
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Neurol. , 10 August 2021
Sec. Stroke
Volume 12 - 2021 | https://doi.org/10.3389/fneur.2021.674398
This article is part of the Research Topic Blood-Based Biomarkers in Acute Ischemic Stroke and Hemorrhagic Stroke View all 36 articles
Background: Uric acid (UA) is proposed as a potential risk factor for stroke in adult, yet the results from published studies are not generally accordant.
Method: We included prospective studies that explored the relationship between serum UA (SUA) and strokes. In this study, strokes include ischemic stroke and hemorrhagic stroke, which consists of intracerebral hemorrhage and subarachnoid hemorrhage. The effect-size estimates were expressed as hazard ratio (HR) and 95% confidence interval (CI). Sensitivity and subgroup analyses were performed to assess the robustness of the pooled estimation and potential sources of heterogeneity between studies.
Results: We meta-analyzed 19 prospective cohort articles, which involve 37,386 males and 31,163 females. Overall analyses results showed a significant association between a 1 mg/dl increase in high levels of SUA and the risk of total stroke (HR = 1.13; 95% CI: 1.09–1.18; P < 0.001), ischemic stroke (HR = 1.15; 95% CI: 1.10–1.21; P < 0.001), and hemorrhagic stroke (HR = 1.07; 95% CI: 1.00 to 1.15; P = 0.046). No significant difference was found between ischemic stroke and hemorrhagic stroke. In the subgroup analyses, the association of high SUA levels and the risk of total stroke was statistically significant in females (HR = 1.19; 95% CI: 1.12–1.26; P < 0.001) and males (HR = 1.11; 95% CI: 1.05–1.17; P < 0.001). Coincidentally, the association was also statistically significant for ischemic stroke, both in females (HR = 1.26; 95% CI: 1.17–1.36; P < 0.001) and in males (HR = 1.12; 95% CI: 1.06–1.19; P < 0.001). However, for hemorrhagic stroke, it was only statistically significant in females (HR = 1.19; 95% CI: 1.04–1.35; P = 0.01). Our dose–response research indicated the J-shaped trend between the ascending SUA levels and the higher risk of suffering from a stroke.
Conclusions: Our findings indicate that elevated SUA is a significant risk factor for adult stroke, both for ischemic stroke and hemorrhagic stroke, and especially in females.
Stroke is believed to be the second leading cause of death and a major contributor to disability-adjusted life-years (DALYs) lost worldwide (1). According to global statistics, together with ischemic heart disease, strokes account for nearly 15.2 million deaths in 2015 (1). In 2017, intracerebral hemorrhage and ischemic stroke caused 57.9 and 47.8 million DALYs lost, separately (2). Stroke is preventable. Multiple modifiable risk factors, such as hypertension, diabetes mellitus, atrial fibrillation, dyslipidemia, smoking, obesity, lack of physical activity, etc., have been widely observed in the prevention and treatment of stroke (1). However, the number of incidents of stroke, survivors, and stroke-related death, as well as DALYs, are still increasing globally (3). Therefore, a better understanding of more potential risk factors are needed to develop additional preventive strategies for stoke.
Uric acid (UA), one metabolic end product of purine, exists in the form of UA salt with high solubility in organisms. Regularly, serum UA (SUA) levels range from 1.5 to 6.0 mg/dl for women and 2.5 to 7.0 mg/dl for men under a healthy status, which is hard upon the upper limit of UA dissolution in serum (4). Up to date, controversial results regarding the correlation between SUA levels and the incidence of stroke have been reported. It was shown that UA is one of the most essential antioxidants in the blood whose concentration is 10 times greater than that of other antioxidants. UA provides an antioxidant defense against oxidant- and radical-caused damage in humans (5). Researches demonstrated that UA is an antioxidant factor to protect nerves from oxidative damage (6, 7), thereby possibly preventing stroke outcomes. Whereas, many studies found that high SUA levels might be a major risk factor for the onset of stroke (8–11). Zhong et al. explored the association between SUA levels and risk of stroke base on a meta-analysis (12). The study revealed that the elevated SUA levels were significantly related to the modestly increased risk of stroke, and there existed no significant gender differences. Meanwhile, the association between SUA and the risk of each subtype of stroke had been developed by different meta-analyses (13, 14). No studies were conducted to compare the effect of SUA levels on ischemic stroke and hemorrhagic stroke. It is widely accepted that hemorrhagic stroke is ascribed to the rupture of a blood vessel, and ischemic stroke is caused by blockage of an artery; both conditions cause local hypoxia that damages brain tissue. Ischemic stroke accounts for the majority of strokes, yet hemorrhagic stroke is responsible for more deaths and DALYs lost (15). Identifying the role of SUA levels in each type of stroke is vital for subsequent targeted treatment and prevention. In our study, we performed a meta-analysis of prospective studies to detect the association between elevated SUA levels and the risk of stroke and explored the differences between ischemic stroke and hemorrhagic stroke.
This meta-analysis was carried out in line with the guidelines of the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) statement (16), which is presented in Supplementary Table 1.
We finished literature search by looking through PubMed, EMBASE, and Web of Science databases as of December 26, 2020. The following medical nomenclature are considered: (uric acid OR ua OR urate OR hyperuricemia OR hyperuric OR ammonium acid urate [Title/Abstract]) AND (stroke OR cerebrovascular OR apoplexy OR brain vascular accident OR cerebral stroke OR ischemic stroke OR ischaemic stroke OR cryptogenic ischemic stroke OR cryptogenic stroke OR embolism stroke OR intracranial embolism OR intracranial infarction OR cerebral embolism OR cerebral infarction OR brain infarction OR intracranial hemorrhage OR brain hemorrhage OR hemorrhagic stroke OR cerebral hemorrhage [Title/Abstract]). In order to avoid underlying missing points, reference lists of retrieved articles and systematic reviews were scanned.
Two researchers (Tianci Qiao and Hongyun Wu) examined all retrieved articles independently, and they seriously assessed preliminary qualification based on the titles, abstracts, and full texts when necessary.
We included the articles when they met the following criteria: (1) the study has a prospective design (prospective cohort or prospective nested case-control study); (2) the study outcomes were stroke, including ischemic stroke and any kinds of hemorrhagic stroke (intracerebral hemorrhage and subarachnoid hemorrhage); (3) enrolled participants were free of stroke at baseline; (4) studies that reported the definition of outcomes in participants with stroke; and (5) hazard ratio (HR) and corresponding 95% confidence interval (CI) of the association between UA and stroke were reported. Articles were excluded if they were reviews, proceedings, letters, case reports, or meta-analyses, or they were not reported in English languages, or the subjects of the studies were not stroke patients, or they were of duplicated publications or studies using overlapping data.
Two investigators (Tianci Qiao and Hongyun Wu) excerpted data from each qualified article and imported them into a standardized Excel spreadsheet independently, including name of the first author, year of publication, location where study was conducted, sample size, sex, baseline age, follow-up period, ascertainment of UA and stroke, type of stroke, levels of UA, effect estimation, adjusted confounders, and other traditional risk factors, if available. The disagreements were resolved by reevaluating original articles jointly and, if necessary, by a third author (Wei Peng).
Stata software version 14.1 for Windows (Stata Corp, College Station, TX, USA) was used to regulate and analyze the data. The random-effects model was employed without considering the magnitude of between-study heterogeneity. Effect size estimates were indicated by HR and its 95% CI. The difference between the two estimates was tested by using Z-test as reported by Altman and Bland (17). Generalized least squares regression proposed by Greenland and Longnecker (18) was used to examine the dose–response association for trend estimation of summarized dose–response data. In addition, non-linearity test between SUA levels and risk of stroke was conduct by restricted cubic splines of exposure distribution with three knots (25, 50, and 75th percentiles).
Heterogeneity between studies was assessed by inconsistency index (I2), which represents the percentage of multiplicity observed between studies whose result is from chance rather than a casual result. A higher I2 value indicates a higher degree of heterogeneity. If the I2 value is higher than 50%, significant heterogeneity would be recorded. As for multiple sources of heterogeneity possibly from clinical and methodological fields, plenty of prespecified subgroups were analyzed according to the baseline age, gender, region, follow-up, factor correction, including whether body mass index (BMI) was adjusted, smoking status, hypertension or blood pressure, diabetes mellitus or blood glucose, hyperlipidemia or lipid, or renal factors.
Begg's funnel plots and Egger regression asymmetry tests were used to evaluate the potential publication bias at a significance level of 10%. In addition, the number of theoretically missing studies was estimated by trim and fill methods, respectively. Sensitivity analysis was conducted to test the stability of results.
A total of 1,522 articles were initially included. After searching the public databases with medical subject terms that were previously defined, there were 19 articles with data on association between SUA and risk of stroke that were eligible for inclusion (11, 19–36), including 37,386 males and 31,163 females in the final analysis. The detailed selection process including specific reasons for exclusion was tabulated in Figure 1.
The baseline characteristics of all cohort studies included in this meta-analysis are displayed in Table 1 and Supplementary Table 1. Only four of 19 qualified articles analyzed the effect of per unit UA increase on stroke (11, 25, 30, 32). Seven articles described the association between different SUA levels and risk of stroke without out separate gender groups (19, 24, 25, 28, 30, 32, 33), and 10 articles specifically reported the effect of different levels of UA on different type of strokes (22, 23, 26–29, 31, 33, 34, 36). Based on geographic regions, all the eligible articles were classified into three categories, namely, America (24, 35), Europe (11, 19, 22, 25–27, 29, 32), and Asia (20, 21, 23, 28, 30, 31, 33, 34, 36). According to sensitive analysis with the exclusion of lower-quality study (30), the outcome was stable (Figure 2).
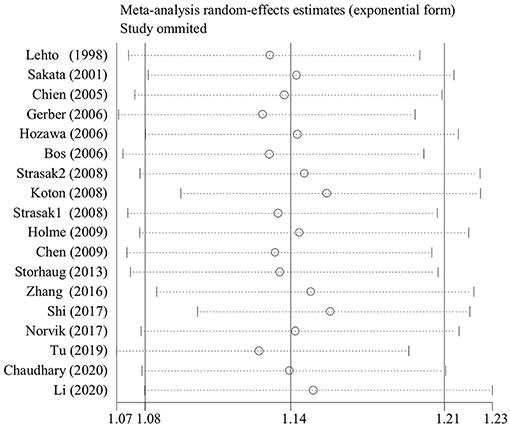
Figure 2. The sensitive plot on the association of uric acid levels and risk of stroke with the exclusion of lower-quality studies.
The Newcastle–Ottawa Scale (NOS) tool was used to assess the quality of the cohort studies, shown in Table 2, with the total scores ranging from 5 to 9 in this meta-analysis.
After pooling the results of all eligible prospective cohorts together (Table 3), there was a statistically significant association between SUA levels and the risk of total stroke (HR = 1.13; 95% CI: 1.09–1.18; P < 0.001), ischemic stroke (HR = 1.15; 95% CI: 1.10–1.21; P < 0.001), and hemorrhagic stroke (HR = 1.07; 95% CI: 1.00–1.15; P = 0.046) (Table 3). This association was obscured by significant between-study heterogeneity, with the corresponding I2 of 59.0, 77.0, and 33.7%. No obvious distinction had been found between ischemic stroke and hemorrhagic stroke (two-sample Z-test P = 0.095).
Begg's funnel plot was used to assess publication bias for the association between SUA levels and risk of stroke, and all of them seemed symmetrical, shown in Figure 3. As exposed by the Egger's test, there were strong evidence of publication bias for total stroke (P = 0.00), ischemic stroke (P = 0.00), and hemorrhagic stroke (P = 0.05). Further filled funnel plots showed that there was one potentially missing study in total stroke, 28 missing studies in ischemic stroke, and 13 missing studies in hemorrhagic stroke due to the publication bias to have a symmetrical plot.

Figure 3. Begg's and filled funnel plots on the association of uric acid levels and risk of stroke. (A) Begg's funnel plot and (B) Filled funnel plots: UA levels and total stroke. (C) Begg's funnel plot and (D) Filled funnel plots: UA levels and ischemic stroke. (E) Begg's funnel plot and (F) Filled funnel plots: UA levels and hemorrhagic stroke.
A sequence of subgroup analyses was conducted to investigate the possible causes of between-study heterogeneity for SUA levels and risk of stroke (Table 3). By gender, the association of SUA levels and risk of total stroke was statistically significant in both women (HR = 1.19; 95% CI: 1.12–1.26; P < 0.001) and men (HR = 1.11; 95% CI: 1.05–1.17; P < 0.001) (two-sample Z-test P = 0.088). It was also statistically significant for ischemic stroke in women (HR = 1.26; 95% CI: 1.17–1.36; P < 0.001) and men (HR = 1.12; 95% CI: 1.06–1.19; P < 0.001) (two-sample Z-test P = 0.015). The association of SUA levels and risk of hemorrhagic stroke was statistically significant in women (HR = 1.19; 95% CI: 1.04–1.35; P = 0.01), but not in men (HR = 1.01; 95% CI: 0.95–1.07; P = 0.81) (two-sample Z-test P = 0.025).
By geographic locations, in Asia, there was a statistically significant in association between SUA levels and risk of total stroke (HR = 1.06; 95% CI: 1.01–1.13; P = 0.03), as well as ischemic stroke (HR = 1.08; 95% CI: 1.02–1.14; P = 0.01) and hemorrhagic stroke (HR = 1.17; 95% CI: 1.03–1.34; P = 0.02). In Europe, however, there was only statistically significant association for SUA levels and risk of total stroke (HR = 1.20; 95% CI: 1.13–1.27; P < 0.001) and ischemic stroke (HR = 1.19; 95% CI: 1.12–1.27; P < 0.001).
By follow-up years, in sector of (0, 10) years, significance was observed for association of the SUA levels and risk of total stroke (HR = 1.13; 95% CI: 1.02–1.25; P = 0.02) and ischemic stroke (HR = 1.10; 95% CI: 1.02–1.19; P = 0.01). For (10, 20) years, total stroke (HR = 1.15; 95% CI: 1.09–1.21; P < 0.001) and ischemic stroke (HR = 1.19; 95% CI: 1.12–1.26; P < 0.001) were observed to be statistically related to a high level of SUA. While for (20, 30) years as well, the association of the SUA levels and risk of total stroke (HR = 1.13; 95% CI: 1.09–1.18; P < 0.001) and ischemic stroke (HR = 1.15; 95% CI: 1.10–1.21; P = 0.02) was statistically significant.
By age, total stroke was significantly associated with SUA levels in all subgroups [(20, 40) years: HR = 1.12; 95% CI: 1.04–1.21; P < 0.001, (40, 50) years: HR = 1.08; 95% CI: 1.02–1.14; P = 0.01, and (50, 90) years: HR = 1.28; 95% CI: 1.17–1.40; P < 0.001]. Similarly, for ischemic stroke, statistically significance was observed [(20, 40) years: HR = 1.18; 95% CI: 1.08–1.30; P < 0.001, (40, 50) years: HR = 1.05; 95% CI: 1.00–1.10; P < 0.001, and (50, 90) years: HR = 1.23; 95% CI: 1.14–1.34; P = 0.04]. While for hemorrhagic stroke, only marginal significance was observed among age group of 50–90 years (HR = 1.23; 95% CI: 1.14–1.34; P = 0.04).
By the stratification for stroke severity, we classified the severity of a stroke as fatal and non-fatal, and we found high SUA levels were significantly associated with both fatal and non-fatal stroke (fatal stroke: HR = 1.17; 95% CI: 1.10–1.25; P < 0.001, non-fatal stroke: HR = 1.16; 95% CI: 1.16–1.23; P < 0.001). The same trend was absorbed in ischemic stroke (fatal stroke: HR = 1.20; 95% CI: 1.13–1.27; P < 0.001, non-fatal stroke: HR = 1.14; 95% CI: 1.07–1.22; P < 0.001) and hemorrhagic stroke (fatal stroke: HR = 1.24; 95% CI: 1.10–1.39; P < 0.001, non-fatal stroke: HR = 1.00; 95% CI: 0.94–1.07; P = 0.98).
It should also be noticed that the significantly positive associations between SUA levels and risk of stroke that remained in subgroups had been found, which adjusted for potential confounders, including BMI, smoking status, hypertension, diabetes mellitus, hyperlipidemia, and renal factors.
Our dose–response research indicated the J-shaped trend between the ascending SUA levels and the higher risk of suffering from stroke. In the dose–response analysis on total stroke, the risk of stroke obviously increased with the higher UA concentration. When the SUA reached 5.35 mg/dl, it started to become statistically significant (Figure 4A). The same pattern was also found in ischemic stroke (the dividing value was 5.25 mg/dl) (Figure 4B) and hemorrhagic stroke (5.5 mg/dl) (Figure 4C).
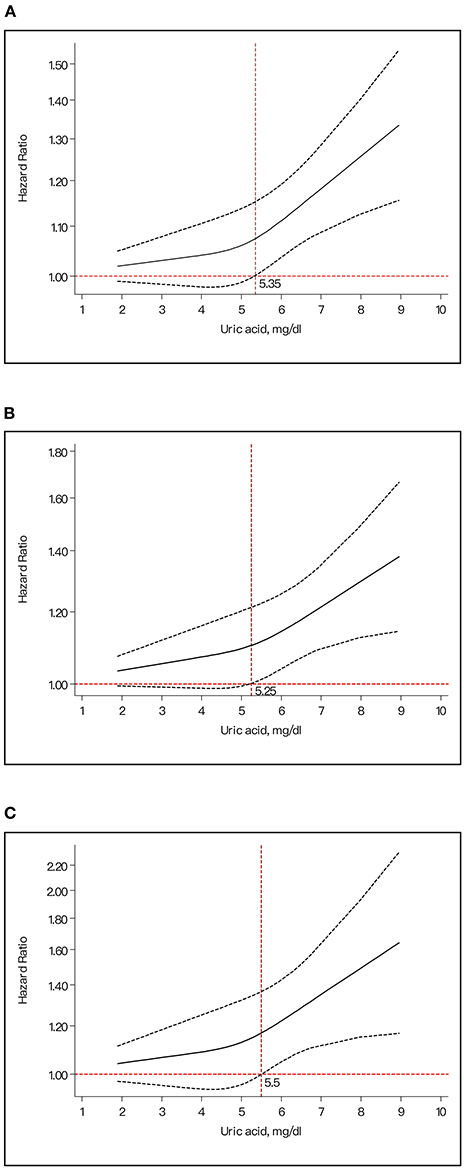
Figure 4. The dose-response plot for the association of uric acid levels and risk of stroke. (A) Uric acid levels and risk of total stroke. (B) Uric acid levels and risk of ischemic stroke. (C) Uric acid levels and risk of hemorrhagic stroke.
In our dose–response dichotomized by gender, it indicated a J-shaped trend between the ascending SUA levels and the higher risk of stroke for males (p for non-linear trend = 0.39) (Figure 5A) and a liner trend (p for non-linear trend = 0.32) for females (Figure 5B).
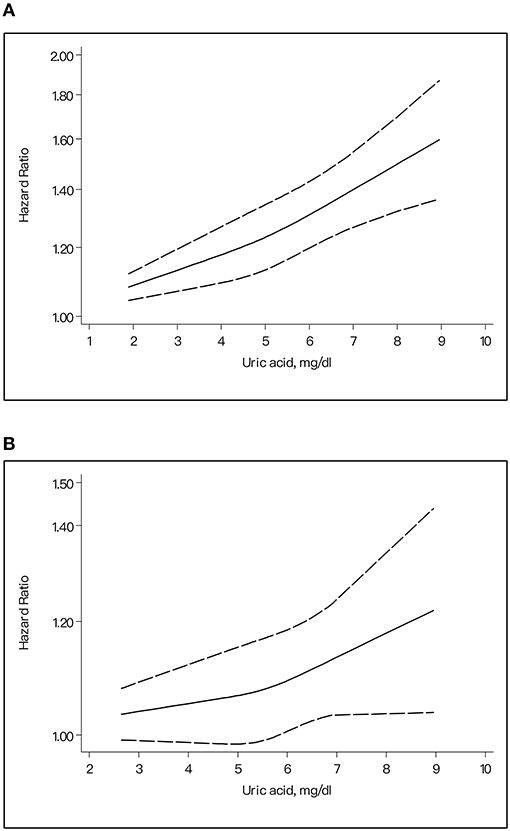
Figure 5. The dose-response plot on the association of uric acid levels and risk of stroke for different gender. (A) Female. (B) Male.
To the best of our knowledge, this is to date the most panoptic meta-analysis that has investigated the association between SUA levels and risk for stroke. The key findings of this study are that elevated SUA is a significant risk factor for adult stroke, both for ischemic stroke and hemorrhagic stroke, and the risk is more evident in females than that in males. Our sensitivity analyses and subgroup analyses also revealed that the relationship between SUA and stroke was robust and not affected by multifactor correction. Moreover, dose–response analysis presented the J-shaped trend between the ascending SUA levels and the higher risk of stroke. However, no obvious distinction was found between ischemic stroke and hemorrhagic stroke. More importantly, we found high SUA levels were significantly associated with an increased risk of fatal stroke. Our findings highlight the prominence and the necessity of closely regulating SUA, especially for elderly females, who have a high risk of suffering from cerebrovascular disease.
Several systematic reviews and meta-analyses have evaluated the impact of high SUA on the onset of stroke. Pooling the results of 13 prospective studies by Zhong et al. (12) showed that elevated serum SUA levels were significantly associated with modestly increased risk of stroke and have similar adverse effects on both sexes, whereas further subsidiary analyses by different types of stroke were lacking. Meanwhile, limited seven studies that involved SUA and the risk of stroke in males and seven studies in females had been included in Zhong et al.s' study. Researchers raised that if 10 or fewer studies are pooled in a meta-analysis, the possibility/capacity to detect statistical significance is low (37). At the same time, the study mixed risk ratio (RR) and HR as effect-size estimates for analysis, which is inaccurate and may affect the conclusions. Our work that was based on high-quality cohort studies have avoided these problems effectively and found the same significant relationship.
The concentration of UA is the key point of the mechanisms underlying the association of UA with development of stroke. As one of the most abundant antioxidant molecules in humans, UA has the valid ability to clear out peroxynitrite, nitric oxide, and hydroxyl radicals; hence, it can prevent protein nitration and lipid peroxidation (38, 39). Studies in animal models have shown that administration of UA or soluble UA analogs that retain the antioxidant properties of UA protects the brain against ischemic injury (40–42). However, once it exceeds the normal range, SUA would impact multiple systems, which in turn lead directly or indirectly to stroke. Possible mechanisms have been reported that elevated UA level was associated with carotid intima media thickness, as reported by the latest meta-analysis; high UA was related to carotid intima thickening (43); and the same trend was found in proximal extracranial artery stenosis (44). Meanwhile, it was demonstrated that elevated UA promoted atherosclerotic progression by increasing production of free radicals and facilitating low-density lipoprotein cholesterol (LDL-C) oxidation and lipid peroxidation (45). In addition, high levels of UA increased vascular endothelial dysfunction (46) and vascular smooth muscle cell proliferation, which could lead to preglomerular vascular disease and high blood pressure (47, 48). Potential mechanisms have also been reported that elevated UA level was involved in microvascular injury (47), increasing platelet aggregation and thrombus formation (49). Studies had revealed that UA could increase inflammatory cytokines such as C-reactive protein, interleukin 6 (IL-6), and tumor necrosis factor α (TNF-α) (50). Simultaneously, clinical studies also suggested that high SUA levels increased the risk of total mortality and cardiovascular and cerebrovascular diseases. In Italy, a national multicenter retrospective cohort study (51) assessed that all-cause mortality was substantially increased when the UA levels were above 4.7 mg/dl (95% CI: 4.3–5.1 mg/dl), and the risk of cardiovascular mortality (CVM) ascended while the value of SUA is over 5.6 mg/dl (95% CI: 4.99–6.21 mg/dl). These findings from experimental, epidemiological, and clinical studies of UA suggested that elevated SUA could be associated with vascular diseases and clarified the important role played by SUA levels in illustrating the possible pathophysiological association with hypertension, atherosclerosis, and stoke.
In our study, we took ischemic stroke and hemorrhagic stroke as the main subtypes, and we found elevated SUA levels have similar adverse effects on the development of stroke in these two subtypes. Evidence showed that ischemic stroke and hemorrhagic stroke both cause local hypoxia that damage brain tissue, and they could be converted to each other. There was a high risk of hemorrhagic transformation during the treatment of ischemic stroke (52). However, the study presented that significant differences existed in body composition between hemorrhagic and ischemic stroke in humans, and individuals with ischemic stroke had significantly worse body composition (53). Further exploration of the molecular mechanisms of SUA and different types of stroke is noteworthy.
Sex differences in the association of elevated SUA with stroke-related risk factors were found in our study. Statistically, females have a higher risk of experiencing a stroke-related fatality than males. Meanwhile, a J-shaped trend between the ascending SUA levels and higher risk of stroke for men and a liner trend for women had been explored. It is universally acknowledged that stroke is a sexually dimorphic disease. For one reason, females have a longer average lifespan, which increases the odds that they will have a stroke. Besides, females suffer greater susceptibility to depression and anxiety and often report higher levels of stress than males do (54–56). Other unique risk factors that females are facing, such as gestational hypertension and climacteric syndrome, may also cause the difference. To conclude, differences in vascular biology, immunity, coagulation, hormonal profiles, lifestyle factors, and societal roles seem to contribute (57).
Some limitations for the present meta-analysis should be acknowledged. Firstly, we were unable to carry out further subgroup comparison of hemorrhagic stroke because the corresponding data were not available in the original articles. The mechanisms and risk factors for subarachnoid hemorrhage and intracerebral hemorrhage are different in important ways, as are treatment and outcomes (58). More clinical and mechanistic studies deserve further research. Secondly, even though the errors of dose–response analysis are unavoidable in secondary analysis, the overall J-shaped trend is worthy of our attention in the relationship of SUA and risk of stroke in this meta-analysis. Thirdly, although a large panel of subgroup analyses were undertaken to account for possible sources of heterogeneity, significance still persisted in some subgroups, limiting the interpretation of pooled effect-size estimates. Finally, similar to any observational studies, a causal relationship could not be fully established.
Our study found that elevated SUA is a significant risk factor for adult stroke, both for ischemic stroke and hemorrhagic stroke, especially in females. Our dose–response research revealed a J-shaped trend between the ascending SUA levels and the higher risk of suffering from stroke. Moreover, high SUA levels are associated with an increased risk of fatal stroke. Further investigations on the molecular mechanisms linking SUA to adult stroke are also warranted.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
TQ reviewed the articles and wrote the manuscript. HW helped with the article review. WP was the editor of the manuscript and helped with the preliminary qualification. All authors contributed to the article and approved the submitted version.
The publication fee was provided by the Applied Research on TCM Community Management of Hypertension (Ji'nan, China).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2021.674398/full#supplementary-material
1. Group GBDNDC. Global, regional, and national burden of neurological disorders during 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet Neurol. (2017) 16:877–97. doi: 10.1016/S1474-4422(17)30299-5
2. Collaborators GBDRF. Global, regional, and national comparative risk assessment of 84 behavioural, environmental and occupational, and metabolic risks or clusters of risks for 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. (2018) 392:1923–94. doi: 10.1016/S0140-6736(18)32225-6
3. Feigin VL, Krishnamurthi RV, Parmar P, Norrving B, Mensah GA, Bennett DA, et al. Update on the global burden of ischemic and hemorrhagic stroke in 1990–2013: the GBD 2013 study. Neuroepidemiology. (2015) 45:161–76. doi: 10.1159/000441085
4. Maiuolo J, Oppedisano F, Gratteri S, Muscoli C, Mollace V. Regulation of uric acid metabolism and excretion. Int J Cardiol. (2016) 213:8–14. doi: 10.1016/j.ijcard.2015.08.109
5. Becker BF. Towards the physiological function of uric acid. Free Radic Biol Med. (1993) 14:615–31. doi: 10.1016/0891-5849(93)90143-I
6. Wang Q, Wen X, Kong J. Recent progress on uric acid detection: a review. Crit Rev Anal Chem. (2020) 50:359–75. doi: 10.1080/10408347.2019.1637711
7. Nieto FJ, Iribarren C, Gross MD, Comstock GW, Cutler RG. Uric acid and serum antioxidant capacity: a reaction to atherosclerosis? Atherosclerosis. (2000) 148:131–9. doi: 10.1016/S0021-9150(99)00214-2
8. Karagiannis A, Mikhailidis DP, Tziomalos K, Sileli M, Savvatianos S, Kakafika A, et al. Serum uric acid as an independent predictor of early death after acute stroke. Circ J. (2007) 71:1120–7. doi: 10.1253/circj.71.1120
9. Khalil MI, Salwa M, Sultana S, Al Mamun MA, Barman N, Haque MA. Role of serum uric acid in ischemic stroke: a case-control study in Bangladesh. PLoS ONE. (2020) 15:e0236747. doi: 10.1371/journal.pone.0236747
10. Tariq MA, Shamim SA, Rana KF, Saeed A, Malik BH. Serum uric acid - risk factor for acute ischemic stroke and poor outcomes. Cureus. (2019) 11:e6007. doi: 10.7759/cureus.6007
11. Storhaug HM, Norvik JV, Toft I, Eriksen BO, Lochen ML, Zykova S, et al. Uric acid is a risk factor for ischemic stroke and all-cause mortality in the general population: a gender specific analysis from The Tromso Study. BMC Cardiovasc Disord. (2013) 13:115. doi: 10.1186/1471-2261-13-115
12. Zhong C, Zhong X, Xu T, Xu T, Zhang Y. Sex-specific relationship between serum uric acid and risk of stroke: a dose-response meta-analysis of prospective studies. J Am Heart Assoc. (2017) 6:5042. doi: 10.1161/JAHA.116.005042
13. Lei Z, Cai J, Hong H, Wang Y. Serum uric acid level and outcome of patients with ischemic stroke: a systematic review and meta-analysis. Neurologist. (2019) 24:121–31. doi: 10.1097/NRL.0000000000000234
14. Zhou Z, Liang Y, Lin J, Zhang X, Qu H, Xu J, et al. Serum uric acid concentrations and risk of intracerebral hemorrhage: a systematic review and meta-analysis. Atherosclerosis. (2018) 275:352–8. doi: 10.1016/j.atherosclerosis.2018.07.002
15. Katan M, Luft A. Global burden of stroke. Semin Neurol. (2018) 38:208–11. doi: 10.1055/s-0038-1649503
16. Moher D, LA TJ, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. (2009) 339:b2535. doi: 10.1136/bmj.b2535
17. Altman DGBJ. Interaction revisited: the difference between two estimates. BMJ. (2003) 326:219. doi: 10.1136/bmj.326.7382.219
18. Greenland SLM. Methods for trend estimation from summarized dose-response data, with applications to meta-analysis. Am J Epidemiol. (1992) 135:1301–9. doi: 10.1093/oxfordjournals.aje.a116237
19. Lehto S, Niskanen L, Rönnemaa T, Laakso M. Serum uric acid is a strong predictor of stroke in patients with non-insulin-dependent diabetes mellitus. Stroke. (1998) 29:635–9. doi: 10.1161/01.STR.29.3.635
20. Sakata K, Hashimoto T, Ueshima H, Okayama A. Absence of an association between serum uric acid and mortality from cardiovascular disease: NIPPON DATA 80, 1980–1994. National integrated projects for prospective observation of non-communicable diseases and its trend in the aged. Eur J Epidemiol. (2001) 17:461–8. doi: 10.1023/a:1013735717961
21. Chien KL, Hsu HC, Sung FC, Su TC, Chen MF, Lee YT. Hyperuricemia as a risk factor on cardiovascular events in Taiwan: the Chin-Shan Community Cardiovascular Cohort Study. Atherosclerosis. (2005) 183:147–55. doi: 10.1016/j.atherosclerosis.2005.01.018
22. Bos MJ, Koudstaal PJ, Hofman A, Witteman JC, Breteler MM. Uric acid is a risk factor for myocardial infarction and stroke: the Rotterdam study. Stroke. (2006) 37:1503–7. doi: 10.1161/01.STR.0000221716.55088.d4
23. Gerber Y, Tanne D, Medalie JH, Goldbourt U. Serum uric acid and long-term mortality from stroke, coronary heart disease and all causes. Eur J Cardiovasc Prev Rehabil. (2006) 13:193–8. doi: 10.1097/01.hjr.0000192745.26973.00
24. Hozawa A, Folsom AR, Ibrahim H, Nieto FJ, Rosamond WD, Shahar E. Serum uric acid and risk of ischemic stroke: the ARIC Study. Atherosclerosis. (2006) 187:401–7. doi: 10.1016/j.atherosclerosis.2005.09.020
25. Koton S, Howard SC, Warlow CP, Murphy MF, Rothwell PM. Serum urate predicts long-term risk of acute coronary events in women after a transient ischaemic attack and stroke. Cerebrovasc Dis. (2008) 26:517–24. doi: 10.1159/000155990
26. Strasak A, Ruttmann E, Brant L, Kelleher C, Klenk J, Concin H, et al. Serum uric acid and risk of cardiovascular mortality: a prospective long-term study of 83,683 Austrian men. Clin Chem. (2008) 54:273–84. doi: 10.1373/clinchem.2007.094425
27. Strasak AM, Kelleher CC, Brant LJ, Rapp K, Ruttmann E, Concin H, et al. Serum uric acid is an independent predictor for all major forms of cardiovascular death in 28,613 elderly women: a prospective 21-year follow-up study. Int J Cardiol. (2008) 125:232–9. doi: 10.1016/j.ijcard.2007.11.094
28. Chen JH, Chuang SY, Chen HJ, Yeh WT, Pan WH. Serum uric acid level as an independent risk factor for all-cause, cardiovascular, and ischemic stroke mortality: a Chinese cohort study. Arthritis Rheum. (2009) 61:225–32. doi: 10.1002/art.24164
29. Holme I, Aastveit AH, Hammar N, Jungner I, Walldius G. Uric acid and risk of myocardial infarction, stroke and congestive heart failure in 417,734 men and women in the Apolipoprotein MOrtality RISk study (AMORIS). J Intern Med. (2009) 266:558–70. doi: 10.1111/j.1365-2796.2009.02133.x
30. Chen Y, Ding X, Teng J, Zou J, Zhong Y, Fang Y, et al. Serum uric acid is inversely related to acute ischemic stroke morbidity in hemodialysis patients. Am J Nephrol. (2011) 33:97–104. doi: 10.1159/000322966
31. Zhang W, Iso H, Murakami Y, Miura K, Nagai M, Sugiyama D, et al. Serum uric acid and mortality form cardiovascular disease: EPOCH-JAPAN study. J Atheroscler Thromb. (2016) 23:692–703. doi: 10.5551/jat.31591
32. Norvik JV, Schirmer H, Ytrehus K, Storhaug HM, Jenssen TG, Eriksen BO, et al. Uric acid predicts mortality and ischaemic stroke in subjects with diastolic dysfunction: the Tromsø Study 1994–2013. ESC heart failure. (2017) 4:154–61. doi: 10.1002/ehf2.12134
33. Shi X, Yang J, Wang L, Zhao M, Zhang C, He M, et al. Prospective study of serum uric acid levels and stroke in a Chinese hypertensive cohort. Clin Exp Hypertens. (2017) 39:527–31. doi: 10.1080/10641963.2017.1281938
34. Tu W, Wu J, Jian G, Lori J, Tang Y, Cheng H, et al. Asymptomatic hyperuricemia and incident stroke in elderly Chinese patients without comorbidities. Eur J Clin Nutr. (2019) 73:1392–402. doi: 10.1038/s41430-019-0405-1
35. Chaudhary NS, Bridges SL Jr, Saag KG, Rahn EJ, Curtis JR, Gaffo A, et al. Severity of hypertension mediates the association of hyperuricemia with stroke in the REGARDS case cohort study. Hypertension. (2020) 75:246–56. doi: 10.1161/HYPERTENSIONAHA.119.13580
36. Li J, Muraki I, Imano H, Cui R, Yamagishi K, Umesawa M, et al. Serum uric acid and risk of stroke and its types: the Circulatory Risk in Communities Study (CIRCS). Hypertens Res. (2020) 43:313–21. doi: 10.1038/s41440-019-0385-5
37. Richardson JL, Koprowski C, Mondrus GT, Dietsch B, Deapen D, Mack TM. Perceived change in food frequency among women at elevated risk of breast cancer. Nutr Cancer. (1993) 20:71–8. doi: 10.1080/01635589309514272
38. Hooper DC, Bagasra O, Marini JC, Zborek A, Ohnishi ST, Kean R, et al. Prevention of experimental allergic encephalomyelitis by targeting nitric oxide and peroxynitrite: implications for the treatment of multiple sclerosis. Proc Natl Acad Sci USA. (1997) 94:2528–33. doi: 10.1073/pnas.94.6.2528
39. Hooper DC, Spitsin S, Kean RB, Champion JM, Dickson GM, Chaudhry I, et al. Uric acid, a natural scavenger of peroxynitrite, in experimental allergic encephalomyelitis and multiple sclerosis. Proc Natl Acad Sci USA. (1998) 95:675–80. doi: 10.1073/pnas.95.2.675
40. Aliena-Valero A, Lopez-Morales MA, Burguete MC, Castello-Ruiz M, Jover-Mengual T, Hervas D, et al. Emergent uric acid treatment is synergistic with mechanical recanalization in improving stroke outcomes in male and female rats. Neuroscience. (2018) 388:263–73. doi: 10.1016/j.neuroscience.2018.07.045
41. Dhanesha N, Vazquez-Rosa E, Cintron-Perez CJ, Thedens D, Kort AJ, Chuong V, et al. Treatment with uric acid reduces infarct and improves neurologic function in female mice after transient cerebral ischemia. J Stroke Cerebrovasc Dis. (2018) 27:1412–6. doi: 10.1016/j.jstrokecerebrovasdis.2017.12.043
42. Cutler RG, Camandola S, Feldman NH, Yoon JS, Haran JB, Arguelles S, et al. Uric acid enhances longevity and endurance and protects the brain against ischemia. Neurobiol Aging. (2019) 75:159–68. doi: 10.1016/j.neurobiolaging.2018.10.031
43. Ma M, Wang L, Huang W, Zhong X, Li L, Wang H, et al. Meta-analysis of the correlation between serum uric acid level and carotid intima-media thickness. PLoS ONE. (2021) 16:e0246416. doi: 10.1371/journal.pone.0246416
44. Yang X, Lv H, Hidru TH, Wu J, Liu H, Wang Y, et al. Relation of serum uric acid to asymptomatic proximal extracranial artery stenosis in a middle-aged Chinese population: a community-based cross-sectional study. BMJ Open. (2018) 8:e020681. doi: 10.1136/bmjopen-2017-020681
45. Wong LK. Global burden of intracranial atherosclerosis. Int J Stroke. (2006) 1:158–9. doi: 10.1111/j.1747-4949.2006.00045.x
46. Otani N, Toyoda S, Sakuma M, Hayashi K, Ouchi M, Fujita T, et al. Effects of uric acid on vascular endothelial function from bedside to bench. Hypertens Res. (2018) 41:923–31. doi: 10.1038/s41440-018-0095-4
47. Lee SW, Kim HC, Nam C, Lee HY, Ahn SV, Oh YA, et al. Age-differential association between serum uric acid and incident hypertension. Hypertens Res. (2019) 42:428–37. doi: 10.1038/s41440-018-0168-4
48. Mazzali M, Hughes J, Kim YG, Jefferson JA, Kang DH, Gordon KL, et al. Elevated uric acid increases blood pressure in the rat by a novel crystal-independent mechanism. Hypertension. (2001) 38:1101–6. doi: 10.1161/hy1101.092839
49. Zapolski T, Wacinski P, Kondracki B, Rychta E, Buraczynska MJ, Wysokinski A. Uric acid as a link between renal dysfunction and both pro-inflammatory and prothrombotic state in patients with metabolic syndrome and coronary artery disease. Kardiol Pol. (2011) 69:319–26.
50. Lyngdoh T, Marques-Vidal P, Paccaud F, Preisig M, Waeber G, Bochud M, et al. Elevated serum uric acid is associated with high circulating inflammatory cytokines in the population-based Colaus study. PLoS ONE. (2011) 6:e19901. doi: 10.1371/journal.pone.0019901
51. Virdis A, Masi S, Casiglia E, Tikhonoff V, Cicero AFG, Ungar A, et al. Identification of the uric acid thresholds predicting an increased total and cardiovascular mortality over 20 years. Hypertension. (2020) 75:302–8. doi: 10.1161/HYPERTENSIONAHA.119.13643
52. Barthels D, Das H. Current advances in ischemic stroke research and therapies. Biochim Biophys Acta Mol Basis Dis. (2020) 1866:165260. doi: 10.1016/j.bbadis.2018.09.012
53. Wilczynski J, Mierzwa-Molenda M, Habik-Tatarowska N. Differences in body composition among patientsafter hemorrhagic and ischemic stroke. Int J Environ Res Public Health. (2020) 17:114170. doi: 10.3390/ijerph17114170
54. Jackson CA, Mishra GD. Depression and risk of stroke in midaged women: a prospective longitudinal study. Stroke. (2013) 44:1555–60. doi: 10.1161/STROKEAHA.113.001147
55. Leffert LR, Clancy CR, Bateman BT, Bryant AS, Kuklina EV. Hypertensive disorders and pregnancy-related stroke: frequency, trends, risk factors, and outcomes. Obstet Gynecol. (2015) 125:124–31. doi: 10.1097/AOG.0000000000000590
56. Sacco S, Merki-Feld GSKLAE, Bitzer J, Canonico M, Kurth T, et al. Correction to: hormonal contraceptives and risk of ischemic stroke in women with migraine: a consensus statement from the European Headache Federation (EHF) and the European Society of Contraception and Reproductive Health (ESC). J Headache Pain. (2018) 19:81. doi: 10.1186/s10194-018-0912-9
57. Cordonnier C, Sprigg N, Sandset EC, Pavlovic A, Sunnerhagen KS, Caso V, et al. Stroke in women - from evidence to inequalities. Nat Rev Neurol. (2017) 13:521–32. doi: 10.1038/nrneurol.2017.95
Keywords: risk factor, meta-analysis, serum uric acid, ischemic stroke, hemorrhagic stroke
Citation: Qiao T, Wu H and Peng W (2021) The Relationship Between Elevated Serum Uric Acid and Risk of Stroke in Adult: An Updated and Dose–Response Meta-Analysis. Front. Neurol. 12:674398. doi: 10.3389/fneur.2021.674398
Received: 01 March 2021; Accepted: 03 June 2021;
Published: 10 August 2021.
Edited by:
Robert G. Kowalski, University of Colorado, United StatesReviewed by:
Raffaele Ornello, University of L'Aquila, ItalyCopyright © 2021 Qiao, Wu and Peng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wei Peng, c3p5cGVuZ3dlaUAxNjMuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.