
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Neurol., 31 May 2021
Sec. Endovascular and Interventional Neurology
Volume 12 - 2021 | https://doi.org/10.3389/fneur.2021.674966
This article is part of the Research TopicAdvances in Flow-diversion Devices for Cerebral AneurysmsView all 20 articles
Background: Few reports have shown the therapeutic outcomes of flow diversion (FD) for intracranial aneurysms beyond the circle of Willis, and the efficacy of this technique remains unclear.
Materials and methods: A retrospective study was performed on 22 consecutive patients, diagnosed with intracranial aneurysms beyond the circle of Willis, and treated with pipeline embolization device (PED) (Medtronic, Irvine, California, USA) between January 2015 and December 2019.
Result: The 22 patients were between 16 and 66 years old (mean 44.5 ± 12.7 years), and six patients were male (27.3%, 6/22). Twenty-two patients had 23 aneurysms. The 23 aneurysms were 3–25 mm in diameter (12.2 ± 7.1 mm on average). The diameter of the parent artery was 1.3–3.0 mm (2.0 ± 0.6 mm on average). The 23 aneurysms were located as follows: 17 (73.9%, 17/23) were in the anterior circulation, and 6 (26.1%, 6/23) were in the posterior circulation. PED deployment was technically successful in all cases. Two overlapping PEDs were used to cover the aneurysm neck in 3 cases. One PED was used to overlap the two tandem P1 and P2 aneurysms. Other cases were treated with single PED. Coil assistance was used to treat 7 aneurysms, including 4 recurrent aneurysms and 3 new cases requiring coiling assistance during PED deployment. There were no cases of complications during PED deployment. All patients were available at the follow-up (mean, 10.9 ± 11.4 months). All patients presented with a modified Rankin Score (mRS) of 0. During angiographic follow-up, complete embolization was observed in 22 aneurysms in 21 patients, and one patient had subtotal embolization with the prolongation of stasis in the arterial phase.
Conclusion: PED deployment for intracranial aneurysms beyond the circle of Willis is feasible and effective, with high rates of aneurysm occlusion.
Currently, flow diversion (FD) has revolutionized the treatment of intracranial aneurysms into a safe and efficacious therapy for large or giant wide-necked aneurysms. However, the off-label uses of FD have increased for intracranial aneurysms, including those in distal locations and bifurcation aneurysms (1).
Currently, FD for aneurysms beyond the circle of Willis is effective, but there are some uncertain factors (2). This is because smaller arteries, the technical challenges of distal navigation, and the coverage of bifurcation branches and perforators may increase the risk of treatment-related complications (3). Thus, these aneurysms remain difficult to treat (4, 5).
Therefore, this study planned to evaluate the safety and efficacy of pipeline embolization device (PED) (Medtronic, Irvine, California, USA) treatment of intracranial aneurysms beyond the circle of Willis, including distal anterior circulation aneurysms and posterior circulation aneurysms.
From January 2015 to December 2019, consecutive 22 patients, who underwent PED treatment for intracranial aneurysms beyond the circle of Willis, were retrospectively reviewed.
(1) The location of intracranial aneurysms was beyond the circle of Willis. (2) These aneurysms, including previously coiled aneurysms, underwent treatment with a PED.
The data collected and recorded included age, sex, clinical presentation, aneurysm side, aneurysm size, number of PED deployments, coiling assistance, and procedural complications.
Dual-antiplatelet medication with aspirin 100 mg and clopidogrel 75 mg was given for at least 5 days before the treatment. In the case of platelet inhibition of 40% to adenosine diphosphate (ADP), an additional 300-mg loading dose of clopidogrel was administered before the procedure. Dual-antiplatelet therapy was maintained for 6 months. Then, aspirin 100 mg was given for a minimum of 6 months or for life.
All patients were treated under general anesthesia via a transfemoral approach. A coaxial system consisting of a Shuttle sheath, a guide catheter, an intermediate catheter and a microcatheter was used. Under roadmap guidance, the 0.027-inch Marksman or Phenom catheter (Medtronic, Irvine, California, USA) was navigated beyond the aneurysm neck. Based on the aneurysm neck and parent artery parameters, a PED was chosen to allow enough wall apposition and coverage of the aneurysm. If the aneurysm was ruptured or when necessary, PED deployment plus coiling was performed. Control angiography was performed at 10 and 20 min intervals after PED deployment to observe platelet aggregation within the stent (5).
The modified Rankin Scale (mRS) was used for clinical outcome assessment. During the follow-up imaging, follow-up angiography was analyzed. If the treatment was incomplete, the degree could be evaluated with the prolongation of stasis, which was divided into arterial, capillary, and venous phases.
Twenty-two patients were identified, with ages ranging from 16 to 66 years (mean, 44.5 ± 12.7 years), and six patients were male (27.3%, 6/22). Seventeen patients were admitted for accidental findings, 1 had subarachnoid hemorrhage (SAH), and 4 recurrent aneurysms were treated with previous coiling with or without stenting assistance.
Twenty-two patients had 23 aneurysms, of which 12 aneurysms were on the left side and 11 were on the right side. In 23 aneurysms, 2 aneurysms were in tandem. The other 21 patients had single aneurysms. The 23 aneurysms were 3–25 mm in diameter (12.2 ± 7.1 mm on average). The diameter of the parent artery was 1.3–3.0 mm (2.0 ± 0.6 mm on average). The locations of 23 aneurysms were as follows: the first segment of the middle cerebral artery (MCA) (M1), 6 aneurysms; the second segment of the MCA (M2), 2 aneurysms; the third segment of the MCA (M3), 6 aneurysms; the second segment of the anterior cerebral artery (ACA) (A2), 3 aneurysms; the first segment of the posterior cerebral artery (PCA) (P1), 3 aneurysms; the second segment of the PCA (P2), 2 aneurysms; and the third segment of the posterior inferior cerebellar artery (PICA) (p3), 1 aneurysm.
PED deployment was technically successful in all cases. Two overlapping PEDs were used to cover the aneurysm neck in 3 cases. One PED was used to overlap the tandem P1 and P2 aneurysms. The other cases were treated with single PED. In 23 aneurysms, coiling assistance was performed for 3 aneurysms, including one ruptured aneurysm. In total, coiling was used to treat 7 aneurysms, including 4 recurrent aneurysms and 3 new cases requiring coiling assistance during PED deployment. During PED deployment, the branches were covered by the PED in 15 cases (68.2%, 15/22), according to the results of immediate angiography.
All patients were available at the clinical follow-up, and the clinical and imaging follow-up ranged from 3 to 48 months (mean, 10.9 ± 11.4 months). All patients presented with a mRS score of 0 (100%). The degree of embolization was 100% occlusion in 22 aneurysms (95.6%, 22/23), and one aneurysm exhibited <90% occlusion (subtotal embolization with the prolongation of stasis in the arterial phase). Representative cases are shown in Figures 1, 2. Clinical data in this study are summarized in Table 1.
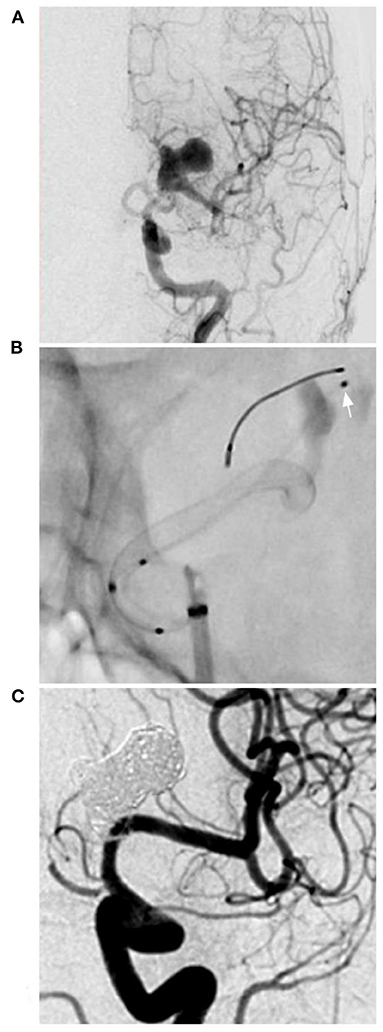
Figure 1. PED for an M1 complex aneurysm. (A) DSA of the anterior-posterior view of the ICA showing a complex lobulated aneurysm on the M1 segment of the middle cerebral artery. (B) X-ray film showing the deployment of the PED and the microcatheter (arrow) in the aneurysm to plan coiling. (C) Follow-up DSA showing complete aneurysm occlusion. DSA, digital subtraction angiography; ICA, internal carotid artery; PED, pipeline endovascular device.
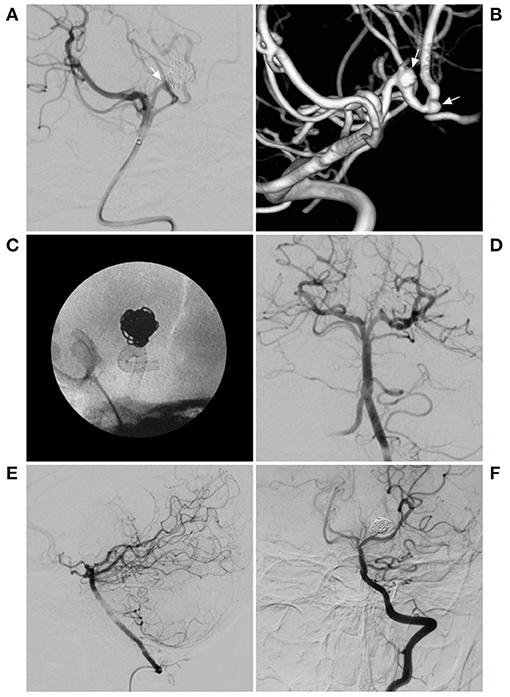
Figure 2. PED for a previously coiled recurrent aneurysm. (A) DSA of the BA showing the previous coiled aneurysm in posterior cerebral artery. The arrow indicates the recurrent neck of the aneurysm. (B) 3D reconstruction of DSA showing the 2 aneurysms, including the previous coiled aneurysm and another aneurysm (arrows). (C) X-ray film showing the deployment of the PED. (D) Immediate angiography showing the deployment of the PED. (E,F) Follow-up DSA of the VA showing complete aneurysm occlusion. BA, basilar artery; DSA, digital subtraction angiography; PED, pipeline endovascular device; VA, vertebral artery.
FD involves 24–55% metal coverage, and after FD deployment, the blood flow within the aneurysm is disturbed, causing stasis that leads to thrombosis, followed by endothelialization of the parent artery (6). Currently, FD technology has revolutionized the treatment of intracranial aneurysms that are suboptimal for surgical or traditional interventional treatment (7). For aneurysms beyond the circle of Willis, classic endovascular approaches to the treatment of these aneurysms include selective coiling or parent artery occlusion, which imparts risks of recurrence and distal infarction (8).
The Pipeline for Uncoilable or Failed Aneurysms (PUFS) trial showed the safety and effectiveness of the use of PEDs in the treatment of large and giant wide-neck aneurysms of the internal carotid artery in adult patients (9). At the same time, based on their ability to reconstruct the parent artery, the off-label uses of FD are constantly extended, including aneurysms beyond the circle of Willis (1). These aneurysms are often dissected and located in sub-2.0-mm vessels, where small-diameter PEDs have been used (5). In this study, we also tried to treat 22 patients with 23 aneurysms with the deployment of PEDs.
The deployment of FDs in arteries beyond the circle of Willis is technically challenging due to the smaller caliber of the parent vessel and the relative stiffness of the high-metal coverage stent. Sometimes, telescoping PEDs with 25–30% overlap is a feasible low-risk treatment option for long-segment aneurysms, using larger-diameter PEDs more proximally (10).
FD among aneurysms beyond the circle of Willis is effective (5). In the Ravindran et al. study of the use of FD for distal circulation aneurysms, complete and near-complete occlusion was noted in 78.2% of aneurysms (11). Our study demonstrates that PED treatment for aneurysms beyond the circle of Willis is effective, with rates of complete occlusion close to 95.6%.
FD can be applied alone or in combination with coiling, which includes the retreatment of previously coiled lesions, theoretically, which allows higher rates of occlusion than treatment with FDs alone, such as the case shown in Figure 2 (11). However, coiling assistance is controversial, especially for large and giant aneurysms, and despite coiling assistance in FD deployment, delayed rupture cannot completely be avoided. Moreover, after coiling assistance, the effect may not be complete, and the coiling could result in the occlusion of a perforating artery.
Intracranial aneurysms beyond the circle of Willis are often dissecting and long but not large. Delayed rupture was uncommon after FD deployment, so the aim of coiling assistance was not to reduce the rupture risk; coiling may increase the degree of aneurysm occlusion. The coiling assistance during FD deployment was the same as that during conventional stent-assisted coiling. For instance, in the case shown in Figure 1, follow-up showed excellent occlusion after coiling assistance. However, coiling assistance is selectively applied for intracranial aneurysms beyond the circle of Willis, because in these aneurysms, the blood flow is not abundant, and FDs alone may be sufficient in most of these cases. In our study, 7 aneurysms were treated with coils in the aneurysms, including 4 recurrent aneurysms and 3 new cases requiring coiling assistance during PED deployment, and complete occlusion was obtained. Coiling assistance was feasible, but whether there is a difference between aneurysms with or without previously coiling requires further study. In our study, due to the small number of cases, it was difficult to identify such a difference. However, PED deployment was safe and effective.
However, the complications associated with FD deployment are not negligible and include ischemic/thromboembolic and hemorrhagic complications (9, 12). In addition, for small vessels, after FD deployment, segmental vasospasm can occur as a frequent vascular reaction, potentially causing symptomatic ischemia or even stroke ~1 month after the procedure (13). Safety concerns regarding FD within small vessels can originate with vessel trauma from robust support to deliver and open the PED in the distal circulation, often in the presence of significant tortuosity, acute stent thrombosis, and delayed in-stent stenosis (5, 14).
The ASPIRe (Aneurysm Study of Pipeline in an Observational Registry) meta-analysis reported outcomes with a major morbidity of 6.8% and mortality of 1.6% across on-label PED treatments (15). In the Bender et al. study of FD for aneurysms in distal vessels measuring <2.0 mm, the major morbidity of 4.5% and mortality of 1.5% observed were lower than the on-label PED series outcomes (5). In the Primiani et al. report of A2, M2, and P2 aneurysms and beyond, the procedural compilation rate of 7.7% indicates a need for further studies as flow diversion technology constantly evolves (16). Our study reported no complications because the choice of cases was appropriate.
To reduce ischemic complications, instead of a PED with 30% metal coverage, an intermediate-porosity braided LEO stent (Balt Extrusion, Montmorency, France) with 14% metal coverage can be used with the help of a flow-diversion effect (17). In the Cagnazzo et al. study of 76 intracranial aneurysms and 98 side branches covered by LEO stents, the rate of flow remodeling on the covered arteries and perforators was 9 and 4%, respectively, and complete occlusion of aneurysms treated with sole stent-placement therapy was 70% (18).
In addition, a new low-profile visualized intraluminal support device (LVIS Blue; MicroVention, Tustin, California, USA) is a braided stent that provides a higher degree of metal coverage (22–28%) than first-generation devices (19). Although the coverage of the LVIS Blue stent is lower than that of FDS, the LVIS Blue stent may be beneficial for complete obliteration of an aneurysm due to not only its support of a high occlusion rate using coils inside of the aneurysm but also its flow-diversion effect (20, 21).
The PED is an effective tool for managing aneurysms beyond the circle of Willis, especially those that are difficult to reconstruct with clipping and residual or recanalizing aneurysms after coiling.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethical review and approval was not required for the study on human participants in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
JY: draft. XL: review, editing, and submitting. All authors read and approved the final version of the manuscript.
This work was supported by the Beijing Municipal Administration of Hospitals Incubating Program (PX2020039), Beijing, China, and the Tsinghua Precision Medicine Foundation (20219990008), Tsinghua University, Beijing, China.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
1. Cagnazzo F, Perrini P, Dargazanli C, Lefevre PH, Gascou G, Morganti R, et al. Treatment of unruptured distal anterior circulation aneurysms with flow-diverter stents: a meta-analysis. Am J Neuroradiol. (2019) 40:687–93. doi: 10.3174/ajnr.A6002
2. Pistocchi S, Blanc R, Bartolini B, Piotin M. Flow diverters at and beyond the level of the circle of willis for the treatment of intracranial aneurysms. Stroke. (2012) 43:1032–8. doi: 10.1161/STROKEAHA.111.636019
3. Atallah E, Saad H, Mouchtouris N, Bekelis K, Walker J, Chalouhi N, et al. Pipeline for distal cerebral circulation aneurysms. Neurosurgery. (2019) 85:E477–84. doi: 10.1093/neuros/nyz038
4. Topcuoglu OM, Akgul E, Daglioglu E, Topcuoglu ED, Peker A, Akmangit I, et al. Flow diversion in middle cerebral artery aneurysms: is it really an all-purpose treatment? World Neurosurg. (2016) 87:317–27. doi: 10.1016/j.wneu.2015.11.073
5. Bender MT, Zarrin DA, Campos JK, Lin LM, Huang J, Caplan JM, et al. Tiny pipes: 67 cases of flow diversion for aneurysms in distal vessels measuring less than 2.0 mm. World Neurosurg. (2019) 127:e193–201. doi: 10.1016/j.wneu.2019.02.204
6. Dandapat S, Mendez-Ruiz A, Martinez-Galdamez M, Macho J, Derakhshani S, Foa Torres G, et al. Review of current intracranial aneurysm flow diversion technology and clinical use. J Neurointerv Surg. (2021) 13:54–62. doi: 10.1136/neurintsurg-2020-015877
7. Cagnazzo F, Fanti A, Lefevre PH, Derraz I, Dargazanli C, Gascou G, et al. Distal anterior cerebral artery aneurysms treated with flow diversion: experience of a large-volume center and systematic review of the literature. J Neurointerv Surg. (2021) 13:42–8. doi: 10.1136/neurintsurg-2020-015980
8. Durst CR, Hixson HR, Schmitt P, Gingras JM, Crowley RW. Endovascular treatment of a fusiform aneurysm at the M3-M4 junction of the middle cerebral artery using the pipeline embolization device. World Neurosurg. (2016) 86:511 e1–4. doi: 10.1016/j.wneu.2015.10.016
9. Becske T, Kallmes DF, Saatci I, McDougall CG, Szikora I, Lanzino G, et al. Pipeline for uncoilable or failed aneurysms: results from a multicenter clinical trial. Radiology. (2013) 267:858–68. doi: 10.1148/radiol.13120099
10. Tonetti DA, Casillo SM, Jankowitz BT. Telescoping flow diverters for a pediatric fusiform distal anterior cerebral artery aneurysm: technical case report. Childs Nerv Syst. (2021) 37:999–1002. doi: 10.1007/s00381-020-04797-y
11. Ravindran K, Enriquez-Marulanda A, Kan PTM, Renieri L, Limbucci N, Mangiafico S, et al. Use of flow diversion for the treatment of distal circulation aneurysms: a multicohort study. World Neurosurg. (2018) 118:e825–33. doi: 10.1016/j.wneu.2018.07.062
12. Wagner KM, Srinivasan VM, Srivatsan A, Ghali MGZ, Thomas AJ, Enriquez-Marulanda A, et al. Outcomes after coverage of lenticulostriate vessels by flow diverters: a multicenter experience. J Neurosurg. (2019) 132:473–80. doi: 10.3171/2018.8.JNS18755
13. Schob S, Richter C, Scherlach C, Lindner D, Planitzer U, Hamerla G, et al. Delayed stroke after aneurysm treatment with flow diverters in small cerebral vessels: A potentially critical complication caused by subacute vasospasm. J Clin Med. (2019) 8:1649. doi: 10.3390/jcm8101649
14. Narata AP, Moura F, Larrabide I, Chapot R, Cognard C, Januel AC, et al. Role of distal cerebral vasculature in vessel constriction after aneurysm treatment with flow diverter stents. J Neurointerv Surg. (2020) 12:818–26. doi: 10.1136/neurintsurg-2019-015447
15. Kallmes DF, Brinjikji W, Boccardi E, Ciceri E, Diaz O, Tawk R, et al. Aneurysm Study of Pipeline in an Observational Registry (ASPIRe). Interv Neurol. (2016) 5:89–99. doi: 10.1159/000446503
16. Primiani CT, Ren Z, Kan P, Hanel R, Pereira VM, Lui WM, et al. A2, M2, P2 aneurysms and beyond: results of treatment with pipeline embolization device in 65 patients. J Neurointerv Surg. (2019) 11:903–7. doi: 10.1136/neurintsurg-2018-014631
17. Geyik S, Yavuz K, Yurttutan N, Saatci I, Cekirge HS. Stent-assisted coiling in endovascular treatment of 500 consecutive cerebral aneurysms with long-term follow-up. Am J Neuroradiol. (2013) 34:2157–62. doi: 10.3174/ajnr.A3574
18. Cagnazzo F, Cappucci M, Dargazanli C, Lefevre PH, Gascou G, Riquelme C, et al. Flow-diversion effect of Leo stents: aneurysm occlusion and flow remodeling of covered side branches and perforators. Am J Neuroradiol. (2018) 39:2057–63. doi: 10.3174/ajnr.A5803
19. Lim YC, Shin YS, Chung J. Flow diversion via LVIS blue stent within enterprise stent in patients with vertebral artery dissecting aneurysm. World Neurosurg. (2018) 117:203–7. doi: 10.1016/j.wneu.2018.06.029
20. Matsuda Y, Chung J, Keigher K, Lopes D. A comparison between the new Low-profile Visualized Intraluminal Support (LVIS Blue) stent and the Flow Redirection Endoluminal Device (FRED) in bench-top and cadaver studies. J Neurointerv Surg. (2018) 10:274–8. doi: 10.1136/neurintsurg-2017-013074
21. Schob S, Hoffmann KT, Richter C, Bhogal P, Kohlert K, Planitzer U, et al. Flow diversion beyond the circle of Willis: Endovascular aneurysm treatment in peripheral cerebral arteries employing a novel low-profile flow diverting stent. J Neurointerv Surg. (2019) 11:1227–34. doi: 10.1136/neurintsurg-2019-014840
Keywords: pipeline embolization device, endovascular treatment, circle of Willis, distal aneurysm, complex aneurysm
Citation: Yu J and Lv X (2021) Flow Diversion for Intracranial Aneurysms Beyond the Circle of Willis. Front. Neurol. 12:674966. doi: 10.3389/fneur.2021.674966
Received: 02 March 2021; Accepted: 28 April 2021;
Published: 31 May 2021.
Edited by:
Hong-Qi Zhang, Capital Medical University, ChinaReviewed by:
Adam A. Dmytriw, Brigham and Women's Hospital and Harvard Medical School, United StatesCopyright © 2021 Yu and Lv. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xianli Lv, bHZ4aWFubGkwMDBAMTYzLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.