
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Neurol. , 10 June 2021
Sec. Stroke
Volume 12 - 2021 | https://doi.org/10.3389/fneur.2021.666919
This article is part of the Research Topic Antiplatelet Agents in Stroke Prevention View all 13 articles
 Gaoting Ma1†
Gaoting Ma1† Shuo Li1†
Shuo Li1† Baixue Jia1
Baixue Jia1 Dapeng Mo1
Dapeng Mo1 Ning Ma1
Ning Ma1 Feng Gao1
Feng Gao1 Xiaochuan Huo1
Xiaochuan Huo1 Gang Luo1
Gang Luo1 Anxin Wang2
Anxin Wang2 Yuesong Pan2
Yuesong Pan2 Ligang Song1
Ligang Song1 Xuan Sun1
Xuan Sun1 Xuelei Zhang1
Xuelei Zhang1 Liqiang Gui3
Liqiang Gui3 Cunfeng Song4
Cunfeng Song4 Ya Peng5
Ya Peng5 Jin Wu6
Jin Wu6 Shijun Zhao7
Shijun Zhao7 Junfeng Zhao8
Junfeng Zhao8 Zhiming Zhou9
Zhiming Zhou9 Zhongrong Miao1* on behalf of ANGEL-ACT study group
Zhongrong Miao1* on behalf of ANGEL-ACT study groupPurpose: Tirofiban administration to acute ischemic stroke patients undergoing mechanical thrombectomy with preceding intravenous thrombolysis remains controversial. The aim of the current study was to evaluate the safety and efficacy of low-dose tirofiban during mechanical thrombectomy in patients with preceding intravenous thrombolysis.
Methods: Patients with acute ischemic stroke undergoing mechanical thrombectomy and preceding intravenous thrombolysis were derived from “ANGEL-ACT,” a multicenter, prospective registry study. The patients were dichotomized into tirofiban and non-tirofiban groups based on whether tirofiban was administered. Propensity score matching was used to minimize case bias. The primary safety endpoint was symptomatic intracerebral hemorrhage (sICH), defined as an intracerebral hemorrhage (ICH) associated with clinical deterioration as determined by the Heidelberg Bleeding Classification. All ICHs and hemorrhage types were recorded. Clinical outcomes included successful recanalization, dramatic clinical improvement, functional independence, and mortality at the 3-month follow-up timepoint. Successful recanalization was defined as a modified Thrombolysis in Cerebral Ischemia score of 2b or 3. Dramatic clinical improvement at 24 h was defined as a reduction in NIH stroke score of ≥10 points compared with admission, or a score ≤1. Functional independence was defined as a Modified Rankin Scale (mRS) score of 0–2 at 3-months.
Results: The study included 201 patients, 81 in the tirofiban group and 120 in the non-tirofiban group, and each group included 68 patients after propensity score matching. Of the 201 patients, 52 (25.9%) suffered ICH, 15 (7.5%) suffered sICH, and 18 (9.0%) died within 3-months. The median mRS was 3 (0–4), 99 (49.3%) achieved functional independence. There were no statistically significant differences in safety outcomes, efficacy outcomes on successful recanalization, dramatic clinical improvement, or 3-month mRS between the tirofiban and non-tirofiban groups (all p > 0.05). Similar results were obtained after propensity score matching.
Conclusion: In acute ischemic stroke patients who underwent mechanical thrombectomy and preceding intravenous thrombolysis, low-dose tirofiban was not associated with increased risk of sICH or ICH. Further randomized clinical trials are needed to confirm the effects of tirofiban in patients undergoing bridging therapy.
Endovascular treatment has proved to be effective for improving functional outcomes and reducing mortality in patients with large-artery occlusive stroke (1–7). However, during the operative procedure, platelet aggregation caused by severe atherosclerotic stenosis or endothelial damage can lead to thrombotic events and early re-occlusion (8, 9). The highly selective glycoprotein IIb/IIIa receptor antagonist tirofiban can efficiently block the final pathway of platelet aggregation and subsequent thrombus formation (10).
A number of studies have reported the effects of tirofiban during mechanical thrombectomy (MT), but outcomes are controversial (11–14). One of the main concerns is whether tirofiban will lead to increased risks of bleeding in patients who have received intravenous thrombolysis (IVT) before MT. Because of this, the use of antiplatelet agents is not recommended within 24 h after IVT in the American Heart Association/American Stroke Association (AHA/ASA) guidelines (15). Few prospective studies have focused on tirofiban administration during MT in patients with preceding IVT, which is also known as bridging therapy. The aim of the current prospective multicenter study was to evaluate the safety of tirofiban during MT with respect to symptomatic intracerebral hemorrhage (sICH) and intracerebral hemorrhage (ICH), as well as its efficacy during artery recanalization, and functional outcomes in patients who underwent bridging IVT.
All patients were enrolled from the registry of “ANGEL-ACT,” which was a nationwide, multicenter, prospective registry study conducted in China from November 2017 to March 2019. Details of the design of the ANGEL-ACT have been reported previously (16). The protocol of the ANGEL-ACT was approved by the Ethics Committee of Beijing Tiantan Hospital and all other participating centers. Written informed consent was obtained from all patients or their representatives. The current study included the following data: (1) Anterior circulation large vessel occlusion (ICA/M1); (2) onset to groin time ≤6 h; (3) the Alberta Stroke Program Early CT Score (ASPECTS) ≥6; and (4) underwent thrombolytic therapy. The main exclusion criterion was incomplete clinical data.
In all MTs a stent retriever or aspiration device was the first recanalization option, in accordance with protocol. In cases in which the first recanalization failed, additional thrombectomy attempts and alternative rescue therapies were used at the discretion of the operator, including intra-arterial or intravenous tirofiban administration, intra-arterial thrombolysis, balloon angioplasty, and emergent stenting. The patients were divided into a tirofiban group and a non-tirofiban group based on tirofiban administration during MT.
All eligible patients underwent endovascular treatment immediately after the assessment of indications. In general, tirofiban was given under the following conditions: (1) Emergency stenting for severe residual stenosis or instant re-occlusion; (2) balloon angioplasty for severe residual stenosis or instant re-occlusion; (3) successful mechanical recanalization with three or more passes with a stent retriever for presumed endothelial damage or instant re-occlusion; and (4) severe in situ atherosclerosis with a high risk of early re-occlusion. Unless an ICH was suspected, a low-dose intra-arterial bolus (0.25–1.00 mg) followed by a continuous intravenous infusion (0.1 μg/kg/min) was administrated for 24 h as a standard procedure. At 4 h prior to the end of the infusion, dual antiplatelet agents (aspirin 100 mg and clopidogrel 75 mg) were administered as bridging therapy if ICH was excluded within 24 h via follow-up computed tomography or magnetic resonance imaging.
The main safety endpoints were sICH, ICH, and mortality within 3-months. sICH was defined as an ICH associated with clinical deterioration according to the Heidelberg Bleeding Classification (17). Hemorrhage types were also recorded. Hemorrhagic outcomes were assessed by a core laboratory, blinded to the clinical data and outcomes. Efficacy outcomes included successful recanalization, dramatic clinical improvement, and functional independence. Successful recanalization was defined as a modified Thrombolysis in Cerebral Ischemia (mTICI) score of 2b or 3 (18). Dramatic clinical improvement was defined at 24 h as a reduction in NIH Stroke Scale (NIHSS) score of ≥ 10 points compared with admission, or a score of ≤1 (19). Functional independence was defined as a modified Rankin Scale (mRS) score of 0–2 at 3-months.
Baseline patient demographic information in the tirofiban and non-tirofiban groups were compared, as were all endpoints. A logistic regression model was used to investigate associations between tirofiban administration and safety and efficacy endpoints. To reduce data bias and confounding variables, propensity score matching (PSM) analysis was performed by matching patients in the two groups at a 1:1 ratio. Age, sex, baseline Modified Rankin Scale score, baseline NIHSS score, ASPECTS score, onset-to-puncture time, and pathogenesis of stroke were used to generate a propensity score for each subject. After PSM the two groups were again compared via the aforementioned statistical methods.
For continuous data, means ± standard deviation or medians and interquartile ranges were used to summarize data, and two-sided t-tests for independent samples or Mann-Whitney U tests were used to assess the significance of differences between groups. Frequencies and percentages were used to summarize binary data, and between-group comparisons were performed via the χ2 test or Fisher's exact tests as appropriate. All analyses were performed using SAS version 9.4 software (SAS Institute, Cary, NC, USA), and p < 0.05 was considered statistically significant.
A total of 1,793 consecutive patients who underwent endovascular treatment were initially recruited from the ANGEL-ACT registry, of which 201 were subsequently shortlisted based on the above-described criteria (Figure 1); 81 in the tirofiban group and 120 in the non-tirofiban group. The median age of the 201 patients was 64 years (range 55–70 years), and 130 (64.7%) were male. Patients in the tirofiban group exhibited a significantly heavier atherosclerotic burden with respect to vascular risk factors such as hypertension (55.6% vs. 40.0%, p = 0.032), and were more likely to have a large-artery atherosclerotic stroke (54.3% vs. 34.2%) (Table 1). Sixty-eight patients from each group were included in the PSM analysis. The comparison of baseline characteristics between the two groups after PSM is shown in Table 2. Both groups were comparable with respect to baseline characteristics. Initial NIHSS score, IV thrombolysis, medical history, and mechanism of stroke were similar in both groups.
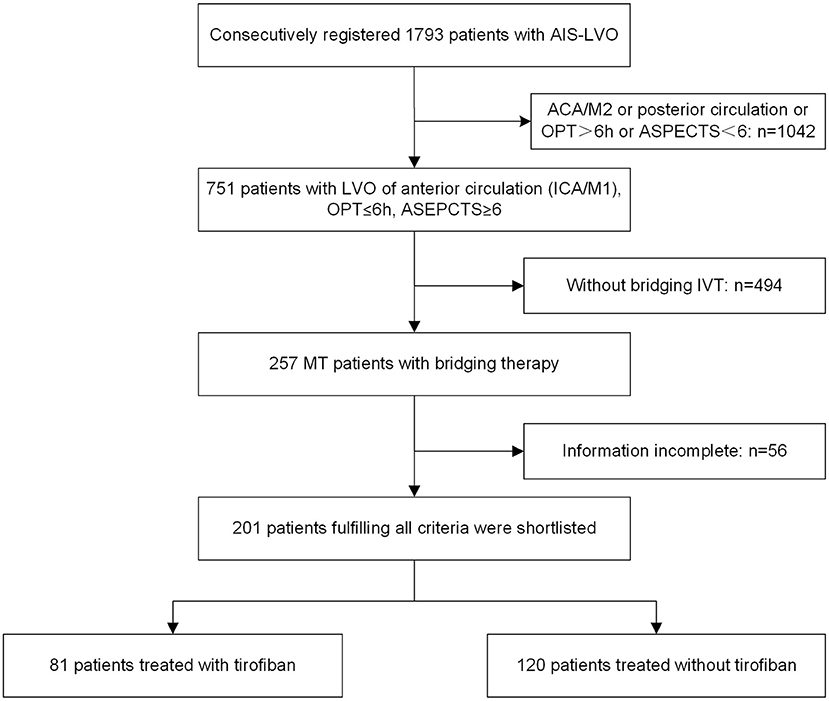
Figure 1. Flowchart. ACA, anterior cerebral artery; AIS, acute ischemic stroke; ASPECTS, Alberta Stroke Program Early CT Score; ICA, internal carotid artery; IVT, intravenous thrombolysis; LVO, large vessel occlusion; M1, M1 segment; M2, M2 segment; MT, mechanical thrombectomy; and OPT, onset-to-puncture time.
Fifteen (7.5%) patients suffered sICH within 24 h after MT, and 52 (25.9%) experienced ICH. There were no significant between-group differences in the incidences of sICH, any ICH, or mortality within 3-months in the entire cohort (all p > 0.05). In the PSM cohort the findings were similar. Three (4.4%) patients in the tirofiban group and seven (10.3%) in the non-tirofiban group suffered sICH (p > 0.05). Fifteen (22.1%) patients in the tirofiban group and 25 (36.8%) in the non-tirofiban group experienced any ICH (p > 0.05). A total of 15 (11.0%) patients died after 3-months, 5 (7.4%) in the tirofiban group and 10 (14.7%) in the non-tirofiban group (p > 0.05) (Tables 3, 4).
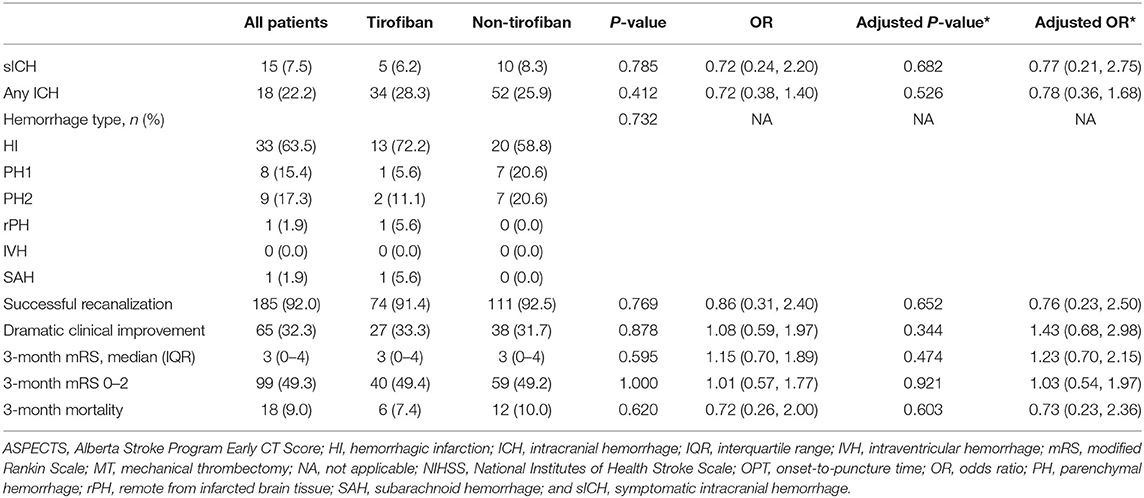
Table 3. Safety and efficacy endpoints of MT patients with preceding intravenous thrombolysis before PSM.
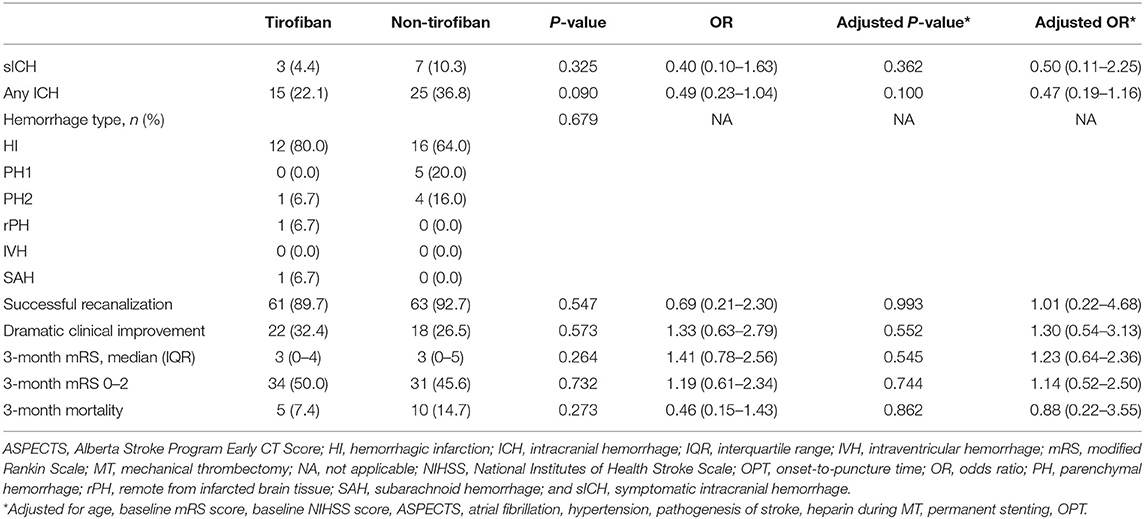
Table 4. Safety and efficacy endpoints of MT patients with preceding intravenous thrombolysis after PSM.
Overall, 185 (92.0%) patients who underwent IVT bridging therapy experienced successful recanalization, 74 (91.4%) in the tirofiban group and 111 (92.5%) in the non-tirofiban group (adjusted p = 0.652). The successful recanalization rates in the tirofiban group and the non-tirofiban group did not differ significantly after PSM (adjusted p = 0.993). In the entire cohort the median NIHSS score at 24 h post-MT was 9 (range 3–14). Sixty-five (32.3%) patients exhibited marked clinical improvement, 27 (33.3%) in the tirofiban group and 38 (31.7%) in the non-tirofiban group. At the 3-month follow-up timepoint, 99 (49.3%) patients had reached functional independence, 40 (49.4%) in the tirofiban group and 59 (49.2%) in the non-tirofiban group (Figure 2). There were no significant differences in any of the above outcomes between the two groups (all p > 0.05). Consistent results were observed in the PSM analysis.
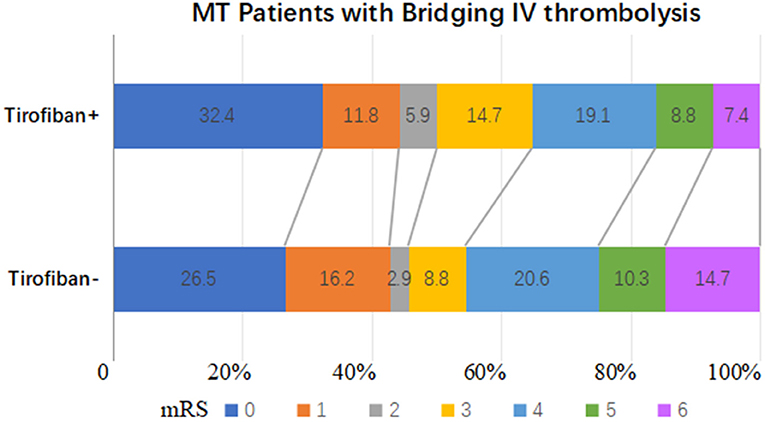
Figure 2. Distributions of the 3-month mRS of patients who underwent mechanical thrombectomy and preceding intravenous thrombolysis after PSM.
In the current prospective registry study, low-dose tirofiban during MT with bridging IVT exhibited acceptable safety with respect to sICH and ICH. The ICH rate was lower in the tirofiban group, but not significantly before or after PSM. This suggested that low-dose tirofiban may be a safe alternative therapy during MT in patients with bridging IVT, especially those with severe in situ atherosclerotic stenosis, permanent stenting, or obvious endothelial damage.
Tirofiban is a non-peptide antagonist of the glycoprotein IIb/IIIa receptor, which regulates the final pathway of platelet aggregation (10). To date little high-quality research has focused on the effects of therapy with glycoprotein IIb/IIIa receptor antagonists during MT in patients with bridging IVT. Huo et al. (20) reported the safety of tirofiban in patients who underwent bridging therapy, but did not detect benefits on long-term functional outcomes. In contrast, Kellert et al. (13) concluded that tirofiban was associated with a higher risk of fatal ICH and poorer outcomes, regardless of whether preceding IVT was administered or not. Notably however, the two studies were observational studies with uncontrolled experimental designs, limited sample sizes, and heterogeneous treatment modalities, thus caution is advised when generalizing from their results.
The use of tirofiban was at the discretion of the treating physician and local practice in the present study. Consistent with previous studies, large-artery atherosclerotic stroke pathogenesis was significantly higher in the tirofiban group (p = 0.011) before PSM. It may be more difficult to achieve successful recanalization in patients with underlying atherosclerotic stenosis, and re-occlusion is more common, so tirofiban with or without angioplasty as an adjuvant rescue strategy may be required. This is concordant with the higher incidence of stent placement in the tirofiban group (23.5% vs. 12.5%, p = 0.055).
In combination therapy with intravenous thrombolysis, Zinkstok et al. (21) suggested that early intravenous administration of aspirin shortly after rt-PA was significantly associated with a higher risk of sICH in the Antiplatelet Therapy in Combination With rt-PA Thrombolysis in Ischemic Stroke trial. Based on this, the use of antiplatelet agents is not recommended within 24 h after IVT in the AHA/ASA guidelines because of the concern of increased hemorrhagic complications (15). Notably however, different inhibition modalities and biologic half-lives influence responses to medication-induced bleeding. Tirofiban is a highly selective and reversible glycoprotein IIb/IIIa receptor antagonist, and has been proven to be safe within the first 24 h after IVT (22). Low-dose tirofiban has been selectively used as rescue therapy during MT in patients with endothelial damage or in situ atherosclerotic stenosis in our clinical practice, and has exhibited acceptable safety. The current study preliminarily confirmed the safety of low-dose tirofiban during MT with respect to sICH and ICH in patients with preceding IVT.
The results of the current study differ from those reported by Kellert et al. (13) and Wu et al. (23) with regard to the safety of rescue tirofiban during MT. This might be due to the following reasons. One pertains to the dosage of tirofiban administration during MT. We reviewed all studies on tirofiban dosage during endovascular treatment of LVO (24). Based on this, we introduced a low-dose intra-arterial bolus of tirofiban (0.25–1.00 mg) for rapid effects on angiographic changes, followed by a continuous intravenous infusion at the lower rate of 0.1 μg/kg/min for 24 h as a standard procedure. Second, according to the specific inhibitory effect on platelet aggregation and atherothrombosis of tirofiban, we prespecified the indications for tirofiban administration during MT in the protocol. Thus, tirofiban was more selectively utilized for large-artery atherosclerotic infarction rather than cardio-embolic stroke (54.3% vs. 34.6%), which might reduce the risk of bleeding. Notably, some of the clinical characteristics of the tirofiban group differed from those of the non-tirofiban group before PSM, which may have affected outcomes. Consequently, PSM was applied to reduce the influence of confounding variables.
The current study had several limitations. First and foremost, all subjects were from an observational study. PSM analysis and a multivariable logistic regression model were used in an effort to reduce selection bias, but potential confounders cannot be ruled out despite adjustment and matching. Therefore, the results of the study need to be interpreted carefully, particularly given that the rate of sICH was lower in the tirofiban group after PSM. Another potential limitation was that all subjects were from China, which has a high prevalence of intracranial atherosclerosis (25). Thus, the results of the study may not be directly generalizable to other populations.
In summary, low-dose tirofiban during MT was not associated with an increased risk of sICH or ICH in patients with preceding IVT. Further dose-escalation trials are needed to confirm its safety and efficacy.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by IRB of Beijing Tiantan Hospital, Capital Medical University. The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
ZM designed, led the study, had full access to all of the data in the study, and takes responsibility for the integrity of the data and the accuracy of the data analysis. GM and SL prepared the first draft of the report. AW and YSP did statistical analyses. All authors except AW and YSP participated in patient enrolment and collection of data. All authors critically reviewed the report and approved the final version.
This study was supported by the National Key Research and Development Program of China (2016 YFC1301500). DM reports grants from National Key Research and Development Program of China (2018YFC1312801). AW reports Grants from Young Elite Scientists Sponsorship Program by CAST (2018QNRC001) and Beijing Municipal Administration of Hospitals Incubating Program (PX2020021). YSP received funding from National Natural Science Foundation of China (81971091) and Beijing Hospitals Authority Youth Programme (QML20190501).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The reviewer WZ declared a shared affiliation, with no collaboration with several of the authors to the handling Editor.
1. Berkhemer OA, Fransen PS, Beumer D, van den Berg LA, Lingsma HF, Yoo AJ, et al. A randomized trial of intraarterial treatment for acute ischemic stroke. N Engl J Med. (2015) 372:11–20. doi: 10.1056/NEJMoa1411587
2. Goyal M, Demchuk AM, Menon BK, Eesa M, Rempel JL, Thornton J, et al. Randomized assessment of rapid endovascular treatment of ischemic stroke. N Engl J Med. (2015) 372:1019–30. doi: 10.1056/NEJMoa1414905
3. Campbell BC, Mitchell PJ, Kleinig TJ, Dewey HM, Churilov L, Yassi N, et al. Endovascular therapy for ischemic stroke with perfusion-imaging selection. N Engl J Med. (2015) 372:1009–18. doi: 10.1056/NEJMoa1414792
4. Jovin TG, Chamorro A, Cobo E, de Miquel MA, Molina CA, Rovira A, et al. Thrombectomy within 8 hours after symptom onset in ischemic stroke. N Engl J Med. (2015) 372:2296–306. doi: 10.1056/NEJMoa1503780
5. Saver JL, Goyal M, Bonafe A, Diener HC, Levy EI, Pereira VM, et al. Stent-retriever thrombectomy after intravenous t-PA vs. t-PA alone in stroke. N Engl J Med. (2015) 372:2285–95. doi: 10.1056/NEJMoa1415061
6. Albers GW, Marks MP, Kemp S, Christensen S, Tsai JP, Ortega-Gutierrez S, et al. Thrombectomy for stroke at 6 to 16 hours with selection by perfusion imaging. N Engl J Med. (2018) 378:708–18. doi: 10.1056/NEJMoa1713973
7. Nogueira RG, Jadhav AP, Haussen DC, Bonafe A, Budzik RF, Bhuva P, et al. Thrombectomy 6 to 24 hours after stroke with a mismatch between deficit and infarct. N Engl J Med. (2018) 378:11–21. doi: 10.1056/NEJMoa1706442
8. Teng D, Pannell JS, Rennert RC, Li J, Li YS, Wong VW, et al. Endothelial trauma from mechanical thrombectomy in acute stroke: in vitro live-cell platform with animal validation. Stroke. (2015) 46:1099–6. doi: 10.1161/STROKEAHA.114.007494
9. Abraham P, Scott Pannell J, Santiago-Dieppa DR, Cheung V, Steinberg J, Wali A, et al. Vessel wall signal enhancement on 3-T MRI in acute stroke patients after stent retriever thrombectomy. Neurosurg Focus. (2017) 42:E20. doi: 10.3171/2017.1.FOCUS16492
10. Schwarz M, Meade G, Stoll P, Ylanne J, Bassler N, Chen YC, et al. Conformation-specific blockade of the integrin GPIIb/IIIa: a novel antiplatelet strategy that selectively targets activated platelets. Circul Res. (2006) 99:25–33. doi: 10.1161/01.RES.0000232317.84122.0c
11. Seo JH, Jeong HW, Kim ST, Kim EG. Adjuvant tirofiban injection through deployed solitaire stent as a rescue technique after failed mechanical thrombectomy in acute stroke. Neurointervention. (2015) 10:22–7. doi: 10.5469/neuroint.2015.10.1.22
12. Zhao W, Che R, Shang S, Wu C, Li C, Wu L, et al. Low-dose tirofiban improves functional outcome in acute ischemic stroke patients treated with endovascular thrombectomy. Stroke. (2017) 48:3289–294. doi: 10.1161/STROKEAHA.117.019193
13. Kellert L, Hametner C, Rohde S, Bendszus M, Hacke W, Ringleb P, et al. Endovascular stroke therapy: tirofiban is associated with risk of fatal intracerebral hemorrhage and poor outcome. Stroke. (2013) 44:1453–5. doi: 10.1161/STROKEAHA.111.000502
14. Yang M, Huo X, Gao F, Wang A, Ma N, Shi H, et al. Low-dose rescue tirofiban in mechanical thrombectomy for acute cerebral large-artery occlusion. Eur J Neurol. (2020) 27:1056–061. doi: 10.1111/ene.14170
15. Powers WJ, Rabinstein AA, Ackerson T, Adeoye OM, Bambakidis NC, Becker K, et al. Guidelines for the early management of patients with acute ischemic stroke: 2019. Update to the 2018 guidelines for the early Management of Acute Ischemic Stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. (2019). 50:e344–418. doi: 10.1161/STR.0000000000000211
16. Jia B, Ren Z, Mokin M, Burgin WS, Bauer CT, Fiehler J, et al. Current status of endovascular treatment for acute large vessel occlusion in China: a Real-World Nationwide Registry. Stroke. (2021) 52:1203–12. doi: 10.1161/STROKEAHA.120.031869
17. von Kummer R, Broderick JP, Campbell BC, Demchuk A, Goyal M, Hill MD, et al. The Heidelberg bleeding classification: classification of bleeding events after ischemic stroke and reperfusion therapy. Stroke. (2015) 46:2981–6. doi: 10.1161/STROKEAHA.115.010049
18. Zaidat OO, Yoo AJ, Khatri P, Tomsick TA, von Kummer R, Saver JL, et al. Recommendations on angiographic revascularization grading standards for acute ischemic stroke: a consensus statement. Stroke. (2013) 44:2650–63. doi: 10.1161/STROKEAHA.113.001972
19. Mazighi M, Meseguer E, Labreuche J, Serfaty JM, Laissy JP, Lavallee PC, et al. Dramatic recovery in acute ischemic stroke is associated with arterial recanalization grade and speed. Stroke. (2012) 43:2998–3002. doi: 10.1161/STROKEAHA.112.658849
20. Huo X, Yang M, Ma N, Gao F, Mo D, Li X, et al. Safety and efficacy of tirofiban during mechanical thrombectomy for stroke patients with preceding intravenous thrombolysis. Clin Interv Aging. (2020) 15:1241–8. doi: 10.2147/CIA.S238769
21. Zinkstok SM, Roos YB. Early administration of aspirin in patients treated with alteplase for acute ischaemic stroke: a randomised controlled trial. Lancet. (2012) 380:731–7. doi: 10.1016/S0140-6736(12)60949-0
22. Wu C, Sun C, Wang L, Lian Y, Xie N, Huang S, et al. Low-dose tirofiban treatment improves neurological deterioration outcome after intravenous thrombolysis. Stroke. (2019) 50:3481–7. doi: 10.1161/STROKEAHA.119.026240
23. Wu Y, Yin C, Yang J, Jiang L, Parsons M, Lin L. Endovascular thrombectomy. Stroke. (2018) 49:2783–5. doi: 10.1161/STROKEAHA.118.022919
24. Yang M, Huo X, Miao Z, Wang Y. Platelet glycoprotein IIb/IIIa receptor inhibitor tirofiban in acute ischemic stroke. Drugs. (2019) 79:515–29. doi: 10.1007/s40265-019-01078-0
Keywords: tirofiban, mechanical thrombectomy, intravenous thrombolysis, large vessel occlusion, propensity score matching
Citation: Ma G, Li S, Jia B, Mo D, Ma N, Gao F, Huo X, Luo G, Wang A, Pan Y, Song L, Sun X, Zhang X, Gui L, Song C, Peng Y, Wu J, Zhao S, Zhao J, Zhou Z and Miao Z (2021) Safety and Efficacy of Low-Dose Tirofiban Combined With Intravenous Thrombolysis and Mechanical Thrombectomy in Acute Ischemic Stroke: A Matched-Control Analysis From a Nationwide Registry. Front. Neurol. 12:666919. doi: 10.3389/fneur.2021.666919
Received: 11 February 2021; Accepted: 22 April 2021;
Published: 10 June 2021.
Edited by:
Gergely Feher, University of Pécs, HungaryReviewed by:
Wenbo Zhao, Capital Medical University, ChinaCopyright © 2021 Ma, Li, Jia, Mo, Ma, Gao, Huo, Luo, Wang, Pan, Song, Sun, Zhang, Gui, Song, Peng, Wu, Zhao, Zhao, Zhou and Miao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhongrong Miao, emhvbmdyb25nbUAxNjMuY29t
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.