- Department of Medicine and Neurology, Royal Melbourne Hospital, University of Melbourne, Parkville, VIC, Australia
One in five ischaemic strokes affects the posterior circulation. Basilar artery occlusion is a type of posterior circulation stroke associated with a high risk of disability and mortality. Despite its proven efficacy in ischaemic stroke more generally, alteplase only achieves rapid reperfusion in ~4% of basilar artery occlusion patients. Tenecteplase is a genetically modified variant of alteplase with greater fibrin specificity and longer half-life than alteplase, which can be administered by intravenous bolus. The single-bolus administration of tenecteplase vs. an hour-long alteplase infusion is a major practical advantage, particularly in “drip and ship” patients with basilar artery occlusion who are being transported between hospitals. Other practical advantages include its reduced cost compared to alteplase. The EXTEND-IA TNK trial demonstrated that tenecteplase led to higher reperfusion rates prior to endovascular therapy (22 vs. 10%, non-inferiority p = 0.002, superiority p = 0.03) and improved functional outcomes (ordinal analysis of the modified Rankin Scale, common odds ratio 1.7, 95% CI 1.0–2.8, p = 0.04) compared with alteplase in large-vessel occlusion ischaemic strokes. We recently demonstrated in observational data that tenecteplase was associated with increased reperfusion rates compared to alteplase prior to endovascular therapy in basilar artery occlusion [26% (n = 5/19) of patients thrombolysed with TNK vs. 7% (n = 6/91) thrombolysed with alteplase (RR 4.0 95% CI 1.3–12; p = 0.02)]. Although randomized controlled trials are needed to confirm these results, tenecteplase can be considered as an alternative to alteplase in patients with basilar artery occlusion, particularly in “drip and ship” patients.
Introduction
One in five ischaemic strokes affects the posterior circulation (1). This type of stroke is associated with a high risk of recurrence, disability, and mortality (2). It has been over 25 years since the publication of the National Institute of Neurological Disorders and Stroke (NINDS) tPA trial (3), the first large positive clinical trial of recombinant tissue plasminogen activator (tPA or alteplase) in ischaemic stroke patients. Despite accounting for 20% of all strokes (1), only 5% of patients from the NINDS study (3) had a posterior circulation stroke and these patients were underrepresented in most of the positive clinical trials of alteplase (4–7). However, several observational studies have demonstrated comparable efficacy and safety profiles in patients with anterior and posterior circulation stroke treated with alteplase. Several studies have also suggested a lower risk of haemorrhagic complications in posterior circulation compared to anterior circulation strokes (8–13). The lower risk of haemorrhagic transformation in posterior circulation stroke may be explained by a stronger tolerance to the ischaemic insult in the posterior circulation territory, likely due to its greater proportion of white matter and collateral pathways, particularly in the brainstem (14). Furthermore, the lower infarct volume in posterior circulation stroke compared to anterior circulation stroke may result in lower bleeding risk in these patients (15). Basilar artery occlusion is a type of posterior circulation stroke associated with a high risk of disability and mortality (16, 17). However, clinical outcomes in basilar artery occlusion improve if reperfusion is achieved. Despite its proven efficacy in ischaemic stroke more generally, alteplase only achieves rapid reperfusion in ~4% of basilar artery occlusion patients (18).
Tenecteplase In Ischaemic Stroke
Tenecteplase is a genetically modified variant of alteplase with greater fibrin specificity and longer half-life than alteplase (22 min for tenecteplase compared to 3–5 min for alteplase) (19), which can be administered by intravenous bolus without the need for the 1-h infusion of alteplase. Tenecteplase has been approved for acute myocardial infarction and was demonstrated to be superior to alteplase (20, 21). The first trial testing tenecteplase in stroke was an open-label, dose-escalation safety study comparing 0.1, 0.2, 0.4, and 0.5 mg/kg in n = 88 ischaemic stroke patients within 3 h (22). Although enrolment into the dose used for myocardial infarction (0.5 mg/kg) was closed prematurely due to the high risk of symptomatic intracranial hemorrhage, the doses of 0.1 to 0.4 mg/kg appeared safe in ischaemic stroke (22). Nonetheless, the 0.4-mg/kg dose tier in a subsequent phase 2b study was terminated due to safety concerns (23). In 2012, Parsons et al. completed a randomized phase IIb study in which they compared tenecteplase 0.1 mg/kg (n = 25 patients) and 0.25 mg/kg (n = 25 patients) to alteplase 0.9 mg/kg (n = 25 patients) in a cohort of ischaemic stroke patients with large-vessel occlusion and visually assessed salvageable tissue on CT perfusion, within 6 h of symptom onset (24). In this trial, which preceded the use of endovascular thrombectomy, the pooled tenecteplase groups had greater reperfusion (p = 0.004) and better outcomes (modified Rankin Score 0–2, 72 vs. 44%, p = 0.02) than the alteplase group. Tenecteplase was associated with increased reperfusion, early neurological improvement, and improved 3-month functional outcome with a strong dose-dependent relationship, with the 0.25-mg/kg dose achieving better efficacy outcomes compared to 0.1 mg/kg, and no increase in symptomatic intracerebral hemorrhage (24). A subsequent phase II trial compared tenecteplase 0.25 mg/kg to alteplase 0.9 mg/kg in n = 104 ischaemic stroke patients within 4.5 h of symptom onset. No significant differences were found for the primary endpoint of percentage of penumbra salvaged (68% [SD 28] in the tenecteplase group vs. 68% [SD 23] in the alteplase group; mean difference 1.3% [95% CI −9.6 to 12.1]; p = 0·81) (25). However, a subsequent pooled analysis of these two trials demonstrated that treatment with tenecteplase was associated with greater early clinical improvement (median National Institutes of Health Stroke Scale score change: tenecteplase, 6; alteplase, 1; p < 0.001) and better functional outcomes (modified Rankin scale score 0–1: odds ratio, 2.33; 95% CI, 1.13–5.94; p = 0.032) than those treated with alteplase, with the greatest benefit seen in patients with a CT perfusion-defined target mismatch (26). Furthermore, patients with anterior circulation large-vessel occlusion treated with tenecteplase had higher recanalization rates at 24 h (71% for tenecteplase vs. 43% for alteplase, p = 0.001) and significantly better functional outcomes (modified Rankin scale score 0–1: odds ratio 4.82, 95% confidence interval 1.02–7.84, p = 0.05) than patients treated with alteplase (27). Patients with basilar artery occlusion were not included in these trials.
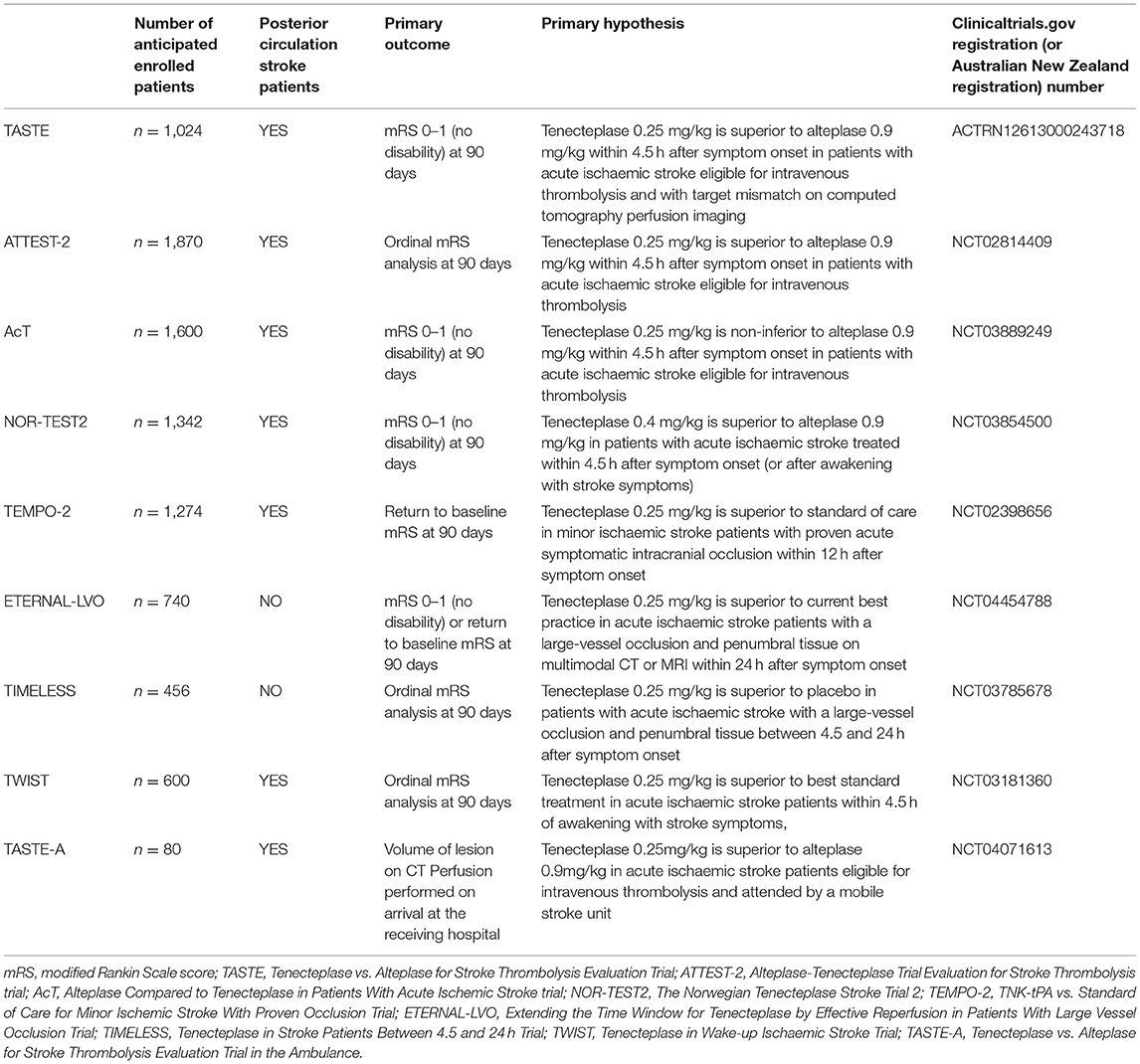
The Tenecteplase vs. Alteplase before Endovascular Therapy for Ischemic Stroke (EXTEND-IA TNK) trial compared tenecteplase 0.25 mg/kg to alteplase prior to endovascular therapy in n = 202 patients with large-vessel occlusion (28). In this trial, tenecteplase led to higher reperfusion rates prior to endovascular therapy (22 vs. 10%, non-inferiority p = 0.002, superiority p = 0.03) and improved functional outcomes (ordinal analysis of the modified Rankin Scale, common odds ratio 1.7, 95% CI 1.0–2.8, p = 0.04) compared with alteplase in large-vessel occlusion ischaemic strokes. Subsequently, the EXTEND-IA TNK part 2 trial compared tenecteplase 0.25 mg/kg with 0.4 mg/kg and did not find any further benefit with the higher dose for vessel recanalization or improved outcomes (29). These results clarified that the optimal dose of tenecteplase for large-vessel occlusion stroke is 0.25 mg/kg. This came after the large phase III Norwegian Tenecteplase Stroke Trial (NOR-TEST) trial, comparing tenecteplase 0.4 mg/kg to alteplase in n = 1,107 stroke patients recruited within 4.5 h of onset or of awakening from sleep with symptoms (30). In this trial, tenecteplase did not show superiority in improving excellent outcome (modified Rankin Scale 0–1, 64 vs. 63%, odds ratio 1.08 [95% CI 0.84–1.38, p = 0.52]). The dose of tenecteplase 0.4 mg/kg was considered safe as the rate of symptomatic haemorrhagic transformation was not increased (p = 0.70). However, this cohort of patients had a very low median baseline severity (National Institutes of Health Stroke Scale score of 4) with a high number of stroke mimics (17%), which significantly reduced the power to detect a meaningful difference between the two thrombolytic agents for both efficacy and safety (30). A subsequent meta-analysis of non-inferiority including five trials of tenecteplase vs. alteplase (31) found that tenecteplase was non-inferior to alteplase for all clinical efficacy measures (modified Rankin Scale 0–1, 0–2, and ordinal analysis) as well as symptomatic haemorrhagic transformation, regardless of the dose being 0.25 mg/kg or 0.4 mg/kg or the need for endovascular therapy for large-vessel occlusion (31). No randomized controlled trial has ever investigated the effect of tenecteplase in a cohort of patients with posterior circulation stroke (with or without large-vessel occlusion). Zhong et al. recently demonstrated that the routine use of tenecteplase for stroke thrombolysis in New Zealand was feasible and had comparable safety profile and outcome to alteplase. This real-world observational study has found that tenecteplase was also safely implemented in two small regional stroke centers less experienced in stroke reperfusion treatment (32). However, the number of posterior circulation stroke patients treated with tenecteplase was not reported in this study. The 2019 American Heart Association/American Stroke Association guidelines endorsed class IIB recommendations for tenecteplase for patients with large-vessel occlusion (33). The Australian Stroke guidelines support tenecteplase as a reasonable alternative to alteplase in patients with large-vessel occlusion (strong recommendation) and non-large-vessel occlusion (weak recommendation) ischaemic stroke who meet specific clinical and brain imaging eligibility criteria (34). Ongoing phase 3 trials comparing 0.25 mg/kg tenecteplase vs. alteplase include Tenecteplase vs. Alteplase for Stroke Thrombolysis Evaluation (TASTE) in stroke patients eligible for intravenous thrombolysis with target mismatch on computed tomography perfusion imaging, the Alteplase-Tenecteplase Trial Evaluation for Stroke Thrombolysis (ATTEST-2) trial, and the Alteplase Compared to Tenecteplase in Patients With Acute Ischemic Stroke (AcT) trial enrolling patients based on non-contrast CT alone (Table 1). In Scandinavia, The Norwegian Tenecteplase Stroke Trial 2 (NOR-TEST 2) is enrolling patients 0–4.5 h on the basis of non-contrast CT using 0.40 mg/kg tenecteplase. These studies will provide Level 1 evidence on the use of tenecteplase in stroke patients within 4.5 h. Although only a small proportion of patients with posterior circulation stroke may be enrolled in these studies, the results of these trials will likely be applied to all stroke patients, regardless of infarct topography (as occurred with previous alteplase trials). Nonetheless, further studies to investigate the safety and efficacy of tenecteplase in posterior circulation stroke patients (with and without large-vessel occlusions) are warranted. Further tenecteplase randomized-controlled trials are ongoing (Table 1).
Tenecteplase In Basilar Artery Occlusion
The use of tenecteplase in basilar artery occlusion has been mostly described in case reports (35, 36). The EXTEND-IA TNK trial (28, 29) was the only tenecteplase trial including patients with basilar artery occlusion. However, it was unclear whether its findings can be extrapolated to basilar artery occlusion as only six patients were included with no difference in the primary outcome (one-third had reperfusion at initial angiography in each treatment arm).
We recently presented the first series of patients with basilar artery occlusion treated with tenecteplase (37). Our findings suggest that tenecteplase may be associated with increased reperfusion rates in comparison with alteplase in patients with basilar artery occlusion, with rates of reperfusion similar to the 22% with tenecteplase and 10% with alteplase reported in the EXTEND-IA TNK trial (28). In our study including n = 110 patients with basilar artery occlusion, reperfusion occurred in 26% (n = 5/19) of patients treated with tenecteplase vs. 7% (n = 6/91) treated with alteplase (RR 4.0, 95% CI 1.3–12; p = 0.02), obviating the need for endovascular therapy. This occurred despite shorter thrombolysis-to-arterial-puncture time in the tenecteplase-treated patients (48[IQR 40–71] min) vs. alteplase-treated patients (110[IQR 51–185]min, p = 0.004). No difference in symptomatic intracranial hemorrhage was observed (0/19(0%) TNK, 1/91(1%) alteplase, p = 0.9). In contrast to EXTEND-IA TNK (28), functional outcomes were similar in the two treatment groups but our study was underpowered to detect such differences. Nonetheless, there was a non-significant trend toward higher 3-month excellent outcomes in patients treated with tenecteplase (modified Rankin Scale 0–1 47 vs. 37%, p = 0.09) compared to alteplase, which did not translate into better outcomes after multivariable analysis adjusted for age and NIHSS (adjusted risk ratio 1.6, 95% CI 0.9–2.7; p = 0.1). However, patients treated with tenecteplase were older than those treated with alteplase, likely due to more recent broader age selection criteria for reperfusion therapies, and tended to have higher baseline NIHSS scores (20 (IQR 5–32) for tenecteplase-treated patients vs. 15 (IQR 7–32) for alteplase-treated patients, p = 0.9). These differences in baseline characteristics would favor improved functional outcomes in the alteplase group. Therefore, our findings may represent a conservative estimate of the clinical benefits associated with tenecteplase. Interestingly, in a recent meta-analysis including five tenecteplase trials (n = 1,585), a greater effect of tenecteplase was detected when excellent outcomes were used as primary endpoint [(modified Rankin scale 0–1, crude cumulative rates of disability-free 57.9% tenecteplase vs. 55.4% alteplase; risk difference 4% (95% CI, −1% to 8%)] compared to good outcomes (modified Rankin Scale score, 0–2, crude cumulative rates of functional independence, 71.9% tenecteplase vs. 70.5% alteplase, risk difference 2% (95% CI, −3 to 6%)] (31). Although no definitive conclusions about the clinical benefit of tenecteplase can be drawn from our findings, the well-established strong relationship between earlier reperfusion and better functional outcomes (28, 32) suggests that tenecteplase could improve outcomes. Nonetheless, larger studies are needed to detect treatment effect differences between the two thrombolytic agents, given the likely larger effect of endovascular therapy. Despite this, our findings corroborate the accumulating evidence that suggests the superiority of tenecteplase compared to alteplase in large-vessel occlusion strokes. Although the alteplase data were extracted from our prospective Basilar Artery Treatment and Management (BATMAN) registry (38) and the use of early-generation thrombectomy devices and learning curve of interventionalists may have influenced our secondary outcomes (e.g., 90 days modified Rankin scale score), these factors should not influence the primary outcome of reperfusion on the initial angiogram prior to endovascular therapy. Other factors such as time from thrombolysis to reperfusion assessment, which in our study was in favor of the alteplase group, thrombus location, and permeability appear to be independently associated with reperfusion (39). A recently published meta-analysis including only patients with large-vessel occlusions (four studies, n = 433 patients) (40) showed that patients receiving tenecteplase had higher successful recanalization (odds ratio, 3.05 [95% CI, 1.73–5.40]), higher odds of good outcomes (modified Rankin Scale scores of 0 to 2, odds ratio, 2.06 [95% CI, 1.15–3.69]), and functional improvement defined as a one-point decrease across all modified Rankin Scale (common odds ratio, 1.84 [95% CI, 1.18–2.87]) at 3 months compared with patients receiving alteplase (40).
Importantly, recent randomized controlled trials failed to show the superiority of endovascular therapy compared to standard medical therapy alone in patients with basilar artery occlusion (41, 42). In the recently completed BASilar artery International Cooperation Study (BASICS) trial, the benefit of endovascular therapy was demonstrated only in patients with moderate-severe clinical syndromes (NIHSS ≥ 10) (43). This suggested that thrombolysis might be the optimal treatment in those with milder deficits.
Advantages Of Tenecteplase Over Alteplase
The reduced cost and single-bolus administration of tenecteplase vs. an hour-long alteplase infusion in patients with basilar artery occlusion who are being transported between hospitals is a major practical advantage. Tenecteplase is given as a single, 5-s intravenous bolus that requires ~2 min to prepare and administer, whereas alteplase requires preparation of both a bolus syringe containing 10% of the dose and an intravenous pump for infusion of the remaining 90% of the dose over 60 min. Moreover, the use of tenecteplase can minimize the risk of error in the preparation and delivery of the thrombolytic drug in the acute setting. Therefore, tenecteplase could be administered more efficiently in patients with basilar artery occlusion, permitting faster commencement of subsequent endovascular therapy, especially in patients treated with intravenous thrombolysis at primary stroke centers, and then transferred for endovascular therapy (“drip and ship patients”). These patients may have tenecteplase administered at the primary hospital and then be immediately transferred by a standard ambulance without having to wait for critical care transport with staff expert in continuous infusion pump management and without risking interruption or discontinuation of the alteplase infusion during transit (31). Tenecteplase also only requires one drug vial compared to potentially multiple for alteplase (patients >55 kg need two vials of alteplase but only ever one vial of tenecteplase, regardless of patient weight) and is cheaper than alteplase in most countries. Economic analysis of the EXTEND-IA TNK trial indicated that tenecteplase was dominant (cost-saving) vs. alteplase in patients with large-vessel occlusion (44). Finally, tenecteplase has been reported as a more practical thrombolytic agent during the COVID-19 pandemic. Eliminating the alteplase 1-h infusion and the required dedicated second intravenous cannula may reduce staff time in proximity to the patient. Moreover, tenecteplase does not need the intravenous infusion pump that accompanies the patient through other hospital departments and wards, presenting an additional surface that could facilitate the transmission of the virus (45).
Endovascular therapy is highly effective but resource-intensive, and access is currently limited in most countries. Endovascular patients with basilar artery occlusion can be referred to a comprehensive stroke center, either from regional hospitals where there are significant barriers to treatment or from metropolitan hospitals that do not have endovascular therapy capacity. Therefore, there may be significant delays to the initiation of endovascular therapy due to inter-hospital transfer times, especially for patients with basilar artery occlusion who often require intubation before endovascular therapy can be performed. Given that each minute reduction in door-to-reperfusion time is associated with a saving of 4.4 disability-adjusted life days (46), tenecteplase may be a safe and effective treatment to “buy some time” until endovascular therapy can be performed in these patients, especially in those transferred from regional areas. The EXTEND-IA TNK (part II trial) (28) demonstrated that longer times between thrombolysis with tenecteplase and commencement of endovascular therapy in rural sites was associated with significantly higher reperfusion rates prior to endovascular therapy compared with metropolitan patients. Therefore, tenecteplase may allow treatment of a higher number of patients with a devastating form of stroke such as basilar artery occlusion in regional areas and obviate the need to transfer some patients if there is rapid recanalization with early clinical improvement. During inter-hospital transfers, tenecteplase will have time to act on the occlusion which may facilitate early recanalization and have beneficial effects during transfer to a comprehensive center for endovascular therapy.
Conclusions
Tenecteplase has several practical advantages compared to alteplase. Although randomized controlled trials are needed to detect treatment effect differences between the two thrombolytic agents in patients with basilar artery occlusion, evidence from observational data suggests that it may be associated with higher reperfusion rates prior to thrombectomy, analogous to anterior circulation large-vessel occlusion stroke. Tenecteplase can be considered as an alternative to alteplase in patients with basilar artery occlusion, particularly in “drip and ship” patients.
Author Contributions
FA has drafted the manuscript and led submission process. BC revised the manuscript for intellectual content. All authors contributed to the article and approved the submitted version.
Funding
BC reported research support from the National Health and Medical Research Council of Australia (GNT1043242, GNT1111972, and GNT1113352), Royal Australasian College of Physicians, Royal Melbourne Hospital Foundation, National Heart Foundation (Future Leaders Fellowship 100782), National Stroke Foundation of Australia and unrestricted Grant funding for the EXTEND-IA trial (Extending the Time for Thrombolysis in Emergency Neurological Deficits – Intra-Arterial) to the Florey Institute of Neuroscience and Mental Health from Covidien (Medtronic).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Bamford J, Sandercock P, Dennis M, Warlow C, Burn J. Classification and natural history of clinically identifiable subtypes of cerebral infarction. Lancet. (1991) 337:1521. doi: 10.1016/0140-6736(91)93206-O
2. Schulz UG, Fischer U. Posterior circulation cerebrovascular syndromes: diagnosis and management. J Neurol Neurosurg Psychiatry. (2017) 88:45–53. doi: 10.1136/jnnp-2015-311299
3. National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group. Tissue plasminogen activator for acute ischemic stroke. N Engl J Med. (1995) 333:1581–7. doi: 10.1056/NEJM199512143332401
4. Hacke W, Kaste M, Fieschi C, Toni D, Lesaffre E, von Kummer R, et al. Intravenous thrombolysis with recombinant tissue plasminogen activator for acute hemispheric stroke. The European cooperative acute stroke study (ECASS). JAMA. (1995) 274:1017–25. doi: 10.1001/jama.274.13.1017
5. Hacke W, Kaste M, Fieschi C, von Kummer R, Davalos A, Meier D, et al. Randomised double-blind placebo-controlled trial of thrombolytic therapy with intravenous alteplase in acute ischaemic stroke (ECASS iI). Second European-Australasian acute stroke study investigators. Lancet. (1998) 352:1245–51. doi: 10.1016/S0140-6736(98)08020-9
6. Clark WM, Wissman S, Albers GW, Jhamandas JH, Madden KP, Hamilton S. Recombinant tissue-type plasminogen activator (Alteplase) for ischemic stroke 3 to 5 hours after symptom onset. The ATLANTIS study: a randomized controlled trial. Alteplase thrombolysis for acute noninterventional therapy in ischemic stroke. JAMA. (1999) 282:2019–26. doi: 10.1001/jama.282.21.2019
7. Clark WM, Albers GW, Madden KP, Hamilton S. The rtPA (alteplase) 0- to 6-hour acute stroke trial, part A (A0276g): results of a double-blind, placebo-controlled, multicenter study. Thromblytic therapy in acute ischemic stroke study investigators. Stroke. (2000) 31:811–6. doi: 10.1161/01.STR.31.4.811
8. Sairanen T, Strbian D, Soinne L, Silvennoinen H, Salonen O, Artto V, et al. Intravenous thrombolysis of basilar artery occlusion: predictors of recanalization and outcome. Stroke. (2011) 42:2175–9. doi: 10.1161/STROKEAHA.110.605584
9. Lindsberg PJ, Soinne L, Tatlisumak T, Roine RO, Kallela M, Happola O, et al. Long-term outcome after intravenous thrombolysis of basilar artery occlusion. JAMA. (2004) 292:1862–6. doi: 10.1001/jama.292.15.1862
10. Sarikaya H, Arnold M, Engelter ST, Lyrer PA, Mattle HP, Georgiadis D, et al. Outcomes of intravenous thrombolysis in posterior versus anterior circulation stroke. Stroke. (2011) 42:2498–502. doi: 10.1161/STROKEAHA.110.607614
11. Lee SH, Han JH, Jung I, Jung JM. Do thrombolysis outcomes differ between anterior circulation stroke and posterior circulation stroke? A systematic review and meta-analysis. Int J Stroke. (2020) 15:849–57. doi: 10.1177/1747493020909634
12. Sung SF, Chen CH, Chen YW, Tseng MC, Shen HC, Lin HJ. Predicting symptomatic intracerebral hemorrhage after intravenous thrombolysis: stroke territory as a potential pitfall. J Neurol Sci. (2013) 335:96–100. doi: 10.1016/j.jns.2013.08.036
13. Keselman B, Gdovinova Z, Jatuzis D, Melo TPE, Vilionskis A, Cavallo R, et al. Safety and outcomes of intravenous thrombolysis in posterior versus anterior circulation stroke: results from the safe implementation of treatments in stroke registry and meta-analysis. Stroke. (2020) 51:876–82. doi: 10.1161/STROKEAHA.119.027071
14. Vergouwen MD, Algra A, Pfefferkorn T, Weimar C, Rueckert CM, Thijs V, et al. Time is brain(stem) in basilar artery occlusion. Stroke. (2012) 43:3003–6. doi: 10.1161/STROKEAHA.112.666867
15. Singer OC, Humpich MC, Fiehler J, Albers GW, Lansberg MG, Kastrup A, et al. Risk for symptomatic intracerebral hemorrhage after thrombolysis assessed by diffusion-weighted magnetic resonance imaging. Ann Neurol. (2008) 63:52–60. doi: 10.1002/ana.21222
16. Archer CR, Horenstein S. Basilar artery occlusion: clinical and radiological correlation. Stroke. (1977) 8:383–90. doi: 10.1161/01.STR.8.3.383
17. Mattle HP, Arnold M, Lindsberg PJ, Schonewille WJ, Schroth G. Basilar artery occlusion. Lancet Neurol. (2011) 10:1002–14. doi: 10.1016/S1474-4422(11)70229-0
18. Bhatia R, Hill MD, Shobha N, Menon B, Bal S, Kochar P, et al. Low rates of acute recanalization with intravenous recombinant tissue plasminogen activator in ischemic stroke: real-world experience and a call for action. Stroke. (2010) 41:2254–8. doi: 10.1161/STROKEAHA.110.592535
19. Tanswell P, Modi N, Combs D, Danays T. Pharmacokinetics and pharmacodynamics of tenecteplase in fibrinolytic therapy of acute myocardial infarction. Clin Pharmacokinet. (2002) 41:1229–45. doi: 10.2165/00003088-200241150-00001
20. Assessment of the Safety and Efficacy of a New Thrombolytic (ASSENT-2) Investigators Van De Werf F Adgey J Ardissino D Armstrong PW Aylward P . Single-bolus tenecteplase compared with front-loaded alteplase in acute myocardial infarction: the ASSENT-2 double-blind randomised trial. Lancet. (1999) 354:716–22. doi: 10.1016/S0140-6736(99)07403-6
21. Binbrek AS, Rao NS, Neimane D, Hatou E, Abdulali S, Sobel BE. Comparison of rapidity of coronary recanalization in men with tenecteplase versus alteplase in acute myocardial infarction. Am J Cardiol. (2004) 93:1465–8. doi: 10.1016/j.amjcard.2004.03.004
22. Haley EC Jr, Lyden PD, Johnston KC, Hemmen TM. Investigators TNKiS. A pilot dose-escalation safety study of tenecteplase in acute ischemic stroke. Stroke. (2005) 36:607–12. doi: 10.1161/01.STR.0000154872.73240.e9
23. Haley EC Jr, Thompson JL, Grotta JC, Lyden PD, Hemmen TG, Brown TL, et al. Phase IIB/III trial of tenecteplase in acute ischemic stroke: results of a prematurely terminated randomized clinical trial. Stroke. (2010) 41:707–11. doi: 10.1161/STROKEAHA.109.572040
24. Parsons M, Spratt N, Bivard A, Campbell B, Chung K, Miteff F, et al. A randomized trial of tenecteplase versus alteplase for acute ischemic stroke. N Engl J Med. (2012) 366:1099–107. doi: 10.1056/NEJMoa1109842
25. Huang X, Cheripelli BK, Lloyd SM, Kalladka D, Moreton FC, Siddiqui A, et al. Alteplase versus tenecteplase for thrombolysis after ischaemic stroke (ATTEST): a phase 2, randomised, open-label, blinded endpoint study. Lancet Neurol. (2015) 14:368–76. doi: 10.1016/S1474-4422(15)70017-7
26. Bivard A, Huang X, McElduff P, Levi CR, Campbell BC, Cheripelli BK, et al. Impact of computed tomography perfusion imaging on the response to tenecteplase in ischemic stroke: analysis of 2 randomized controlled trials. Circulation. (2017) 135:440–8. doi: 10.1161/CIRCULATIONAHA.116.022582
27. Bivard A, Huang X, Levi CR, Spratt N, Campbell BCV, Cheripelli BK, et al. Tenecteplase in ischemic stroke offers improved recanalization: analysis of 2 trials. Neurology. (2017) 89:62–7. doi: 10.1212/WNL.0000000000004062
28. Campbell BCV, Mitchell PJ, Churilov L, Yassi N, Kleinig TJ, Dowling RJ, et al. Tenecteplase versus alteplase before thrombectomy for ischemic stroke. N Engl J Med. (2018) 378:1573–82. doi: 10.1056/NEJMoa1716405
29. Campbell BCV, Mitchell PJ, Churilov L, Yassi N, Kleinig TJ, Dowling RJ, et al. Effect of intravenous tenecteplase dose on cerebral reperfusion before thrombectomy in patients with large vessel occlusion ischemic stroke: the EXTEND-IA tNK part 2 randomized clinical trial. JAMA. (2020) 323:1257–65. doi: 10.1001/jama.2020.1511
30. Logallo N, Novotny V, Assmus J, Kvistad CE, Alteheld L, Ronning OM, et al. Tenecteplase versus alteplase for management of acute ischaemic stroke (NOR-TEST): a phase 3, randomised, open-label, blinded endpoint trial. Lancet Neurol. (2017) 16:781–8. doi: 10.1016/S1474-4422(17)30253-3
31. Burgos AM, Saver JL. Evidence that tenecteplase is noninferior to alteplase for acute ischemic stroke: meta-analysis of 5 randomized trials. Stroke. (2019) 50:2156–62. doi: 10.1161/STROKEAHA.119.025080
32. Zhong CS, Beharry J, Salazar D, Smith K, Withington S, Campbell BCV, et al. Routine use of tenecteplase for thrombolysis in acute ischemic stroke. (2021) 52:1087–90. doi: 10.1161/STROKEAHA.120.030859
33. Powers WJ, Rabinstein AA, Ackerson T, Adeoye OM, Bambakidis NC, Becker K, et al. Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 guidelines for the early management of acute ischemic stroke. Stroke. (2019) 50:e344–418. doi: 10.1161/STR.0000000000000211
34. Stroke Foundation Australia. Australian Clinical Guidelines for Management. (2020). Available online at: https://informme.org.au/en/Guidelines/Clinical-Guidelines-for-Stroke-Management (accessed May 1, 2020).
35. Switzer JA, Forseen SE, Bruno A, Hess DC. Serendipitous recanalization of basilar artery occlusion. J Stroke Cerebrovasc Dis. (2013) 22:e671–3. doi: 10.1016/j.jstrokecerebrovasdis.2013.06.019
36. Smadja D, Olindo S, Saint-Vil M, Chausson N. Sequential combination of two intravenous thrombolytics (recombinant tissue plasminogen activator/tenecteplase) in a patient with stroke and cardioembolic basilar artery occlusion. J Stroke Cerebrovasc Dis. (2009) 18:68–71. doi: 10.1016/j.jstrokecerebrovasdis.2008.08.001
37. Alemseged F, Ng FC, Williams C, Puetz V, Boulouis G, Kleinig TJ, et al. BATMAN study group and EXTEND IA TNK study group. Tenecteplase vs alteplase before endovascular therapy in basilar artery occlusion. Neurology. (2021) 96:e1272–7. doi: 10.1212/WNL.0000000000011520
38. Alemseged F, Van der Hoeven E, Di Giuliano F, Shah D, Sallustio F, Arba F, et al. Response to late-window endovascular revascularization is associated with collateral status in basilar artery occlusion. Stroke. (2019) 50:1415–22. doi: 10.1161/STROKEAHA.118.023361
39. Menon BK, Al-Ajlan FS, Najm M, Puig J, Castellanos M, Dowlatshahi D, et al. Association of clinical, imaging, and thrombus characteristics with recanalization of visible intracranial occlusion in patients with acute ischemic stroke. JAMA. (2018) 320:1017–26. doi: 10.1001/jama.2018.12498
40. Katsanos AH, Safouris A, Sarraj A, Magoufis G, Leker RR, Khatri P, et al. Intravenous thrombolysis with tenecteplase in patients with large vessel occlusions: systematic review and meta-analysis. Stroke. (2021) 52:308–12. doi: 10.1161/STROKEAHA.120.030220
41. Katsanos AH, Safouris A, Nikolakopoulos S, Mavridis D, Goyal N, Psychogios MN, et al. Endovascular treatment for basilar artery occlusion: a systematic review and meta-analysis. Eur J Neurol. (2021) 28:2106–10. doi: 10.1111/ene.14751
42. Liu X, Dai Q, Ye R, Zi W, Liu Y, Wang H, et al. Endovascular treatment versus standard medical treatment for vertebrobasilar artery occlusion (BEST): an open-label, randomised controlled trial. Lancet Neurol. (2020) 19:115–22. doi: 10.1016/S1474-4422(19)30395-3
43. Langezaal LCM, van der Hoeven EJRJ, Mont'Alverne FJA, de Carvalho JJF, Lima FO, Dippel DWJ, et al. Endovascular therapy for stroke due to basilar-artery occlusion. N Engl J Med. (2021) 384:1910–20. doi: 10.1056/NEJMoa2030297
44. Gao L, Moodie M, Mitchell PJ, Churilov L, Kleinig TJ, Yassi N, et al. Cost-effectiveness of tenecteplase before thrombectomy for ischemic stroke. Stroke. (2020) 51:3681–9. doi: 10.1161/STROKEAHA.120.029666
45. Warach SJ, Saver JL. Stroke thrombolysis with tenecteplase to reduce emergency department spread of coronavirus disease 2019 and shortages of Alteplase. JAMA Neurol. (2020) 77:1203–4. doi: 10.1001/jamaneurol.2020.2396
46. Emberson J, Lees KR, Lyden P, Blackwell L, Albers G, Bluhmki E, et al. Effect of treatment delay, age, and stroke severity on the effects of intravenous thrombolysis with alteplase for acute ischaemic stroke: a meta-analysis of individual patient data from randomised trials. Lancet. (2014) 384:1929–35. doi: 10.1016/S0140-6736(14)60584-5
Keywords: tenecteplase, basilar artery occlusion, alteplase, posterior circulation stroke, thrombolytic agent
Citation: Alemseged F and Campbell BCV (2021) Tenecteplase Thrombolysis in Posterior Circulation Stroke. Front. Neurol. 12:678887. doi: 10.3389/fneur.2021.678887
Received: 10 March 2021; Accepted: 07 June 2021;
Published: 06 August 2021.
Edited by:
Thanh Nguyen, Boston University, United StatesReviewed by:
Aristeidis H. Katsanos, McMaster University, CanadaFabricio Oliveira Lima, Hospital Geral de Fortaleza, Brazil
Copyright © 2021 Alemseged and Campbell. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Fana Alemseged, ZmFuYS5hbGVtc2VnZWRAbWgub3JnLmF1
 Fana Alemseged
Fana Alemseged Bruce C. V. Campbell
Bruce C. V. Campbell