. (2016) 11:807–22. doi: 10.1177/1747493016660102
132. Ong PH, Koh GC. Caregiver factors in stroke: are they the missing piece of the puzzle? Arch Phys Med Rehabil. (2016) 97:1223–5. doi: 10.1016/j.apmr.2016.02.001
133. Dewey HM, Thrift AG, Mihalopoulos C, Carter R, Macdonell RA, McNeil JJ, et al. Informal care for stroke survivors: results from the North East Melbourne Stroke Incidence Study (NEMESIS). Stroke. (2002) 33:1028–33. doi: 10.1161/01.STR.0000013067.24300.B0
134. Wang TC, Tsai AC, Wang JY, Lin YT, Lin KL, Chen JJ, et al. Caregiver-mediated intervention can improve physical functional recovery of patients with chronic stroke: a randomized controlled trial. Neurorehabil Neural Repair. (2015) 29:3–12. doi: 10.1177/1545968314532030
135. Dhand A, Luke DA, Lang CE, Lee JM. Social networks and neurological illness. Nat Rev Neurol. (2016) 12:605–12. doi: 10.1038/nrneurol.2016.119
136. Dhand A, Luke D, Lang C, Tsiaklides M, Feske S, Lee JM. Social networks and risk of delayed hospital arrival after acute stroke. Nat Commun. (2019) 10:1206. doi: 10.1038/s41467-019-09073-5
137. Medin J, Larson J, von Arbin M, Wredling R, Tham K. Elderly persons' experience and management of eating situations 6 months after stroke. Disabil Rehabil. (2010) 32:1346–53. doi: 10.3109/09638280903514747
138. Cacioppo JT, Cacioppo S. The growing problem of loneliness. Lancet. (2018) 391:426. doi: 10.1016/S0140-6736(18)30142-9
139. Lowry CA, Jin AY. Improving the social relevance of experimental stroke models: social isolation, social defeat stress and stroke outcome in animals and humans. Front Neurol. (2020) 11:427. doi: 10.3389/fneur.2020.00427
140. Friedler B, Crapser J, McCullough L. One is the deadliest number: the detrimental effects of social isolation on cerebrovascular diseases and cognition. Acta Neuropathol. (2015) 129:493–509. doi: 10.1007/s00401-014-1377-9
141. Klamroth-Marganska V. Stroke rehabilitation: therapy robots and assistive devices. Adv Exp Med Biol. (2018) 1065:579–87. doi: 10.1007/978-3-319-77932-4_35
142. Westerlind E, Persson HC, Törnbom K, Sunnerhagen KS. Return to work predicts perceived participation and autonomy by individuals with stroke. Disabil Rehabil. (2019) 42:3673–8. doi: 10.1080/09638288.2019.1608324
143. Aarnio K, Rodríguez-Pardo J, Siegerink B, Hardt J, Broman J, Tulkki L, et al. Return to work after ischemic stroke in young adults: a registry-based follow-up study. Neurology. (2018) 91:e1909–17. doi: 10.1212/WNL.0000000000006510
144. Edwards JD, Kapoor A, Linkewich E, Swartz RH. Return to work after young stroke: a systematic review. Int J Stroke. (2018) 13:243–56. doi: 10.1177/1747493017743059
145. Schwarz B, Claros-Salinas D, Streibelt M. Meta-synthesis of qualitative research on facilitators and barriers of return to work after stroke. J Occup Rehabil. (2018) 28:28–44. doi: 10.1007/s10926-017-9713-2
146. Robison J, Wiles R, Ellis-Hill C, McPherson K, Hyndman D, Ashburn A. Resuming previously valued activities post-stroke: who or what helps? Disabil Rehabil. (2009) 31:1555–66. doi: 10.1080/09638280802639327
147. Howard G, Till JS, Toole JF, Matthews C, Truscott BL. Factors influencing return to work following cerebral infarction. JAMA. (1985) 253:226–32. doi: 10.1001/jama.253.2.226
148. Kripalani S, LeFevre F, Phillips CO, Williams MV, Basaviah P, Baker DW. Deficits in communication and information transfer between hospital-based and primary care physicians: implications for patient safety and continuity of care. JAMA. (2007) 297:831–41. doi: 10.1001/jama.297.8.831
149. Bashshur RL, Shannon GW, Smith BR, Alverson DC, Antoniotti N, Barsan WG, et al. The empirical foundations of telemedicine interventions for chronic disease management. Telemed J E Health. (2014) 20:769–800. doi: 10.1089/tmj.2014.9981
150. Joubert J, Joubert LB, de Bustos EM, Ware D, Jackson D, Harrison T, et al. Telestroke in stroke survivors. Cerebrovasc Dis. (2009) 27(Suppl 4):28–35. doi: 10.1159/000213056
151. Lehnerer S, Hotter B, Padberg I, Knispel P, Remstedt D, Liebenau A, et al. Social work support and unmet social needs in life after stroke: a cross-sectional exploratory study. BMC Neurol. (2019) 19:220. doi: 10.1186/s12883-019-1451-y
152. Friedland JF, McColl M. Social support intervention after stroke: results of a randomized trial. Arch Phys Med Rehabil. (1992) 73:573–81.
153. Walsh ME, Galvin R, Loughnane C, Macey C, Horgan NF. Community re-integration and long-term need in the first five years after stroke: results from a national survey. Disabil Rehabil. (2015) 37:1834–8. doi: 10.3109/09638288.2014.981302
154. Wolfenden B, Grace M. Returning to work after stroke: a review. Int J Rehabil Res. (2009) 32:93–7. doi: 10.1097/MRR.0b013e328325a358
155. Jellema S, van Hees S, Zajec J, van der Sande R. Nijhuis-van der Sanden MW, Steultjens EM. What environmental factors influence resumption of valued activities post stroke: a systematic review of qualitative and quantitative findings. Clin Rehabil. (2017) 31:936–47. doi: 10.1177/0269215516671013
156. Thrift AG, Dewey HM, Sturm JW, Paul SL, Gilligan AK, Srikanth VK, et al. Greater incidence of both fatal and nonfatal strokes in disadvantaged areas: the Northeast Melbourne Stroke Incidence Study. Stroke. (2006) 37:877–82. doi: 10.1161/01.STR.0000202588.95876.a7
157. Hammel J, Jones R, Gossett A, Morgan E. Examining barriers and supports to community living and participation after a stroke from a participatory action research approach. Top Stroke Rehabil. (2006) 13:43–58. doi: 10.1310/5X2G-V1Y1-TBK7-Q27E
158. Logan PA, Dyas J, Gladman JR. Using an interview study of transport use by people who have had a stroke to inform rehabilitation. Clin Rehabil. (2004) 18:703–8. doi: 10.1191/0269215504cr742oa
159. Culler KH, Wang YC, Byers K, Trierweiler R. Barriers and facilitators of return to work for individuals with strokes: perspectives of the stroke survivor, vocational specialist, and employer. Top Stroke Rehabil. (2011) 18:325–40. doi: 10.1310/tsr1804-325
Keywords: cerebrovascular disease, ischemic stroke, endovascular treatment, long-term outcome, post-acute care
Citation: Ganesh A, Ospel JM, Marko M, van Zwam WH, Roos YBWEM, Majoie CBLM and Goyal M (2021) From Three-Months to Five-Years: Sustaining Long-Term Benefits of Endovascular Therapy for Ischemic Stroke. Front. Neurol. 12:713738. doi: 10.3389/fneur.2021.713738
Received: 24 May 2021; Accepted: 28 June 2021;
Published: 26 July 2021.
Edited by:
Marios K. Georgakis, LMU Munich University Hospital, GermanyReviewed by:
Gian Marco De Marchis, University of Basel, SwitzerlandCopyright © 2021 Ganesh, Ospel, Marko, van Zwam, Roos, Majoie and Goyal. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mayank Goyal, bWdveWFsQHVjYWxnYXJ5LmNh
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.