
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Neurol. , 21 September 2021
Sec. Neuromuscular Disorders and Peripheral Neuropathies
Volume 12 - 2021 | https://doi.org/10.3389/fneur.2021.704928
Nusinersen is the first oligonucleotide-based drug that is approved for the treatment of spinal muscular atrophy. In January 2020, the WHO declared COVID-19 a pandemic and nusinersen-provider centers had to postpone planned infusions for some children along with other related interventions. Considering the important contribution that the intrathecal infusions and other support activities could have on the quality of life of spinal muscular atrophy patients and their families, this emergency could have a relevant impact on the course of the pathology. The present work aims to assess the clinical and social issues that arise for spinal muscular atrophy children in care at the referral pediatric palliative care Centre of Padua (Veneto) from a delay in nusinersen infusions, resulting from the contingent COVID-19 restrictions. This evaluation has been carried out in both the short and long term after the first lockdown period and can be considered as a “proxy” of a situation of a possible delay in administration or management of infusions, due to other different causes.
Spinal muscular atrophy (SMA) is a relatively rare neuromuscular disorder, which could lead to infant mortality (1). It is caused by the loss or mutation of the “survival of motor neuron” gene, termed SMN1. This induces the degeneration of motor neurons, with progressive muscle weakness and atrophy (2). There are five subtypes of SMA, characterized by different clinical severity (3).
Nusinersen is the first oligonucleotide-based drug that is approved for the treatment of SMA (4); it has been available in the market in Italy since October 2017 (5, 6). This molecule has shown to be effective in patients with SMA1 and SMA2 in pivotal trials (NCT02193074 and NCT02292537) (7, 8). However, nusinersen requires an intrathecal administration, and the benefits potentially associated with this treatment can be disrupted using invasive procedures (9, 10). Indeed, some patients treated with nusinersen, especially those who started treatment at an older age, do not actually improve their clinical status and remain disabled with a continuous requirement of intensive care, thus raising ethical implications related to this treatment (11, 12).
In January 2020, the World Health Organization (WHO) declared COVID-19 a pandemic (13). Due to the COVID-19 emergency, nusinersen-provider centers had to postpone planned infusions for some children (14).
In the Veneto region of Italy, children diagnosed with SMA are cared for from birth in the pediatric palliative care (PPC) program, which provides coordination of follow-up, enrollment therapy, parental qualification and network coordination (15). In the referral PPC Centre of Padua (Veneto), during the first lockdown period, some activities had to be changed or suspended.
Considering the important contribution that the intrathecal infusions and other support activities (nursing interventions, physiotherapy activity, and medical intervention) could have on the quality of life of patients and their families, stopping them could have a relevant impact.
The present work aims to assess the clinical and social issues that arise for PPC children and their families from a delay in nusinersen infusions, resulting from the contingent COVID-19 restrictions. This evaluation has been carried out both in the short and long term after the first lockdown period and can be considered as a “proxy” of a situation of a possible delay in administration or management of infusions due to other different causes.
In the referral PPC Centre of Padua (Veneto), during the first lockdown period and accordingly to a shared decision taken by all the national nusinersen-provider centers, the nusinersen loading doses were configured as a health emergency and therefore respected, maintenance doses instead were moved in compliance with the restrictions, but not exceeding an interval of 6 months (16).
Consequent to the COVID-19 emergency, clinical and social issues for SMA patients were evaluated through a medical record review that has been carried out during these time points: (1) first diagnosis of SMA; (2) last nusinersen administration before the first lockdown period; (3) first nusinersen administration after the first lockdown period (on time or delayed; short-term evaluation); and (4) second nusinersen administration after the first lockdown period (on time; long-term evaluation).
In addition, a cross-sectional survey has been conducted at the moment of the first nusinersen administration after the lockdown period.
Both tools involved children in care at the regional referral center for PPC of Padua in the Veneto region and their families.
Children (0–18 years) with a genetic diagnosis of SMA in maintenance therapy with nusinersen who consent to participate in the study have been considered. Children performing loading doses, children for whom it has been decided to not continue the therapy with nusinersen, and families with a language barrier have been excluded from the study. The Local Ethical Committee has approved this study (protocol number: 0049524), and all patients, or their caregivers, have signed an informed consent to use the data for research purposes.
Support for SMA patients and families is routinely guaranteed by the PPC center with a telephone availability 24/7 and a nursing telephone monitoring intervention every 15–30 days. During the first lockdown period, extraordinary support was provided by converting physiotherapy activity into video call interventions. Home interventions (for medical need or physiotherapy activity) have been provided only in cases of necessity in compliance with the safety standards.
For each time point, the following data were collected in a clinical practice setting: weight, height, chest circumference, head circumference (only for children <3 years age), thoracic circumference (17), motor function scale values (CHOP: Children's Hospital of Philadelphia Infant Test of Neuromuscular Disorders, 0–64-point scale composed of 16 items used to assess motor skills (18); HINE: Hammersmith Infant Neurological Examination, a 0- to 26-point scale to evaluate motor skills in children from 2 months to 2 years of age (19); HFMSE: Hammersmith Functional Motor Scale—Expanded, a 0–66-point scale to evaluate motor function in children (20)), and responder or non-responder condition according to the pivotal trials (21–23).
In addition, the number of children for whom the infusion was postponed and how long it was postponed (days) and the number of patients who discontinue infusions have been recorded.
The interventions performed at home or remotely during lockdown by a nurse, physiotherapist, and doctor were also documented for each child.
An ad hoc questionnaire was administered by two operators of the Center to children (only if ≥6 years) and parents, at the first administration of therapy after the temporary suspension.
The survey contains questions about the perception of muscle strength, swallowing, and breathing functions, along with some questions about personal feelings and fears during the lockdown period.
Improvements relative to muscle strength, swallowing, and breathing have been assessed through a Likert scale, from “not perceived” to “great.”
Descriptive statistics were used for the present study, which was performed with an explorative intent.
At the date of discontinuation of intrathecal infusions at the Padua PPC center (27/02/2020), 31 children were on nusinersen therapy. Five out of 31 (16%) patients decided during the lockdown to not resume the therapy and were therefore excluded from the study. One patient has been excluded because of a language barrier. Consequently, a total of 25 children were included in the study. The baseline and clinical characteristics of these children are summarized in Table 1.
The data for every single patient are reported in Table 2. In total, 11 patients (44%) were responders to nusinersen at the last infusion before lockdown. Of them, one patient had a diagnosis of SMA1a, one of SMA1b, two of SMA1c, five of SMA2, and two of SMA3. Consequently, responder rates were 100% (1/1) for SMA1a, 100% (1/1) for SMA1b, 28% (2/7) for SMA1c (24), 55% (5/9) for SMA2, and 28% (2/7) for SMA3. Among all responders, eight (73%) received early treatment (i.e., within 1 week since the genetic diagnosis of SMA); seven (64%) were sitters, and four (36%) were layers; three (27%) were on autonomous ventilation, seven (64%) were on NIV (non-invasive ventilation), and one (9%) required invasive mechanical ventilation. Four of the responders (36%) had poor compliance to the standard of care, defined according to Finkel et al. (25).
Considering all 25 patients, 9 patients (36%) showed a reduction in the functional score at the first visit after the lockdown period, compared with the last visit before the lockdown period, whereas five patients (20%) showed an improvement. Among the 9 patients who complained of a reduction in the functional score, no one was a responder. Only in three cases was the reduction in the functional score >-2: one patient with poor compliance to the standard of care (patient number 6, −5 points on the CHOP scale), one patient aged 4 years with a natural history of more severe disease (patient number 21, −3 points on the HFMSE scale), and one patient aged 14 years with a marked worsening of scoliosis (patient number 13, −7 points on the HFMSE scale).
A total of eight children (32%) experienced a delay in the infusion schedule (median delay, 58 days; range, 26–91). Among them, only marginal changes in the functional scores were reported, except for patient number 13 (see above) who had a 59-day delay in the administration of nusinersen.
During the lockdown, each family received two phone calls a month from the nurses. A total of 100 nursing calls have been carried out.
The physiotherapist made a total of 64 video calls to patients with a median of two calls per patient (range: 1–5) and six home visits.
During the lockdown, no children needed to access the emergency room or were hospitalized.
Long-term evaluations were collected at the second infusion after lockdown, which has been administered 4 months after the previous infusion without delay and has been compared with the last visit before the lockdown period. A total of 10 patients (40%) showed a reduction in the functional score, whereas 8 patients (32%) showed an improvement. Only in three cases was the reduction in the functional score >-2: patient 13, who did not improve his score compared with the previous evaluation (−7 points on the HFMSE scale, see above), one patient with a single and fatigued parent as a sole caregiver (patient 8, −4 points on the CHOP scale), and one patient with a difficult social situation and parents who experienced depression due to the loss of their jobs (patient 30, −4 points on the CHOP scale). Both patients 8 and 30 got worse between the first and second infusions after lockdown.
Patient 26 experienced a great improvement in his score, with a 7-point increase since the pre-lockdown evaluations. This was due to the presence of two caregivers during the lockdown.
Of note, patient 6, who experienced a great score loss at the first visit after lockdown (see above), improved his score by 4 points at this second evaluation: this was due to better compliance with supportive therapies (cough machine).
In total, 25 parents were interviewed; the responder was primarily the mother (23 out of 25, 92%).
Among the parents interviewed, 5 out of 25 (20%) did not perceive a change in their child's muscle strength, 12 out of 25 (48%) felt a worsening (one rated the worsening as great, five as quiet, and the other as small/very small), and 8 out of 25 (32%) felt an improvement (two rated the improvement as great, five as quiet, and one as very small) (Figure 1).
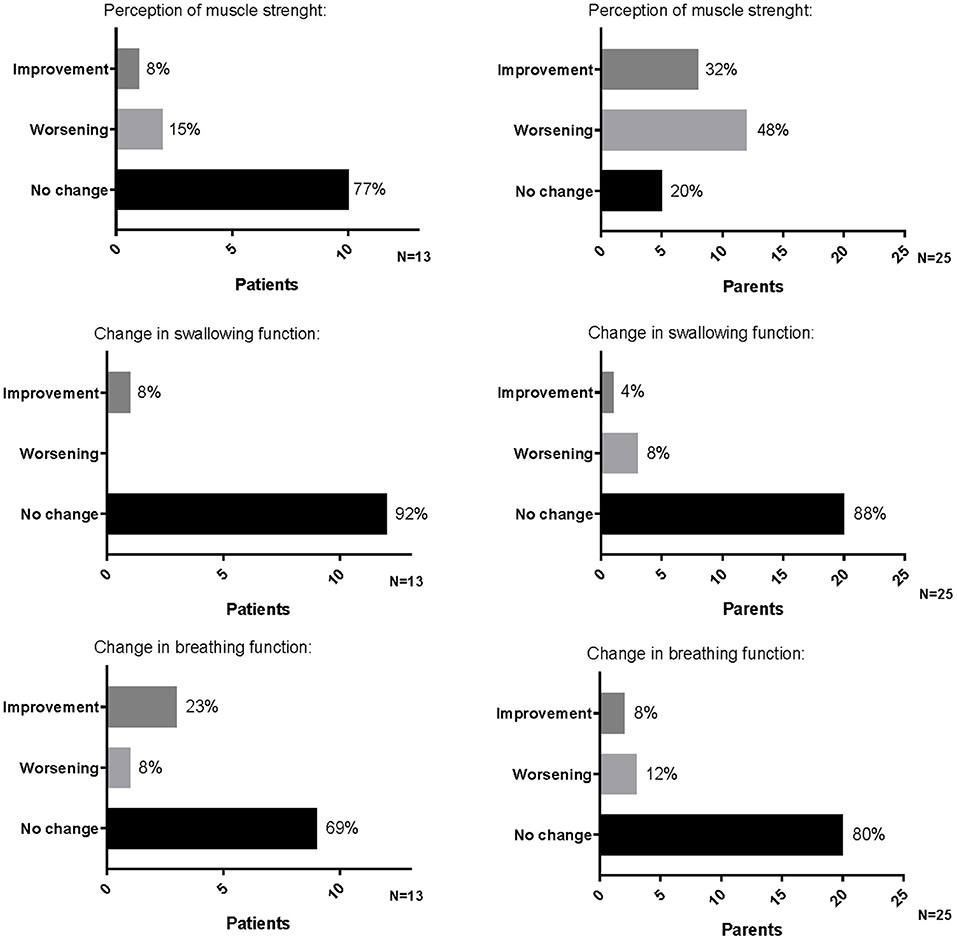
Figure 1. Perception of muscle strength, swallowing, and breathing functions reported by patients and parents on the occasion of the first administration of therapy after the temporary suspension.
A total of 22 parents (88%) did not perceive a change in the child swallowing function, 2 parents (8%) felt a worsening (one rated as great and one as very small), and 1 (4%) felt a great improvement (Figure 1).
A total of 20 parents (80%) did not perceive a change in child breathing function, 2 parents (8%) felt a great improvement, and 3 (12%) felt a worsening rating as “quiet” and had to intensify the respiratory support to their children (Figure 1).
Parents retain that worsening changes are mainly due to the suspension of outpatient physiotherapy (10 out of 25, 40%) or to a delay in nusinersen infusion (6 out of 25, 24%). Six out of 25 (24%) are unable to identify a cause, two (8%) parents attribute the worsening to the impossibility of changing aids, and one (4%) attributes it to less home help.
Improving changes are attributed to less fatigue for the child in six cases (24%), to the time spent with the family (six cases, 24%), and to the adjunctive home respiratory physiotherapy (six cases, 24%) or stretching (five cases, 20%); two parents (8%) failed to identify a reason.
Three parents (12%) said that they were not worried about the interruption of therapy; the rest said they were quite worried (three cases, 12%), slightly worried (five cases, 20%), very worried (eight cases, 32%), and extremely worried (six cases, 24%).
The final question concerns the assistance received during the lockdown period: 12 parents (48%) report to have not requested any additional help compared to what is normally provided and 13 parents (52%) report to have benefited from the extraordinary support of the PPC center.
Among the 25 children of the study, 14 were >6 years. One did not consent to participate; consequently, a total of 13 children were interviewed. The median age was 10 years (range: 7–15); 1 was functionally a layer, 11 were sitters, and 1 was a walker.
Among them, 10 (77%) did not perceive a change in their muscle strength, 2 (15%) felt a worsening (reported as small worsening), and 1 (8%) felt a great improvement (Figure 1).
In total, 12 children (92%) did not perceive a change in swallowing function; the other one (8%) felt an improvement, reported as great (Figure 1).
Nine children (69%) did not perceive a change in breathing function, three (23%) felt an improvement (two reported as quite an improvement and one as a great improvement), and one (8%) felt a small worsening with a consequent increase of the assisted ventilation (Figure 1).
Seven children out of 13 (53%) said they were not worried about the interruption of intrathecal therapy; the other said they were quite worried (3/13, 23%), slightly worried (1/13, 8%), very worried (1/13, 8%), and extremely worried (1/13, 8%).
What children missed more during the lockdown period was going to school and seeing their friends in eight cases (61%) or taking lessons in three cases (23%). Two children (16%) missed going to physiotherapy or hydrotherapy more.
The present work aimed to investigate the effects of the delay of nusinersen infusions due to the COVID-19 pandemic on children with SMA, in the context of global care at the PPC center.
Although a detailed statistical analysis was not possible due to the small sample size, no correlation between a delayed treatment and changes of functional scores emerged over the short-period evaluations (~2 months after the end of the first lockdown) and the immediately following months (long-term evaluations).
Furthermore, three patients showed a relevant (>-2) change in functional scores in the short period. This worsening had a strong clinical reason; one patient was not compliant with the standard of care due to a poor social context, the second was a 14-year-old patient with scoliosis, which severely worsened during the last months before the lockdown period, and the third presents a more severe disease trajectory.
When assessing the proportion of responders before the lockdown period, it emerged that the majority of them (approximately three out of four) had an early initiation of treatment, immediately after the genetic confirmation of diagnosis. For the others, the diagnosis preceded the approval of nusinersen therapy for SMA patients. However, no correlation with the changes in functional scores was possible since all score variations reported over the period of the first lockdown were of modest magnitude.
Considering long-term evaluations, 10 patients reported an overall worsening of their functional score compared to evaluations before the lockdown. The reduction was >-2 in three cases. One was the same patient who reported a worsening already in the short term; the other two patients got worse between the first and second infusions after lockdown.
Consequently, the great worsening was seen in both the short- and long-term evaluations and cannot be attributed to the delay in infusions but to a difficult family situation of the patients.
Of note, two patients experienced a global great improvement in the long-term evaluation (+5 points) compared to evaluations before the lockdown, and this was due to great family support.
Considering the survey results, the global patient's perception is to not have suffered a worsening in muscle strength, swallowing, and breathing functions. Otherwise, a worsening in muscle strength is perceived by 48% of parents. This discordant perception is mainly due to a state of anxiety of parents related to the suspension of physiotherapy (reported by 40% of parents).
On the other hand, a worsening of breathing functions is perceived in a minority of cases.
This is because, in our reality, parents are enabled to the home management of the respiratory aspect, supported with 24/7 nurse telephone availability and the possibility of video calls supporting the respiratory physiotherapy.
Of note, considering that a direct consequence of the worsening of muscle strength is an impairment of the breathing function, this underlines the importance of home physiotherapy.
This is confirmed by the fact that children who had both parents caring for them and helping each other during the lockdown showed the best improvement in motor function, while in the presence of social issues, above all in single-parent families, children suffered a lack of home institutional help.
On the other hand, patients who were facing worsening scoliosis during the lockdown showed even more detrimental evolution in motor function.
For instance, the study period is too short to perceive a change in swallowing functions, as reported in the literature (21).
The PPC center is equipped to provide remote assistance if necessary, and during the pandemic, this kind of assistance was implemented. For most parents (52%), the extraordinary support implemented during the pandemic has been the main source of help. For the other families, the assistance measures routinely carried out were satisfactory even in this emergency period.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by the Comitato Etico per la Sperimentazione Clinica e Servizio di Bioetica dell'Azienda Ospedale Università Padova and all patients, or their caregivers, have signed an informed consent to use the data for research purposes. Written informed consent to participate in this study was provided by the participants' legal guardian/next of kin.
CA, ES, FBene, and FBeni: study design. CA, LG, and SP: manuscript writing. All authors: manuscript editing, approval to submit, data collection, and interpretation.
LG and SP were employed by the company Polistudium Srl.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Editorial assistance was provided by Aashni Shah (Polistudium SRL, Milan, Italy). This assistance was supported by internal funds.
1. Verhaart IEC, Robertson A, Wilson IJ, Aartsma-Rus A, Cameron S, Jones CC, et al. Prevalence, incidence and carrier frequency of 5q-linked spinal muscular atrophy - a literature review. Orphanet J Rare Dis. (2017) 12:124. doi: 10.1186/s13023-017-0671-8
2. Mercuri E, Pera MC, Scoto M, Finkel R, Muntoni F. Spinal muscular atrophy - insights and challenges in the treatment era. Nat Rev Neurol. (2020) 16:706–15. doi: 10.1038/s41582-020-00413-4
3. Kolb SJ, Kissel JT. Spinal muscular atrophy. Neurol Clin. (2015) 33:831–46. doi: 10.1016/j.ncl.2015.07.004
4. Li Q. Nusinersen as a therapeutic agent for spinal muscular atrophy. Yonsei Med J. (2020) 61:273–83. doi: 10.3349/ymj.2020.61.4.273
5. Messina S, Pane M, Sansone V, Bruno C, Catteruccia M, Vita G, et al. Expanded access program with Nusinersen in SMA type I in Italy: strengths and pitfalls of a successful experience. Neuromuscul Disord. (2017) 27:1084–6. doi: 10.1016/j.nmd.2017.09.006
6. Wang CH, Finkel RS, Bertini ES, Schroth M, Simonds A, Wong B, et al. Consensus statement for standard of care in spinal muscular atrophy. J Child Neurol. (2007) 22:1027–49. doi: 10.1177/0883073807305788
7. Finkel RS, Mercuri E, Darras BT, Connolly AM, Kuntz NL, Kirschner J, et al. Nusinersen versus sham control in infantile-onset spinal muscular atrophy. N Engl J Med. (2017) 377:1723–32. doi: 10.1056/NEJMoa1702752
8. Mercuri E, Darras BT, Chiriboga CA, Day JW, Campbell C, Connolly AM, et al. Nusinersen versus sham control in later-onset spinal muscular atrophy. N Engl J Med. (2018) 378:625–35. doi: 10.1056/NEJMoa1710504
9. Pechmann A, Langer T, Wider S, Kirschner J. Single-center experience with intrathecal administration of Nusinersen in children with spinal muscular atrophy type 1. Eur J Paediatr Neurol. (2018) 22:122–7. doi: 10.1016/j.ejpn.2017.11.001
10. Aragon-Gawinska K, Seferian AM, Daron A, Gargaun E, Vuillerot C, Cances C, et al. Nusinersen in patients older than 7 months with spinal muscular atrophy type 1: a cohort study. Neurology. (2018) 91:e1312–8. doi: 10.1212/WNL.0000000000006281
11. Audic F, de la Banda MGG, Bernoux D, Ramirez-Garcia P, Durigneux J, Barnerias C, et al. Effects ofnusinersen after one year of treatment in 123 children with SMA type 1 or 2: a French real-life observational study. Orphanet J Rare Dis. (2020) 15:148. doi: 10.1186/s13023-020-01414-8
12. Agosto C, Salamon E, Divisic A, Benedetti F, Giacomelli L, Shah A, et al. Do we always need to treat patients with spinal muscular atrophy? A personal view and experience. Orphanet J Rare Dis. (2021) 16:78. doi: 10.1186/s13023-020-01593-4
13. Livingston E, Bucher K. Coronavirus disease 2019 (COVID-19) in Italy. JAMA. (2020) 323:1335. doi: 10.1001/jama.2020.4344
14. Mercuri E, Finkel RS, Muntoni F, Wirth B, Montes J, Main M, et al. Diagnosis and management of spinal muscular atrophy: Part 1: recommendations for diagnosis, rehabilitation, orthopedic and nutritional care. Neuromuscul Disord. (2018) 28:103–15. doi: 10.1016/j.nmd.2017.11.005
15. Di Pede C, Salamon E, Motta M, Agosto C, Benini F, Ferrari A. Spinal bracing and lung function in type-2 spinal muscular atrophy. Eur J Phys Rehabil Med. (2019) 55:505–9. doi: 10.23736/S1973-9087.18.05046-3
16. Veerapandiyan A, Connolly AM, Finkel RS, Arya K, Mathews KD, Smith EC, et al. Spinal muscular atrophy care in the COVID-19 pandemic era. Muscle Nerve. (2020) 62:46–9. doi: 10.1002/mus.26903
17. Ropars J, Barnerias C, Hully M, Chabalier D, Peudenier S, Barzic A, et al. Thoracic circumference: a new outcome measure in spinal muscular atrophy type 1? Neuromuscul Disord. (2019) 29:415–21. doi: 10.1016/j.nmd.2019.03.003
18. Glanzman AM, Mazzone E, Main M, Pelliccioni M, Wood J, Swoboda KJ, et al. The children's hospital of philadelphia infant test of neuromuscular disorders (CHOP INTEND): test development and reliability. Neuromuscul Disord. (2010) 20:155–61. doi: 10.1016/j.nmd.2009.11.014
19. Romeo DM, Ricci D, Brogna C, Mercuri E. Use of the hammersmith infant neurological examination in infants with cerebral palsy: a critical review of the literature. Dev Med Child Neurol. (2016) 58:240–5. doi: 10.1111/dmcn.12876
20. The Pediatric Neuromuscular Clinical Research Network for SMA. Expanded Hammersmith Functional Motor Scale for SMA (HFMSE). Available online at: http://columbiasma.org/docs/HFMSE_2019_Manual.pdf (accessed March 7, 2009).
21. McGraw S, Qian Y, Henne J, Jarecki J, Hobby K, Yeh WS. A qualitative study of perceptions of meaningful change in spinal muscular atrophy. BMC Neurol. (2017) 17:68. doi: 10.1186/s12883-017-0853-y
22. Pane M, Coratti G, Sansone VA, Messina S, Bruno C, Catteruccia M, et al. Nusinersen in type 1 spinal muscular atrophy: twelve-month real-world data. Ann Neurol. (2019) 86:443–51. doi: 10.1002/ana.25533
23. Mercuri E, Finkel R, Montes J, Mazzone ES, Sormani MP, Main M, et al. Patterns of disease progression in type 2 and 3 SMA: implications for clinical trials. Neuromuscul Disord. (2016) 26:126–31. doi: 10.1016/j.nmd.2015.10.006
24. Finkel R, Bertini E, Muntoni F, Mercuri E, ENMC SMA Workshop Study Group. 209th ENMC International Workshop: Outcome Measures and Clinical Trial Readiness in Spinal Muscular Atrophy 7–9 November 2014, Heemskerk, The Netherlands. Neuromuscul Disord. (2015) 25:593–602. doi: 10.1016/j.nmd.2015.04.009
Keywords: SMA, nusinersen, COVID-19 pandemic, nusinersen infusion delay, PPC
Citation: Agosto C, Salamon E, Giacomelli L, Papa S, Benedetti F and Benini F (2021) Effect of the COVID-19 Pandemic on Children With SMA Receiving Nusinersen: What Is Missed and What Is Gained? Front. Neurol. 12:704928. doi: 10.3389/fneur.2021.704928
Received: 04 May 2021; Accepted: 16 August 2021;
Published: 21 September 2021.
Edited by:
Jens Schmidt, Universitätsklinikum Göttingen, GermanyReviewed by:
Zhiyv Neal Niu, Mayo Clinic, United StatesCopyright © 2021 Agosto, Salamon, Giacomelli, Papa, Benedetti and Benini. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Caterina Agosto, Y2F0ZXJpbmEuYWdvc3RvQGFvcGQudmVuZXRvLml0
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.