- 1Neurology Unit O.S.A., Azienda Ospedale-Università di Padova, Padova, Italy
- 2Ludwig Boltzmann Institute for Experimental and Clinical Traumatology Donaueschingenstraße 13 A-1200 Vienna, Vienna, Austria
- 3Department of Neurology, Medical University Vienna, Vienna, Austria
- 4Neurology Unit, Ospedale S Chiara, Azienda Provinciale per i Servizi Sanitari (APSS), Trento, Italy
- 5Department of Neurology, University of Trieste, Trieste, Italy
The diagnostic criteria published by the PNS (Paraneoplastic Neurological Syndromes) Euronetwork in 2004 provided a useful classification of PNS, including paraneoplastic neuropathies. Subacute sensory neuronopathy (SSN) was the most frequently observed peripheral PNS, whereas other forms of neuropathy, as sensory polyneuropathy, sensorimotor polyneuropathy, demyelinating neuropathies, autonomic neuropathies, and focal nerve or plexus lesions, were less frequent. At the time of publication, the main focus was on onconeural antibodies, but knowledge regarding the mechanisms has since expanded. The antibodies associated with PNS are commonly classified as onconeural (intracellular) and neuronal surface antibodies (NSAbs). Since 2004, the number of antibodies and the associated tumors has increased. Knowledge has grown on the mechanisms underlying the neuropathies observed in lymphoma, paraproteinemia, and multiple myeloma. Moreover, other unrevealed mechanisms underpin sensorimotor neuropathies and late-stage neuropathies, where patients in advanced stages of cancer—often associated with weight loss—experience some mild sensorimotor neuropathy, without concomitant use of neurotoxic drugs. The spectrum of paraneoplastic neuropathies has increased to encompass motor neuropathies, small fiber neuropathies, and autonomic and nerve hyperexcitability syndromes. In addition, also focal neuropathies, as cranial nerves, plexopathies, and mononeuropathies, are considered in some cases to be of paraneoplastic origin. A key differential diagnosis for paraneoplastic neuropathy, during the course of cancer disease (the rare occurrence of a PNS), is chemotherapy-induced peripheral neuropathy (CIPN). Today, novel complications that also involve the peripheral nervous system are emerging from novel anti-cancer therapies, as targeted and immune checkpoint inhibitor (ICH) treatment. Therapeutic options are categorized into causal and symptomatic. Causal treatments anecdotally mention tumor removal. Immunomodulation is sometimes performed for immune-mediated conditions but is still far from constituting evidence. Symptomatic treatment must always be considered, consisting of both drug therapy (e.g., pain) and attempts to treat disability and neuropathic pain.
Introduction
The paper published in 2004 by Graus et al. (1) on behalf of the PNS (Paraneoplastic Neurological Syndromes) Euronetwork provided a useful classification of PNS, including neuropathies. At that time, subacute sensory neuronopathy (SSN) was the most frequently observed neuropathy (“classical” paraneoplastic neuropathy) associated with cancer, while other entities, as sensory polyneuropathy, sensorimotor polyneuropathy, and demyelinating neuropathies, were less frequent. The other classical peripheral syndrome defined in 2004 was chronic gastrointestinal pseudo-obstruction. Several subsequent works have reported novel antibody associations, further types of tumor associations, and different types of neuropathy, several of which were not contained in the former classification. A recent update of the classification (2) confirmed the range of clinical presentations of neurological syndromes typically associated with cancer (“high-risk neurologic phenotypes”), including SSN. In this review we aim to provide an update on mechanisms, antibodies, clinical presentation, and management of paraneoplastic neuropathies focusing on the pathological entities.
Mechanisms
The mechanisms underlying paraneoplastic neuropathies (PN) are manifold and not uniform for individual neuropathies or tumor types. Although paraneoplastic neuropathies have been known for a long time, the autoimmune hypothesis only appeared in 1965 when Wilkinson and Zeromski described antibodies against neurons in paraneoplastic sensory neuropathy for the first time (3). At the core of the autoimmune hypothesis is the production of antibodies against neural antigens determined by an immune response to cancer.
The main focus of the 2004 classification was on onconeural antibodies, targeting intracellular antigens shared by neuronal and tumoral tissues. The pathogenic role of onconeural antibodies remains unresolved. The most widely recognized hypothesis is that T-cell cytotoxicity accounts for neuronal cell loss in these conditions (4). Today, however, the mechanisms causing paraneoplastic neuropathies by far exceed the spectrum of onconeural antibodies (Table 1). Some peripheral conditions, e.g., peripheral nerve hyperexcitability syndromes (5), are mediated by neuronal surface antibodies (NSAbs). The term “surface antibodies” indicates antibodies targeting antigens present on the membrane of neurons, thus producing a potential direct effect, such as ion channel dysfunction.
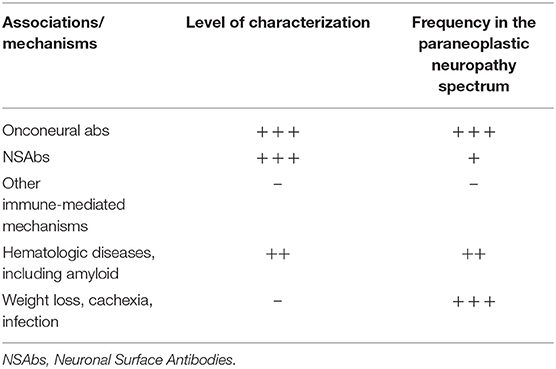
Table 1. Level of characterization and frequency of mechanisms underlying paraneoplastic neuropathies.
Immune-mediated mechanisms determine also the rare presentations of Guillain-Barré syndrome (GBS), chronic inflammatory demyelinating polyneuropathy (CIDP), and vasculitis. Reference is occasionally made to these large groups of diseases in a paraneoplastic context. The exact trigger and relationship between the immune response and cancer is not clear, but it is doubtful that the same mechanisms apply to all these entities.
Plasma cell dyscrasia and other hematological entities present with a spectrum of different neuropathies of both axonal and demyelinating forms, like anti-myelin-associated glycoprotein (MAG) neuropathy, POEMS (polyneuropathy, organomegaly, endocrinopathy, monoclonal-protein and skin changes) syndrome, and immunoglobulin light-chain (AL) amyloidosis. MAG and other antibodies targeting myelin-associated glycoprotein and glycolipids are deemed to be pathogenic (6). Hyperproduction of light chains could have a direct toxic effect but overall leads to the formation of amyloid deposits with extracellular accumulation of fibrils and consequent axonal damage. In addition, hyperviscosity effects have been claimed in Bing-Neel syndrome with peripheral involvement in Waldendström's macroglobulinemia (7).
A number of causes for paraneoplastic sensorimotor neuropathies, especially in advanced stages of cancer, remain obscure and resemble the development of neuropathies in some general diseases, as infections and critically ill conditions (See the paragraph on “Sensory neuromyopathy and terminal neuropathy”).
By enhancing antitumor immunity, the emerging immune therapies, particularly immune checkpoint inhibitors (ICI), have been associated with a spectrum of immune-mediated diseases, including neuropathies and rapidly progressive polyradiculoneuropathies (8, 9).
Antibody and Tumor Association
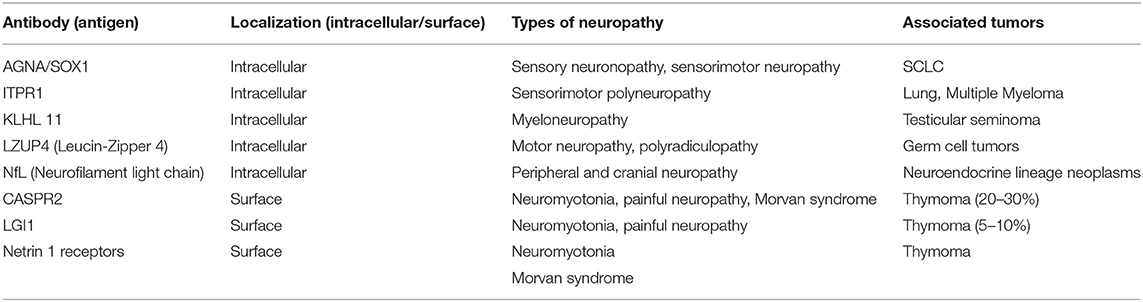
Several antibodies targeting neural antigens have been described in patients with PN, although this condition is well-known to occur also without antibodies (1, 10). The 2004 diagnostic criteria established that onconeural antibodies were consistently associated with PNS. Still they are now a fundamental marker supporting diagnosis of paraneoplastic neuropathy, commonly detected using commercial and in-house tests on cerebellar tissue with specific patterns and recombinant protein-based dot/line blotting (11). In 2004, onconeural antibodies were classified as “well-characterized” or “partially characterized” antibodies. This distinction was based on clinical relevance, number of published cases, a recognizable pattern on immunohistochemistry, availability of immunoblotting confirmation, and absence/low frequency in patients without cancer. The 2021 update of diagnostic criteria (2) classifies the antibodies associated with paraneoplastic diseases in three groups according to the frequency of association with cancer (high- >70%, intermediate-, and low- <30% risk antibodies), but in this review, we use the former nomenclature.
Well-characterized antibodies associated with paraneoplastic neuropathies included anti-Hu (ANNA-1), anti-CV2 (CRMP5), and anti-amphiphysin antibodies. Since 2004 the literature has confirmed that these three markers are consistently associated with the PN spectrum (12–19) (Table 2). SSN with anti-Hu antibodies is considered the most frequent PN (10). It is often the predominant manifestation of anti-Hu multifocal encephalomyelitis (paraneoplastic encephalomyelitis), which in 10% cases can also manifest with dysautonomic symptoms, as hypotension, dysrhythmia, or intestinal pseudo-obstruction (20). Two extensive case series have compared anti-Hu with anti-CV2 neuropathy (12, 13). Anti-CV2 neuropathy seems to be characterized more frequently by sensorimotor involvement and by asymmetric polyradicular involvement. SCLC has been confirmed as the most frequent cancer associated with both anti-Hu and anti-CV2 antibodies. Anti-amphiphysin antibodies are rare and associated with a broad spectrum of neurological manifestations, especially in women with breast cancer or SCLC. Peripheral involvement includes sensory neuronopathy, sensorimotor neuropathy, polyradiculopathy, and neuromyotonia (14, 15).
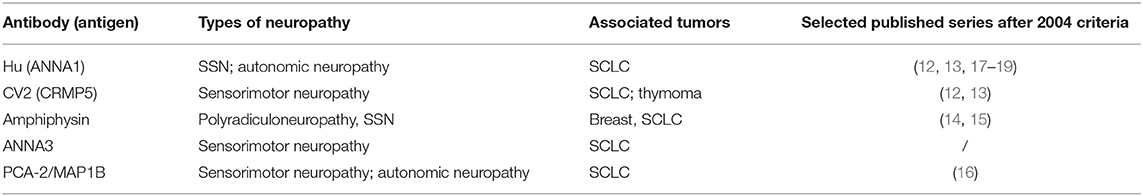
Table 2. Onconeural antibodies reported in 2004 classification and associated with paraneoplastic neuropathy.
Partially characterized antibodies in 2004 included ANNA-3, a very rare reactivity reported in some cases of sensory, sensorimotor and autonomic neuropathy (21), anti-Zic4—a marker of cerebellar degeneration described in some cases of neuropathy, usually with concomitant antibodies (anti-Hu and/or anti-CV2) (22)—and anti-PCA2, which in the earlier large case series (23) was described in both central and peripheral syndromes, including LEMS and neuropathy. The real target of PCA-2 was identified in 2017, consisting of a microtubule-associated protein (MAP1B). PN is the most frequent presentation and was reported in about a half of anti-MAP1B patients with SCLC (16).
In the last 15 years, several novel onconeural antibodies related to PN have been reported. Most have been described in individual works, have not yet been confirmed by other research groups, and have a limited number of patients. The strength of these associations thus needs further characterization and confirmation before being considered relevant in the diagnostic approach to PN (Table 3).
In 2016, antibodies against 1,4,5-trisphosphate receptor type 1 (ITPR1) were characterized in patients with autoimmune cerebellar ataxia; this antibody was also found in three patients with sensorimotor polyneuropathy, associated in two cases with malignancy (adenocarcinoma of the lung and multiple myeloma) (24).
Antibodies targeting neurofilament light chain (NfL) have been reported as markers of ataxia and encephalopathy accompanying various cancers, especially neuroendocrine lineage neoplasms (25). Six out of 21 of the reported patients manifested with peripheral or cranial neuropathy.
Anti-KLHL11 is a recently described antibody identified in patients with brainstem encephalitis or cerebellar symptoms related to testicular and ovarian cancer (26, 27). A recent retrospective study of 32 patients manifesting a distinctive pattern of subacute paraneoplastic myeloneuropathy, associated with several neoplasms, identified the presence of anti-amphiphysin (8), anti-Hu (5), anti-CV2 (6), anti-Yo (1), anti-PCA2 (2), and one case of anti-KLHL11, or combinations of these (28).
Very recently, a further antibody reaction against Leucine Zipper 4 (LZUP4) was described in 28 patients with germ cell tumors (e.g., seminoma); four patients were affected with motor neuronopathy and polyradiculopathy (29).
In this context, the characterization of antibodies against Sry-like high mobility group box 1 (SOX1) deserves mention. In 2005, anti-glial nuclear antibody (AGNA) was identified as a marker of PNS-related lung cancer. The most frequent clinical association was LEMS, but SSN and sensorimotor neuropathy were also reported (30, 31). SOX1 was identified as the antigen in 2008 (32). As anti-SOX1 antibody is not a rare finding in patients with SCLC without paraneoplastic accompaniments (33), it can more properly be considered a serological marker of SCLC. Recently, the presence of SOX-1 was also advocated in non-paraneoplastic neuropathies (34, 35), but this finding was not confirmed in a later work (36).
In recent years several antibodies directed against NsAbs have been described, greatly expanding the interest in autoimmune neurological diseases. Antibodies against voltage-gated potassium channels (VGKC) were first discovered in the 1990s in patients with motor nerve hyperexcitability (neuromyotonia) and hyperhidrosis (37, 38). Anti-VGKC antibodies were later described in limbic encephalitis, with most cases being of non-paraneoplastic origin (39, 40). In 2010, it was established that anti-VGKC antibodies actually target two different associated proteins associated with ion channels, namely, LGI1 and CASPR2 (41, 42). These antibodies determine a continuous disease spectrum, often dominated by central nervous system involvement (limbic encephalitis) but frequently with relevant peripheral manifestations, including neuromyotonia, dysautonomia, and pain (43). Peripheral involvement, especially when isolated, is more frequent in CASPR2 than in LGI1 patients; this is probably due to the higher expression of CASPR2 in the peripheral nervous system, in the juxta-paranodal region (44). Morvan syndrome is another clinical picture associated with CASPR2 antibodies, in which peripheral hyperexcitability and dysautonomia coexist with psychiatric disturbances and sleep dysfunction. Recently, painful manifestations of LGI1 and CASPR2 autoimmunity have been highlighted. Sometimes pain is the cardinal symptom, especially in CASPR2 patients (45–47). As regards the associated tumors, anti-LGI1 diseases are rarely paraneoplastic, whereas a tumor, mostly a thymoma, can be detected in 20–30% of patients with CASPR2 autoimmunity. In patients with double positivity (anti-LGI1/anti-CASPR2), the likelihood of cancer is higher (up to 44%) (47).
Finally, a further surface reaction against Netrin-1 receptor has recently been described in patients with thymoma affected by Morvan syndrome or neuromyotonia and myasthenia gravis; the clinical relevance of this reactivity, which can be found together with CASPR2, needs further specification (48, 49).
Tumor association reflects antibody association (see Tables 2, 3). The most frequent malignancy associated with PN is SCLC. However, other lung cancers, like adenocarcinoma, are not rare. Other associated malignancies are breast and ovarian cancer, and thymoma. Prostate cancer is an infrequent finding in the neurological paraneoplastic context, considering the frequency of the neoplasm, although cases of SSN have been reported with Hu antibodies (50). When the diagnosis of PNS is performed in a patient with an unknown or occult malignancy, oncological screening must be performed as recommended (51).
Types of Neuropathies
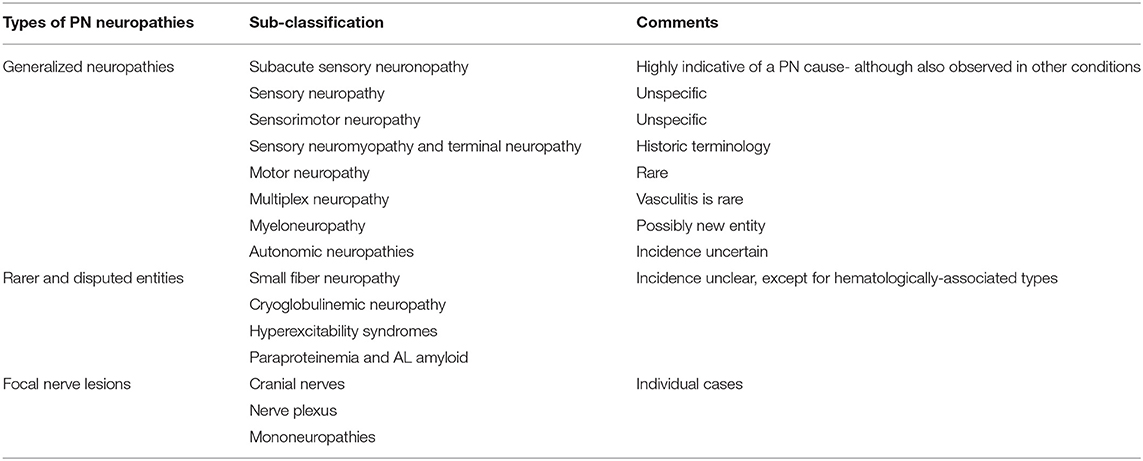
Over the decades, numerous individual observations have been published. Gradually, a core of generalized neuropathies has emerged, in particular, SSN. Several rarer types need to be considered, as do focal peripheral nerve lesions. The spectrum is probably still incomplete but reflects the present level of knowledge and experience (Table 4).
Generalized Neuropathies
Subacute Sensory Neuronopathy
SSN is the prototype of PN. It is characterized by asymmetry, acute/subacute onset in the upper extremities, and pain. The main clinical feature is sensory ataxia. Even though the motor system is not impaired in terms of strength, sensory loss results in ataxia and severe disability. The disease quickly progresses to a plateau phase, and has little or no tendency to improve. Electrophysiologic study reveals absent sensory responses; motor responses can be minimally altered (52). The neuropathology consists of inflammation in the dorsal root ganglia (DRG) and is often associated with posterior column degeneration. The therapeutic approaches are manifold (53) and usually immune modulation is recommended, although no evidence-based recommendations exist. SSN is a disabling condition and persons affected remain completely dependent. SSN is not entirely specific and has been observed as an idiopathic occurrence or in other autoimmune conditions as Sjogren's syndrome. The DRG can be affected by toxicity, as through platinum compounds and pyridoxine overdosage, but the extent of the clinical symptoms is not the same as in Hu-associated paraneoplastic SSN. SSN has also been observed in association with other PNS as cerebellar degeneration, PEM, brainstem involvement, limbic encephalitis, Lambert–Eaton myasthenic syndrome, and motor neuropathy. Patients may have evidence of autonomic involvement. A rare combination with myositis has been described (54). For the clinician, the appearance of an SSN constitutes a strong recommendation to search for cancer, particularly SCLC.
Sensory Neuropathy
A pure sensory neuropathy is less specific for a paraneoplastic disease, and opinions regarding paraneoplastic etiology are controversial. Patients present with sensory symptoms in the typical glove-stocking distribution, often with neuropathic pain and a variable degree of incoordination (55). Onset is usually more insidious, following the length-dependent distribution. The most likely causes of a pure sensory neuropathy are autoimmune diseases (e.g., Sjögren) and toxic and metabolic neuropathies. The distinction between SSN and sensory neuropathy can be difficult; even in the 2004 classification it was not always certain how precise the distinction could be. Subacute or acute onset may resemble SSN. An attempt to differentiate ataxic from more painful sensory neuropathies was made by Oki et al. (18) and may help in differentiation.
Sensorimotor Neuropathy
Sensorimotor neuropathy (SMN) is generally the most frequent yet enigmatic neuropathy in terms of specific characteristics and etiology. The term implies a combination of sensory and motor symptoms of varying degrees. No specific or characteristic clinical items have been defined. Patients with SMN usually do not have severe neurological impairment. SMN has been observed as a paraneoplastic condition, also in association with onconeural antibodies (55, 56). The differential diagnosis is wide and ranges from other causes as alcohol, diabetes, and chronic idiopathic axonal polyneuropathy, particularly in individuals aged over 55 years (57).
Yet individual cases with SMN presenting as the first sign of cancer have been described. Practically speaking, SMN is uncharacteristic and the suggestion is to first exclude other possibilities before embarking on an extensive tumor search. At a later stage of the cancer, chemotherapy-induced peripheral neuropathy (CIPN) is the most likely differential diagnosis. In plasma cell dyscrasia and associated hematological diseases, a spectrum of neuropathies has been described, including SMN (see below).
Sensory Neuromyopathy and Terminal Neuropathy
Paraneoplastic sensory neuromyopathy is usually a late effect of cancer on the peripheral nervous system. It presents as a symmetric sensorimotor neuropathy, is usually mild, and can be associated with type 2 fiber muscle atrophy. It is not specific for individual cancers, is often associated with weight loss, and can be a general sign of advanced cancer. The sensory loss affects all qualities. Muscle weakness occurs in proximal and distal muscles (with so-called “intermediate sparing”). It is slowly progressing. Concomitant factors as chemotherapy or diabetes need to be ruled out. There is no specific antibody association at present. The nomenclature is historic and was not contained in the 2004 classification. However, in clinical practice, this type of neuropathy can be observed in advanced cancer patients.
The term “terminal neuropathy” refers to mild sensorimotor neuropathies observed in progressive general diseases, in advanced stages, including cancer, with or without concomitant use of neurotoxic drugs. This type of neuropathy can be likened to a common occurrence in patients with severe infections, i.e., weight loss, and its origin is probably different. For example, sarcopenia and cachectic neuropathy have been described in association with diabetic neuropathy (58, 59).
Motor Neuropathy
Pure motor neuropathy is rare. The term “lower motor neuron disease” has been used to identify patients with subacute development of generalized flaccid paresis with sparing of long tracts and bulbar muscles (60). This entity has been described infrequently and is not well-characterized. Only a few cases were collected in the PNS Euronetwork database (61), corresponding to <1% of identified patients. The term “lower motor neuropathy” has been also used in conjunction with plasma cell dyscrasia and myeloma (62, 63), in hematological malignancies, and as a sequelae of local RT.
Multiplex Neuropathy
Despite being described in individual cases, paraneoplastic mononeuropathy multiplex is a rarePNS (64, 65). Mononeuropathy multiplex is generally associated with vasculitis. Chronic dysimmune neuropathies, as multifocal motor neuropathy (MMN), have been reported as PN (66), while rare cases of asymmetric neuropathies have been observed in AL amyloidosis (67) and in association with Waldenstrom's disease (68). Recently observed complications of ICI therapies also include reports of vasculitis (69). Notably, also other tissues can be involved by vasculitis as a clinical sign, e.g., skin vasculitis (70) and digital ischemia (71).
Myeloneuropathy
The simultaneous involvement of the spinal cord and peripheral nerves is termed myeloneuropathy. The usual underlying etiologies include B12 or copper deficiency, inflammatory/infectious diseases, and toxic diseases. This is a fairly new term in conjunction with PNS, which is not contained in classical descriptions. A recent paper (28) has described a number of cases that could be the basis for further considering its inclusion in the PNS classification. The series consists of 34 patients with various antibodies and cancers, especially SCLC and breast adenocarcinoma. Clinically they had a subacute, asymmetric presentation with sensory (e.g., paresthesias and impaired proprioception) and motor signs, as asymmetric weakness, and long tract signs. Pain and bladder dysfunction were frequent clinical findings. The concomitant presence of hyporeflexia and hyperreflexia at different sites was found in 81% of patients. MRI imaging showed spinal hyperintensity, which in about a half of patients was longitudinally extended. One third of patients showed lumbar nerve-root enhancement. Previous descriptions of similar syndromes are available (72, 73).
Autonomic Neuropathy
Autonomic features can be associated with several types of neuropathy. Symptoms can result in orthostatic hypotension, urinary symptoms, sweating abnormalities, intestinal pseudo-obstruction, gastrointestinal dysmotility, and subacute dysautonomia. Dysautonomia is typical of AL amyloid neuropathy. It has likewise been observed in several paraneoplastic forms of PN (74) and small fiber neuropathy (75). Another important aspect is the association of autonomic symptoms with other PNS, in particular with LEMS, but also in conjunction with paraneoplastic hyperexcitability syndromes as Morvan syndrome. In addition to areflexia, stocking-like sensory loss, and areflexia, autonomic symptoms also appear. Autonomic ganglionopathies have been observed in conjunction with ICI therapy (76). Notably, autonomic disturbances are also well-described in other common causes of neuropathy, as diabetes or neuropathies associated with systemic autoimmune diseases.
Rarer and Disputed Entities
Small Fiber Neuropathy
Small fiber sensory neuropathy (SFN) has not been part of the classical paraneoplastic spectrum. A recent study suggests that paraneoplastic causes can amount to 3% of SFN in association with onconeural antibodies that were not further specified (77). SFN has been documented in cases of Morvan syndrome (78). Although examples of SF involvement have been described in PN in solid cancers (79) and hematological diseases (80), it remains unclear whether the SF involvement is exclusive, or part of a general neuropathy syndrome.
Immune-Mediated Neuropathies
From the large, growing number of immune-mediated neuropathies, GBS, CIDP, and also rarely MMN have been mentioned to appear as a paraneoplastic phenomenon. In the PNS Euronetwork database, the incidence of this conjunction was low. The occurrence of both GBS and CIDP seems higher in hematological conditions (81, 82) and has also been described to be associated with other cancer types (74, 83) and with axonal loss. The occurrence of MMN as a PNS has been reported but seems to be extremely rare (84, 85).
Practically speaking, the appearance of either GBS or CIDP does not necessarily point to an underlying malignancy. Conversely, GBS may occur during the course of the disease in cancer patients with solid tumors (86) but can be the presenting phenotype in lymphoma or leukemia, and may be called “paraneoplastic” in these circumstances.
As a new development and based on immune mechanisms, several immune-mediated neuropathies, also resembling GBS, have been described to occur in conjunction with immune therapies, in particular with ICIs (9, 87).
Cryoglobulinemia
Cryoglobulinemia can occur as part of lymphoproliferative disease as in MGUS, macroglobulinemia, multiple myeloma (MM), leukemia, CLL, and immunoblastic lymphadenopathy. In addition to acrocyanosis, digital necrosis and purpura may occur. Likewise, signs of hyperviscosity, as thrombosis, can be associated. Cryoglobulins are monoclonal IgG, often IgM, and rarely light chain. Cryoglobulinemic neuropathy has been described as paraneoplastic, with IgM precipitation (88) and other conditions. Except for lymphoproliferative diseases, where the coexistence has been noted, it does not seem to be a frequent association.
Hyperexcitability Syndromes
Abnormal muscular activity with cramps, twitching and stiffness characterizes neuromyotonia and hyperexcitability syndromes. Electromyographic findings are typical (89). Acquired forms are considered immune mediated and can be found with underlying neoplasms, including in seronegative cases. Neuromyotonia can also present with symptoms and signs of sensorimotor and motor neuropathy, with no clear mechanisms (90). Neuropathic pain is a frequent finding in Morvan syndrome (see above) (91).
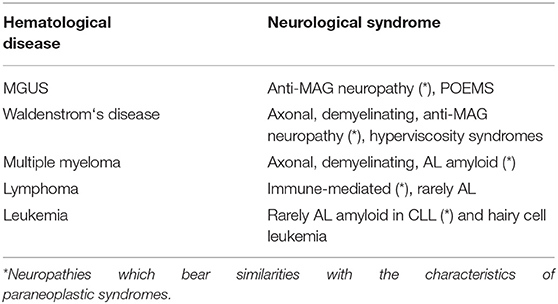
Plasma Cell Dyscrasia and Paraproteinemia
Plasma cell dyscrasias and paraproteinemias are usually not classified among paraneoplastic neuropathies. This is probably due to the inhomogeneous presentations within the different groups with paraproteinemia. Despite this, several types of neuropathies occur in association with the hematological disease by indirect involvement (not directly neoplastic), resembling the pattern of paraneoplastic disease. At least three conditions, such as POEMS syndrome, anti-MAG neuropathy, and AL amyloidosis, have characteristics of paraneoplastic disorders. The mechanisms are diverse and range from immunoglobulin (IgG) deposition to axonal and demyelinating neuropathies, hyperviscosity issues, and AL amyloid deposition.
Neuropathies related to MGUS are usually sensory-motor, axonal, or demyelinating. Commonly, IgG or IgA cause axonal lesions, whereas IgM tends to be associated with demyelinating features.
Waldenstrom's disease can develop from MGUS and is considered as a low-grade lymphoma (lymphocytoplasmatic lymphoma). It can be associated with demyelinating neuropathies, sometimes with MAG antibodies. Hyperviscosity mechanisms can also result in a multifocal neuropathy (92). Cryoglobulinemia can be associated; AL amyloidosis is rare.
Multiple myeloma presents with a variety of PN, including axonal and demyelinating types with no typical clinical features. AL amyloidosis is responsible for 30–40% of neuropathies in myeloma, in particular with λ light chain.
Three phenotypes further illustrate the relationship between PNS and plasma cell dyscrasia. Anti-MAG neuropathy results in a painful sensory neuropathy, predominantly in the legs with impaired balance. Progression is slow and leads to sensory ataxia. Tremor action is frequently reported. Numerous investigations have discussed the effects of anti-MAG and the role of sulfoglucuronyl-glycosphingolipid (SGPG) antibodies (6). Therapy suggestions are controversial, among them anti-CD20 drugs, like rituximab, and attempt to neutralize the MAG antibodies (93).
POEMS syndrome is considered by many to be a PNS. The disease is often rapidly devastating, resulting in neuropathy with tetraparesis and multi-organ involvement. The neuropathy is M-protein related (Ig A or G) and can cause axonal, demyelinating, or mixed neuropathy (61). Often, an isolated osteosclerotic lesion can be detected. The distinction between CIDP and POEMS can be difficult and neurophysiological criteria have been suggested (94). A CIDP-like presentation with pain could be a useful indicator for POEMS. Elevated levels of vascular endothelial growth factor (VEGF) are found in about two-thirds of patients (95).
Light-chain (AL) amyloidosis is an important differential diagnosis in paraproteinemia, Waldenstrom's disease, multiple myeloma, and several other entities. Clinically, a combination of fatigue, renal impairment, sensory and autonomic neuropathy, and often also carpal tunnel syndrome (CTS) is suggestive. In addition to the characteristic sensory, often painful neuropathy, frequently including autonomic features, focal lesions due to deposits of amyloid are also described. Macroglossia, periorbital purpura, congestive heart failure, and orthostatic hypotension are other typical (but not specific) clinical findings (96) (Table 5).
Focal Nerve Lesions
Focal paraneoplastic neurological syndromes are rare and usually not contained in the classifications. The list of entities provided here is incomplete and based on observations and case reports, lacking consistency and systematic approach. Focal presentations include cranial nerve lesions, plexopathies, mononeuropathies (e.g., CTS) (97), and also muscle involvement.
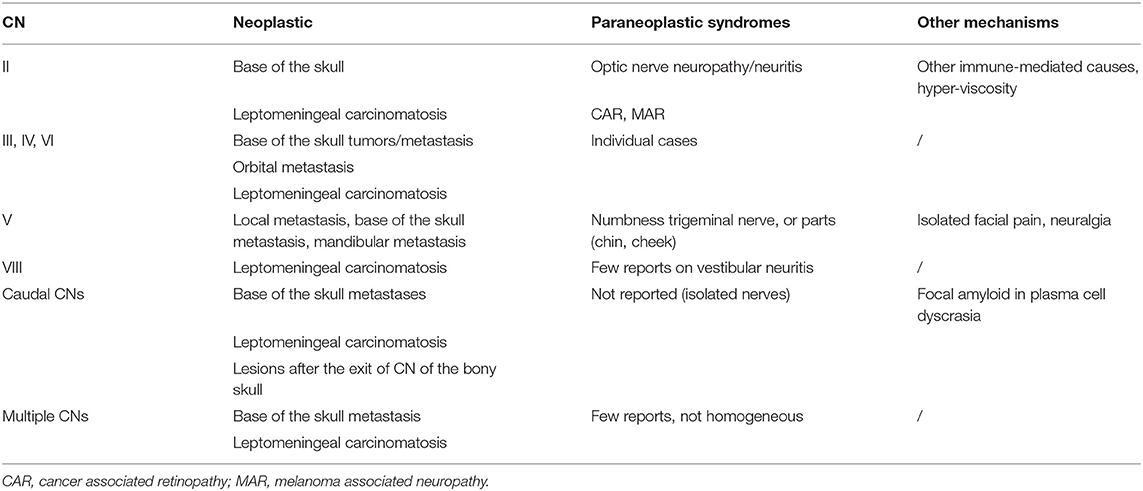
Cranial Neuropathies
There are numerous reports on paraneoplastic cranial nerve (CN) lesions (Table 6). Three CNs appear to be preferably affected: the optic, trigeminal, and vestibular nerves. In addition to lesions of the optic nerve (98, 99), also in combination with spinal cord lesions (100), there are several visual conditions, as cancer-associated retinopathy (101), melanoma-associated retinopathy (102), acquired night blindness, bilateral diffuse uveal melanotic proliferation, bilateral diffuse uveal melanotic proliferation, cone dystrophy and achromatopsia, and photophobia (103).
Oculomotor nerve lesions are rarely described as a PN. Local neoplastic causes and orbital myositis need to be excluded (104). However, extraocular muscle involvement has been described in AL amyloidosis, and local AL deposits in the lid may cause ptosis.
The trigeminal nerve can be affected in autoimmune and rheumatoid arthritis. Similarly, sensory trigeminal neuropathy, or the “numb chin” (105) and “numb cheek” (106) syndrome subtypes, have been reported to be caused by paraneoplastic mechanisms (107, 108). The presence of orofacial pain (109–111) and neuralgia have been suggested to be of paraneoplastic origin, among other causes. Vestibular damage and neuritis have been described as paraneoplastic phenomena in a few selected cases (112–114). There is a paucity of reports on possible damage to the caudal cranial nerves. Local amyloid depositions (115) in the soft palate and tongue (116) need to be considered under plasma cell dyscrasia. Several cases of possible multiple CN lesions, caused by a potential paraneoplastic mechanism, have been mentioned (117, 118). A similar distribution of affected CNs has been reported as a complication of treatment with immune checkpoint inhibitors (119) (see below).
Plexopathies
PN causing plexopathies are rare but have been reported in individual cases. Most reports date back some years, and new investigations, in particular with the aid of imaging techniques, could potentially detect a symptomatic cause. The cervical plexus is mentioned in regard to the phrenic nerve (120–122). Although there are reports that inflammatory paraneoplastic causes can affect the brachial and lumbosacral plexus, the evidence is based on individual case reports (123).
Mononeuropathies
In plasma cell dyscrasia, CTS could be a sign of amyloidosis. The issue of other isolated paraneoplastic mononeuropathies is uncertain. One report suggests a paraneoplastic ulnar nerve lesion (124); there is also mention of a peroneal nerve lesion (125). Multiple enlarged nerves (126) have been described in ultrasound as a PNS, but may lack a clinical correlate. Deposition of light chains causing individual nerve lesions has been described (97). In summary, the appearance of a mononeuropathy of paraneoplastic origin is not likely, except in the case of CTS associated with AL amyloid.
Cancer Therapy in Differential Diagnosis
CIPN is the commonest form of neurotoxicity in cancer patients, causing a chronic neuropathy in about 30% and milder/temporary symptoms in up to 70% of those undergoing traditional chemotherapy (127). In addition to the burden on patients' quality of life, CIPN can lead to modifications or discontinuation of oncologic therapy, thus impacting on their overall prognosis. Even after cessation of chemotherapy, symptoms impacting the quality of life persist in half of patients (128). Alteration of cancer treatment due to CIPN involves 10–65% of patients; in up to one third of patients, chemotherapy needs to be discontinued (129).
Despite significant variability in susceptibility [depending on concurrent diabetes, alcohol consumption, and other pre-existing neuropathies, along with genetic factors and older age at onset (130)], the development of CIPN is generally dose-dependent and thus influenced by different drug schedules and combinations, as well as route of administration. Traditionally, CIPN involves breast and colon cancer patients undergoing toxic doses of intravenous (IV) cisplatin and oxaliplatin. The cumulative toxicity mainly affects the DRG and their axons, causing a sensory neuronopathy or dying-back neuropathy; small fiber neuropathies can also ensue, while motor, autonomic, and cranial involvement is less common.
Most patients develop CIPN in the first three or four cycles of treatment, with gradual progression of symptoms and subsequent stabilization at or soon after treatment completion. Patients typically display length-dependent symmetric sensory loss (sometimes even leading to ataxia) with prominent acral sensory symptoms, pain episodes, and reduced dexterity. A notable exception is platinum-associated neuropathy, as oxaliplatin may also cause distinctive acute, transient, cold-induced dysesthesias after the first infusions, while both oxaliplatin and cisplatin neurotoxicity usually worsens in the months following the end of chemotherapy (i.e., the coasting phenomenon). CIPN then slowly improves with time, sometimes leaving a chronic pain syndrome with dysesthesia, hyperalgesia, tactile and thermal allodynia, and spontaneous pain. Acute pain syndrome related to nerve pathology has also been reported with paclitaxel (131). New anti-microtubule drugs can induce axonal, mainly sensory polyneuropathy, as can older taxanes [e.g., eribulin and ixabepilone (132)].
Recently, advancements in more selective targeted therapies and newer agents have improved chemotherapy tolerability and overall cancer survival but have not translated into a reduction of CIPN cases. Besides having to differentiate CIPN from other conditions that do not dictate premature treatment discontinuation, the advent of biological agents, known to have more idiosyncratic and off-target side-effects, has further added to the neurologist's diagnostic conundrum. Indeed, CIPN pathogenesis largely remains to be defined and is still one of the most common dose-limiting complications of antitumor treatment, considering the growing number of cancer survivors who develop late drug-resistant chronic pain syndromes that may heavily impact on their quality of life.
Targeted Therapies
Tumor-specific antibodies cause similar, unexpected nerve injury ranging from mild, predominantly sensory neuropathies (i.e., polatuzumab vedotin and enfortumab vedotin) to potentially severe motor neuropathy, as seen in lymphoma patients undergoing brentuzumab (133, 134); these generally improve slowly upon discontinuation. Neuropathies have also been reported in high numbers during adotrastuzumab emtansine treatment (135). Regarding the latest drugs, such as neurotrophic-tyrosine-receptor-kinase gene and anaplastic-lymphoma-kinase inhibitors, neuropathy has been described with both larotrectinib [including grade III and IV reactions within 3 months of treatment (136)] and lorlatinib (137), respectively.
Immune Checkpoint Inhibitors
Immune checkpoint inhibitors (ICIs) are monoclonal antibodies directed against specific molecules on activated immune cells whose role is to maintain self-tolerance and prevent autoimmunity, thus enhancing anti-tumor immunity. Targets include programmed death-1 receptor (nivolumab, pembrolizumab, cemiplimab), its ligand (atezolizumab, avelumab, durvalumab), and cytotoxic T lymphocyte antigen-4 (ipilimumab). Alongside, ICIs may cause off-target toxicities called immune-related adverse events (irAEs) (138, 139). Neurological irAEs represent a minor proportion, with an estimated incidence of 1–3%, more common after combination checkpoint blockade (140, 141). Nevertheless, they are clinically relevant complications with high-grade toxicity, carrying significant morbidity and mortality (142), and thus requiring prompt identification. Immune-related peripheral nerve disorders induced by ICI are highly heterogeneous (8, 9). Cranial neuropathies and polyradiculoneuropathies have emerged as the most common phenotypes (9). Cranial neuropathies may be isolated or associated with other neurological manifestations, frequently involving facial and abducens nerves, although any CN may be affected (143) Acute and chronic polyradiculoneuropathies mostly include GBS or CIDP and usually develop within the first three cycles of therapy (9, 144). Unlike classical GBS, cerebrospinal fluid analysis often reveals lymphocytic pleocytosis besides hyperproteinorrachia (142) and benefits from steroid administration. Other reported ICI-related neuropathies are mononeuritis multiplex/vasculitic neuropathy (8, 9), small fiber/autonomic neuropathy (145), sensory neuronopathies, and neuralgic amyotrophy (146, 147).
Despite these findings, ICIs are generally considered safer than conventional chemotherapy for the peripheral nerve, and ICI-induced neuropathies are more likely to have an acute/subacute and non-length-dependent presentation (9, 146).
According to current guidelines (148), management requires ICI discontinuation and high-dose IV steroid administration, followed by slow steroid tapering to avoid relapses related to the long-lasting half-life of ICIs (146). Up to 50% of cases (149) could be resistant to steroid therapy and, due to their severity, require IV immunoglobin (IG), plasma exchange, or immunosuppressants.
CAR T Cell Therapy
Chimeric antigen receptor T-cell therapies have become standard treatments of relapsed or refractory hematological malignancies. Acute neurotoxicity is well-documented and moderate-to-severe neurological events are estimated to occur in 20–30% of patients, with a median time of onset of 5–6 days after infusion (150–152). Neurological manifestations are associated with cytokine release syndrome, referred to as “immune effector cell-associated neurotoxicity syndrome,” which identifies a distinctive encephalopathy manifesting with stereotypic evolution of a specific set of symptoms (152). Very little is known about potential long-term adverse events; however, a case of peripheral neuropathy has been described as a late complication (153).
Therapy
The general therapeutic strategy for PNS is based on the assumption that the detection of cancer and its removal can improve the neurological syndrome. This is seldom obtained in PN associated with onconeural antibodies. Numerous attempts have been published for the treatment of SSN. A systematic review in 2012 concluded that only class IV evidence was available for the effect of IVIG, plasma-exchange, steroids, or immune-suppressive chemotherapy (53). Most individual recommendations suggest immune-modulating therapies can stop the progression of the PN. Given the severe debilitating outcomes in patients with SSN, these interventions are justified but should be performed very early (e.g., 2 months) after neurological symptoms onset (154). On the other hand, diseases associated with NSAbs are usually responsive to immunotherapy. This also seems to be confirmed for peripheral involvement in syndromes with anti-LGI1 anti-CASPR2 antibodies (47). Steroids are often the first option, followed by or associated with IVIG and plasma exchange. Therapies with anti-CD20 treatment (e.g., rituximab), or immunosuppressors like cyclophosphamide, have less supporting evidence than do other NSAbs associated diseases (e.g., NMDAR encephalitis) (155). Nevertheless, these options can be considered in non-responsive cases and their efficacy has been reported (44, 78, 156).
The smaller group of acute immune-mediated neuropathies, as GBS, CIDP, and vasculitis, usually respond to conventional immune therapies, approved for the non-neoplastic entity of the given neuropathy. Paraproteinemic neuropathies and AL amyloidosis are also treatable to some extent, according to current hematological guidelines.
The appearance of PN has implications not only for cancer and cancer therapy but also significantly impacts patient performance, quality of life, and disability. Symptomatic therapy is therefore a cardinal feature of treatment. Pain control is a key goal. This can be achieved by antidepressants (tricyclic antidepressants and serotonin-noradrenaline reuptake inhibitors) and by GABA-mimetic drugs (first-line therapy). Second- and third-line drugs for neuropathic pain include topical lidocaine and opioids (157). Neuromyotonia can respond to antiepileptics as carbamazepine, phenytoin, lamotrigine, and sodium valproate (158).
Cancer rehabilitation is an important initiative to promote specific therapies for patients affected by neurological conditions in cancer. Therapy effects and, due to increased long-term survival, persisting effects need to be specifically treated. The rehabilitation of neuropathies is generally also heterogeneous, depending on the focus of deficit and disability. The reference level could be at best adapted and copied from the rehabilitation of cancer patients with CIPN, which bears similarities with the most common phenotype.
Conclusion
In summary, since the 2004 classification, several peripheral manifestations have been described, mainly with new antibodies, in patients affected by cancer. Whereas novel intracellular antibodies need more robust evidence to become relevant in clinical practice, the true novelty has been the discovery of NSAbs in diseases with prominent, or coexisting, peripheral involvement, which can be paraneoplastic.
Circulating autoantibodies are commonly considered a valuable tool for cancer diagnosis and are frequently requested in clinical practice for patients with unclear neurological peripheral symptoms. However, proper adherence to the use of biomarkers is critical in translating recommendations into clinical practice. At present, circulating classical onconeural antibodies and only a few NSAbs are considered a valuable tool for cancer diagnosis for patients with paraneoplastic neuropathy.
Finally, new cancer therapies seem to evoke immune-mediated neurological syndromes, which may mimic PN, but appear at different times during treatment. In the coming years, the study of the mechanisms and effects of these therapies will provide new insights into the relationship between cancer, immunity and the nervous system.
Author Contributions
WG, MZ, AG, and BG conceived and drafted the review. VP and FB contributed to draft the review. WG and BG revised the manuscript for important intellectual content. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
The authors thank Joanne Fleming for English editing.
References
1. Graus F, Delattre JY, Antoine JC, Dalmau J, Giometto B, Grisold W, et al. Recommended diagnostic criteria for paraneoplastic neurological syndromes. J Neurol Neurosurg Psychiatry. (2004) 75:1135–40. doi: 10.1136/jnnp.2003.034447
2. Graus F, Vogrig A, Muñiz-Castrillo S, Antoine J-CG, Desestret V, Dubey D, et al. Updated diagnostic criteria for paraneoplastic neurologic syndromes. Neurol Neuroimmunol Neuroinflamm. (2021) 8:e1014. doi: 10.1212/NXI.0000000000001014
3. Wilkinson PC, Zeromski J. Immunofluorescent detection of antibodies against neurones in sensory carcinomatous neuropathy. Brain. (1965) 88:529–38. doi: 10.1093/brain/88.3.529
4. Zuliani L, Graus F, Giometto B, Bien C, Vincent A. Central nervous system neuronal surface antibody associated syndromes: review and guidelines for recognition. J Neurol Neurosurg Psychiatry. (2012) 83:638–45. doi: 10.1136/jnnp-2011-301237
5. Sawlani K, Katirji B. Peripheral nerve hyperexcitability syndromes. Contin Lifelong Learn Neurol. (2017) 23:1437–50. doi: 10.1212/CON.0000000000000520
6. Dalakas MC. Advances in the diagnosis, immunopathogenesis and therapies of IgM-anti-MAG antibody-mediated neuropathies. Ther Adv Neurol Disord. (2018) 11:175628561774664. doi: 10.1177/1756285617746640
7. Simon L, Fitsiori A, Lemal R, Dupuis J, Carpentier B, Boudin L, et al. Bing-Neel syndrome, a rare complication of Waldenstrom macroglobulinemia: analysis of 44 cases and review of the literature. A study on behalf of the French Innovative Leukemia Organization (FILO). Haematologica. (2015) 100:1587–94. doi: 10.3324/haematol.2015.133744
8. Johansen A, Christensen SJ, Scheie D, Højgaard JLS, Kondziella D. Neuromuscular adverse events associated with anti-PD-1 monoclonal antibodies. Neurology. (2019) 92:663–74. doi: 10.1212/WNL.0000000000007235
9. Dubey D, David WS, Amato AA, Reynolds KL, Clement NF, Chute DF, et al. Varied phenotypes and management of immune checkpoint inhibitor-associated neuropathies. Neurology. (2019) 93:e1093–103. doi: 10.1212/WNL.0000000000008091
10. Antoine JC, Camdessanché JP. Paraneoplastic neuropathies. Curr Opin Neurol. (2017) 30:513–20. doi: 10.1097/WCO.0000000000000475
11. Zoccarato M, Gastaldi M, Zuliani L, Biagioli T, Brogi M, Bernardi G, et al. Diagnostics of paraneoplastic neurological syndromes. Neurol Sci. (2017) 38:237–42. doi: 10.1007/s10072-017-3031-5
12. Camdessanché JP, Jousserand G, Ferraud K, Vial C, Petiot P, Honnorat J, et al. The pattern and diagnostic criteria of sensory neuronopathy: a case-control study. Brain. (2009) 132:1723–33. doi: 10.1093/brain/awp136
13. Dubey D, Lennon VA, Gadoth A, Pittock SJ, Flanagan EP, Schmeling JE, et al. Autoimmune CRMP5 neuropathy phenotype and outcome defined from 105 cases. Neurology. (2018) 90:e103–10. doi: 10.1212/WNL.0000000000004803
14. Pittock SJ, Lucchinetti CF, Parisi JE, Benarroch EE, Mokri B, Stephan CL, et al. Amphiphysin autoimmunity: paraneoplastic accompaniments. Ann Neurol. (2005) 58:96–107. doi: 10.1002/ana.20529
15. Dubey D, Jitprapaikulsan J, Bi H, Campo RV. Do, McKeon A, Pittock SJ, et al. Amphiphysin-IgG autoimmune neuropathy: a recognizable clinicopathologic syndrome. Neurology. (2019) 93:E1873–80. doi: 10.1212/WNL.0000000000008472
16. Gadoth A, Kryzer TJ, Fryer J, McKeon A, Lennon VA, Pittock SJ. Microtubule-associated protein 1B: novel paraneoplastic biomarker. Ann Neurol. (2017) 81:266–77. doi: 10.1002/ana.24872
17. Oh SJ, Gürtekin Y, Dropcho EJ, King P, Claussen GC. Anti-Hu antibody neuropathy: a clinical, electrophysiological, and pathological study. Clin Neurophysiol. (2005) 116:28–34. doi: 10.1016/j.clinph.2004.07.012
18. Oki Y, Koike H, Iijima M, Mori K, Hattori N, Katsuno M, et al. Ataxic vs Painful Form of Paraneoplastic Neuropathy. (2007). Available online at: www.neurology.org
19. Schwenkenbecher P, Chacko LP, Wurster U, Pars K, Pul R, Sühs KW, et al. Intrathecal synthesis of anti-Hu antibodies distinguishes patients with paraneoplastic peripheral neuropathy and encephalitis. BMC Neurol. (2016) 16:136. doi: 10.1186/s12883-016-0657-5
20. Graus F, Keime-Guibert F, Reñe R, Benyahia B, Ribalta T, Ascaso C, et al. Anti-Hu-associated paraneoplastic encephalomyelitis: analysis of 200 patients. Brain. (2001) 124:1138–48. doi: 10.1093/brain/124.6.1138
21. Chan KH, Vernino S, Lennon VA. ANNA-3 anti-neuronal nuclear antibody: marker of lung cancer-related autoimmunity. Ann Neurol. (2001) 50:301–11. doi: 10.1002/ana.1127
22. Bataller L, Wade DF, Graus F, Stacey HD, Rosenfeld MR, Dalmau J. Antibodies to Zic4 in paraneoplastic neurologic disorders and small-cell lung cancer. Neurology. (2004) 62:778–82. doi: 10.1212/01.WNL.0000113749.77217.01
23. Vernino S, Lennon VA. New Purkinje cell antibody (PCA-2): marker of lung cancer–related neurological autoimmunity. Ann Neurol. (2000) 47:297–305. doi: 10.1002/1531-8249(200003)47:3<297::AID-ANA4>3.0.CO;2-4
24. Jarius S, Ringelstein M, Haas J, Serysheva II, Komorowski L, Fechner K, et al. Inositol 1,4,5-trisphosphate receptor type 1 autoantibodies in paraneoplastic and non-paraneoplastic peripheral neuropathy. J Neuroinflamm. (2016) 13:278. doi: 10.1186/s12974-016-0737-x
25. Basal E, Zalewski N, Kryzer TJ, Hinson SR, Guo Y, Dubey D, et al. Paraneoplastic neuronal intermediate filament autoimmunity. Neurology. (2018) 91:E1677–89. doi: 10.1212/WNL.0000000000006435
26. Mandel-Brehm C, Dubey D, Kryzer TJ, O'Donovan BD, Tran B, Vazquez SE, et al. Kelch-like protein 11 antibodies in seminoma-associated paraneoplastic encephalitis. N Engl J Med. (2019) 381:47–54. doi: 10.1056/NEJMoa1816721
27. Maudes E, Landa J, Muñoz-Lopetegi A, Armangue T, Alba M, Saiz A, et al. Clinical significance of Kelch-like protein 11 antibodies. Neurol Neuroimmunol Neuroinflamm. (2020) 7:e666. doi: 10.1212/NXI.0000000000000666
28. Shah S, Vazquez Do Campo R, Kumar N, McKeon A, Flanagan EP, Klein C, et al. Paraneoplastic myeloneuropathies: clinical, oncologic, and serologic accompaniments. Neurology. (2021) 96:e632–9. doi: 10.1212/WNL.0000000000011218
29. Dubey D, Kryzer T, Guo Y, Clarkson B, Cheville JC, Costello BA, et al. LUZP4 autoantibody: A novel germ cell tumor and paraneoplastic biomarker. Ann Neurol. (2021) doi: 10.1002/ana.26050
30. Graus F, Vincent A, Pozo-Rosich P, Sabater L, Saiz A, Lang B, et al. Anti-glial nuclear antibody: marker of lung cancer-related paraneoplastic neurological syndromes. J Neuroimmunol. (2005) 165:166–71. doi: 10.1016/j.jneuroim.2005.03.020
31. Stich O, Klages E, Bischler P, Jarius S, Rasiah C, Voltz R, et al. SOX1 antibodies in sera from patients with paraneoplastic neurological syndromes. Acta Neurol Scand. (2012) 125:326–31. doi: 10.1111/j.1600-0404.2011.01572.x
32. Sabater L, Titulaer M, Saiz A, Verschuuren J, Güre AO, Graus F. SOX1 antibodies are markers of paraneoplastic Lambert-Eaton myasthenic syndrome. Neurology. (2008) doi: 10.1212/01.wnl.0000281663.81079.24
33. Titulaer MJ, Klooster R, Potman M, Sabater L, Graus F, Hegeman IM, et al. SOX antibodies in small-cell lung cancer and lambert-eaton myasthenic syndrome: frequency and relation with survival. J Clin Oncol. (2009) 27:4260–7. doi: 10.1200/JCO.2008.20.6169
34. Tschernatsch M, Singh P, Gross O, Gerriets T, Kneifel N, Probst C, et al. Anti-SOX1 antibodies in patients with paraneoplastic and non-paraneoplastic neuropathy. J Neuroimmunol. (2010) 226:177–80. doi: 10.1016/j.jneuroim.2010.07.005
35. Berger B, Dersch R, Ruthardt E, Rasiah C, Rauer S, Stich O. Prevalence of anti-SOX1 reactivity in various neurological disorders. J Neurol Sci. (2016) 369:342–6. doi: 10.1016/j.jns.2016.09.002
36. Ruiz-García R, Martínez-Hernández E, García-Ormaechea M, Español-Rego M, Sabater L, Querol L, et al. Caveats and pitfalls of SOX1 autoantibody testing with a commercial line blot assay in paraneoplastic neurological investigations. Front Immunol. (2019) 10:1–5. doi: 10.3389/fimmu.2019.00769
37. Shillito P, Molenaar PC, Vincent A, Leys K, Zheng W, van den Berg RJ, et al. Acquired neuromyotonia: evidence for autoantibodies directed against K+ channels of peripheral nerves. Ann Neurol. (1995) 38:714–22. doi: 10.1002/ana.410380505
38. Hart IK, Waters C, Vincent A, Newland C, Beeson D, Pongs O, et al. Autoantibodies detected to expressed K+channels are implicated in neuromyotonia. Ann Neurol. (1997) doi: 10.1002/ana.410410215
39. Buckley C, Oger J, Clover L, Tüzün E, Carpenter K, Jackson M, et al. Potassium channel antibodies in two patients with reversible limbic encephalitis. Ann Neurol. (2001) 50:73–8. doi: 10.1002/ana.1097
40. Vincent A, Buckley C, Schott JM, Baker I, Dewar B-K, Detert N, et al. Potassium channel antibody-associated encephalopathy: a potentially immunotherapy-responsive form of limbic encephalitis. Brain. (2004) 127:701–12. doi: 10.1093/brain/awh077
41. Irani SR, Alexander S, Waters P, Kleopa KA, Pettingill P, Zuliani L, et al. Antibodies to Kv1 potassium channel-complex proteins leucine-rich, glioma inactivated 1 protein and contactin-associated protein-2 in limbic encephalitis, Morvan's syndrome and acquired neuromyotonia. Brain. (2010) 133:2734–48. doi: 10.1093/brain/awq213
42. Lai M, Huijbers MGM, Lancaster E, Graus F, Bataller L, Balice-Gordon R, et al. Investigation of LGI1 as the antigen in limbic encephalitis previously attributed to potassium channels: a case series. Lancet Neurol. (2010) 9:776–85. doi: 10.1016/S1474-4422(10)70137-X
43. Sonderen A Van, Ariño H, Petit-pedrol M, Leypoldt F, Körtvélyessy P, Lancaster E, et al. The clinical spectrum of Caspr2 antibody – associated disease. (2016) 87:521–8. doi: 10.1212/WNL.0000000000002917
44. Binks SNM, Klein CJ, Waters P, Pittock SJ, Irani SR. LGI1, CASPR2 and related antibodies: a molecular evolution of the phenotypes. J Neurol Neurosurg Psychiatry. (2018) 89:526–34. doi: 10.1136/jnnp-2017-315720
45. Klein CJ, Lennon VA, Aston PA, Mckeon A, Pittock SJ. Chronic pain as a manifestation of potassium channel-complex autoimmunity. Neurology. (2012) 79:1136–44. doi: 10.1212/WNL.0b013e3182698cab
46. Ellwardt E, Geber C, Lotz J, Birklein F. Heterogeneous presentation of caspr2 antibody-associated peripheral neuropathy – a case series. Eur J Pain. (2020) 24:1411–8. doi: 10.1002/ejp.1572
47. Gadoth A, Pittock SJ, Dubey D, McKeon A, Britton JW, Schmeling JE, et al. Expanded phenotypes and outcomes among 256 LGI1/CASPR2-IgG–positive patients. Ann Neurol. (2017) 82:79–92. doi: 10.1002/ana.24979
48. Ohkawa T, Fukata Y, Yamasaki M, Miyazaki T, Yokoi N, Takashima H, et al. Autoantibodies to epilepsy-related LGI1 in limbic encephalitis neutralize LGI1-ADAM22 interaction and reduce synaptic AMPA receptors. J Neurosci. (2013) 33:18161–74. doi: 10.1523/JNEUROSCI.3506-13.2013
49. Torres-Vega E, Mancheño N, Cebrián-Silla A, Herranz-Pérez V, Chumillas MJ, Moris G, et al. Netrin-1 receptor antibodies in thymoma-associated neuromyotonia with myasthenia gravis. Neurology. (2017) 88:1235–42. doi: 10.1212/WNL.0000000000003778
50. Storstein A, Raspotnig M, Vitaliani R, Giometto B, Graus F, Grisold W, et al. Prostate cancer, Hu antibodies and paraneoplastic neurological syndromes. J Neurol. (2016) 263:1001–7. doi: 10.1007/s00415-016-8090-7
51. Titulaer MJ, Soffietti R, Dalmau J, Gilhus NE, Giometto B, Graus F, et al. Screening for tumours in paraneoplastic syndromes: report of an EFNS Task Force. Eur J Neurol. (2011) 18:19–e3. doi: 10.1002/9781444346268.ch21
52. Camdessanché JP, Antoine JC, Honnorat J, Vial C, Petiot P, Convers P, et al. Paraneoplastic peripheral neuropathy associated with anti-Hu antibodies. A clinical and electrophysiological study of 20 patients. Brain. (2002) 125:166–75. doi: 10.1093/brain/awf006
53. Giometto B, Vitaliani R, Lindeck-Pozza E, Grisold W, Vedeler C. Treatment for paraneoplastic neuropathies. Cochrane Database Syst Rev. (2012) doi: 10.1002/14651858.CD007625.pub2
54. França MC, Faria AV., Queiroz LS, Nucci A. Myositis with sensory neuronopathy. Muscle Nerve. (2007) 36:721–25. doi: 10.1002/mus.20783
55. Mendell JR, Sahenk Z. Painful sensory neuropathy. N Engl J Med. (2003) 348:1243–55. doi: 10.1056/NEJMcp022282
56. Malandrini A, Gambelli S, Muglia M, Berti G, Patitucci A, Sugie K, et al. Motor-sensory neuropathy with minifascicle formation in a woman with normal karyotype. Neurology. (2005) 65:776. doi: 10.1212/01.wnl.0000174516.41417.b9
57. Notermans NC, Jansen GH, Wokke JHJ, Said G, Vrancken AFJE. Progressive idiopathic axonal neuropathy. J Neurol. (2004) 251:269–78. doi: 10.1007/s00415-004-0275-9
58. Yusof NA, Idris NS, Zin FM. Diabetic neuropathic cachexia in a young woman. Korean J Fam Med. (2019) 40:194–98. doi: 10.4082/kjfm.17.0127
59. Yasemin Ö, Seydahmet A, Özcan K. Relationship between diabetic neuropathy and sarcopenia. Prim Care Diabetes. (2019) 13:521–8. doi: 10.1016/j.pcd.2019.04.007
60. Graus F, Dalmau J. Paraneoplastic neuropathies. Curr Opin Neurol. (2013) 26:489–95. doi: 10.1097/WCO.0b013e328364c020
61. Giometto B, Grisold W, Vitaliani R, Graus F, Honnorat J, Bertolini G, et al. Paraneoplastic neurologic syndrome in the PNS Euronetwork database. Arch Neurol. (2010) 67:330. doi: 10.1001/archneurol.2009.341
62. Lavrnić D, Vidaković A, Miletić V, Trikić R, Marinković Z, Rakočević V, et al. Motor neuron disease and monoclonal gammopathy. Eur Neurol. (1995) 35:104–7. doi: 10.1159/000117102
63. Koc F, Paydas S, Yerdelen D, Demirkiran M. Motor neuron disease associated with Multiple. Myeloma Int J Neurosci. (2008) 118:337–41. doi: 10.1080/00207450701242644
64. Sheikh AAE, Sheikh AB, Tariq U, Siddiqui FS, Malik WT, Rajput HM, et al. Paraneoplastic mononeuritis multiplex: a unique presentation of non-Hodgkin lymphoma. Cureus. (2018) 10:e2885. doi: 10.7759/cureus.2885
65. Ekiz E, Ozkok A, Ertugrul NK. Paraneoplastic Mononeuritis multiplex as a presenting feature of adenocarcinoma of the lung. Case Rep Oncol Med. (2013) 2013:1–3. doi: 10.1155/2013/457346
66. Rigamonti A, Lauria G, Stanzani L, Piamarta F, Agostoni E. A case of multifocal motor neuropathy with conduction block associated with gastric and lung adenocarcinoma. J Peripher Nerv Syst. (2012) 17:226–28. doi: 10.1111/j.1529-8027.2012.00401.x
67. Liao J, El-Sadi F, Nikonova A, Yang S, Jakate K, Micieli J, et al. AL-Amyloidosis presenting with painful mononeuropathy multiplex and bilateral cranial nerve 3 palsies (4349). Neurology. (2020) 94:4349
68. Leschziner GD, Roncaroli F, Moss J, Guiloff RJ. Nineteen-year follow-up of Waldenström's-associated neuropathy and Bing-Neel syndrome. Muscle Nerve. (2009) 39:95–100. doi: 10.1002/mus.21112
69. Aya F, Ruiz-Esquide V, Viladot M, Font C, Prieto-González S, Prat A, et al. Vasculitic neuropathy induced by pembrolizumab. Ann Oncol. (2017) 28:433–34. doi: 10.1093/annonc/mdw613
70. Nozawa K, Kaneko H, Itoh T, Katsura Y, Noguchi M, Suzuki F, et al. Synchronous malignant B-cell lymphoma and gastric tubular adenocarcinoma associated with paraneoplastic cutaneous vasculitis: hypereosinophilic syndrome with mixed cryoglobulinemia is an important sign of paraneoplastic syndrome. Rare Tumors. (2009) 1:128–31. doi: 10.4081/rt.2009.e42
71. Woei-A-Jin FJSH, Tamsma JT, Khoe LV, den Hartog WCE, Gerritsen JJ, Brand A. Lymphoma-associated paraneoplastic digital ischemia. Ann Hematol. (2014) 93:355–7. doi: 10.1007/s00277-013-1806-1
72. Murphy SM, Khan U, Alifrangis C, Hazell S, Hrouda D, Blake J, et al. Anti Ma2-associated myeloradiculopathy: expanding the phenotype of anti-Ma2 associated paraneoplastic syndromes. J Neurol Neurosurg Psychiatry. (2012) 83:232–33. doi: 10.1136/jnnp.2010.223271
73. Verma R, Lalla R, Patil T, Babu S. “Person in the barrel” syndrome: unusual heralding presentation of squamous cell carcinoma of the lung. Ann Indian Acad Neurol. (2016) 19:152. doi: 10.4103/0972-2327.167693
74. Vernino S, Adamski J, Kryzer TJ, Fealey RD, Lennon VA. Neuronal nicotinic ACH receptor antibody in subacute autonomic neuropathy and cancer-related syndromes. Neurology. (1998) 50:1806–13. doi: 10.1212/WNL.50.6.1806
75. Seneviratne U, Gunasekera S. Acute small fibre sensory neuropathy: another variant of Guillain-Barré syndrome? J Neurol Neurosurg Psychiatry. (2002) 72:540–2. doi: 10.1136/jnnp.72.4.540
76. Gao CA, Weber UM, Peixoto AJ, Weiss SA. Seronegative autoimmune autonomic ganglionopathy from dual immune checkpoint inhibition in a patient with metastatic melanoma. J Immunother Cancer. (2019) 7:262. doi: 10.1186/s40425-019-0748-0
77. Pál E, Fülöp K, Tóth P, Deli G, Pfund Z, Janszky J, et al. Small fiber neuropathy: clinicopathological correlations. Behav Neurol. (2020) 2020:1–7. doi: 10.1155/2020/8796519
78. Laurencin C, Andre-Obadia N, Camdessanche JP, Mauguiere F, Ong E, Vukusic S, et al. Peripheral small fiber dysfunction and neuropathic pain in patients with Morvan syndrome. Neurology. (2015) 85:2076–78. doi: 10.1212/WNL.0000000000002037
79. Waheed W, Boyd J, Khan F, Mount SL, Borden NM, Tandan R. Double trouble: para-neoplastic anti-PCA-2 and CRMP-5-mediated small fibre neuropathy followed by chorea associated with small cell lung cancer and evolving radiological features. BMJ Case Rep. (2016). 2016:bcr2016215158. doi: 10.1136/bcr-2016-215158
80. Liu Y, Magro C, Loewenstein JI, Makar RS, Stowell CP, Dzik WH, et al. A man with paraneoplastic retinopathy plus small fiber polyneuropathy associated with Waldenström macroglobulinemia (lymphoplasmacytic lymphoma): insights into mechanisms. Ocul Immunol Inflamm. (2015) 23:405–9. doi: 10.3109/09273948.2014.884599
81. Briani C, Vitaliani R, Grisold W, Honnorat J, Graus F, Antoine JC, et al. Spectrum of paraneoplastic disease associated with lymphoma. (2011). 76:705–10. doi: 10.1212/WNL.0b013e31820d62eb
82. Grisold W, Grisold A, Marosi C, Meng S, Briani C. Neuropathies associated with lymphoma. Neuro-Oncology Pract. (2015) 2:167–78. doi: 10.1093/nop/npv025
83. Antoine JC, Mosnier JF, Lapras J, Convers P, Absi L, Laurent B, et al. Chronic inflammatory demyelinating polyneuropathy associated with carcinoma. J Neurol Neurosurg Psychiatry. (1996) 60:188–90. doi: 10.1136/jnnp.60.2.188
84. Garcia-Moreno JM, Castilla JM, Garcia-Escudero A, Izquierdo G. Multifocal motor neuropathy with conduction blocks and prurigo nodularis. A paraneoplastic syndrome in a patient with non-Hodgkin B-cell lymphoma?. Neurologia. (2004). 19:220–4.
85. Stern BV., Baehring JM, Kleopa KA, Hochberg FH. Multifocal motor neuropathy with conduction block associated with metastatic lymphoma of the nervous system. J Neurooncol. (2006). 78:81–84. doi: 10.1007/s11060-005-9060-6
86. Vigliani M-C, Magistrello M, Polo P, Mutani R, Chiò A. Piemonte and Valle d'Aosta Register for Guillain-Barré Syndrome. Risk of cancer in patients with Guillain-Barrlain-Barré Syndroa population-based study. J Neurol. (2004) 251:321–6. doi: 10.1007/s00415-004-0317-3
87. Graus F, Dalmau J. Paraneoplastic neurological syndromes in the era of immune-checkpoint inhibitors. Nat Rev Clin Oncol. (2019). 16:535–48. doi: 10.1038/s41571-019-0194-4
88. Prior R, Schober R, Scharffetter K, Wechsler W. Occlusive microangiopathy by immunoglobulin (IgM-kappa) precipitation: pathogenetic relevance in paraneoplastic cryoglobulinemic neuropathy. Acta Neuropathol. (1992) 83:423–6. doi: 10.1007/BF00713536
89. Maddison P. Neuromyotonia. Clin Neurophysiol. (2006). 117:2118–27. doi: 10.1016/j.clinph.2006.03.008
90. Rubio-Agusti I, Perez-Miralles F, Sevilla T, Muelas N, Chumillas MJ, Mayordomo F, et al. Peripheral nerve hyperexcitability: a clinical and immunologic study of 38 patients. Neurology. (2011) 76:172–8. doi: 10.1212/WNL.0b013e3182061b1e
91. Irani SR, Pettingill P, Kleopa KA, Schiza N, Waters P, Mazia C, et al. Morvan syndrome: clinical and serological observations in 29 cases. Ann Neurol. (2012). 72:241–55. doi: 10.1002/ana.23577
92. Carr A, D'Sa S, Arasaretnam A, Boyd K, Johnston R, Jaunmuktane Z, et al. Peripheral nerve Bing-Neel syndrome. J Neurol Neurosurg Psychiatry. (2015) 86:e4.59–e4. doi: 10.1136/jnnp-2015-312379.151
93. Herrendorff R, Hänggi P, Pfister H, Yang F, Demeestere D, Hunziker F, et al. Selective in vivo removal of pathogenic anti-MAG autoantibodies, an antigen-specific treatment option for anti-MAG neuropathy. Proc Natl Acad Sci. (2017) 114:E3689–98. doi: 10.1073/pnas.1619386114
94. Mauermann ML, Sorenson EJ, Dispenzieri A, Mandrekar J, Suarez GA, Dyck PJ, et al. Uniform demyelination and more severe axonal loss distinguish POEMS syndrome from CIDP. J Neurol Neurosurg Psychiatry. (2012) 83:480–6. doi: 10.1136/jnnp-2011-301472
95. D'Souza A, Hayman SR, Buadi F, Mauermann M, Lacy MQ, Gertz MA, et al. The utility of plasma vascular endothelial growth factor levels in the diagnosis and follow-up of patients with POEMS syndrome. Blood. (2011) 118:4663–5. doi: 10.1182/blood-2011-06-362392
96. Vaxman I, Gertz M. When to suspect a diagnosis of amyloidosis. Acta Haematol. (2020) 143:304–11. doi: 10.1159/000506617
97. Luigetti M, Frisullo G, Laurenti L, Conte A, Madia F, Profice P, et al. Light chain deposition in peripheral nerve as a cause of mononeuritis multiplex in Waldenström's macroglobulinaemia. J Neurol Sci. (2010) 291:89–91. doi: 10.1016/j.jns.2010.01.018
98. Cross SA, Salomao DR, Parisi JE, Kryzer TJ, Bradley EA, Mines JA, et al. Paraneoplastic autoimmune optic neuritis with retinitis defined by CRMP-5-IgG. Ann Neurol. (2003). 54:38–50. doi: 10.1016/j.ajo.2003.09.031
99. Xu Q, Du W, Zhou H, Zhang X, Liu H, Song H, et al. Distinct clinical characteristics of paraneoplastic optic neuropathy. Br J Ophthalmol. (2019). 103:797–801. doi: 10.1136/bjophthalmol-2018-312046
100. Carette T, Mulquin N, van Pesch V, London F. Simultaneous bilateral optic neuropathy and myelitis revealing paraneoplastic neurological syndrome associated with multiple onconeuronal antibodies. Mult Scler Relat Disord. (2021) 49:102789. doi: 10.1016/j.msard.2021.102789
101. Hoogewoud F, Butori P, Blanche P, Brézin AP. Cancer-associated retinopathy preceding the diagnosis of cancer. BMC Ophthalmol. (2018). 18:285. doi: 10.1186/s12886-018-0948-2
102. Bussat A, Langner-Lemercier S, Salmon A, Mouriaux F. Paraneoplastic syndromes in ophthalmology. J Fr Ophtalmol. (2018) 41:e181–5. doi: 10.1016/j.jfo.2018.03.002
103. Alessandro L, Schachter D, Farez MF, Varela F. Cerebellar ataxia with extreme photophobia associated with anti-SOX1 antibodies. The Neurohospitalist. (2019) 9:165–8. doi: 10.1177/1941874418802130
104. Harris GJ. Orbital myositis as a paraneoplastic syndrome. Arch Ophthalmol. (1994) 112:380. doi: 10.1001/archopht.1994.01090150110032
105. Lossos A, Siegal T. Numb chin syndrome in cancer patients: etiology, response to treatment, and prognostic significance. Neurology. (1992) 42:1181. doi: 10.1212/WNL.42.6.1181
106. Raaphorst J, Vanneste J. Numb cheek syndrome as the first manifestation of anti-Hu paraneoplastic neuronopathy. J Neurol. (2006). 253:664–65. doi: 10.1007/s00415-005-0047-1
107. Gabrielli GB, Bonetti F, Tognella P, Corrocher R, De Sandre G. Trigeminal neuropathy in a case of mesenteric localized Castleman's disease. Haematologica. (1991). 76:245–7.
108. De Schamphelaere E, Sieben A, Heyndrickx S, Lammens M, Geboes K, De Bleecker JL. Long lasting trigeminal neuropathy, limbic encephalitis and abdominal ganglionitis without primary cancer: an atypical case of Hu-antibody syndrome. Clin Neurol Neurosurg. (2020). 194:105849. doi: 10.1016/j.clineuro.2020.105849
109. Kalanie H, Harandi AA, Mardani M, Shahverdi Z, Morakabati A, Alidaei S, et al. Trigeminal neuralgia as the first clinical manifestation of anti-Hu paraneoplastic syndrome induced by a borderline ovarian mucinous tumor. Case Rep Neurol. (2014) 6:7–13. doi: 10.1159/000357971
110. Benoliel R, Epstein J, Eliav E, Jurevic R, Elad S. Orofacial pain in cancer: part I—mechanisms. J Dent Res. (2007) 86:491–505. doi: 10.1177/154405910708600604
111. Seidel E, Hansen C, Urban PP, Vogt T, Müller-Forell W, Hopf HC. Idiopathic trigeminal sensory neuropathy with gadolinium enhancement in the cisternal segment. Neurology. (2000). 54:1191–92. doi: 10.1212/WNL.54.5.1191
113. Strupp M, Brandt T. Review: current treatment of vestibular, ocular motor disorders and nystagmus. Ther Adv Neurol Disord. (2009) 2:223–39. doi: 10.1177/1756285609103120
114. Greco A, Macri GF, Gallo A, Fusconi M, De Virgilio A, Pagliuca G, et al. Is vestibular neuritis an immune related vestibular neuropathy inducing vertigo? J Immunol Res. (2014) 2014:1–8. doi: 10.1155/2014/459048
115. Yoshida T, Yazaki M, Gono T, Tazawa K ichi, Morita H, Matsuda M, et al. Severe cranial nerve involvement in a patient with monoclonal anti-MAG/SGPG IgM antibody and localized hard palate amyloidosis. J Neurol Sci. (2006). doi: 10.1016/j.jns.2006.01.018
116. Finsterer J, Wogritsch C, Pokieser P, Vesely M, Ulrich W, Grisold W, et al. Light chain myeloma with oro-pharyngeal amyloidosis presenting as bulbar paralysis. J Neurol Sci. (1997) 147:205–8. doi: 10.1016/S0022-510X(96)05326-9
117. Fujimoto S, Kumamoto T, Ito T, Sannomiya K, Inuzuka T, Tsuda T, et al. clinicopathological study of a patient with anti-Hu-associated paraneoplastic sensory neuronopathy with multiple cranial nerve palsies. Clin Neurol Neurosurg. (2002) 104:98–102. doi: 10.1016/S0303-8467(01)00190-1
118. Nomiyama K, Uchino A, Yakushiji Y, Kosugi M, Takase Y, Kudo S. Diffuse cranial nerve and cauda equina lesions associated with breast cancer. Clin Imaging. (2007). 31:202–5. doi: 10.1016/j.clinimag.2007.01.006
119. Vogrig A, Muñiz-Castrillo S, Joubert B, Picard G, Rogemond V, Skowron F, et al. Cranial nerve disorders associated with immune checkpoint inhibitors. Neurology. (2021). 96:e866–75. doi: 10.1212/WNL.0000000000011340
120. Thomas NE, Passamonte PM, Sunderrajan E V., Andelin JB, Ansbacher LE. Bilateral diaphragmatic paralysis as a possible paraneoplastic syndrome from renal cell carcinoma. Am Rev Respir Dis. (1984). 129:507–9. doi: 10.1164/arrd.1984.129.3.507
121. Otrock ZK, Barada WM, Sawaya RA, Saab JF, Bazarbachi AA. Bilateral phrenic nerve paralysis as a manifestation of paraneoplastic syndrome. Acta Oncol. (2010). 49:264–5. doi: 10.3109/02841860903373716
122. Grisold A, Brandl I, Lindeck-Pozza E, Pöhnl R, Pratschner T, Schmaldienst S, et al. Transient paralysis of diaphragm in Waldenstroms disease; a focal variant of Guillain-Barré syndrome? J Neurol Sci. (2016) 366:1–2. doi: 10.1016/j.jns.2016.04.011
123. Sharp L, Vernino S. Paraneoplastic neuromuscular disorders. Muscle Nerve. (2012) 46:839–40. doi: 10.1002/mus.23502
124. Sharief MK, Robinson SFD, Ingram DA, Geodes JF, Swash M. Paraneoplastic painful ulnar neuropathy. Muscle and Nerve. (1999). 22:952–5. doi: 10.1002/(SICI)1097-4598(199907)22:7<952::AID-MUS24>3.0.CO;2-J
125. Koehler PJ, Buscher M, Rozeman CAM, Leffers P, Twijnstra A. Peroneal nerve neuropathy in cancer patients: a paraneoplastic syndrome? J Neurol. (1997). 244:328–32. doi: 10.1007/s004150050096
126. Leypoldt F, Friese MA, Böhm J, Bäumer T. Multiple enlarged nerves on neurosonography: an unusual paraneoplastic case. Muscle and Nerve. (2011). 43:756–7. doi: 10.1002/mus.22010
127. Flatters SJL, Dougherty PM, Colvin LA. Clinical and preclinical perspectives on Chemotherapy-Induced Peripheral Neuropathy (CIPN): a narrative review. Br J Anaesth. (2017). 119:737–49. doi: 10.1093/bja/aex229
128. Beijers A, Mols F, Dercksen W, Driessen C, Vreugdenhil G. Chemotherapy-induced peripheral neuropathy and impact on quality of life 6 months after treatment with chemotherapy. J Community Support Oncol. (2014) 12:401–6. doi: 10.12788/jcso.0086
129. Hertz DL, Childs DS, Park SB, Faithfull S, Ke Y, Ali NT, et al. Patient-centric decision framework for treatment alterations in patients with Chemotherapy-induced Peripheral Neuropathy (CIPN). Cancer Treat Rev. (2021). 99:102241. doi: 10.1016/j.ctrv.2021.102241
130. Argyriou AA, Kyritsis AP, Makatsoris T, Kalofonos HP. Chemotherapy-induced peripheral neuropathy in adults: a comprehensive update of the literature. Cancer Manag Res. (2014) doi: 10.2147/CMAR.S44261
131. Loprinzi CL, Reeves BN, Dakhil SR, Sloan JA, Wolf SL, Burger KN, et al. Natural history of paclitaxel-associated acute pain syndrome: prospective cohort study NCCTG N08C1. J Clin Oncol. (2011) 29:1472–8. doi: 10.1200/JCO.2010.33.0308
132. Carlson K, Ocean AJ. Peripheral neuropathy with microtubule-targeting agents: occurrence and management approach. Clin Breast Cancer. (2011) 11:73–81. doi: 10.1016/j.clbc.2011.03.006
133. Sehn LH, Herrera AF, Flowers CR, Kamdar MK, McMillan A, Hertzberg M, et al. Polatuzumab vedotin in relapsed or refractory diffuse large B-cell lymphoma. J ClinOncol. (202) 38:155–65. doi: 10.1200/JCO.19.00172
134. Rosenberg JE, O'Donnell PH, Balar A V, McGregor BA, Heath EI Yu EY, et al. Pivotal trial of enfortumab vedotin in urothelial carcinoma after platinum and anti-programmed death 1/programmed death ligand 1 therapy. J Clin Oncol. (2019) 37:2592–600. doi: 10.1200/JCO.19.01140
135. Krop IE, Modi S, LoRusso PM, Pegram M, Guardino E, Althaus B, et al. Phase 1b/2a study of trastuzumab emtansine (T-DM1), paclitaxel, and pertuzumab in HER2-positive metastatic breast cancer. Breast Cancer Res. (2016) 18:1–0. doi: 10.1186/s13058-016-0691-7
136. B. Dunn, PharmD D. Larotrectinib and Entrectinib: TRK Inhibitors for the treatment of pediatric and adult patients with NTRK gene fusion. J Adv Pract Oncol. (2020) 11:418. doi: 10.6004/jadpro.2020.11.4.9
137. Shaw AT, Bauer TM, de Marinis F, Felip E, Goto Y, Liu G, et al. First-line lorlatinib or crizotinib in advanced ALK -positive lung cancer. N Engl J Med. (2020) 383:2018–29. doi: 10.1056/NEJMoa2027187
138. Postow MA, Sidlow R, Hellmann MD. Immune-related adverse events associated with immune checkpoint blockade. N Engl J Med. (2018) doi: 10.1056/NEJMra1703481
139. Johnson DB, Chandra S, Sosman JA. Immune checkpoint inhibitor toxicity in 2018. JAMA J Am Med Assoc. (2018) 320:1702–3. doi: 10.1001/jama.2018.13995
140. Spain L, Diem S, Larkin J. Management of toxicities of immune checkpoint inhibitors. Cancer Treat Rev. (2016) 44:51–60. doi: 10.1016/j.ctrv.2016.02.001
141. Dubey D, David WS, Reynolds KL, Chute DF, Clement NF, Cohen J V, et al. Severe neurological toxicity of immune checkpoint inhibitors: growing spectrum. Ann Neurol. (2020) 87:659–69. doi: 10.1002/ana.25708
142. Cuzzubbo S, Javeri F, Tissier M, Roumi A, Barlog C, Doridam J, et al. Neurological adverse events associated with immune checkpoint inhibitors: review of the literature. Eur J Cancer. (2017) 73:1–8. doi: 10.1016/j.ejca.2016.12.001
143. Johnson DB, Manouchehri A, Haugh AM, Quach HT, Balko JM, Lebrun-Vignes B, et al. Neurologic toxicity associated with immune checkpoint inhibitors: a pharmacovigilance study. J Immunother Cancer. (2019) 7:134. doi: 10.1186/s40425-019-0617-x
144. Supakornnumporn S, Katirji B. Guillain-Barré syndrome triggered by immune checkpoint inhibitors: a case report and literature review. J Clin Neuromuscul Dis. (2017) 19:80–3. doi: 10.1097/CND.0000000000000193
145. Appelbaum J, Wells D, Hiatt JB, Steinbach G, Stewart FM, Thomas H, et al. Fatal enteric plexus neuropathy after one dose of ipilimumab plus nivolumab: a case report. J Immunother Cancer. (2018) 6:82. doi: 10.1186/s40425-018-0396-9
146. Psimaras D, Velasco R, Birzu C, Tamburin S, Lustberg M, Bruna J, et al. Immune checkpoint inhibitors-induced neuromuscular toxicity: from pathogenesis to treatment. J Peripher Nerv Syst. (2019) 24:S74–85. doi: 10.1111/jns.12339
147. Alhammad RM, Dronca RS, Kottschade LA, Turner HJ, Staff NP, Mauermann ML, et al. Brachial plexus neuritis associated with anti–programmed cell death-1 antibodies: report of 2 cases. Mayo Clin Proc Innov Qual Outcomes. (2017) 1:192–7. doi: 10.1016/j.mayocpiqo.2017.07.004
148. Haanen JBAG, Carbonnel F, Robert C, Kerr KM, Peters S, Larkin J, et al. Management of toxicities from immunotherapy: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. (2017) 28:iv119–42. doi: 10.1093/annonc/mdx225
149. Vogrig A, Muñiz-Castrillo S, Joubert B, Picard G, Rogemond V, Marchal C, et al. Central nervous system complications associated with immune checkpoint inhibitors. J Neurol Neurosurg Psychiatry. (2020) 91:772–8. doi: 10.1136/jnnp-2020-323055
150. Prudent V, Breitbart WS. Chimeric antigen receptor T-cell neuropsychiatric toxicity in acute lymphoblastic leukemia. Palliat Support Care. (2017) 15:499–503. doi: 10.1017/S147895151600095X
151. Maude SL, Laetsch TW, Buechner J, Rives S, Boyer M, Bittencourt H, et al. Tisagenlecleucel in children and young adults with B-cell lymphoblastic leukemia. N Engl J Med. (2018) 378:439–48. doi: 10.1056/NEJMoa1709866
152. Berzero G, Picca A, Psimaras D. Neurological complications of chimeric antigen receptor T cells and immune-checkpoint inhibitors: ongoing challenges in daily practice. Curr Opin Oncol. (2020) 32:603–12. doi: 10.1097/CCO.0000000000000681
153. Cordeiro A, Bezerra ED, Hirayama A V, Hill JA, Wu Q V, Voutsinas J, et al. Late events after treatment with CD19-targeted chimeric antigen receptor modified T cells. Biol Blood Marrow Transplant. (2020) 26:26–33. doi: 10.1016/j.bbmt.2019.08.003
154. Antoine J-C, Robert-Varvat F, Maisonobe T, Créange A, Franques J, Mathis S, et al. Identifying a therapeutic window in acute and subacute inflammatory sensory neuronopathies. J Neurol Sci. (2016) 361:187–91. doi: 10.1016/j.jns.2015.12.044
155. Zuliani L, Nosadini M, Gastaldi M, Spatola M, Iorio R, Zoccarato M, et al. Management of antibody-mediated autoimmune encephalitis in adults and children: literature review and consensus-based practical recommendations. Neurol Sci. (2019) 40:2017–30. doi: 10.1007/s10072-019-03930-3
156. Irani SR, Gelfand JM, Bettcher BM, Singhal NS, Geschwind MD. Effect of rituximab in patients with leucine-rich, glioma-inactivated 1 antibody–associated encephalopathy. JAMA Neurol. (2014) 71:896. doi: 10.1001/jamaneurol.2014.463
157. Fornasari D. Pharmacotherapy for neuropathic pain: a review. Pain Ther. (2017). 26:25–33. doi: 10.1007/s40122-017-0091-4
Keywords: cancer, tumor type, paraneoplastic neuropathy, onconeural antibodies, surface antibodies, mechanisms, therapy
Citation: Zoccarato M, Grisold W, Grisold A, Poretto V, Boso F and Giometto B (2021) Paraneoplastic Neuropathies: What's New Since the 2004 Recommended Diagnostic Criteria. Front. Neurol. 12:706169. doi: 10.3389/fneur.2021.706169
Received: 06 May 2021; Accepted: 30 August 2021;
Published: 01 October 2021.
Edited by:
John Greenlee, University of Utah, United StatesReviewed by:
Aksel Siva, Istanbul University Cerrahpasa, TurkeyKelsey Barrell, The University of Utah, United States
Copyright © 2021 Zoccarato, Grisold, Grisold, Poretto, Boso and Giometto. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Marco Zoccarato, bWFyY296b2NjYXJhdG8mI3gwMDA0MDtnbWFpbC5jb20=
†These authors have contributed equally to this work
 Marco Zoccarato
Marco Zoccarato Wolfgang Grisold
Wolfgang Grisold Anna Grisold3
Anna Grisold3 Bruno Giometto
Bruno Giometto