- 1Department of Neurology, University Hospital, Ludwig-Maximilians-University, Munich, Germany
- 2Institute of Neuropathology, University Hospital, Ludwig-Maximilians-University, Munich, Germany
- 3Department of Radiotherapy and Radiation Oncology, Faculty of Medicine and University Hospital Carl Gustav Carus, Technische Universität Dresden, Dresden, Germany
- 4Department of Neurosurgery, University Hospital, Ludwig-Maximilians-University, Munich, Germany
Objectives: We describe two new cases of acute hemorrhagic leucoencephalitis (AHLE), who survived with minimal sequelae due to early measures against increased intracranial pressure, particularly craniotomy. The recently published literature review on treatment and outcome of AHLE was further examined for the effect of craniotomy.
Methods: We present two cases from our institution. The outcome of 44 cases from the literature was defined either as good (no deficit, minimal deficit/no daily help) or poor outcome (severe deficit/disabled, death). Patients with purely infratentorial lesions (n = 9) were excluded. Fisher's exact test was applied.
Results: Two cases are presented: A 43-year-old woman with rapidly progressive aphasia and right hemiparesis due to a huge left frontal white matter lesion with rim contrast enhancement. Pathology was consistent with AHLE. The second case was a 56-year-old woman with rapidly progressive aphasia and right hemiparesis. Cranial MRI showed a huge left temporo-occipital white matter lesion with typical morphology for AHLE. Both patients received craniotomy within the first 24 h and consequent immunosuppressive-immunomodulatory treatment and survived with minimal deficits. Out of 35 supratentorial reported AHLE cases, seven patients received decompressive craniotomy. Comparing all supratentorial cases, patients who received craniotomy were more likely to have a good outcome (71 vs. 29%).
Conclusion: Due to early control of the intracranial pressure, particularly due to early craniotomy; diagnosis per biopsy; and immediate start of immunosuppressive-immunomodulatory therapies (cortisone pulse, plasma exchanges), both patients survived with minimal sequelae. Craniotomy plays an important role and should be considered early on in patients with probable AHLE.
Introduction
Acute hemorrhagic leucoencephalitis (Weston–Hurst, AHLE) is a rare, rapidly progressive, demyelinating disease, commonly considered to be a severe variant of an acute disseminated encephalomyelitis (ADEM) (1, 2). The disease is often preceded by a respiratory tract infection (3–5), and in some cases, AHLE also occurred shortly after seasonal influenza vaccinations (6, 7). Most patients die within a couple of days due to fulminant brain edema. It more commonly affects the supratentorial regions, but there are also cases documented with infratentorial lesions (6, 8–10). Mortality is still high, but there are cases in the literature with a good outcome (11–14). There are no standard operating procedures concerning the treatment (12). A detailed systematic review from Grzonka (15) shows that most of the patients were treated immunosuppressive-immunomodulatory, mostly with glucocorticoids. There was no relationship between the delay between the start of the treatment and the outcome. With our two successfully treated fulminant AHLE cases, our aim was, in combination with the reviewed cases from the literature, to find out what may have attributed to the good outcome.
Methods
We present two cases from our institution with AHLE seen in the last 7 years. The systematically reviewed AHLE case collective, including the presented case of Grzonka et al. (15), was examined for their outcomes, depending on whether they were treated with craniotomy. Purely infratentorial localized cases were excluded.
Outcome was defined either as no deficit, minimal deficit (no influence on everyday life), severe deficit (disabled), or death. Fisher's exact test was applied.
Results
Case Presentation
Case 1
A 43-year-old woman with a preceding unspecific respiratory infection was admitted to the hospital due to rapidly progressive aphasia and right hemiparesis. Brain MRI revealed a left frontal white matter lesion with rim contrast enhancement (Figures 1A,B). In the cerebrospinal fluid, 112 cells/μl (granulocytic) and oligoclonal IgG-bands were found. Neuroinfectious pathogens were not detected. Under the suspected diagnosis of an ADEM, glucocorticoid pulse therapy was initiated (1 g per day). As the patient still rapidly worsened and imaging revealed progressive edema with a midline shift (Figures 1C,D), hemicraniectomy was performed (<24 h after admission). Histopathology of cortical biopsy was consistent with AHLE (Weston–Hurst, Figures 1I–IV). The patient sequentially received another cortisone pulse (methylprednisolone 1 g for 5 days), immunoglobulins (120 g for 5 days), and seven cycles of therapeutic plasma exchange until day 36 after symptom onset. Under this treatment, the maximum of the swelling was reached on day 15 (Figures 1E,F), from that time on, the patient improved in both the imaging finding and clinically. Four months after symptom onset, the bone flap could be reimplanted (Figure 1G). The patient survived with a minimal weakness of the right hand and slight neurocognitive deficits.
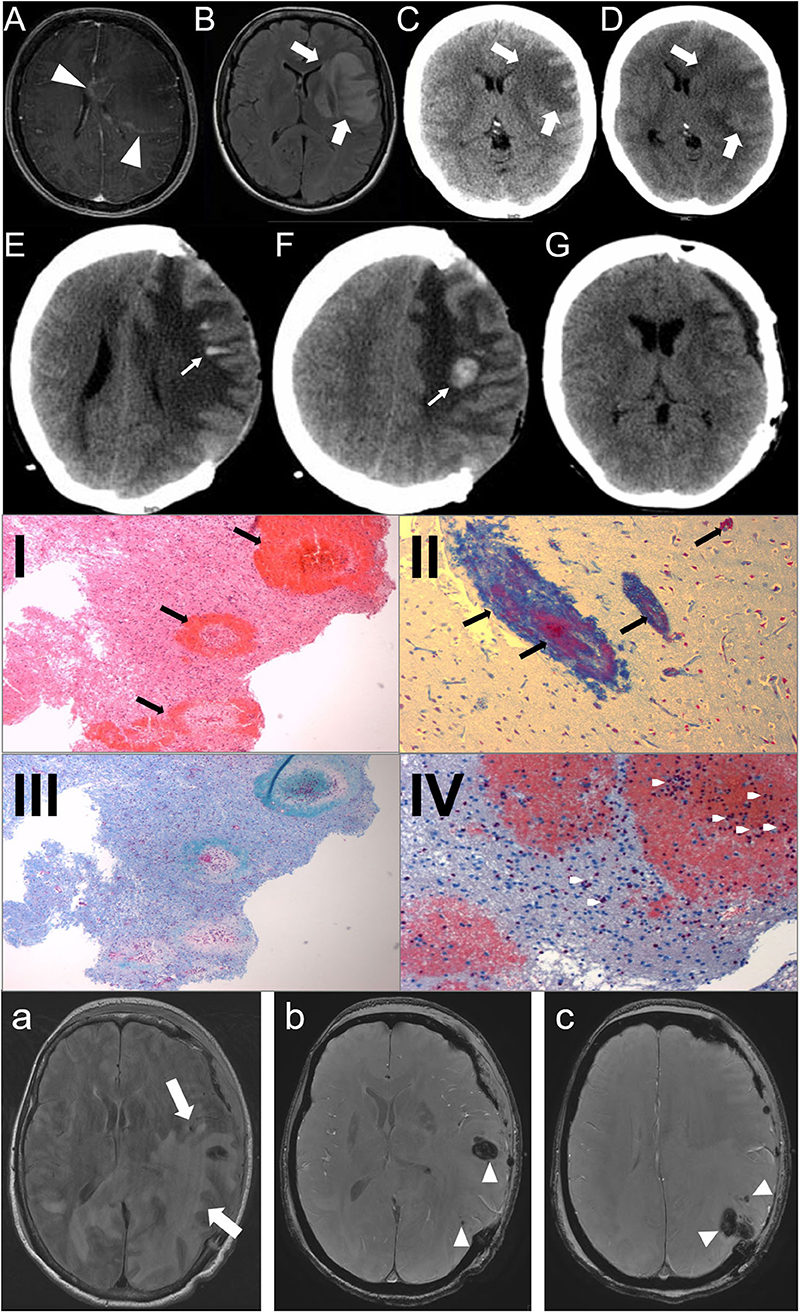
Figure 1. (A–G): Case 1: Day 1: axial MRI, T1-weighted with contrast (A), FLAIR-sequence (B), CT-scan (C) show a left frontal white matter lesion (big arrows) with rim contrast enhancement (arrowheads) and midline shift; Day 2 (D): CT-scan: progressive edema; Day 15 (E,F): CT-scan: bleedings (small arrows); Day 80 (G): reimplantation of bone plate. (I–IV): Histopathology of the brain biopsy (Case 1). Multiple perivascular bleedings [(I), HE-stain], fibrin accumulation [(II), Ladewig-stain], demyelinization [(III), LFB-PAS] and granulocystic infiltration [(IV), Chloracetatesterase]. (a–c): Case 2: Day 3: axial MRI, FLAIR-sequence (a) shows a subcortical left temporooccipital white matter lesion (big arrows) with midline shift and multiple hemorrhages (arrowheads) in SWAN-sequence (b,c).
Case 2
The second case was a 56-year-old woman who also presented with aphasia and right hemiparesis. She also had a history of a recent unspecific respiratory infection over the last days. CT scan of the brain revealed a huge left temporo-occipital lesion with midline shift. As the patient rapidly worsened and the lesion was progressive in the CT scan, the patient immediately (within 24 h after admission) underwent decompressive craniotomy. Cranial MRI also showed a huge left temporo-occipital white matter lesion with multiple small hemorrhages. The lesion was located predominantly subcortical without affecting the temporal pole, typical for AHLE (Figures 1a-c). Due to the rapid course of the disease and the morphology of the lesion, AHLE was immediately suspected. Glucocorticoid pulse therapy was initiated (methylprednisolone 1 g for 5 days) followed by tapering with prednisolone, immunoglobulins (20 g/day for 5 days) and six cycles of immunoabsorption. The bone flap was reimplanted 4 months after symptom onset. The patient also survived with minimal sequelae.
In the literature from January 1, 2000, until the publication of Grzonka et al. in 2020, 44 adult patients are reported. One case with a spinal localization of the lesion was excluded. Out of 43 cases, 30 are males (70%) and 13 females (30%); the mean age was 38 years. Eight patients had only infratentorial lesions (seven males, 88%; one female, 12%, mean age 44 years). Out of 35 supratentorial reported AHLE cases [23 males, 66%, 12 females, 34%, mean age 38 years; Table 1; (1, 2, 5, 7–46)], seven patients received decompressive craniotomy [Table 2; (2, 5, 11–13, 18, 42)]. In four of the operated patients, hemorrhages were detected in neuroimaging prior to surgery (2, 11, 13, 42). In the other patients, the diagnosis of AHLE was confirmed by biopsy (12, 18) or postmortem (5). Patients who received craniotomy, like our two cases, were more likely to have a good outcome (71%, n = 5; Table 3, Fisher's exact test: p = 0.0754). Three of the seven patients who received craniotomy had no deficit, two had minimal sequelae, and only two patients died (29%). The initial biopsy of one of those two patients (5) showed a neutrophil predominance and did not lead to the direct diagnosis of AHLE. Thus, a differential diagnosis of pneumonia with hematogenous spread to the brain was suspected, and the craniectomy was combined with antibiotics instead of a combination with immunosuppressive therapy. The other patient who died despite craniectomy had a 2-week history of illness and refused to go to the hospital at first (42). Also, the MRI could not be obtained on the first day after admission, which led to a delay in immunosuppressive treatment. In the non-operated group, 71% (n = 20) of the patients had an unfavorable outcome (7 patients severely disabled, 13 died), and 29% survived with minimal (n = 6) to no deficit (n = 2). This difference, however, just did not reach statistical significance (Fisher's exact test: p = 0.0754).
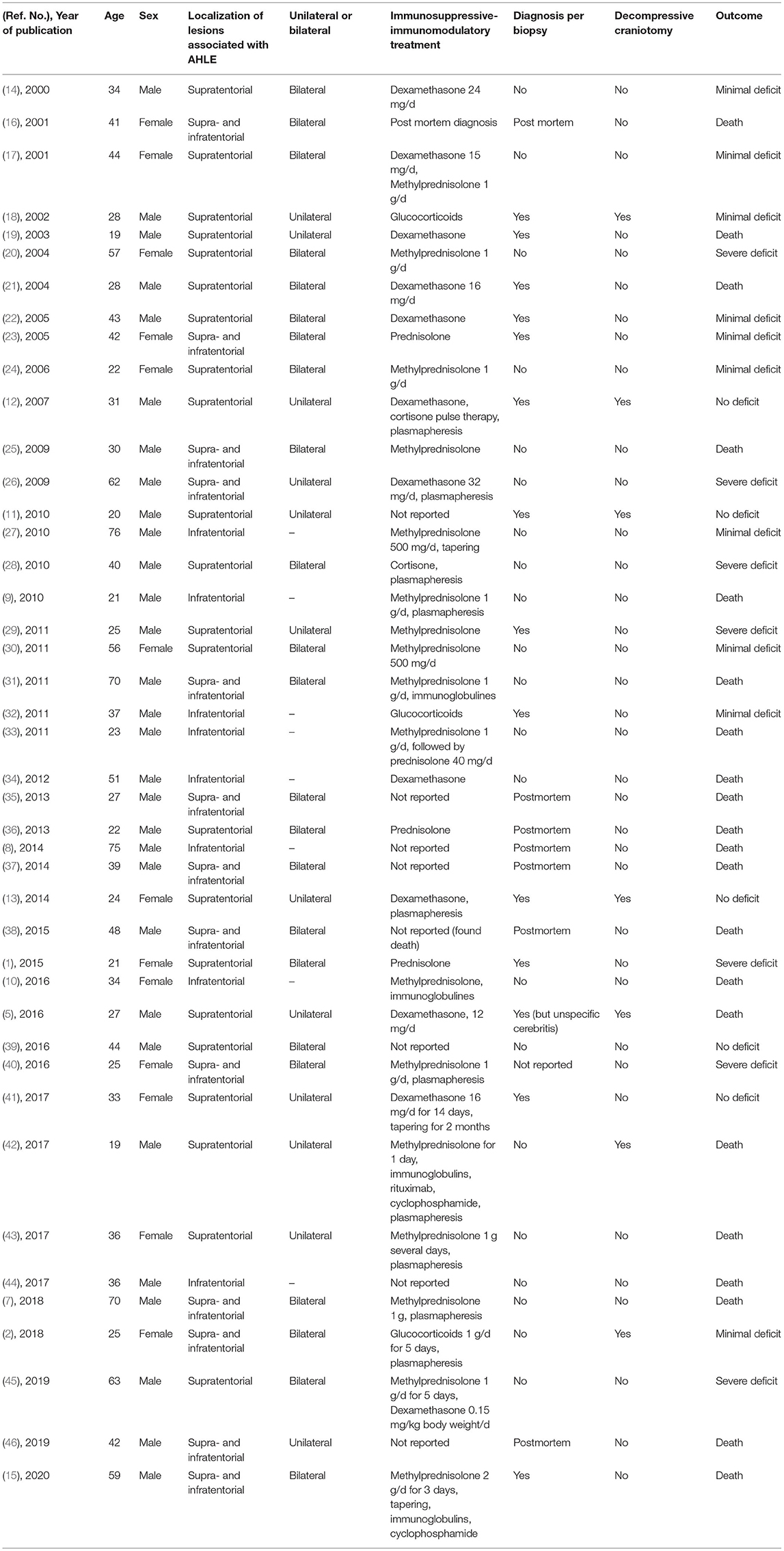
Table 1. Clinical, neuroradiologic, and neuropathologic characteristics of adult patients with AHLE.
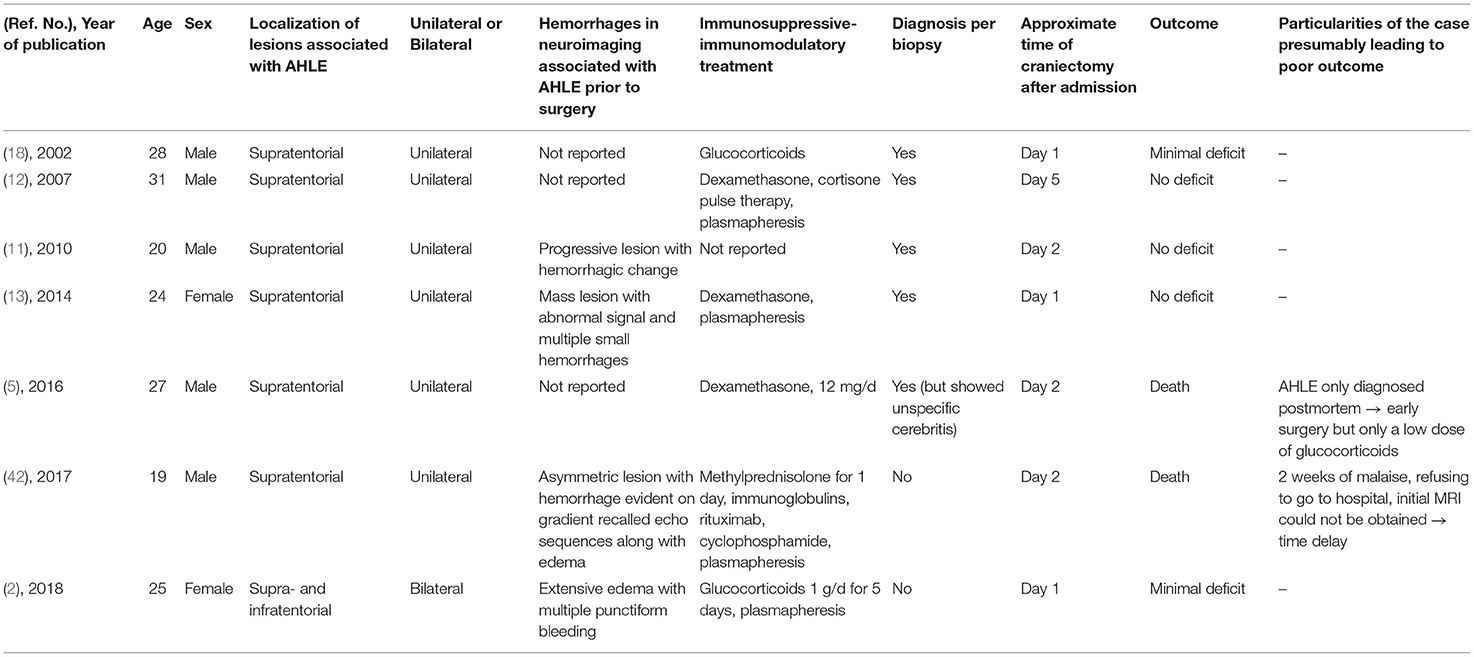
Table 2. Clinical, neuroradiologic, and neuropathologic characteristics of adult patients who received craniectomy with AHLE.

Table 3. Statistical analysis of the outcome of adult AHLE patients regarding craniectomy, Fisher's exact test, p-value = 0.0754.
Discussion
AHLE is still a rare and fulminant disease with a mostly life-threatening outcome and a high mortality. The disease mostly affects young males but is also reported in patients of all ages (16). Even with early aggressive immunosuppressive treatment, AHLE can be a devastating condition in terms of mortality and severe neurological sequelae. One of the most detailed reviews on AHLE by Grzonka et al. (15) shows that, looking only at immunosuppressive and immunomodulatory treatments, there was no clear relationship between different treatments and outcome.
Thus, based on our literature review, we consider that, early interventions against the increased intracranial pressure due to the rapid increasing brain edema, especially craniotomy, can change the fulminant course of the disease. We suggest that better prognosis can be expected when craniotomy is performed early, together with a consequent immunosuppressive-immunomodulatory treatment at the same time than medical treatment alone. Despite the worry that sudden decompression could aggravate intracerebral bleedings of the hemorrhagic encephalitis, the mass effect of the disease seems to be the life-limiting factor. This is in line with a review on decompressive craniotomy in viral encephalitis patients with brain herniation (47) and also in patients with spontaneous intracerebral hemorrhage (48). They also suggest that, in those cases, a better prognosis without increasing the hemorrhage can be expected when craniotomy is performed in addition to medical treatment alone.
We believe that the present data support the decision for an early surgical decompression in patients with severe and rapid AHLE and should be considered early on. Control of the intracranial pressure seems to be an important part of the therapy concept in addition to early immunosuppression. Still, this is the conclusion based on a review of literature with a relative low number of cases and, therefore, only a low level of evidence. Otherwise, we don't expect that, in such a rare and severe disease, a prospective controlled study can be done.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author Contributions
AL-B contributed by executing the literature search, collected the data, and drafted and revised the manuscript. AJ, JL, NT, SH, CO, and AS helped with the data collection and manuscript revision. AJ also contributed with the histopathological images. JL provided the radiological images. AS also helped with the literature search. HP interpreted the data, helped with the literature search, and revised the manuscript for intellectual content. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Yildiz Ö, Pul R, Raab P, Hartmann C, Skripuletz T, Stangel M. Acute hemorrhagic leukoencephalitis (Weston-Hurst syndrome) in a patient with relapse-remitting multiple sclerosis. J Neuroinflammation. (2015) 12:175. doi: 10.1186/s12974-015-0398-1
2. Bonduelle T, Stricker J, Minéo JF, Massri A, Guesdon C, Barroso B, et al. Weston-Hurst syndrome with acute hemorrhagic cerebellitis. Clin Neurol Neurosurg. (2018) 173:118–19. doi: 10.1016/j.clineuro.2018.08.007
3. Hofer M, Weber A, Haffner K, Berlis A, Klingel K, Krüger M, et al. Acute hemorrhagic leukoencephalitis (Hurst's disease) linked to Epstein-Barr virus infection. Acta Neuropathol. (2005) 109:226–30. doi: 10.1007/s00401-004-0930-3
4. An SF, Groves M, Martinian L, Kuo LT, Scaravilli F. Detection of infectious agents in brain of patients with acute hemorrhagic leukoencephalitis. J Neurovirol. (2002) 8:439–46. doi: 10.1080/13550280260422749
5. Magun R, Verschoor CP, Bowdish DME, Provias J. Mycoplasma pneumoniae a trigger for Weston Hurst syndrome. Neurol Neuroimmunol Neuroinflamm. (2016) 3:e187. doi: 10.1212/NXI.0000000000000187
6. Wu CY, Riangwiwat T, Nakamoto BK. Hemorrhagic longitudinally extensive transverse myelitis. Case Rep Neurol Med. (2016) 2016:1–3. doi: 10.1155/2016/1596864
7. Sinzobahamvya E, Borrelli S, Rutgers MP, Clause D, Gille M. Acute hemorrhagic leukoencephalitis after seasonal influenza vaccination. Acta Neurol Belg. (2018) 118:127–129. doi: 10.1007/s13760-017-0861-0
8. Dos Santos MP, Martin J, Woulfe J, Lim SP, Chakraborty S. Autopsy-proven acute hemorrhagic leukoencephalitis in an elderly patient. Can J Neurol Sci. (2014) 41:99–102. doi: 10.1017/S031716710001636X
9. Abou Zeid NE, Burns JD, Wijdicks EFM, Giannini C, Keegan BM. Atypical acute hemorrhagic leukoencephalitis (Hurst's disease) presenting with focal hemorrhagic brainstem lesion. Neurocrit Care. (2010) 12:95–7. doi: 10.1007/s12028-009-9293-x
10. Atherton DS, Perez SR, Gundacker ND, Franco R, Han X. Acute disseminated encephalomyelitis presenting as a brainstem encephalitis. Clin Neurol Neurosurg. (2016) 143:76–9. doi: 10.1016/j.clineuro.2016.02.014
11. Takeuchi S, Takasato Y, Masaoka H, Hayakawa T, Otani N, Yoshino Y, et al. Hemorrhagic encephalitis associated with Epstein-Barr virus infection. J Clin Neurosci. (2010) 17:153–4. doi: 10.1016/j.jocn.2009.03.043
12. Ryan LJ, Bowman R, Zantek ND, Sherr G, Maxwell R, Clark HB, et al. Use of therapeutic plasma exchange in the management of acute hemorrhagic leukoencephalitis: a case report and review of the literature. Transfusion. (2007) 47:981–6. doi: 10.1111/j.1537-2995.2007.01227.x
13. Duggal N. Acute hemorrhagic leukoencephalitis associated with autoimmune myopathy. J Vasc Interv Neurol. (2014) 7:19–22.
14. Klein CJ, Wijdicks EFM, Earnest F IV. Full recovery after acute hemorrhagic leukoencephalitis (Hurst's disease) J Neurol. (2000) 247:977–9. doi: 10.1007/s004150070060
15. Grzonka P, Scholz MC, De Marchis GM, Tisljar K, Rüegg S, Marsch S, et al. Acute hemorrhagic leukoencephalitis: a case and systematic review of the literature. Front Neurol. (2020) 11:899. doi: 10.3389/fneur.2020.00899
16. Tanser SJ, Walker MB, Hilton DA. Acute haemorrhagic leucoencephalitis complicating sepsis. Anaesth Intensive Care. (2001) 29:54–7. doi: 10.1177/0310057X0102900111
17. Meilof JF, Hijdra A, Vermeulen M. Successful recovery after high-dose intravenous methylprednisolone in acute hemorrhagic leukoencephalitis. J. Neurol. (2001) 898–9. doi: 10.1007/s004150170076
18. Pfausler B, Engelhardt K, Kampfl A, Spiss H, Taferner E, Schmutzhard E. Post-infectious central and peripheral nervous system diseases complicating Mycoplasma pneumoniae infection: report of three cases and review of the literature. Eur J Neurol. (2002) 9:93–6. doi: 10.1046/j.1468-1331.2002.00350.x
19. Kuperan S, Ostrow P, Landi MK, Bakshi R. Acute hemorrhagic leukoencephalitis vs ADEM: FLAIR MRI and neuropathology findings. Neurology. (2003) 60:721–2. doi: 10.1212/01.WNL.0000048493.82053.4C
20. Milhomem Martins H, Teixeira AL, Lana-Peixoto MA. Acute hemorrhagic leukoencephalitis mimicking herpes simplex encephalitis: case report. Arq Neuropsiquiatr. (2004) 62:139–43.
21. Francisci D, Sensini A, Fratini D, Moretti MV, Luchetta ML, Caro AD, et al. Acute fatal necrotizing hemorrhagic encephalitis caused by Epstein-Barr virus in a young adult immunocompetent man. J Neurovirol. (2004) 10:414–7. doi: 10.1080/13550280490521050
22. Gibbs WN, Kreidie MA, Kim RC, Hasso AN. Acute hemorrhagic leukoencephalitis: Neuroimaging features and neuropathologic diagnosis. J Comput Assist Tomogr. (2005) 29:689–93. doi: 10.1097/01.rct.0000173843.82364.db
23. Lee HY, Chang KH, Kirn JH, Na DG, Kwon BJ, Lee KW, et al. Serial MR imaging findings of acute hemorrhagic leukoencephalitis: a case report. Am J Neuroradiol. (2005) 26:1996–9.
24. Alemdar M, Selekler HM, Iseri P, Demirci A, Komsuoglu SS. The importance of EEG and variability of MRI findings in acute hemorrhagic leukoencephalitis. Eur J Neurol. (2006). 13:e1–3. doi: 10.1111/j.1468-1331.2006.01465.x
25. Kumar RS, Kuruvilla A. Teaching NeuroImages: Acute hemorrhagic leukoencephalitis after mumps. Neurology. (2009) 73:e98. doi: 10.1212/WNL.0b013e3181c2eee3
26. Catalan M, Naccarato M, Grandi FC, Capozzoli F, Koscica N, Pizzolato G. Acute hemorrhagic leukoencephalitis with atypical features. Neurol Sci. (2009) 30:55–7. doi: 10.1007/s10072-008-0003-9
27. Befort P, Gaillard N, Roubille C, Quellec A Le. Hemorrhagic leukoencephalitis linked to Epstein-Barr virus in an adult patient. Clin Neurol Neurosurg. (2010) 112:829–31. doi: 10.1016/j.clineuro.2010.06.017
28. Fugate JE, Lam EM, Rabinstein AA, Wijdicks EFM. Acute hemorrhagic leukoencephalitis and hypoxic brain injury associated with H1N1 influenza. Arch Neurol. (2010) 67:756–8. doi: 10.1001/archneurol.2010.122
29. Virmani T, Agarwal A, Klawiter EC. Clinical reasoning: a young adult presents with focal weakness and hemorrhagic brain lesions. Neurology. (2011) 76:e106–9. doi: 10.1212/WNL.0b013e31821d748a
30. Cisse FA, Antoine JC, Pillet S, Jousserand G, Reynaud-Salard M, Camdessanche JP. Acute hemorrhagic leukoencephalopathy associated with influenza A (H1N1) virus. J Neurol. (2011) 513–4. doi: 10.1007/s00415-010-5772-4
31. Pinto PS, Taipa R, Moreira B, Correia C, Melo-Pires M. Acute hemorrhagic leukoencephalitis with severe brainstem and spinal cord involvement: MRI features with neuropathological confirmation. J Magn Reson Imag. (2011) 33:957–61. doi: 10.1002/jmri.22505
32. Lee NK, Lee BH, Hwang YJ, Kim SY, Lee JY, Joo M. Serial computed tomography and magnetic resonance imaging findings of biphasic acute hemorrhagic leukoencephalitis localized to the brain stem and cerebellum. Jpn J Radiol. (2011) 29:212–6. doi: 10.1007/s11604-010-0523-0
33. Hashim HZ, Ibrahim NM, Wanyahya NS, Tan HJ, Zainun KA, SA MA, et al. A case of biopsy proven acute demyelinating encephalomyelitis (ADEM) with haemorrhagic leucoencephalitis. Ann Acad Med Singapore. (2011) 40:197–200.
34. Kao HW, Alexandru D, Kim R, Yanni D, Hasso AN. Value of susceptibility-weighted imaging in acute hemorrhagic leukoencephalitis. J Clin Neurosci. (2012) 19:1740–1. doi: 10.1016/j.jocn.2011.04.034
35. Jeganathan N, Fox M, Schneider J, Gurka D, Bleck T. Acute hemorrhagic leukoencephalopathy associated with influenza A (H1N1) virus. Neurocrit Care. (2013) 19:218–21. doi: 10.1007/s12028-013-9880-8
36. Venugopal V, Haider M. First case report of acute hemorrhagic leukoencephalitis following plasmodium vivax infection. Indian J Med Microbiol. (2013) 31:79–81. doi: 10.4103/0255-0857.108736
37. Robinson CA, Adiele RC, Tham M, Lucchinetti CF, Popescu BFG. Early and widespread injury of astrocytes in the absence of demyelination in acute haemorrhagic leukoencephalitis. Acta Neuropathol Commun. (2014) 2:52. doi: 10.1186/2051-5960-2-52
38. Kitulwatte ID, Kim PJH, Pollanen MS. Acute hemorrhagic leukoencephalomyelitis in a man with viral myocarditis. Forensic Sci Med Pathol. (2015) 11:416–20. doi: 10.1007/s12024-015-9692-6
39. Prabhakar AT, Kamanahalli R, Sivadasan A, Joseph E, Viggeswarpu S. Non-fatal acute haemorrhagic leukoencephalitis following snake bite: a case report. Trop Doct. (2016) 46:57–9. doi: 10.1177/0049475515577987
40. Nabi S, Badshah M, Ahmed S, Nomani AZ. Weston-Hurst syndrome: a rare fulminant form of acute disseminated encephalomyelitis (ADEM). BMJ Case Rep. (2016). doi: 10.1136/bcr-2016-217215
41. Solis WG, Waller SE, Harris AK, Sugo E, Hansen MA, Lechner-Scott J. Favourable outcome in a 33-year-old female with acute haemorrhagic leukoencephalitis. Case Rep Neurol. (2017) 9:106–13. doi: 10.1159/000472706
42. Sureshbabu S, Babu R, Garg A, Peter S, Sobhana C, Mittal G. Acute hemorrhagic leukoencephalitis unresponsive to aggressive immunosuppression. Clin Exp Neuroimmunol. (2017) 8:63–6. doi: 10.1111/cen3.12361
43. George IC, Youn TS, Marcolini EG, Greer DM. Clinical reasoning: acute onset facial droop in a 36-year-old pregnant woman. Neurology. (2017) 88:e240–4. doi: 10.1212/WNL.0000000000004030
44. Peerani R, Berggren M, Herath JC. Sudden death of a young man by acute hemorrhagic leukoencephalitis. Acad Forensic Pathol. (2017) 7:487–93. doi: 10.23907/2017.041
45. Mondia MWL, Reyes NGD, Espiritu AI, Pascual V JLR. Acute hemorrhagic leukoencephalitis of Weston Hurst secondary to herpes encephalitis presenting as status epilepticus: a case report and review of literature. J Clin Neurosci. (2019) 67:265–70. doi: 10.1016/j.jocn.2019.06.020
46. Ashraf Z, Todnam N, Morgan J, Rojiani AM. 42-year-old man with worsening headache. Brain Pathol. (2019) 29:305–6. doi: 10.1111/bpa.12706
47. Pérez-Bovet J, Garcia-Armengol R, Buxó-Pujolràs M, Lorite-Díaz N, Narváez-Martínez Y, Caro-Cardera JL, et al. Decompressive craniectomy for encephalitis with brain herniation: case report and review of the literature. Acta Neurochir. (2012) 154:1717–24. doi: 10.1007/s00701-012-1323-3
Keywords: Weston-Hurst syndrome, decompressive craniectomy, outcome, autoimmun disease, acute hemorrhagic leukoencephalitis
Citation: Loesch-Biffar AM, Junker A, Linn J, Thon N, Heck S, Ottomeyer C, Straube A and Pfister HW (2021) Case Report: Minimal Neurological Deficit of Two Adult Patients With Weston–Hurst Syndrome Due to Early Craniectomy: Case Series and Review of Literature on Craniectomy. Front. Neurol. 12:673611. doi: 10.3389/fneur.2021.673611
Received: 04 March 2021; Accepted: 30 July 2021;
Published: 31 August 2021.
Edited by:
Rajeev Kumar Garg, Rush University, United StatesReviewed by:
Luis Rafael Moscote-Salazar, University of Cartagena, ColombiaAlejandro Vargas, Rush University Medical Center, United States
Copyright © 2021 Loesch-Biffar, Junker, Linn, Thon, Heck, Ottomeyer, Straube and Pfister. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Anna Mira Loesch-Biffar, YW5uYW1pcmEubG9lc2NoQG1lZC51bmktbXVlbmNoZW4uZGU=
 Anna Mira Loesch-Biffar
Anna Mira Loesch-Biffar Andreas Junker2
Andreas Junker2 Andreas Straube
Andreas Straube Hans Walter Pfister
Hans Walter Pfister