- 1Department of Neurology, Beijing Tiantan Hospital, Capital Medical University, Beijing, China
- 2China National Clinical Research Center for Neurological Diseases, Beijing, China
- 3Research Unit of Artificial Intelligence in Cerebrovascular Disease, Chinese Academy of Medical Sciences, Beijing, China
Background: The relationship between serum lipids levels and prognosis after spontaneous intracerebral hemorrhage (ICH) is still unclear. We aim to examine the association between lipid levels and 3-month ICH prognosis in women.
Method: We went through a registry of spontaneous ICH cases and selected female patients to study according to our criteria. We collected demographic, clinical, and laboratory information and evaluated serum triglyceride (TG) levels, total cholesterol (TC) levels, low-density cholesterol (LDLC) levels, high-density cholesterol (HDLC) levels, non-high-density cholesterol (non-HDLC) levels, and 3-month modified Rankin Scale (mRS). Multivariate logistic regression was performed, and receiver operating characteristic (ROC) curves were plotted to explore the relationship between serum lipid levels and 3-month ICH clinical outcomes.
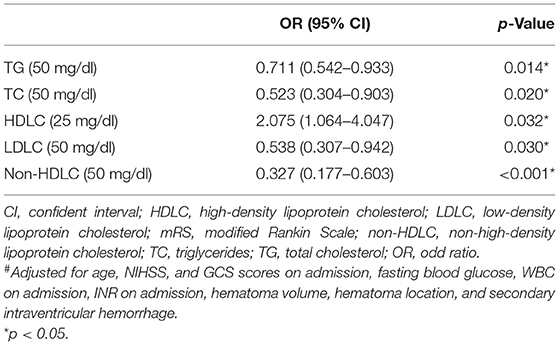
Results: Two hundred six female patients were included in this study, and 96 (46.6%) of them had poor functional outcomes. In the univariate analysis, low TG (p = 0.006), TC (p = 0.025), LDLC (p = 0.001), non-HDLC (p < 0.001) levels, and high HDL (p = 0.036) levels were associated with poor 3-month clinical outcomes in women. In the multivariate logistic regression, low levels of TG (OR = 0.711, 95% CI = 0.542–0.933, p = 0.014), TC (OR = 0.523, 95% CI = 0.304–0.903, p = 0.020), LDLC (OR = 0.538, 95% CI = 0.307–0.942, p = 0.030), non-HDLC (OR = 0.327, 95% CI = 0.177–0.603, p < 0.001), and a high level of HDLC (OR = 2.075, 95% CI = 1.064–4.047, p = 0.032) with area under the curve (AUC) of 0.610, 0.590, 0.630, 0.645, and 0.415, respectively, remained as independent indicators of poor prognosis at 3 months after adjusting for confounding factors.
Conclusion: Low levels of TG, TC, LDLC, non-HDLC, and high levels of HDLC were independently associated with poor prognosis of spontaneous ICH in women.
Introduction
Spontaneous intracerebral hemorrhage (ICH) is a neurological disease with a high mortality and morbidity (1). The incidence rate was about 10 to 30 per 100,000 people (2). According to the global, regional, and country-specific lifetime risk of stroke 1990–2016, Chinese men have the greatest lifetime stroke risk [41.1% (39.2–42.9)], and the difference in risk between men [41.1% (39.2–42.9)] and women [36.7% (35.0–38.6]) was also the largest (3). Stroke is the third leading cause of death in China (4). All over the world, ICH accounts for 10–15% of total strokes each year. The fatality rate within 1 month after ICH can be as high as 40.4% (1). Only a small proportion (12–39%) of survivors could achieve functional independence (5).
While high levels of serum total cholesterol (TC) and low-density cholesterol (LDLC) are closely related to an increased risk of ischemic stroke (6–8), the association between lipoprotein cholesterol levels and intracerebral hemorrhage (ICH) remains controversial. In the Korea Medical Insurance Corporation Study, a low TC level was not an independent risk factor for ICH in 114,793 men (9). However, in a prospective study of the relationship between serum total cholesterol and stroke incidence, it was found that higher serum TC levels in women often indicated a lower risk of ICH. After adjusting for confounding factors, the hazard ratios (HR) for different levels of TC [<5(reference), 5–5.9, 6–6.0, and ≥7.0 mmol/L) were 1.00, 0.58, 0.61, and 0.50, respectively (p trend = 0.02) (10). Since lipid-lowering strategies are widely used for the prevention of cardiovascular diseases, we are interested in whether low lipid levels will increase ICH risk. Previous studies observed that low serum lipid levels may increase the mortality of ICH patients (11–14); however, a few studies have focused on the relationship between women's serum lipid levels and the occurrence and prognosis of ICH. Studies have shown that women have a higher burden of stroke compared with men (15), which means that it is particularly important to be concerned of the risk factors among women. Currently, there has not been any research on the association between serum lipid level and the prognosis of female patients.
Therefore, in this study, we aim to identify the association between serum lipid levels, including TC, LDLC, triglycerides (TG), non-high-density cholesterol (non-HDLC), and HDLC, and the prognosis of ICH in a prospective cohort of women in this study.
Methods
All patients participating in this study provided written informed consent. The study protocol was approved by the Institutional Review Board of the Beijing Tiantan Hospital affiliated to Capital Medical University, and this study was approved by the Beijing Tiantan Hospital Ethics Committee.
Our analysis was performed using data from a multicenter, prospective, and observational cohort study (Registration Study on the Medical Quality Evaluation of Cerebral Hemorrhage Based on Etiology in the Beijing Area. The main aim of that cohort study was to analyze the gender and age distribution of different ICH etiologies and the medical conditions of ICH in the Beijing area). A total of 13 hospitals in Beijing participated in the study. Eventually, 1,964 eligible ICH patients were included in the database (16). The design of the database was outlined as described by Feng et al. (16). For our study, we specifically focused on female patients in the ICH database.
Inclusion and Exclusion Criteria
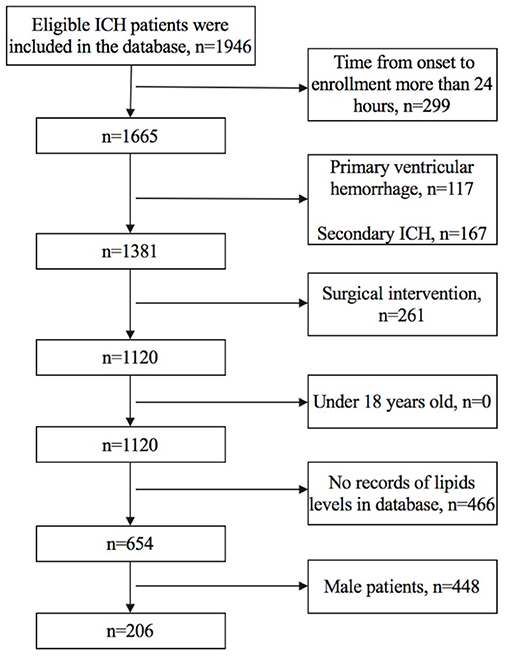
We included female patients whose (1) time from onset to enrollment was not more than 24 h, (2) TG, TC, LDLC, HDLC, and non-HDLC levels were all documented in the database, and (3) age was older than 18 years old. We excluded patients who had (1) primary ventricular hemorrhage; (2) diagnosis of secondary ICH, including head trauma, brain tumor, aneurysm, cavernous hemangioma, arteriovenous malformations, acute thrombolysis, coagulopathy, and moyamoya disease; (3) surgical intervention, including extraventricular drainage, craniotomy, hematoma puncture, and aspiration during the follow-up; and (4) anticoagulant therapy before the onset of symptoms. Figure 1 shows details regarding the selection of patients.
Baseline Information
By using standard questionnaires, trained stroke physicians collected demographic information, past medical history, and past medications for each enrolled patient, and conducted clinical evaluations of the patients' neurological function. Arterial hypertension was diagnosed when documented in medical records or when at least two blood pressure readings were higher than 140 mmHg (systolic) or 90 mmHg (diastolic) after the acute phase of stroke. Diabetes was recorded when a patient had known diabetes mellitus at presentation, or the plasma glucose level was higher than 11 mmol/L on admission or during hospital stay. Cigarette smoking was classified as never, previous, or current smokers based on daily tobacco consumption. Alcohol intake was classified as current drinker or non-drinker.
The variables that were collected included age, past medical history, National Institutes of Health Stroke Scale (NIHSS) at admission, Glasgow Coma Scale (GCS) at admission, blood pressure level at admission, and secondary intraventricular hemorrhage. On the initial CT scan, we evaluated ICH localization and volume (ABC/2 method). Routine blood sampling and testing were performed within 1 h of the patients' arrival. Fasting blood samples were collected from an antecubital vein on the morning after arrival following an overnight fasting (>8 h), and serum TG, TC, HDLC, and LDLC levels were recorded. Non-HDLC levels were calculated by subtracting serum HDLC levels from serum TC levels. We also documented prior antiplatelet/statin use and statin use after admission.
Follow-Up Information and Intracerebral Hemorrhage Clinical Outcomes
The 3-month modified Rankin Scale (mRS) scores of the patients were assessed via telephone interviews. The interviewers were trained on standard protocol and were blinded to the patients' clinical, laboratory, and imaging information during the follow-up evaluation. If we were not able to reach the patients on the first telephone interview attempt, we then telephoned them once a week for up to three more weeks. The patient was considered unreachable if none of the four telephone interview attempts were successful. An mRS ≥3 was regarded as a poor prognosis, and mRS <3 was regarded as a good prognosis.
Statistical Analyses
For continuous variables, we use mean values together with their corresponding standard deviations (SDs) to describe the normally distributed data, and median [interquartile range (IQR)] to describe non-normally distributed data. Continuous variables were compared using Student's t-test or Mann–Whitney U-test as appropriate. Chi-squared test was applied to compare categorical variables. Multivariate analysis with the enter method logistic regression model was performed to determine the independent predictors. Receiver operating characteristic curves (ROC) were plotted to assess the discriminative predictive value of the lipid levels. The areas under the receiver operating characteristic curves (AUC) were calculated and compared. All tests of significance were two tailed. A p-value < 0.05 was set as the significance level for all analyses. SPSS software (version 24.0; IBM, Armonk, NY, USA) was the software program used to perform the statistical analyses.
Results
We finally included 206 female patients with spontaneous ICH (Figure 1). The median age was 62 (52–71) years old, ranging from 25 to 91 years old, 96 patients (46.6%) had poor prognosis at 3 months (mRS score of 3–6), the median time from onset to admission was 2.87 (1.50–5.67) h, 18 patients (8.7%) had hyperlipidemia, and 10 (4.9%) patients had prior statin use before admission. The median volume of hematoma was 8.85 (4.10–18.68) ml, and 178 (86.4%) subjects had supratentorial ICH (Table 1).
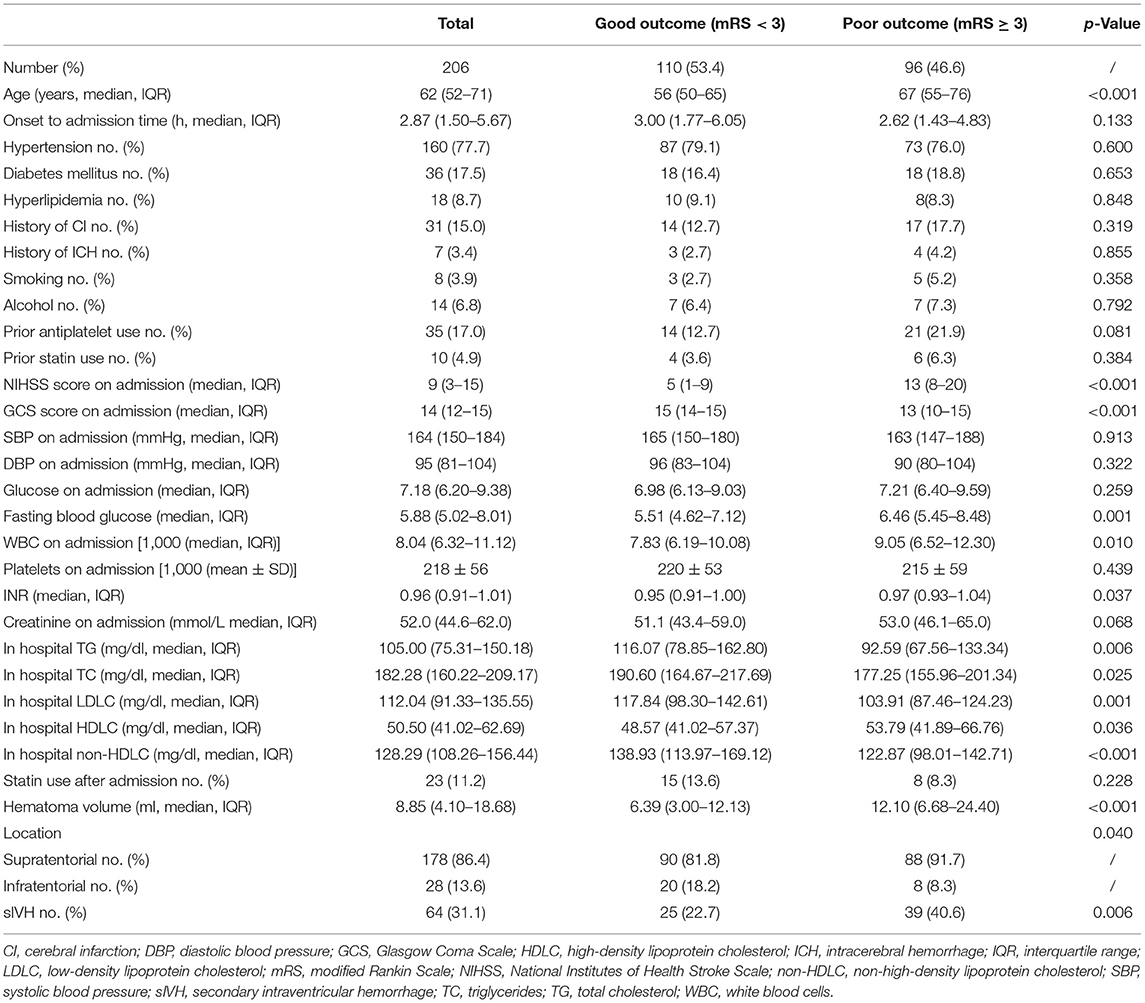
Table 1. Comparison of characteristics between female patients with good and poor 3-month clinical outcome.
Predictors of Poor 3-Month Outcome in Female Patients With Spontaneous Intracerebral Hemorrhage Patients
Female ICH patients who had poor 3-month prognosis (mRS ≥ 3) were older (p < 0.001) and had higher NIHSS (p < 0.001), lower GCS (p < 0.001), larger hematoma volume (p < 0.001), higher proportion of secondary intraventricular hemorrhage (p < 0.001), higher levels of fasting blood glucose (p = 0.001), higher levels of white blood cell count (WBC) on admission (p = 0.010), and higher international normalized ratio (INR) (p = 0.037). We found that female ICH patients with mRS ≥ 3 had increased levels of HDLC (p = 0.036) and decreased levels of TC (p = 0.025), TG (p = 0.006), LDLC (p = 0.001), and non-HDLC (p < 0.001). There was no significant association between statin use and 3-month prognosis (Table 1).
In multivariate logistic analysis (Table 2), low levels of TG (OR = 0.711, 95% CI = 0.542–0.933, p = 0.014), TC (OR = 0.523, 95% CI = 0.304–0.903, p = 0.020), LDLC (OR = 0.538, 95% CI = 0.307–0.942, p = 0.030), non-HDLC levels (OR = 0.327, 95% CI = 0.177–0.603, p < 0.001), and high levels of HDLC (OR = 2.075, 95% CI = 1.064–4.047, p < 0.032) remained as independent indicators of poor clinical outcomes after adjusting for age, NIHSS, GCS scores on admission, fasting blood glucose, WBC on admission, INR on admission, hematoma volume, hematoma location, and secondary intraventricular hemorrhage.
Sensitivity and Specificity Tests of Lipid Levels for 3-Month Clinical Outcome in Female Patients With Spontaneous Intracerebral Hemorrhage Patients
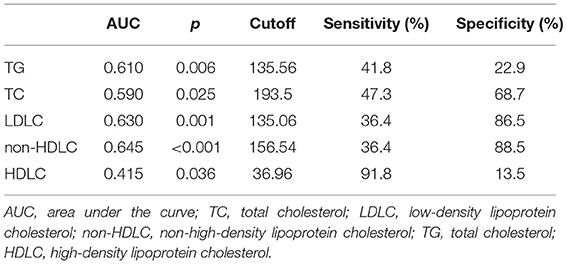
We further, studied the accuracy of the predictive value and the cutoff value of the clinical outcomes (Table 3). The sensitivity and specificity of TG, TC, LDLC, non-HDLC, and HDLC were 41.8, 47.3, 36.4, 36.4, and 91.8%, and 22.9, 68.7, 86.5, 88.5, and 13.5%, respectively. The ROC was generated (Figure 2), and the AUCs were calculated for TG, TC, LDLC, non-HDLC, and HDLC in relation to clinical outcomes (0.610, 0.590, 0.630, 0.645, and 0.415, respectively; Table 3). Although, the AUC of TG reached 0.61, its sensitivity and specificity were very low (41.8 and 22.9%). The sensitivity of HDLC was as high as 91.8%, but its specificity was very low (13.5%). Compared with LDLC levels and TC levels, non-HDLC levels have higher predictive value for 3-month clinical outcomes in female patients with spontaneous ICH.
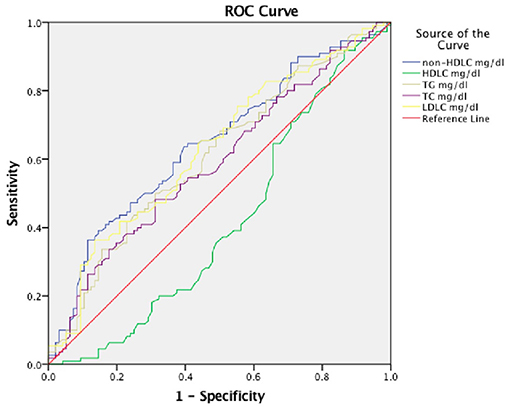
Figure 2. Receiver operating characteristic (ROC) curves for 3-month prognosis according to lipid profiles. TC, total cholesterol; LDLC, low-density lipoprotein cholesterol; non-HDLC, non-high-density lipoprotein cholesterol; TG, total cholesterol; HDLC, high-density lipoprotein cholesterol.
Discussion
Our study evaluated the association between serum lipid levels in female ICH patients and the 3-month prognosis. Our results suggested that low TG, TC, LDLC, non-HDLC levels, and high HDLC levels in female patients indicated a poor prognosis at 3 months.
Although, previous literatures have reported the relationship between serum lipid levels and the risk of ICH (13, 14, 17), a few studies have explored whether serum lipid levels affect the prognosis of patients with ICH. In a previous study of 2,444 ICH patients from Taiwan, patients with low TC levels (<160 mg/dl) were more likely to have a poor 90-day prognosis (OR = 1.41, 95% CI = 1.11–1.78) (18). Another study found that ICH patients with low serum TC levels (≤ 150 mg/dl) had their mRS scores worsen (OR = 3.3, 95% CI = 1.33–8.00) at 30 days from onset (19). Excessive lowering of serum TC levels may cause unfavorable clinical outcomes in ICH recovery (19). In our study, the cutoff TC predictive value for ICH poor prognosis was 156.54 mg/dl, and this finding suggested that patients with TC levels lower than 156.54 mg/dl may have a poor prognosis. Our results were consistent with previous studies, which found that low serum lipid levels were associated with poor ICH outcome.
In 2009, Ramirez-Moreno et al. (11) suggested that low LDLC levels were independently associated with death after ICH (HR = 3.07, 95% CI = 1.04–9.02, p = 0.042). The association between low LDLC levels and the death after ICH was confirmed by other studies (12, 20, 21). A previous study suggested that low LDLC levels were not associated with poor mRS after ICH (12); however, we found that low-level LDLC was an independent risk factor for poor prognosis in female patients after ICH. The difference could be caused by differences in population and gender. In our study, we also reported the relationship between low non-HDLC levels and poor prognosis in female patients with ICH, which was consistent with our previous study where we analyzed ICH patients of both genders (16). In our study, we found that low TG levels and high HDLC levels were associated with poor prognosis in female patients, and this result conflicted with previous studies (12, 18, 22, 23). The reason might be that TG and HDLC only affected the prognosis of female ICH patients and had no association with the prognosis of male ICH patients, while previous studies did not specifically analyze female patients.
A study by Zhang et al. (10) showed that TC levels were positively correlated with the risk of all types of stroke and ischemic stroke in men, and were negatively correlated with the risk of ICH in women. They did not find any association between TC levels and ICH risk in men. This reminded us that the effect of cholesterol levels on blood vessels in men and women may vary, and the mechanism deserves further study. The most common cause of ICH was microaneurysm ruptures caused by hypertension, and the most vulnerable arteries were the penetrating arteries because those arterial walls sustain higher stresses. Previous studies had put forward the inference that low levels of serum cholesterol may reduce atherosclerosis in large proximal intracranial arteries, which exposed the distal penetrating arteries to higher wall stress (24). Based on the above theory, we can speculate that the distal penetrating artery around the hematoma in ICH patients with low baseline cholesterol levels is more prone to ischemic damage, which leads to a poorer clinical prognosis.
We demonstrated that low non-HDLC levels and low LDLC levels predicted poor clinical outcomes in female ICH patients with high specificity. Although, HDLC had a high sensitivity, its specificity was so low that we surmised that it had no clinical predictive value. The sensitivity and specificity of TG were very low; thus, we considered that it did not have high clinical value in predicting prognosis.
In our study, we did not find significant differences in the statin use between the poor and good prognosis groups. Statins were mainly used for the prevention of ischemic cerebrovascular diseases. Lipid-lowering therapy was associated with an increased ICH risk in secondary prevention trials (OR = 1.18, 95% CI = 1.00–1.38), but not in primary prevention trials (OR = 1.01; 95% CI = 0.78–1.30). The benefits of lipid-lowering therapy in preventing ischemic stroke greatly outweighed the possible increased risk of ICH. Based on our study results, stroke clinicians do not need to refrain from prescribing lipid-lowering treatments for secondary prevention of ischemic stroke due to concerns about increased risk of cerebral hemorrhage is not recommended (25).
There were some potential limitations in our study. First, we did not continuously monitor changes in serum lipid levels during follow-up continuously. The patients' cholesterol levels may have varied over time and that may have impacted the prognosis. Second, the percentage of pre-ICH lipid-lowering therapy was too small. Finally, we recruited patients from the emergency department, and we were unable to complete the collection of the blood lipid information for some patients due to the deterioration of their condition or transfer to other hospitals, which might affect the patients' selection for our analyses.
Conclusion
Our study suggested that lipid levels at the presentation of ICH were associated with 3-month prognosis in women, especially when the TG <135.56, TC <193.50, LDLC <135.06, non-HDLC <156.54, and HDLC > 36.96 mg/dl. Low levels of TG, TC, LDLC, non-HDLC, and high levels of HDLC are independently associated with the poor prognosis of spontaneous ICH in women.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.
Ethics Statement
The studies involving human participants were reviewed and approved by the study protocol was approved by the Institutional Review Board of the Beijing Tiantan Hospital affiliated to Capital Medical University and the Beijing Tiantan Hospital Ethics Committee approved this study. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
HF, XW, and XZ designed the study and drafted the manuscript. HF and WW collected the clinical data and managed the database. All authors approved for the manuscript submitted.
Funding
This study is supported by the National Science and Technology Major Project (2017ZX09304018), CAMS Innovation Fund for Medical Sciences (2019-I2M-5-029), Beijing Municipal Committee of Science and Technology (Z201100005620010), and National Key Research and Development Program of China (Grant Nos. 2018YFC1312200 and 2018YFC1312204). XW was supported by China Postdoctoral Science Foundation (No. 2017M620835) and Beijing Postdoctoral Research Foundation (2017-22-119).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank all the participants for their important contributions to our study. This manuscript has not been published elsewhere in whole or in part.
References
1. van Asch CJ, Luitse MJ, Rinkel GJ, van der Tweel I, Algra A, Klijn JC. Incidence, case fatality, and functional outcome of intracerebral haemorrhage over time, according to age, sex, and ethnic origin: a systematic review and meta-analysis. Lancet Neurol. (2010) 9:167–76. doi: 10.1016/S1474-4422(09)70340-0
2. Qureshi AI, Mendelow AD, Hanley DF. Intracerebral haemorrhage. Lancet. (2009) 373:1632–44. doi: 10.1016/S0140-6736(09)60371-8
3. Feigin VL, Nguyen G, Cercy K, Johnson CO, Alam T, Parmar PG, et al. Global, regional, and country-specific lifetime risks of stroke, 1990 and 2016. N Engl J Med. (2018) 379:2429–37. doi: 10.1056/NEJMoa1804492
4. Wang YJ, Li ZX, Gu HQ, Zhai Y, Jiang Y, Zhao XQ, et al. China stroke statistics writing: China stroke statistics 2019: a report from the National Center for Healthcare Quality Management in neurological diseases, China National Clinical Research Center for neurological diseases, the Chinese Stroke Association, National Center for Chronic and Non-communicable Disease Control and Prevention, Chinese Center for Disease Control and Prevention and Institute for Global Neuroscience and Stroke Collaborations. Stroke Vasc Neurol. (2020) 5:211–39. doi: 10.1136/svn-2020-000457
5. An SJ, Kim TJ, Yoon BW. Epidemiology, risk factors, and clinical features of intracerebral hemorrhage: an update. J Stroke. (2017) 19:3–10. doi: 10.5853/jos.2016.00864
6. Kurth T, Everett BM, Buring JE, Kase CS, Ridker PM, Gaziano JM. Lipid levels and the risk of ischemic stroke in women. Neurology. (2007) 68:556–62. doi: 10.1212/01.wnl.0000254472.41810.0d
7. Berger JS, McGinn AP, Howard BV, Kuller L, Manson JE, Otvos J, et al. Lipid and lipoprotein biomarkers and the risk of ischemic stroke in postmenopausal women. Stroke. (2012) 43:958–66. doi: 10.1161/STROKEAHA.111.641324
8. Amarenco P, Kim JS, Labreuche J, Charles H, Abtan J, Bejot Y, et al. Treat stroke to target: a comparison of two LDL cholesterol targets after ischemic stroke. N Engl J Med. (2020) 382:9. doi: 10.1056/NEJMoa1910355
9. Suh I Jee SH Kim HC Nam CM Kim IS Appel LJ. Low serum cholesterol and haemorrhagic stroke in men: Korea Medical Insurance Corporation Study. Lancet. (2001) 357:922–5. doi: 10.1016/S0140-6736(00)04213-6
10. Zhang Y, Tuomilehto J, Jousilahti P, Wang Y, Antikainen R, Hu G. Total and high-density lipoprotein cholesterol and stroke risk. Stroke. (2012) 43:1768–74. doi: 10.1161/STROKEAHA.111.646778
11. Ramirez-Moreno JM, Casado-Naranjo I, Portilla JC, Calle ML, Tena D, Falcon A, et al. Serum cholesterol LDL and 90-day mortality in patients with intracerebral hemorrhage. Stroke. (2009) 40:1917–20. doi: 10.1161/STROKEAHA.108.536698
12. Rodriguez-Luna D, Rubiera M, Ribo M, Coscojuela P, Pagola J, Pineiro S, et al. Serum low-density lipoprotein cholesterol level predicts hematoma growth and clinical outcome after acute intracerebral hemorrhage. Stroke. (2011) 42:2447–52. doi: 10.1161/STROKEAHA.110.609461
13. Phuah L, Raffeld MR, Ayres AM, Viswanathan A, Greenberg SM, Biffi A, et al. Subacute decline in serum lipids precedes the occurrence of primary intracerebral hemorrhage. Neurology. (2016) 86:2034–41. doi: 10.1212/WNL.0000000000002716
14. Wieberdink RG, Poels MMF, Vernooij MW, Koudstaal PJ, Hofman A, van der Lugt A, et al. Serum lipid levels and the risk of intracerebral hemorrhage: the Rotterdam Study. Arterioscler Thromb Vasc Biol. (2011) 31:2982–9. doi: 10.1161/ATVBAHA.111.234948
15. Reeves MJ, Bushnell CD, Howard G, Gargano JW, Duncan PW, Lynch G, et al. Sex differences in stroke: epidemiology, clinical presentation, medical care, and outcomes. Lancet Neurol. (2008) 7:915–26. doi: 10.1016/S1474-4422(08)70193-5
16. Feng H, Wang X, Wang W, Zhao X. Association between non-high-density lipoprotein cholesterol and 3-month prognosis in patients with spontaneous intracerebral hemorrhage. Front Neurol. (2020) 11:920. doi: 10.3389/fneur.2020.00920
17. Wang X, Dong Y, Qi X, Huang C, Hou L. Cholesterol levels and risk of hemorrhagic stroke: a systematic review and meta-analysis. Stroke. (2013) 44:1833–9. doi: 10.1161/STROKEAHA.113.001326
18. Chen YW, Li CH, Yang CD, Liu CH, Chen CH, Sheu JJ, et al. Taiwan stroke registry: low cholesterol level associated with severity and outcome of spontaneous intracerebral hemorrhage: results from Taiwan Stroke Registry. PLoS ONE. (2017) 12:e0171379. doi: 10.1371/journal.pone.0171379
19. Miura K, Yoshii Y, Nakamura Y, Ikeda K. Clinicoradiological profile and serum lipid levels of intracerebral hemorrhage in prior statin users. Intern Med. (2011) 50:1385–91. doi: 10.2169/internalmedicine.50.5144
20. Chang JJ, Katsanos AH, Khorchid Y, Dillard K, Kerro A, Burgess LG, et al. Higher low-density lipoprotein cholesterol levels are associated with decreased mortality in patients with intracerebral hemorrhage. Atherosclerosis. (2018) 269:14–20. doi: 10.1016/j.atherosclerosis.2017.12.008
21. Mustanoja S, Strbian D, Putaala J, Meretoja A, Curtze S, Haapaniemi E, et al. Association of prestroke statin use and lipid levels with outcome of intracerebral hemorrhage. Stroke. (2013) 44:2330–2. doi: 10.1161/STROKEAHA.113.001829
22. Liu Q, Zhao W, Xing Y, Hong Y, Zhou G. Low triglyceride levels are associated with unfavorable outcomes in patients with spontaneous intracerebral hemorrhage. Neurocrit Care. (2020) 34:218–26. doi: 10.1007/s12028-020-01023-0
23. Liu J, Wang D, Yuan R, Xiong Y, Liu M. Prognosis of 908 patients with intracerebral hemorrhage in Chengdu, Southwest of China. Int J Neurosci. (2017) 127:586–91. doi: 10.1080/00207454.2016.1216414
24. Masawa N, Yoshida Y, Yamada T, Joshita T, Sato S, Mihara B. Morphometry of structural preservation of tunica media in aged and hypertensive human intracerebral arteries. Stroke. (1994) 25:122–7. doi: 10.1161/01.STR.25.1.122
Keywords: spontaneous intracerebral hemorrhage, 3-month prognosis, women, total cholesterol, non-high-density cholesterol, low-density cholesterol, triglycerides, high-density cholesterol
Citation: Feng H, Wang X, Wang W and Zhao X (2021) Lipid Levels and 3-Month Prognosis After Spontaneous Intracerebral Hemorrhage in Women. Front. Neurol. 12:690194. doi: 10.3389/fneur.2021.690194
Received: 02 April 2021; Accepted: 19 May 2021;
Published: 17 June 2021.
Edited by:
Nishant K. Mishra, University of California, Los Angeles, United StatesReviewed by:
Archana Hinduja, The Ohio State University, United StatesTom Moullaali, University of Edinburgh, United Kingdom
Copyright © 2021 Feng, Wang, Wang and Zhao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xingquan Zhao, enhxQHZpcC4xNjMuY29t
†These authors have contributed equally to this work
 Hao Feng
Hao Feng Xin Wang
Xin Wang Wenjuan Wang
Wenjuan Wang Xingquan Zhao
Xingquan Zhao