
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Neurol. , 26 May 2021
Sec. Multiple Sclerosis and Neuroimmunology
Volume 12 - 2021 | https://doi.org/10.3389/fneur.2021.667651
This article is part of the Research Topic Prognostic and Predictive Factors in Multiple Sclerosis and Related Disorders View all 9 articles
 Jinwei Zhang†
Jinwei Zhang† Yanfei Li†
Yanfei Li† Yongyan Zhou
Yongyan Zhou Yi Zhao
Yi Zhao Haojie Xie
Haojie Xie Ranran Duan
Ranran Duan Yaobing Yao
Yaobing Yao Zhe Gong
Zhe Gong Junfang Teng
Junfang Teng Yanjie Jia*
Yanjie Jia*Background: Many patients with neuromyelitis optica spectrum disorders (NMOSD) experience the adverse consequences of relapse and disability aggravation. Thus, it is necessary to identify sensitive and reliable biomarkers for early prognosis. This study investigated whether serum homocysteine (Hcy) level was associated with the risk of relapse or poor prognosis in first-attack NMOSD patients.
Methods: We enrolled 161 first-attack NMOSD patients in this retrospective study. We reviewed their medical records and evaluated their initial Expanded Disability Status Scale (EDSS). Clinical outcomes were measured by the final EDSS and the relapse rate. The association between Hcy levels and EDSS score at last follow-up was analyzed by binary logistic regression. The association between Hcy levels and relapse rate was assessed by Cox regression analysis. Receiver operating characteristic (ROC) curve analysis was used to predict the target value of Hcy reduction.
Results: Compared with the high Hcy group, the final EDSS score in the low Hcy group was significantly lower (median: 0.5 vs. 2.5, P < 0.001). The relapse rate differed significantly between these groups (30.6 vs. 50.0%, P = 0.023). Multivariate analysis showed that the initial EDSS score (odds ratio [OR] 3.03, 95% confidence interval [CI] 2.07–4.45, P < 0.001) and serum Hcy level (OR 1.13, 95%CI 1.04–1.22, P = 0.002) were significantly associated with poor prognosis in NMOSD patients. Additionally, multivariate analysis showed that serum Hcy level (hazard ratio 1.06, 95%CI 1.04–1.09, P < 0.001) was an independent predictor of the risk for relapse in NMOSD. The 12-month relapse rate of the high Hcy group was 34.8%, and 50% of high Hcy patients relapsed within 35 months after the first onset. A serum Hcy level exceeding 14.525 μmol/L indicated a high risk of relapse, with a sensitivity of 43.7%, specificity of 90.0%, and area under the ROC curve of 0.674 (95%CI 0.59–0.76, P < 0.001).
Conclusion: Serum Hcy level is an independent predictor of relapse and poor prognosis in first-attack NMOSD patients. Early monitoring and reduction of serum Hcy levels may be of great significance in the prevention of disease relapse and severe disability.
Neuromyelitis optica spectrum disorder (NMOSD) is a rare and severe, inflammatory, immune-mediated, demyelinating disease of the central nervous system (CNS). It is commonly characterized by monophasic or recurrent optic neuritis, longitudinal extensive transverse myelitis, or posterior region syndrome (1, 2). The pathogenesis of diseases is closely related to a circulating antibody against the astrocytic aquaporin 4 (AQP4) water channel protein (3, 4). At present, there is no validated consensus on NMOSD treatment, although empirical use of corticosteroids, immunosuppressants, and plasma exchange are considered effective treatments for both the first-attack and relapses of the disease (5, 6). However, many patients still face the adverse consequences of disease relapse and increased disability (7–9). Therefore, the determination of sensitive and reliable biomarkers for predicting the prognosis of the disease and implementing early treatment measures to reduce the risk of relapse is of great significance (10).
Homocysteine (Hcy) is a non-essential sulfur-containing amino acid and is an important intermediate product of folic acid, vitamin B12, and single carbon metabolism (11). Hcy level is considered as an independent risk factor for many cardiovascular and cerebrovascular diseases (12). The nervous system is particularly sensitive to extracellular Hcy, which induces oxidative stress by increasing the production of reactive oxygen species (ROS), and promotes excitotoxicity by stimulating the N-methyl-D-aspartate (NMDA) receptor, causing neuronal damage (13, 14). In addition, Hcy is seen to be able to induce the destruction of the blood–brain barrier (BBB) and to regulate the function of adaptive immune system cells (15–17). Recently, the role of Hcy in many autoimmune diseases has attracted great attention of researchers, particularly demyelinating diseases of the nervous system, such as multiple sclerosis (18). Hcy levels in patients with multiple sclerosis was higher than that of healthy people, and hyperhomocysteinemia may be related to disease progression and disability severity in patients with multiple sclerosis (19, 20).
A previous study has shown that serum Hcy levels were increased significantly in patients with NMOSD with an Expanded Disability Status Scale (EDSS) score ≥ 4 in the acute stage, which indicates that Hcy may play a role in the pathogenesis or progression of NMOSD (21). However, it is not clear whether Hcy levels are related to relapse and poor prognosis in this disease. In our study, we investigated the association of Hcy levels with relapse risk and prognosis of first-attack NMOSD, and sought to determine the target value of Hcy level for predicting risk of relapse in NMOSD patients.
In this retrospective study, we collected 544 cases of NMOSD treated in the First Affiliated Hospital of Zhengzhou University from June 2011 to December 2019. The inclusion criteria were as follows: (1) meeting the 2006 or 2015 internationally recognized diagnostic criteria for NMOSD (22, 23); (2) confirmed as first-attack NMOSD patients; (3) no diseases involving renal dysfunction, vitamin B12 dysfunction, hypothyroidism, or hemolysis; (4) no homocysteine-lowering drug treatment before admission; (5) no use of drugs affecting Hcy concentration, such as phenytoin, isoniazid, medroxyprogesterone, and levodopa, before admission (24); (6) no immunosuppressive therapy in the 6 months before admission, although use of immunomodulators (such as interferon-beta) was not excluded; (7) no relapse within 6 months before the last follow-up; (8) availability of complete data. In total, 161 NMOSD patients met these criteria and were included in the study. The detailed selection process is shown in Figure 1.
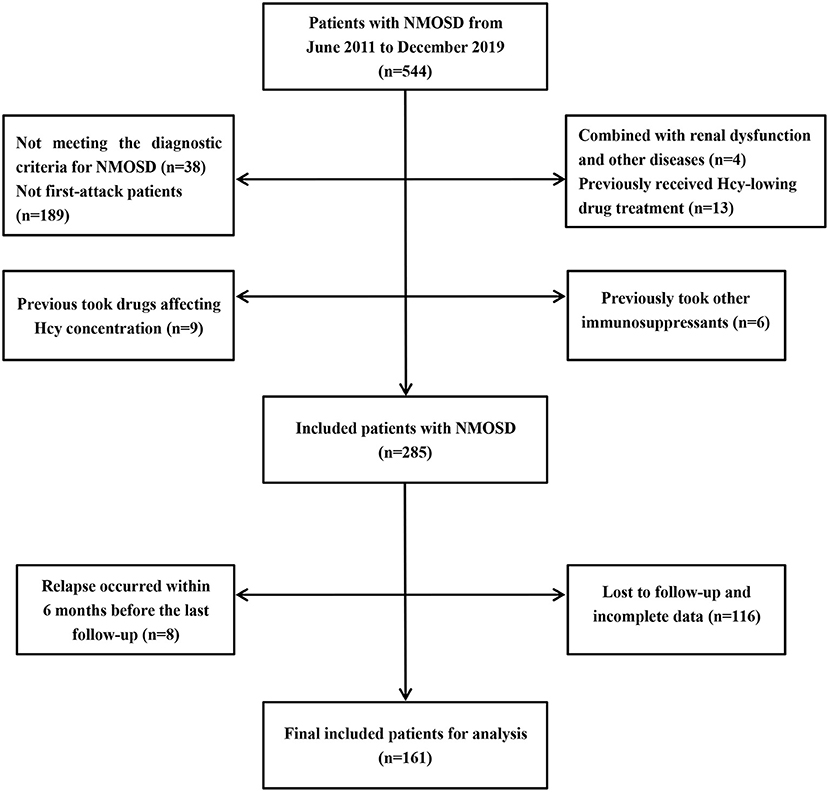
Figure 1. Patients selection process. NMOSD, neuromyelitis optica spectrum disorders; Hcy, homocysteine.
This study was approved by the Ethics Committee of Zhengzhou University (2019-KY-018). Written informed consent was provided by all patients or their proxy.
We reviewed medical records and collected individual clinical data, including gender, onset age, medical history, anti-AQP4 status, treatment plan, and serum Hcy, folic acid, and vitamin B12 levels. The EDSS was preformed and recorded as the initial EDSS scores.
Blood samples were collected from peripheral veins of all NMOSD patients after overnight fasting at 7:00–8:00 am the next day and were kept on ice prior to separation (maximum 30 min after drawing). Hcy, folic acid, and vitamin B12 levels in these samples were determined in the coagulation function laboratory of the First Affiliated Hospital of Zhengzhou University. Hcy levels were assessed by rate method with an automated chemistry analyzer (Roche Group, Sweden). Hyperhomocysteinemia was defined as homocysteine concentration ≥10 μmol/L (25, 26). Serum folic acid and vitamin B12 levels were measured with a chemiluminescent immunoassay on the Maglumi 2000 Plus analyzer (Shenzhen New Industries Biomedical Engineering Company Limited, China). Serum or cerebrospinal fluid samples were used to detect anti-AQP4 status at the Neurology Laboratory of the First Affiliated Hospital of Zhengzhou University using an assay of live cells transfected with AQP4. All tests were performed according to the manufacturers' instructions, and the investigators were blinded to the patients' diagnoses and clinical symptoms.
The primary outcome of this study was the relapse rate. Relapse referred to the occurrence of new, progressive, or recurrent neurological symptoms in patients with NMOSD, which lasted for at least 24 h, and which increased the existing EDSS score by 0.5 points (27). The relapse rate and first relapse duration were obtained by clinical or telephonic follow-up every 6 months.
The secondary outcome was clinical disability, determined according to the final EDSS score, which was determined by an experienced neurologist at the last follow-up. To reduce the error of transition of EDSS score from 0 to 1.0, patients with an initial EDSS score of 0 needed an increase of 1.5 points, patients with score of 1.0–5.0 needed an increase of 1.0 points, and patients with a score > 5.0 needed an increase of 0.5 points. The final EDSS were converted into categorical variables. Patients with EDSS score > 3 were defined as showing poor recovery, and those with EDSS scores ≤ 3 were defined as showing good recovery.
The data were analyzed using SPSS 26.0 software (International Business Machines Corporation, Chicago, IL, USA). The graphs were generated by using GraphPad Prism 8.3 (Graphpad Inc, Harvey Motulsky, USA). Although Hcy levels could be regarded as a continuous variable, they were stratified into high Hcy levels (≥10 μmol/L) and normal Hcy levels (<10 μmol/L) (25, 26). We compared the demographic and clinical characteristics between patients with high or normal Hcy levels. If the Kolmogorov–Smirnov test showed that continuous variables were normally distributed; they were expressed as means and standard deviations (SD) and were compared by t-tests. Otherwise, data were expressed as medians (interquartile range) and were compared using a Mann–Whitney U-test. Classification variables were expressed as frequency (percentage, %) and were compared by a chi-square test or Fisher's exact test. Spearman's correlation analysis was used to evaluate the relationship between Hcy level and EDSS progression. A univariate logistic regression analysis was used to evaluate the factors potentially related to poor recovery in first-attack NMOSD patients. The variables with P < 0.2, which were clinically considered to be closely related to the dependent variables, were included in a multivariate model. A multivariate logistic regression was preformed to analyze the independent effect of Hcy level on recovery. The results were expressed using odds ratios (ORs). A Kaplan–Meier analysis and log-rank test were used to analyze the effect of Hcy levels in different groups on the timing of first relapse. A univariate Cox proportional hazards model was used to screen variables with P ≤ 0.2, and a multivariate Cox regression model was used to analyze the predictive strength of Hcy levels on the timing of the first relapse of NMOSD. The results were expressed by hazard ratios (HRs). Receiver operating characteristic (ROC) curve analysis was used to test the predictive ability of Hcy for disease relapse and to predict the optimal cutoff value for reduction of Hcy level in patients with first-attack NMOSD. A two-tailed P < 0.05 was considered statistically significant.
In this cohort study, 161 NMOSD patients were included. The detailed inclusion and exclusion criteria are shown in Figure 1. The average onset age was 45.17 ± 15.17 years. More than three-quarter patients were women. The median follow-up time was 42.2 (27.2–55.9) months. Anti-AQP4-positive cerebrospinal fluid or serum was identified in almost half of the patients.
Patients were divided into a normal Hcy group (serum Hcy level < 10 μmol/L; n = 49) and a high Hcy group (serum Hcy level ≥ 10 μmol/L; n = 112). Compared with the high Hcy group, the proportion of female patients and the level of folic acid in the normal Hcy group were significantly higher (P = 0.002). Other parameters, including onset age and vitamin B12 level, showed no significant difference between these groups. Additionally, the number of patients with hypertension, diabetes, or autoimmune diseases were not statistically significantly different between the groups. Patients received different treatments according to clinical symptoms and economic status, such as corticosteroids, immunosuppressants (mycophenolate mofetil, azathioprine, methotrexate, tacrolimus, and rituximab), and received rehabilitation training, but there was no statistically significant differences in these parameters between the two groups. There was also no significant difference in the initial EDSS score between the two groups. At the last follow-up, the final EDSS score of the normal Hcy group was significantly lower than that of high Hcy group (P < 0.001), while the proportion of good recovery was higher in the former than in the latter group (P = 0.018). The relapse rate differed significantly between the two groups (P = 0.023). The demographic and clinical features of the patients are summarized in Table 1.
Spearman's correlation analysis (Figure 2) was used to investigate the relationship between Hcy level and clinical recovery (assessed as final EDSS minus initial EDSS in the same patient). We found that the change in EDSS was negatively correlated with Hcy level (r2 = 0.302, P < 0.001), and the lower the level of Hcy, the greater the change in EDSS.
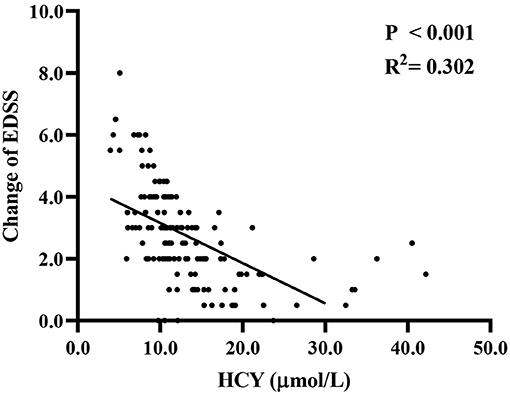
Figure 2. The association of Hcy level with changes between final EDSS and initial EDSS. EDSS, expanded disability status scale; Hcy, homocysteine.
Univariate analysis (Table 2) showed that serum Hcy level was positively correlated with the final EDSS score (OR 1.07, 95%CI 1.01–1.13, P = 0.018). Initial EDSS score (OR 2.77, 95%CI 1.96–3.90, P < 0.001) and hypertension (OR 2.86, 95%CI 1.14–7.16, P = 0.025) were significantly correlated with prognosis. However, there was no correlation of age, gender, anti-AQP4 antibody status, other complications, folic acid or vitamin B12 levels, and treatment methods with the risk of poor prognosis in NMOSD patients. After selecting variables, and adjusting for age, hypertension, and initial EDSS score, multivariate logistic regression analysis showed that the initial EDSS score (OR 3.03, 95%CI 2.07–4.45, P < 0.001) and serum Hcy level (OR 1.13, 95%CI 1.04–1.22, P = 0.002) remained significantly correlated with poor prognosis in patients with NMOSD. The risk of poor recovery increased 1.13 times with every 1 μmol/L increase in Hcy level.
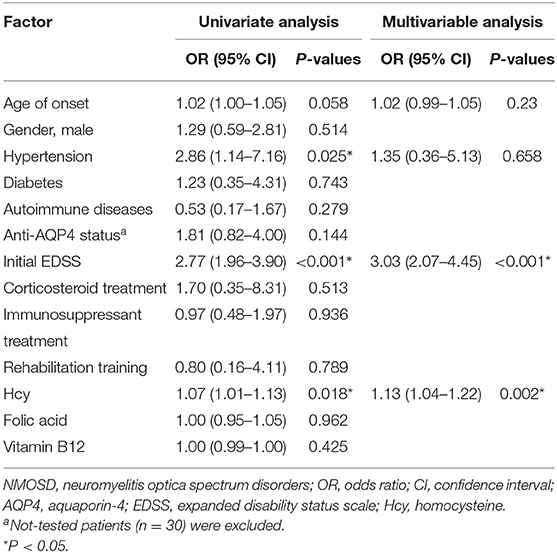
Table 2. Predictors of outcomes for NMOSD patients by univariate and multivariable logistic regression models.
Kaplan–Meier survival analysis (Figure 3) showed that Hcy level was a significant predictor of relapse rate in NMOSD patients (log rank test P = 0.035). The median relapse interval in the high Hcy group was 46.7 months, and the relapse rate within 12 months was 34.8%. Half of the high Hcy group patients relapsed within 35 months of the first onset.
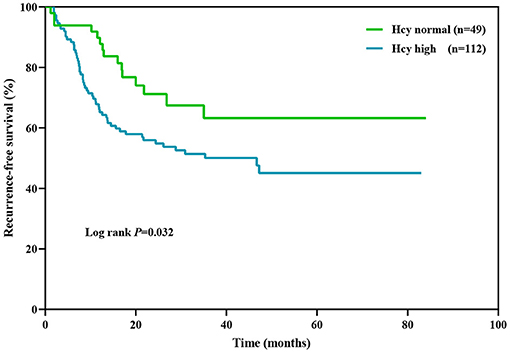
Figure 3. Kaplan–Meier analysis demonstrating the cumulative proportion of patients without relapse. Hcy, homocysteine.
The Cox proportional hazards model was used to test the independent effect of Hcy level on the relapse rate in NMOSD patients (Table 3). Univariate analysis showed that Hcy level (HR 1.07, 95%CI 1.04–1.09, P < 0.001) was significantly correlated with the risk of relapse. Hypertension (HR 1.58, 95%CI 0.86–2.89, P = 0.138), concomitant autoimmune disease (HR 0.43, 95%CI 0.17–1.07, P = 0.068), initial EDSS score (HR 1.11, 95%CI 0.98–1.27, P = 0.098), and folic acid level (HR 0.97, 95%CI 0.93–1.01, P = 0.193) had moderate effects, while age, gender, diabetes, anti-AQP4 antibody status, treatment methods, and serum vitamin B12 level had no correlation with relapse. After selecting variables, a multivariate Cox proportional hazards model showed that Hcy level (HR 1.06, 95%CI 1.04–1.09, P < 0.001) remained a risk factor for relapse in NMOSD patients.
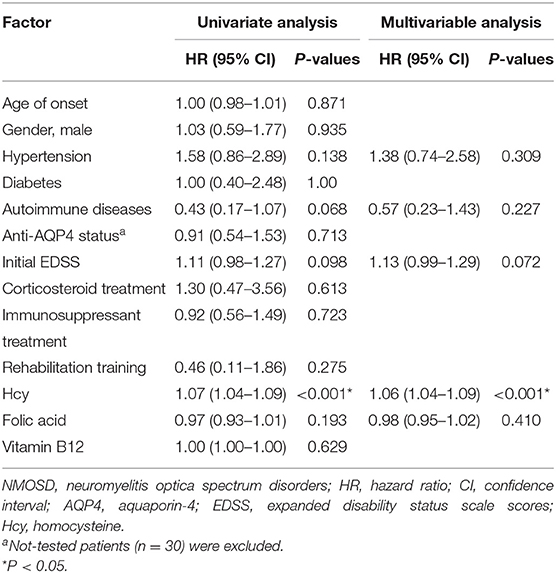
Table 3. Predictors of relapse in NMOSD patients by univariate and multivariable Cox proportional hazards models.
ROC curve analysis was used to determine the target value for reduction of Hcy in NMOSD patients (Figure 4). At an Hcy cut-off value of 14.525 μmol/L, the sensitivity for predicting relapse was 43.7%, the specificity was 90.0%, and the area under ROC curve (AUC) was 0.674 (95%CI 0.59–0.76, P < 0.001).
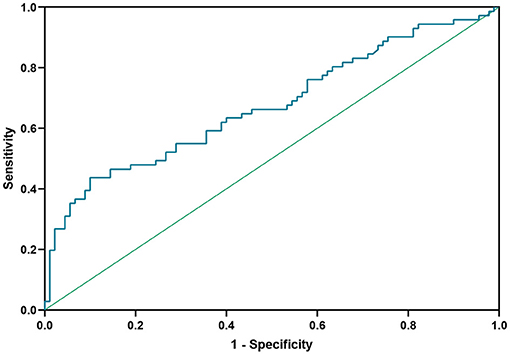
Figure 4. Receiver operating characteristic (ROC) curve showing the predictive ability of Hcy for disease relapse. Hcy, homocysteine.
In this cohort study, we investigated the possible association between Hcy level and disease prognosis through a large NMOSD data set. We found that compared with those with normal Hcy levels, patients with high Hcy levels had a higher relapse rate and worse recovery. The lower the Hcy level, the greater the change between the final and initial EDSS scores. Hcy level may thus be an independent risk factor for further relapse and poor prognosis. The optimal cut-off value of Hcy level for predicting relapse was 14.525 μmol/L.
There is increasing awareness of the role of serum Hcy in autoimmune demyelinating diseases of the CNS (13). Recent studies have shown that the serum Hcy levels of multiple sclerosis patients were higher than that of a healthy control group, by evaluating baseline levels and follow-up EDSS (19, 20). Compared with multiple sclerosis patients with a lower Hcy level, those patients with hyperhomocysteinemia showed more disease progression (28, 29). However, no studies to date had evaluated any association between serum Hcy and the progression and relapse of NMOSD. In our study, we found that patients with high serum Hcy showed a higher relapse rate and worse prognosis, and the relapse rate within 12 months was 34.8%. The changes of final EDSS scores from the initial EDSS scores were negatively correlated with Hcy levels, and the lower the level of Hcy, the greater the change in EDSS. Recently, it was reported that, in patients with acute NMOSD, the serum Hcy level of patients with severe initial symptoms (EDSS score ≥ 4) was significantly increased as compared with patients with mild initial symptoms (EDSS score < 4) (21). This finding, together with our data, supported the potential role of serum Hcy levels in the pathogenesis and disease progression of NMOSD and confirmed serum Hcy level as an independent risk factor of relapse and poor prognosis in patients with first-attack NMOSD.
The nervous system is particularly sensitive to extracellular Hcy, which may influence the prognosis of diseases through various mechanisms (30). Hcy in blood could induce oxidative stress and mitochondrial dysfunction by increasing ROS production (14, 31), and could stimulate NMDAR to promote excitotoxicity, which would lead to an increase in intracellular calcium levels and the activation of caspases, damaging neuronal DNA, and inducing apoptosis (19, 32, 33). In addition, Hcy is an important intermediate in the methionine cycle (13). High Hcy levels could reduce the availability of S-adenosylmethionine, which is an important methyl donor for many biochemical processes (29, 34). In the CNS, myelin basic protein (MBP) is the main component of myelin, and its methylation is very important for the maintenance and repair of the myelin sheath (35, 36). Hyperhomocysteinemia may lead to hypomethylation of MBP, which reduces its hydrophobicity and stability, thus leading to structural instability and degeneration and loss of the myelin sheath, with an adverse impact on disease progression (37, 38).
The pro-inflammatory effect of Hcy could also be mediated by regulation of cellular function in the adaptive immune system and destruction of the BBB. This could interfere with the reaction of T lymphocytes and B lymphocytes, natural killer cells, and adhesion molecules (15, 39, 40). Th17 cells interfere with the BBB by producing pro-inflammatory IL-17 and migrate to the CNS by expression of the chemokine receptor CCR-6 (CD196) (18). Hcy could indirectly influence the function of the BBB by promoting the differentiation of Th17 cells (41). The central event in NMOSD is the entry of anti-AQP4 antibody into the CNS through the damaged BBB, and its binding to AQP4 on astrocytes, which leads to inflammatory lesions and CNS injuries (42). There is evidence that serum Hcy itself could induce BBB destruction, thereby aggravating disease progression (17). At the same time, Hcy is also produced and secreted by astrocytes, and astrocyte activation increases secretion of Hcy, which may be one of the reasons for its abnormal levels in NMOSD patients (30).
Hypertension is closely related to CNS diseases. In our study, the univariate analysis showed that hypertension was an independent risk factor for poor recovery in NMOSD patients (OR 2.86, 95%CI 1.14–7.16, P = 0.025), but there was no significant correlation in multivariate analysis, similar to the findings of previous studies (43). Previous studies showed that the homocysteine concentration in male patients was significantly higher than that in female patients (P < 0.001) (39). In our study, the gender ratio of NMOSD patients differed significantly between the normal and high Hcy groups (female, 93.9 vs. 67.9%, P < 0.001). The proportion of men in the high Hcy group was significantly higher than that in the other group, which requires further investigation. In addition, the univariate and multivariate analysis showed that the initial EDSS score, that is, the severity of the first attack, significantly affected the prognosis of the disease (OR 2.77, 95%CI 1.96–3.90, P < 0.001), which was consistent with previous research results, suggesting the importance of early treatment to improve the prognosis (44).
Previous studies have shown that, compared with a healthy control group, multiple sclerosis patients have higher Hcy levels, lower vitamin B12 and folic acid levels. Severe disability is significantly correlated with high Hcy and low vitamin B12 levels in multiple sclerosis patients (20, 36, 45, 46). In our study, folic acid in the high Hcy NMOSD group was significantly lower than in the normal Hcy group (median 6.98 vs. 9.74, P = 0.002). Univariate analysis showed that folic acid level had a moderate but non-significant effect on disease relapse (HR 0.97, 95%CI 0.93–1.01, P = 0.193), and vitamin B12 and folic acid level had no effect on the prognosis of NMOSD. This may be related to different living habits, eating habits, and drug intake of patients. The relationship between serum folic acid and vitamin B12 levels and the progress of NMOSD requires further exploration. Under the mediation of methionine synthetase, folic acid and vitamin B12 are necessary cofactors in the process of transforming Hcy into methionine (47). Vitamin B12 and folic acid supplementation could reduce serum Hcy levels (48). In our study, when serum Hcy level was higher than 14.525 μmol/l, the risk of relapse was high. This suggested that in clinical practice by reducing Hcy levels below the target value with administration of supplementary vitamin B12 and folic acid, clinicians may improve the prognosis of NMOSD and reduce the risk of relapse; this could also be used as a potential treatment strategy for NMOSD.
This research had some limitations. First, because this study was a single-center cohort study with few subjects, it is necessary to verify our results in a large, multi-center study. Second, only a small number of our patients were included in the cohort (161/544), which may have led to selection bias. Third, some patients were not tested for anti-AQP4 antibodies as our hospital started conducing anti-AQP4 antibody tests only after June 2013. Finally, some other confounding factors may affect the correlation of Hcy, vitamin B12, and folic acid, with NMOSD, such as vitamin D level, drug intake, living habits (smoking, drinking, excessive intake of coffee), etc. The inaccuracy of these data may have influenced results.
In conclusion, no previous study had reported investigation of the correlation between serum Hcy level and NMOSD progression. Our results show that serum Hcy levels are an independent risk factor for relapse and poor prognosis of NMOSD. Early monitoring and reducing the serum Hcy level may be of great significance for preventing disease relapse and severe disability. The mechanism underlying the effect of serum Hcy, folic acid, and vitamin B12 on disease progression requires further elucidation.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
The studies involving human participants were reviewed and approved by the Ethics Committee of Zhengzhou University. Written informed consent to participate in this study was provided by the participants' legal guardian/next of kin. Written informed consent was obtained from the individual(s), and minor(s)' legal guardian/next of kin, for the publication of any potentially identifiable images or data included in this article.
JZ and YL contributed to conception and design of the research and conducted regular follow-up of all cases. YZho, YZha, and HX organized the database. RD, YY, and JZ performed the statistical analysis. JZ wrote the first draft of the manuscript. ZG, JT, and YJ undertook the task of revising the manuscript critically. All authors contributed to manuscript revision, read, and approved the submitted version.
This work was supported by the Joint Funds of the National Natural Science Foundation of China (Grant No. U1604170).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2021.667651/full#supplementary-material
1. Bruscolini A, Sacchetti M, La Cava M, Gharbiya M, Ralli M, Lambiase A, et al. Diagnosis and management of neuromyelitis optica spectrum disorders-an update. Autoimmun Rev. (2018) 17:195–200. doi: 10.1016/j.autrev.2018.01.001
2. Jarius S, Paul F, Weinshenker BG, Levy M, Kim HJ, Wildemann B. Neuromyelitis optica. Nat Rev Dis Primers. (2020) 6:85. doi: 10.1038/s41572-020-0214-9
3. Chang VTW, Chang HM. Review: recent advances in the understanding of the pathophysiology of neuromyelitis optica spectrum disorder. Neuropathol Appl Neurobiol. (2020) 46:199–218. doi: 10.1111/nan.12574
4. Papadopoulos MC, Verkman AS. Aquaporin 4 and neuromyelitis optica. Lancet Neurol. (2012) 11:535–44. doi: 10.1016/S1474-4422(12)70133-3
5. Trebst C, Jarius S, Berthele A, Paul F, Schippling S, Wildemann B, et al. Update on the diagnosis and treatment of neuromyelitis optica: recommendations of the Neuromyelitis Optica Study Group (NEMOS). J Neurol. (2014) 261:1–16. doi: 10.1007/s00415-013-7169-7
6. Kim SH, Hyun JW, Joung A, Park EY, Joo J, Kim HJ. Predictors of response to first-line immunosuppressive therapy in neuromyelitis optica spectrum disorders. Mult Scler. (2017) 23:1902–8. doi: 10.1177/1352458516687403
7. Poupart J, Giovannelli J, Deschamps R, Audoin B, Ciron J, Maillart E, et al. Evaluation of efficacy and tolerability of first-line therapies in NMOSD. Neurology. (2020) 94:e1645–e56. doi: 10.1212/WNL.0000000000009245
8. Stellmann JP, Krumbholz M, Friede T, Gahlen A, Borisow N, Fischer K, et al. Immunotherapies in neuromyelitis optica spectrum disorder: efficacy and predictors of response. J Neurol Neurosurg Psychiatry. (2017) 88:639–47. doi: 10.1136/jnnp-2017-315603
9. Shi Z, Du Q, Chen H, Zhang Y, Qiu Y, Zhao Z, et al. Effects of immunotherapies and prognostic predictors in neuromyelitis optica spectrum disorder: a prospective cohort study. J Neurol. (2020) 267:913–24. doi: 10.1007/s00415-019-09649-7
10. Wu K, Wen L, Duan R, Li Y, Yao Y, Jing L, et al. Triglyceride level is an independent risk factor in first-attacked neuromyelitis optica spectrum. Front Neurol. (2019) 10:1230. doi: 10.3389/fneur.2019.01230
11. Jakubowski H. Homocysteine Modification in protein structure/function and human disease. Physiol Rev. (2019) 99:555–604. doi: 10.1152/physrev.00003.2018
12. Price BR, Wilcock DM, Weekman EM. Hyperhomocysteinemia as a risk factor for vascular contributions to cognitive impairment and dementia. Front Aging Neurosci. (2018) 10:350. doi: 10.3389/fnagi.2018.00350
13. Ansari R, Mahta A, Mallack E, Luo JJ. Hyperhomocysteinemia and neurological disorders: a review. J Clin Neurol. (2014) 10: 281–8. doi: 10.3988/jcn.2014.10.4.281
14. Škovierová H, Vidomanová E, Mahmood S, Sopková J, Drgová A, Cervenová T, et al. The molecular and cellular effect of homocysteine metabolism imbalance on human health. Int J Mol Sci. (2016) 17:1733. doi: 10.3390/ijms17101733
15. Deng J, Lü S, Liu H, Liu B, Jiang C, Xu Q, et al. Homocysteine activates B cells via regulating PKM2-dependent metabolic reprogramming. J Immunol. (2016) 198:170–83. doi: 10.4049/jimmunol.1600613
16. Dawson H, Collins G, Pyle R, Deep-Dixit V, Taub DD. The immunoregulatory effects of homocysteine and its intermediates on T-lymphocyte function. Mech Ageing Dev. (2004) 125:107–10. doi: 10.1016/j.mad.2003.11.013
17. Kamath AF, Chauhan AK, Kisucka J, Dole VS, Loscalzo J, Handy DE, et al. Elevated levels of homocysteine compromise blood-brain barrier integrity in mice. Blood. (2006) 107:591–3. doi: 10.1182/blood-2005-06-2506
18. Dubchenko E, Ivanov A, Spirina N, Smirnova N, Melnikov M, Boyko A, et al. Hyperhomocysteinemia and endothelial dysfunction in multiple sclerosis. Brain Sci. (2020) 10:637. doi: 10.3390/brainsci10090637
19. Oliveira SR, Flauzino T, Sabino BS, Kallaur AP, Alfieri DF, Kaimen-Maciel DR, et al. Elevated plasma homocysteine levels are associated with disability progression in patients with multiple sclerosis. Metab Brain Dis. (2018) 33:1393–9. doi: 10.1007/s11011-018-0224-4
20. Li X, Yuan J, Han J, Hu W. Serum levels of homocysteine, vitamin B12 and folate in patients with multiple sclerosis: an updated meta-analysis. Int J Med Sci. (2020) 17:751–61. doi: 10.7150/ijms.42058
21. Liang J, Liu J, Fan R, Chen Z, Chen X, Tong J, et al. Plasma homocysteine level is associated with the expanded disability status scale in neuromyelitis optica spectrum disorder. Neuroimmunomodulation. (2019) 26:258–64. doi: 10.1159/000503426
22. Wingerchuk DM, Banwell B, Bennett JL, Cabre P, Carroll W, Chitnis T, et al. International consensus diagnostic criteria for neuromyelitis optica spectrum disorders. Neurology. (2015) 85:177–89. doi: 10.1212/WNL.0000000000001729
23. Wingerchuk DM, Lennon VA, Pittock SJ, Lucchinetti CF, Weinshenker BG. Revised diagnostic criteria for neuromyelitis optica. Neurology. (2006) 66:1485–9. doi: 10.1212/01.wnl.0000216139.44259.74
24. Panunzio MF, Pisano A, Antoniciello A, Di Martino V, Frisoli L, Cipriani V, et al. Supplementation with fruit and vegetable concentrate decreases plasma homocysteine levels in a dietary controlled trial. Nutr Res. (2003) 23:1221–8. doi: 10.1016/S0271-5317(03)00133-7
25. Malinow MR, Bostom AG, Krauss RM. Homocyst(e)ine, diet, and cardiovascular diseases: a statement for healthcare professionals from the Nutrition Committee, American Heart Association. Circulation. (1999) 99:178–82. doi: 10.1161/01.cir.99.1.178
26. Sacco RL, Adams R, Albers G, Alberts MJ, Benavente O, Furie K, et al. Guidelines for prevention of stroke in patients with ischemic stroke or transient ischemic attack: a statement for healthcare professionals from the American Heart Association/American Stroke Association Council on Stroke: cosponsored by the Council on Cardiovascular Radiology and Intervention: the American Academy of Neurology affirms the value of this guideline. Circulation. (2006) 113:e409–49. doi: 10.1161/STR.0b013e3181f7d043
27. Mcdonald WI, Compston A, Edan G, Goodkin D, Hartung HP, Lublin FD, et al. Recommended diagnostic criteria for multiple sclerosis: guidelines from the International Panel on the diagnosis of multiple sclerosis. Ann Neurol. (2001) 50:121–7. doi: 10.1002/ana.1032
28. Jamroz-Wiśniewska A, Bełtowski J, Bartosik-Psujek H, Wójcicka G, Rejdak K. Processes of plasma protein N-homocysteinylation in multiple sclerosis. Int J Neurosci. (2017) 127:709–15. doi: 10.1080/00207454.2016.1241782
29. Teunissen CE, Killestein J, Kragt JJ, Polman CH, Dijkstra CD, Blom HJ. Serum homocysteine levels in relation to clinical progression in multiple sclerosis. J Neurol Neurosurg Psychiatry. (2008) 79:1349–53. doi: 10.1136/jnnp.2008.151555
30. Ramsaransing GS, Fokkema MR, Teelken A, Arutjunyan AV, Koch M, De Keyser J. Plasma homocysteine levels in multiple sclerosis. J Neurol Neurosurg Psychiatry. (2006) 77:189–92. doi: 10.1136/jnnp.2005.072199
31. Faraci FM, Lentz SR. Hyperhomocysteinemia, oxidative stress and cerebral vascular dysfunction. Stroke. (2004) 35:345–7. doi: 10.1161/01.STR.0000115161.10646.67
32. Ho PI, Ortiz D, Rogers E, Shea TB. Multiple aspects of homocysteine neurotoxicity: glutamate excitotoxicity, kinase hyperactivation and DNA damage. J Neurosci Res. (2002) 70:694–702. doi: 10.1002/jnr.10416
33. Darendelioglu E, Aykutoglu G, Tartik M, Baydas G. Turkish propolis protects human endothelial cells in vitro from homocysteine-induced apoptosis. Acta Histochem. (2016) 118:369–76. doi: 10.1016/j.acthis.2016.03.007
34. Li H, Lu H, Tang W, Zuo J. Targeting methionine cycle as a potential therapeutic strategy for immune disorders. Expert Opin Ther Targets. (2017) 23:1–17. doi: 10.1080/14728222.2017.1370454
35. Škovierová H, Mahmood S, Blahovcová E, Hatok J, Lehotský J, Murín R. Effect of homocysteine on survival of human glial cells. Physiol Res. (2015) 64:747–54. doi: 10.33549/physiolres.932897
36. van Rensburg SJ, Kotze MJ, Hon D, Haug P, Kuyler J, Hendricks M, et al. Iron and the folate-vitamin B12-methylation pathway in multiple sclerosis. Metab Brain Dis. (2006) 21:121–37. doi: 10.1007/s11011-006-9019-0
37. Pan L, Yin Y, Chen J, Ma Z, Chen Y, Deng X, et al. Homocysteine, vitamin B12, and folate levels in patients with multiple sclerosis in Chinese population: a case-control study and meta-analysis. Mult Scler Relat Disord. (2019) 36:101395. doi: 10.1016/j.msard.2019.101395
38. Kim JK, Mastronardi FG, Wood DD, Lubman DM, Zand R, Moscarello MA. Multiple sclerosis: an important role for post-translational modifications of myelin basic protein in pathogenesis. Mol Cell Proteomics. (2003) 2:453–62. doi: 10.1074/mcp.M200050-MCP200
39. Zoccolella S, Tortorella C, Iaffaldano P, Direnzo V, D'Onghia M, Paolicelli D, et al. Elevated plasma homocysteine levels in patients with multiple sclerosis are associated with male gender. J Neurol. (2012) 259:2105–10. doi: 10.1007/s00415-012-6464-z
40. Barroso M, Florindo C, Kalwa H, Silva Z, Turanov AA, Carlson BA, et al. Inhibition of cellular methyltransferases promotes endothelial cell activation by suppressing glutathione peroxidase 1 protein expression. J Biol Chem. (2014) 289:15350–62. doi: 10.1074/jbc.M114.549782
41. Melnikov M, Rogovskii V, Boyko A, Pashenkov M. Dopaminergic therapeutics in multiple sclerosis: focus on Th17-cell functions. J Neuroimmune Pharmacol. (2019) 15:37–47. doi: 10.1007/s11481-019-09852-3
42. Huda S, Whittam D, Bhojak M, Chamberlain J, Noonan C, Jacob A. Neuromyelitis optia spectrum disorders. Clin Med. (2019) 19:169–76. doi: 10.7861/clinmedicine.19-2-169
43. He Q, Li L, Li Y, Lu Y, Wu K, Zhang R, et al. Free thyroxine level is associated with both relapse rate and poor neurofunction in first-attack Neuromyelitis Optica Spectrum Disorder (NMOSD) patients. BMC Neurol. (2019) 19:329. doi: 10.1186/s12883-019-1560-7
44. Palace J, Lin DY, Zeng D, Majed M, Elsone L, Hamid S, et al. Outcome prediction models in AQP4-IgG positive neuromyelitis optica spectrum disorders. Brain. (2019) 142:1310–23. doi: 10.1093/brain/awz054
45. Fahmy EM, Elfayoumy NM, Abdelalim AM, Sharaf SA, Ismail RS, Elshebawy H. Relation of serum levels of homocysteine, vitamin B12 and folate to cognitive functions in multiple sclerosis patients. Int J Neurosci. (2018) 128:835–41. doi: 10.1080/00207454.2018.1435538
46. Tas Kilic D, Akdogan A, Kilic L, Sari A, Erden A, Armagan B, et al. Evaluation of vitamin B12 deficiency and associated factors in patients with systemic sclerosis. J Clin Rheumatol. (2018) 24:250–4. doi: 10.1097/RHU.0000000000000686
47. MacFarlane AJ, Greene-Finestone LS, Shi Y. Vitamin B-12 and homocysteine status in a folate-replete population: results from the Canadian Health Measures Survey. Am J Clin Nutr. (2011) 94:1079–87. doi: 10.3945/ajcn.111.020230
Keywords: expanded disability status scale, neuromyelitis optica spectrum disorders, prognosis, relapse, serum homocysteine
Citation: Zhang J, Li Y, Zhou Y, Zhao Y, Xie H, Duan R, Yao Y, Gong Z, Teng J and Jia Y (2021) Serum Homocysteine Level Is a Predictor of Relapse and Prognosis in Patients With First-Attack Neuromyelitis Optica Spectrum Disorders. Front. Neurol. 12:667651. doi: 10.3389/fneur.2021.667651
Received: 14 February 2021; Accepted: 26 April 2021;
Published: 26 May 2021.
Edited by:
Francesco Patti, University of Catania, ItalyReviewed by:
Jelena Srbislav Drulovic, University of Belgrade, SerbiaCopyright © 2021 Zhang, Li, Zhou, Zhao, Xie, Duan, Yao, Gong, Teng and Jia. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yanjie Jia, amlheWFuamllMTk3MUB6enUuZWR1LmNu
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.