
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
BRIEF RESEARCH REPORT article
Front. Neurol. , 27 October 2021
Sec. Applied Neuroimaging
Volume 12 - 2021 | https://doi.org/10.3389/fneur.2021.702185
This article is part of the Research Topic Accelerated Brain Aging: Different Diseases - Different Imaging Patterns View all 9 articles
 Qihui Zhang1
Qihui Zhang1 Xiaobin Zhao2
Xiaobin Zhao2 Peng Lei3
Peng Lei3 Hank F. Kung4
Hank F. Kung4 Zhi Yang5
Zhi Yang5 Lin Zhu6
Lin Zhu6 Shujing Wang5
Shujing Wang5 Hua Zhu5
Hua Zhu5 Xiangxi Meng5
Xiangxi Meng5 Yunyun Duan7
Yunyun Duan7 Li Sun8
Li Sun8 Jianwei Pan9
Jianwei Pan9 Ruixue Ma10
Ruixue Ma10 Haiyan Hong6
Haiyan Hong6 Xingquan Zhao1
Xingquan Zhao1 Andrew Demchuk11
Andrew Demchuk11 Eric E. Smith11
Eric E. Smith11 Yongjun Wang1*
Yongjun Wang1*Objective: We aimed to investigate the distribution of [68Ga]Ga-p14-032, a novel PET ligand that binds to vascular amyloid, in patients diagnosed clinically with probable cerebral amyloid angiopathy (CAA) compared with patients with Alzheimer's disease (AD) and normal controls (NC).
Methods: This longitudinal cohort study was composed of 10 subjects (three probable CAA patients, two AD patients, five NC subjects), recruited from a clinic in China. CAA patients had a history of lobar intracerebral hemorrhage (ICH) and met modified Boston criteria for probable CAA. All participants were aged at least 55 years and underwent [68Ga] Ga-p14-032 PET/CT or/and PET/MRI, and the Montreal Cognitive Assessment on initial assessment. Demographics were measured at baseline (diabetes, hypertension, hypercholesterolemia, ischemic stroke, and ICH). Two PET imaging experts reviewed the PET images with cortical standardized uptake value ratio (SUVr) displayed on a color scale and visually classified the images as positive or negative. The mean of SUVr was calculated using the pons as reference.
Results: In CAA patients, PET scans were positive in regions with higher numbers of CMBs. No significant signal was seen in AD subjects or controls. The relative [68Ga]Ga-p14-032 retention in the cortex was stronger in patients with CAA than AD and NC (median SUVr 2.68 ± 1.53 vs. 1.77 ± 0.08 and 0.83 ± 0.24).
Conclusions: Our results provide early evidence that the [68Ga] Ga-p14-032 PET probe binds preferentially to vascular amyloid and may be a useful tracer for diagnosing CAA.
Cerebral amyloid angiopathy (CAA) is a common cerebrovascular disease in the elderly (1). Because amyloid-β (Aβ) deposits in the walls of small arteries and arterioles in the cerebral cortex and leptomeninges (2), it can lead to lobar hemorrhage and cognitive decline (3, 4). The definitive diagnosis of CAA relies on biopsy or autopsy (5), but this is often not available clinically. Amyloid imaging has become an important tool in the study of CAA (6). It has been used to explore the spatial and quantitative correlation between the brain injury related to CAA and vascular amyloid (7, 8). Although current ligands can show the deposition of vascular Aβ protein, they also pass through the blood-brain barrier and bind to the Aβ protein deposited in brain parenchyma (9). Therefore, it is still difficult to differentiate CAA and Alzheimer's disease (AD) by specific binding of the tracer to Aβ protein. Although patients with CAA exhibit a relatively higher degree of posterior binding compared with overall binding than patients with AD, these differences in relative binding are not large enough to make accurate diagnoses in individual patients. What is needed are new tracers with higher specificity for vascular β amyloid.
A series of studies suggests the relationship between vascular amyloid and lobar bleeds in CAA (10–12). In vitro autoradiography suggests that [68Ga]Ga-p14-032 may be a useful PET imaging agent for selectively detecting Aβ associated with cerebral vessels in the living human brain (13). Therefore, we designed a study to address whether [68Ga]Ga-p14-032-PET imaging can predict sites of microbleeds in CAA patients and whether the overall [68Ga]Ga-p14-032 burden provides information about the currency of CAA.
We enrolled 10 subjects at least 55 years old: three probable CAA patients, two AD patients, and five normal controls (NC). The subjects were recruited from an ongoing single-center prospective registered study of evaluating [68Ga]Ga-p14-032 as a novel PET tracer for diagnosis of CAA (Beijing Tiantan Hospital, Beijing China). They underwent [68Ga]Ga-p14-032 PET/CT and/or PET/MRI at Beijing Cancer Hospital. Two NC subjects did not complete the PET/MRI scan because of intolerance to noise. They had brain MRI before and had no history of stroke. The three CAA subjects after symptomatic intracerebral hemorrhage (ICH) history met the criteria for probable CAA according to modified Boston criteria. The AD cases met NINCDS-ADRDA criteria. The NC subjects have no stroke or allergic history or cognitive impairment. Detailed information, including demographics, characteristics, and the Montreal Cognitive Assessment (MoCA) score were assessed as the baseline.
This study was performed with the approval of ethics committees of the participating institutions (Beijing Tiantan hospital, and Beijing Cancer Hospital) and with the informed consent of all subjects or family members.
Using the freeze-drying kit containing the labeled precursor, 68Ga solution washed from the 68Ga/68Ga generator was used for labeling with testing of the quality of the product through pH value, radiochemical purity (thing-layer chromatography), sterility, and pyrogen. A labeled drug meeting the clinical application quality standard was used in the PET/CT or PET/MRI clinical imaging research.
PET/CT images of subjects were collected with the Siemens Biograph mCT flow PET/CT scanner after intravenous injection of 3–6 mCi [68Ga]Ga-p14-032 for 30 min and 1 h. PET images and PET/CT fusion images were obtained after reconstruction by the ordered subset expectation maximization (OSEM) algorithm. PET/MRI imaging was performed on the head with a United Imaging uPMR 790 PET/MRI scanner 2 h after the injection of [68Ga] Ga-p14-032. Raw image data were reconstructed using MR attenuation correction and the OSEM algorithm. The PET acquisition time was 15 min for both PET/CT and PET/MRI.
The relative retention in the cortex was expressed as SUVr. VOIs were manually delineated on the cortex and the pon of each patient. Then, the SUVmean was calculated in the voxels of each VOI. Finally, the SUVmean in the cortex was divided by the SUVmean in the pon to yield SUVr.
The hemorrhagic lesions (Hem) and the pons were manually segmented on the susceptibility weighted image (SWI) sequence for the patient. The radioactivity and volumes of these segmented areas were obtained on the registered PET image. The concentration ratio (R) was them obtained as
where α denotes the total activity of the segmented region (Bq), and V denotes the volume of the region.
MRI factors included diffusion-weighted imaging hyperintense lesions (DWIHLs) with associated hypointensity, or white matter hyperintensity (WMH) was evaluated visually on fluid-isointensity on apparent diffusion coefficients. White attenuated inversion recovery images were evaluated using the Fazekas scale.
The images were read and interpreted by two doctors in nuclear medicine and imaging. They were blinded to the diagnosis.
The statistical results were expressed in mean ± standard deviation.
Demographic and imaging data for the 10 subjects are summarized in Table 1. As shown in the Tables 1, 2, three CAA patients and two NC subjects had microbleeds. The CAA and AD groups had a lower MoCA score (13.67 ± 5.03, 19.00 ± 2.83) than normal controls (29.20 ± 0.45). Two NC patients had incidentally discovered CMBs; one participant had two CMBs in the white matter near the border of the left lateral ventricle, and the other participant had one CMB in the left basal ganglia. The results of the two visually raters were identical.
All AD and NC subjects were negative for [68Ga] Ga-p14-032 binding, and all CAA subjects were [68Ga] Ga-p14-032 positive by a visual read of [68Ga]Ga-p14-032 PET scans. In all three CAA patients, the regions with CMBs largely overlapped with regions that showed increased [68Ga] Ga-p14-032 uptake. It is distributed in the venous sinus, scalp, and cortex but not in the pituitary gland, white matter, brain stem, and skull. [68Ga]Ga-p14-032 images are shown for individual patients (Figure 1). Slices from a representative patient with probable CAA, AD, and a cognitive healthy control are shown. Global [68Ga]Ga-p14-032 retention was stronger in patients with CAA than those in the AD or NC groups (median SUVr 2.68 ± 1.53 vs. 1.77±0.08 and 0.08 ± 0.24) (Table 3).
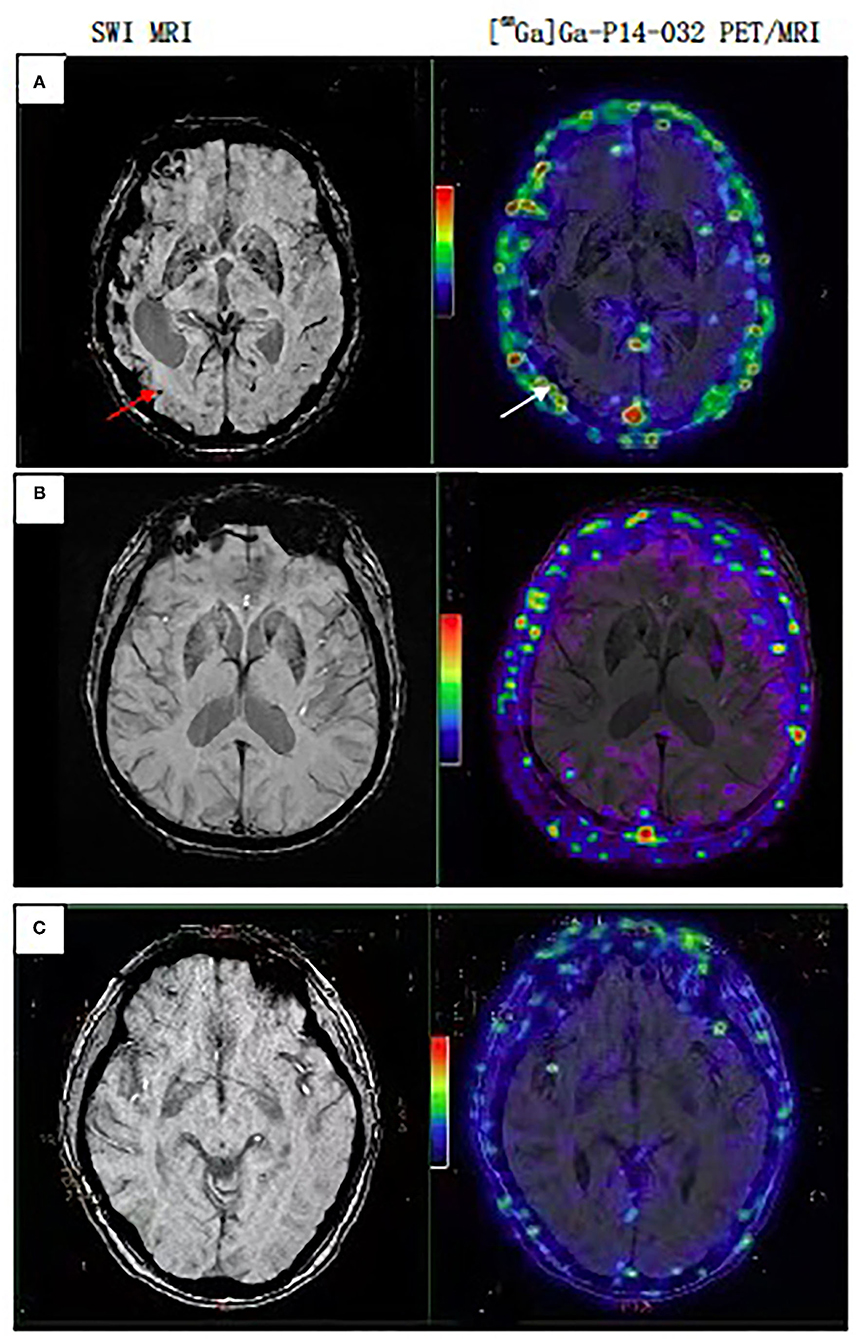
Figure 1. PET/MRI scan sets representative of CAA, AD, Normal subjects. (A) Case 7, female, 70 years, probable CAA patient, multiple CMBs on MRI, white arrow point to the same point to the same area with [68Ga]Ga-p14-032 retention on PET/MRI. (B) Case 5, male, 84 years, AD patient. (C) Case 3, female, 57 years, cognitive normal subject. Cases (B,C) show only non-specific binding in extracerebral tissues.
This was the first clinical evaluation of [68Ga]Ga-p14-032, a novel PET ligand for vascular amyloid aggregates associated with CAA. Three CAA patients, two AD patients, and five elderly NC subjects received [68Ga]Ga-p14-032. We chose the pons as the reference region to calculate SUVr instead of using the cerebellum, a more widely used region in calculating amyloid PET SUVr, to avoid the confounding from cerebellar amyloid angiopathy. We found that [68Ga]Ga-p14-032 retention was higher in regions with CMBs in CAA patients, in which vascular amyloid deposition is expected to be the highest. Additionally, the overall retention of [68Ga]Ga-p14-032 in the cerebral cortex was significantly higher in patients with CAA. AD and NC subjects did not show positive regional binding of [68Ga]Ga-p14-032.
Case 7 had a right hemispheric dominant distribution of CMBs, and the other two CAA patients had CMBs in both hemispheres. Our preliminary study raises the possibility that [68Ga]Ga-p14-032 identifies vascular Aβ in areas prone to vascular rupture (14). Other studies report that CAA and associated lobar hemorrhages exhibit a preferential posterior distribution, in which vascular amyloid deposits tend to be highest (15, 16). The local accumulations of amyloid may trigger future vessel rupture and bleeding after their initial clinical presentation (17).
This study has some limitations. First, because of the sample size, the findings should be regarded as proof-of-concept. If the sample size were larger, we should be able to get more data to analysis the differences among AD and CAA via the conventional amyloid scan (18). Second, we did not have pathological confirmation of CAA, but used the modified Boston criteria, which have high sensitivity and specificity. Third, two of the NC subjects had incidental CMBs, which are common in older populations; however, neither had a pattern highly suggestive of CAA. If incidental CAA were present, it would tend to bias toward the null, whereas we instead found a significant difference between CAA and NC. Third, our results suggest that [68Ga] Ga-p14-032 is specific for vascular amyloid rather than parenchymal amyloid because retention was seen in CAA patients, but we did not have autopsy tissue with which this could be correlated. In future studies, we plan to show in the same patients that there is a differential binding of the CAA tracer vs. a non-specific beta-amyloid tracer (such as 11C-PiB or Florbetapir). 11C-PiB or Florbetapir should produce a strong signal in AD and a moderate signal in CAA, and the CAA tracer should do the opposite. Nevertheless, our findings raised the possibility that [68Ga] Ga-p14-032 PET, which is characteristic marker of vascular amyloid, may provide new ideas or methods for diagnosis of CAA.
Aβ, amyloid-β; CAA, cerebral amyloid angiopathy; SWI, susceptibility-weighted imaging; MoCA, Montreal Cognitive Assessment; PET/MRI, Positron Emission Tomography/Magnetic Resonance Imaging; AD, Alzheimer's disease.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
The studies involving human participants were reviewed and approved by Beijing Tiantan Hospital, Beijing Cancer Hospital. The patients/participants provided their written informed consent to participate in this study.
QZ, ES, and AD studied concept, designed, revised the manuscript for intellectual content. HK, LZ, and HH labelled the PET tracer. PL obtained the funding. ZY, SW, and HZ labelled the PET tracer and analyzed the data. XZ, XM, YD, and RM analyzed the images. XZ revised the manuscript for intellectual content. LS and JP studied coordination, contributed vital reagents, tools, and patents. YW obtained the funding and revised the manuscript for intellectual content. All authors contributed to the article and approved the submitted version.
This study was carried out as a collaborative study supported by the Ministry of Science and Technology of the People's Republic of China (Grant Nos. 2018YFC1312300 and 2016YFC0901002). The funding source had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and the decision to submit the manuscript for publication.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2021.702185/full#supplementary-material
1. Greenberg SM, Bacskai BJ, Hernandez-Guillamon M, Pruzin J, Sperling R, van Veluw SJ. Cerebral amyloid angiopathy and Alzheimer disease-one peptide, two pathways. Nat Rev Neurol. (2020) 16:30–42. doi: 10.1038/s41582-019-0281-2
2. Smith EE, Maas MB. To predict recurrence in cerebral amyloid angiopathy, look to the subarachnoid space. Neurology. (2020) 94:375–6. doi: 10.1212/WNL.0000000000009029
3. Reijmer YD, van Veluw SJ, Greenberg SM. Ischemic brain injury in cerebral amyloid angiopathy. J Cereb Blood Flow Metab. (2016) 36:40–54. doi: 10.1038/jcbfm.2015.88
4. Miller-Thomas MM, Sipe AL, Benzinger TL, McConathy J, Connolly S, Schwetye KE. Multimodality review of amyloid-related diseases of the central nervous system. Radiographics. (2016) 36:1147–63. doi: 10.1148/rg.2016150172
5. Greenberg SM, Charidimou A. Diagnosis of cerebral amyloid angiopathy: evolution of the boston criteria. Stroke. (2018) 49:491–7. doi: 10.1161/STROKEAHA.117.016990
6. Johnson KA, Gregas M, Becker JA, Kinnecom C, Salat DH, Moran EK, et al. Imaging of amyloid burden and distribution in cerebral amyloid angiopathy. Ann Neurol. (2007) 62:229–34. doi: 10.1002/ana.21164
7. Yilmaz P, Ikram MA, Ikram MK, Niessen WJ, Viswanathan A, Charidimou A, et al. Application of an imaging-based sum score for cerebral amyloid angiopathy to the general population: risk of major neurological diseases and mortality. Front Neurol. (2019) 10:1276. doi: 10.3389/fneur.2019.01276
8. Charidimou A, Frosch MP, Al-Shahi Salman R, Baron JC, Cordonnier C, Hernandez-Guillamon M, et al. Advancing diagnostic criteria for sporadic cerebral amyloid angiopathy: Study protocol for a multicenter MRI-pathology validation of Boston criteria v2.0. Int J Stroke. (2019) 14:956–71. doi: 10.1177/1747493019855888
9. Charidimou A, Farid K, Baron JC. Amyloid-PET in sporadic cerebral amyloid angiopathy: a diagnostic accuracy meta-analysis. Neurology. (2017) 89:1490–8. doi: 10.1212/wnl.0000000000004539
10. Yamada M. Cerebral amyloid angiopathy: emerging concepts. J Stroke. (2015) 17:17–30. doi: 10.5853/jos.2015.17.1.17
11. Gokcal E, Horn MJ, van Veluw SJ, Frau-Pascual A, Das AS, Pasi M, et al. Lacunes, microinfarcts and vascular dysfunction in cerebral amyloid angiopathy. Neurology. (2021) 96:e1646–54. doi: 10.1212/WNL.0000000000011631
12. Castellani RJ, Smith MA, Perry G, Friedland RP. Cerebral amyloid angiopathy: major contributor or decorative response to Alzheimer's disease pathogenesis. Neurobiol Aging. (2004) 25:599–602; discussion 603–4. doi: 10.1016/j.neurobiolaging.2003.12.019
13. Zha Z, Song J, Choi SR, Wu Z, Ploessl K, Smith M, et al. 68Ga-bivalent polypegylated styrylpyridine conjugates for imaging Aβ plaques in cerebral amyloid angiopathy. Bioconjug Chem. (2016) 27:1314–23. doi: 10.1021/acs.bioconjchem.6b00127
14. Zha Z, Choi SR, Ploessl K, Lieberman BP, Qu W, Hefti F, et al. Multidentate (18)F-polypegylated styrylpyridines as imaging agents for Aβ plaques in cerebral amyloid angiopathy (CAA). J Med Chem. (2011) 54:8085–98. doi: 10.1021/jm2009106
15. Grothe MJ, Barthel H, Sepulcre J, Dyrba M, Sabri O, Teipel SJ, et al. In vivo staging of regional amyloid deposition. Neurology. (2017) 89:2031–8. doi: 10.1212/WNL.0000000000004643
16. Planton M, Saint-Aubert L, Raposo N, Payoux P, Salabert AS, Albucher JF, et al. Florbetapir regional distribution in cerebral amyloid angiopathy and Alzheimer's disease: a PET study. Alzheimers Dis. (2020) 73:1607–14. doi: 10.3233/JAD-190625
17. Gurol ME, Dierksen G, Betensky R, Gidicsin C, Halpin A, Becker A, et al. Predicting sites of new hemorrhage with amyloid imaging in cerebral amyloid angiopathy. Neurology. (2012) 79:320–6. doi: 10.1212/WNL.0b013e31826043a9
Keywords: cerebral amyloid angiopathy, amyloid-β, PET tracer, Alzheimer's disease, PET/MRI
Citation: Zhang Q, Zhao X, Lei P, Kung HF, Yang Z, Zhu L, Wang S, Zhu H, Meng X, Duan Y, Sun L, Pan J, Ma R, Hong H, Zhao X, Demchuk A, Smith EE and Wang Y (2021) Evaluating [68Ga]Ga-p14-032 as a Novel PET Tracer for Diagnosis Cerebral Amyloid Angiopathy. Front. Neurol. 12:702185. doi: 10.3389/fneur.2021.702185
Received: 29 April 2021; Accepted: 23 September 2021;
Published: 27 October 2021.
Edited by:
Dusko Kozic, University of Novi Sad, SerbiaReviewed by:
Qing Ye, Nanjing Drum Tower Hospital, ChinaCopyright © 2021 Zhang, Zhao, Lei, Kung, Yang, Zhu, Wang, Zhu, Meng, Duan, Sun, Pan, Ma, Hong, Zhao, Demchuk, Smith and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yongjun Wang, eW9uZ2p1bndhbmdAbmNyY25kLm9yZy5jbg==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.