- 1Department of Vascular Neurology, University Clinical Centre Ljubljana, Faculty of Medicine, University of Ljubljana, Ljubljana, Slovenia
- 2Department of Internal Medicine, Faculty of Medicine, School of Health Sciences, University of Thessaly, Larissa, Greece
- 3Department of Vascular Disorders, University Clinical Centre Ljubljana, Ljubljana, Slovenia
Background and Purpose: Idarucizumab achieves instant reversal of anticoagulation and enables intravenous thrombolysis (IVT) in dabigatran-treated acute ischemic stroke (AIS) patients. AIS in dabigatran-treated patients is a rare event, therefore the experience is limited. A review of all published cases was performed to evaluate the safety and effectiveness of this therapeutic strategy.
Methods: We searched PubMed and Scopus for all published cases of IVT after reversal with idarucizumab in dabigatran-treated AIS patients. The outcomes were safety assessed by hemorhagic transformation (HT), symptomatic intracranial hemorrhage (SICH) and death, and efficacy assessed by National Institutes of Health Stroke Scale (NIHSS) reduction.
Results: We identified 251 AIS patients (39,9% females) with an average age of 74 years. HT, SICH, and death were reported in 19 (7.6%), 9 (3.6%), and 21 (8.4%) patients, respectively. Patients experiencing HT presented with more severe strokes (median NIHSS on admission: 21 vs. 8, p < 0.001; OR: 1.12, 95% CI: 1.05–1.20). After IVT there was a significant NIHSS reduction of 6 points (IQR:3–10, p < 0.001) post-stroke and linear regression revealed a correlation of admission NIHSS to NIHSS reduction (p < 0.001).
Conclusions: In this systematic review of all published cases of IVT in dabigatran-treated AIS patients after reversal with idarucizumab the rates of HT, SICH and mortality, as well as NIHSS reduction, were comparable with previous studies in non-anticoagulated patients. This provides reassuring evidence about the safety and efficacy of this therapeutic strategy.
Introduction
Idarucizumab is a specific reversal agent for dabigatran, which achieves reversal of anticoagulation within a few minutes after application without thrombotic or other side-effects (1, 2). The use of idarucizumab is indicated in dabigatran-treated patients with life-threatening and uncontrolled bleeding and those who need urgent surgery or intervention (2, 3). The updated 2018 European Heart Rhythm Association guidelines and other expert panels recommend reversal with idarucizumab for dabigatran-treated patients with acute ischemic stroke (AIS) if they are eligible candidates for intravenous thrombolysis (IVT) (4, 5).
AIS in dabigatran-treated patients is a rare event (6, 7), with yearly incidences of 0.9% for the standard dose and 1.3% for the reduced dose (8), therefore the experience is expected to be limited. The first case reports describing IVT in dabigatran-treated AIS patients who received idarucizumab were published in 2014. A previous systematic review reported lower rates of symptomatic intracranial hemorrhage (SICH) and death as well as higher rates of favorable outcome after IVT among 44 dabigatran/idarucizumab treated patients compared to 108 dabigatran treated patients without idarucizumab reversal (9). During the recent years the number of case reports and case series grew, providing more data on patients treated with IVT after dabigatran reversal with idarucizumab (1, 10–57). It is expected that the use of anticoagulants, including dabigatran, will continue to progressively rise.
The aim of the present systematic review was to summarize all published cases of dabigatran-treated patients with AIS who were treated with IVT after reversal with idarucizumab. This could be very useful to stroke physicians when treating dabigatran-treated patients with AIS.
Methodology
Search Strategy and Inclusion Criteria
We searched PubMed and Scopus until 27/10/2020 for studies reporting AIS patients treated with IVT after dabigatran reversal with idarucizumab using the terms: “idarucizumab” or “reversal” or “dabigatran” and “thrombolysis” or “tpa” or “alteplase” or “tissue plasminogen activator” or “tenecteplase” and “stroke” or “cerebrovascular.” The search was limited from 01/01/2013 to 27/10/2020, since the term “idarucizumab” was first reported during 2014. In addition, we searched the references of related letters, reviews and editorials to identify other potentially eligible studies. We also contacted experts in the field to identify potential missed studies. To be eligible for the present analysis, the studies had to be published full-text articles in English language, providing data about the characteristics and outcomes of interest in patients treated with IVT after dabigatran reversal with idarucizumab. This work was reported according to the PRISMA statement (58) and registered in PROSPERO (CRD42020207198).
Outcomes and Data Extraction
The outcomes assessed were HT, SICH (both as defined in the included case reports/series), death and the change in the National Institutes of Health Stroke Scale (NIHSS). A follow-up head computed tomography (CT) scan or magnetic resonance imaging (MRI) was obtained 24 h after IVT in each included case. Additional CT (or MRI) was obtained only if neurological deterioration occurred. HT was defined as the presence of any blood in the ischemic region obtained on the control CT. Precise definitions of SICH are presented in Supplementary Table 1 if applicable.
Eligible studies were assessed independently by the first two authors. Individual patient data were extracted from single-case reports and case series whenever possible using prespecified forms. Quality assessment of the included case reports and case series performed with a tool made to evaluate the methodological quality of case reports and case series (59).
Methodology and Statistical Analysis
Patient characteristics and outcomes were described based on two different prespecified approaches: individual patient data from single case-reports or case-series that provided individual patient data, were pooled together to form a unified group of patients. Case-series that provided aggregate data rather than individual patient data, were reported separately. Discrete variables reported using proportions and continuous variables as means with standard deviations (SD) or medians with interquartile range (IQR). Univariate comparisons were performed from non-parametric Kruskall Wallis test for continuous variable's or Pearson's chi-squared test for categorical variables. The non-parametrical Wilcoxon signed-rank test was used to compare admission NIHSS to NIHSS at 24 h or at discharge. We performed logistic regression analysis to evaluate the association between admission NIHSS and the risk of hemorrhagic transformation and death. Estimates are provided as odds ratio (OR) and 95% confidence intervals (CI). The level of statistical significance was set at 5%. Statistical analyses were performed with the StataCorp. 2015. Stata Statistical Software: Release 14. College Station, TX: StataCorp LP.
Results
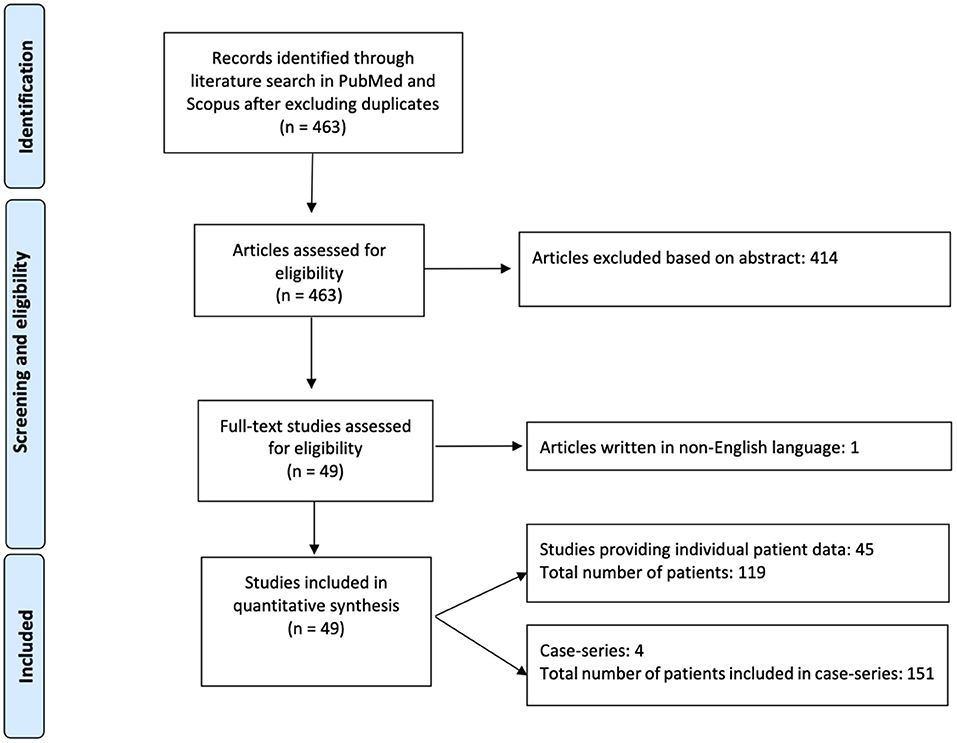
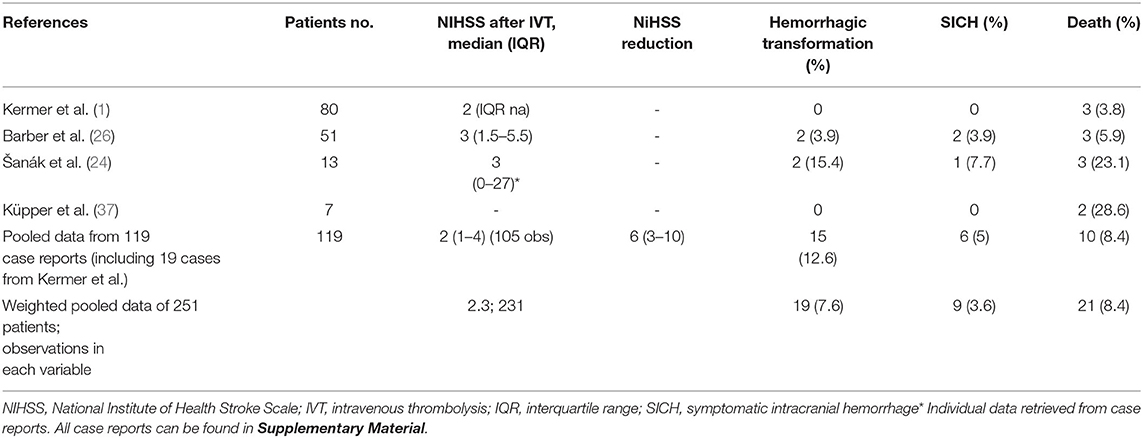
Among 463 articles identified from the initial literature search, 49 studies including 251 patients were eligible and included in the analysis (1, 13–57, 60–63) (Figure 1). Forty-five studies provided individual patient data of 119 AIS patients (13–23, 25, 27–36, 38–57, 60–63), whereas four case-series reported results on overall 151 patients (1, 24, 26, 37).
Data about 19 patients included in a paper from Kermer et al. (13) were published later again in a paper from Kermer et al. (1). Therefore, in the overall weighted analysis of individual patient data and case-series, we did not include the first study (13).
The case-series which reported individual patient data was used in the individual patient analysis (13) and the case-series which reported overall results was used in the aggregate patient analysis (1).
The baseline characteristics of the included studies and the quality assessment can be found in Supplementary File (Supplementary Tables 1, 2).
The average age was 74 years and 97 (38.6%) were females. Overall, 19 patients (7.6%) experienced a HT and among them 9 (3.6%) were complicated with SICH. Twenty-one patients (8.4%) died.
Individual Patient Data Subanalysis
Among 119 patients included in single case reports or case-series that provided individual patient data, the mean age was 73.8 ± 11.3 years and 50 (42%) patients were females. The median stroke-to-needle time was 155 (IQR: 120–210) min and the median NIHSS at admission was 10 (IQR: 6–16). Thirteen patients (10.9%) were treated with endovascular thrombectomy (EVT) additionally to IVT. The baseline characteristics of patients are summarized in Table 1.
HT was reported in 15 patients (12.6%) and among them, six patients (5%) experienced SICH. Patients experiencing a HT presented with more severe strokes (median NIHSS on admission: 21 vs. 8, p < 0.001; OR: 1.12, 95% CI: 1.05–1.20).
Patients who were treated with IVT after idarucizumab reversal of dabigatran had a significant median NIHSS reduction of six (IQR: 3–10, Wilcoxon sign-ranked test, p < 0.001; Figure 2A). In the sub analysis a linear correlation between initial stroke severity (NIHSS at admission) and the outcome (evaluated as NIHSS improvement), controlling for age and gender was found (p < 0.001) (Figure 2B). Death was reported in 10 patients (8.4%) who presented with significantly higher admission NIHSS compared to stroke survivors (23 vs. 9, OR: 1.21, 95% CI: 1.09–1.33) (Table 2).
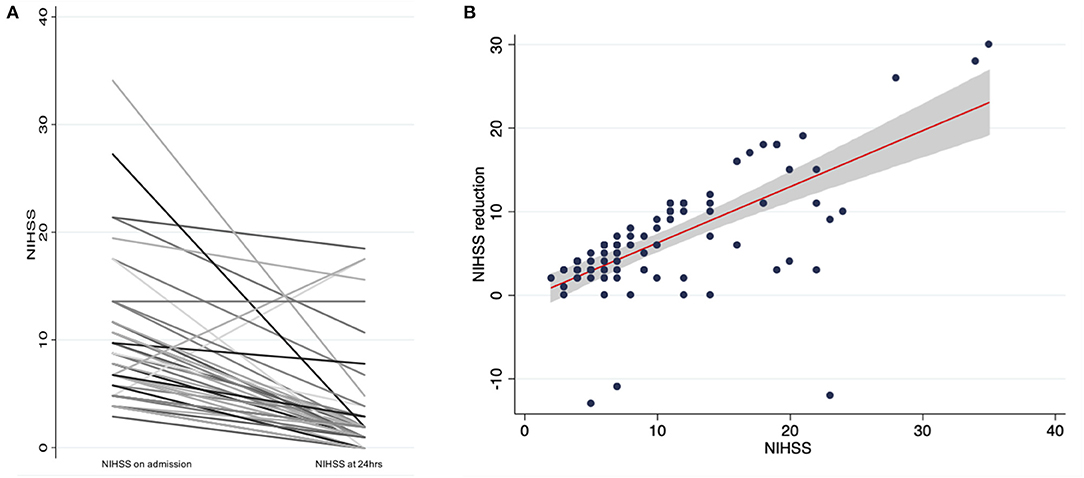
Figure 2. (A) NIHSS change in thrombolysed patients after dabigatran reversal with idarucizumab, (B) Linear regression analysis and 95% confidence interval of NIHSS reduction based on admission NIHSS.
Aggregate Patient Data Subanalysis
Four studies reported aggregate data for 151 patients who were treated with IVT after dabigatran reversal with idarucizumab (1, 24, 26, 37). The baseline characteristics of each case series are summarized in Table 1. Hemorrhagic transformation was reported in 4 patients (2.7%) and only one patient (0.7%) was complicated with SICH. Death was reported in 11 patients (7.2%) (Table 2).
Discussion
In a systematic review of all published cases of IVT in idarucizumab/dabigatran-treated AIS patients published up until the end of October, 2020 (1, 13–57, 60–63), we confirmed the safety and efficacy of this treatment. IVT after dabigatran reversal with idarucizumab resulted in similar rate of HT, SICH and death, as well as similar reduction of NIHSS compared with previous studies in non-anticoagulated patients (64, 65). HT and death occurred in patients presenting with severe AIS. Importantly, effectiveness was the same regardless of stroke severity and age.
We have performed a comprehensive review of all published cases of IVT (up until October, 2020) after dabigatran reversal in patients with AIS with the aim to explore the safety and efficacy of idarucizumab reversal. Forty-seven studies were eligible and included in the analysis, with 251 AIS patients, who have been treated with IVT after reversal. So far the safety and efficacy of IVT after dabigatran reversal with idarucizumab has not been evaluated in large studies. In 2017 a small meta-analysis of all published cases (n = 21) with the review was done by Pikija et al. (15) suggesting good efficacy and safety of idarucizumab reversal. In 2020, so far the largest cohort of dabigatran-treated patients receiving IVT after reversal with idarucizumab (n = 80) in Germany was published (1). A significant clinical improvement was found in 78% of patients, neither bleeding nor thrombotic complications were associated with IVT after idarucizumab treatment. Thus, meta-analysis of all published cases from different regions and including patients of all ethnicities could be clinically very relevant and helpful to clinicians all around the world when treating dabigatran-treated patients with AIS.
Our analysis of safety revealed HT in 19 (7.6%) of patients and SICH in only 9 (3.6%) patients. Twenty-one patients (8.4%) died. The results are in line with the safety observed in studies of IVT in patients without previous anticoagulation (64, 65). Thus, pretreatment with dabigatran and idarucizumab at stroke onset and IVT is not accompanied with an increased risk of hemorrhage or death.
A significant median NIHSS reduction of six points was achieved, confirming the efficacy of this treatment being at least similar, if not better, than obtained in non-anticoagulated patients (64, 65). IVT after idarucizumab reversal was effective regardless of stroke severity and age. In the sub analysis a linear correlation between initial stroke severity and the outcome (adjusted for age and gender) was found.
In our analysis we recorded a high success rate of IVT after reversal in dabigatran-treated patients which was also described in two previous smaller analyses (1, 15). Although it is highly speculative this was previously presumed to be a possible consequence of higher sensitivity of thrombi in dabigatran-treated patients to lysis (11). Indeed, in vitro studies showed the ability of dabigatran to enhance the susceptibility of plasma clots to rt-PA-induced lysis by reducing thrombin-activatable fibrinolysis inhibitor (TAFI) activation and by altering the clot structure (66).
Our review has several limitations. Missing and uncomplete data on times of stroke onset to IVT and on discharge NIHSS, discharge mRS and mRS at 3 months in big cohorts are the main limitation and did not allow the analysis of these parameters. The lack of comparator group and the fact that efficacy and safety could not be directly compared with the results of alteplase trials are additional limitations. Clearly, further studies are needed to definitively confirm the findings of our meta-analysis. Registry such as Registry of Acute Stroke Under Novel Oral Anticoagulants-Prime (RASUNOA-Prime) (67), where patients are systematically followed-up for management, clinical course and outcome, will provide detailed insights into outcome of these patients.
In conclusion, our meta-analysis of all published cases of dabigatran-treated patients, who received idarucizumab as a reversal agent, confirmed the safety and efficacy of treatment in all AIS patients, treated with dabigatran, receiving IVT after reversal, regardless of age and stroke severity on admission. Nonetheless, admission NIHSS score appeared to be an independent predictor of mortality.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
Author Contributions
SF and DS: study design, data acquisition, statistical analysis and interpretation, and manuscript preparation. JP and MŠ: data acquisition, statistical analysis and interpretation, and critical revision of the manuscript. GN: study concept and design, statistical analysis and interpretation, manuscript preparation, and study supervision. All authors contributed to the article and approved the submitted version.
Conflict of Interest
In the past 2 years, SF, MŠ, and JP have received honoraria for oral presentations from Bayer, Boehringer Ingelheim and Pfizer. GN reports Speaker fees/Advisory Boards/Research support from Abbott; Amgen; Bayer; Boehringer-Ingelheim; Elpen; Pfizer outside the submitted work.
The remaining author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2021.666086/full#supplementary-material
Abbreviations
AIS, acute ischemic stroke; IVT, intravenous thrombolysis; SICH, symptomatic intracranial hemorrhage; HT, hemorrhagic transformation; NIHSS, National Institutes of Health Stroke Scale; OR, odds ratio; CI, confidence interval.
References
1. Kermer P, Eschenfelder CC, Diener HC, Grond M, Abdalla Y, Abraham A, et al. Antagonizing dabigatran by idarucizumab in cases of ischemic stroke or intracranial hemorrhage in germany—updated series of 120 cases. Int J Stroke. (2020) 15:609–18. doi: 10.1177/1747493019895654
2. Pollack CV, Reilly PA, van Ryn J, Eikelboom JW, Glund S, Bernstein RA, et al. Idarucizumab for dabigatran reversal — full cohort analysis. N Engl J Med. (2017) 377:431–41. doi: 10.1056/NEJMoa1707278
3. Thibault N, Morrill AM, Willett KC. Idarucizumab for reversing dabigatran-induced anticoagulation: a systematic review. Am J Ther. (2018) 25:e333–e8. doi: 10.1097/MJT.0000000000000460
4. Steffel J, Verhamme P, Potpara TS, Albaladejo P, Antz M, Desteghe L, et al. The 2018 european heart rhythm association practical guide on the use of non-vitamin k antagonist oral anticoagulants in patients with atrial fibrillation. Europ Heart J. (2018) 39:1330–93. doi: 10.5603/KP.2018.0180
5. Diener H, Bernstein R, Butcher K, Campbell B, Cloud G, Davalos A, et al. Thrombolysis and thrombectomy in patients treated with dabigatran with acute ischemic stroke: expert opinion. Int J Stroke. (2017) 12:9–12. doi: 10.1177/1747493016669849
6. Ruff CT, Giugliano RP, Braunwald E, Hoffman EB, Deenadayalu N, Ezekowitz MD, et al. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta-analysis of randomised trials. Lancet. (2014) 383:955–62. doi: 10.1016/S0140–6736(13)62343–0
7. Ntaios G, Papavasileiou V, Diener HC, Makaritsis K, Michel P. Nonvitamin-k-antagonist oral anticoagulants versus warfarin in patients with atrial fibrillation and previous stroke or transient ischemic attack: an updated systematic review and meta-analysis of randomized controlled trials. Int J Stroke. (2017) 12:589–96. doi: 10.1177/1747493017700663
8. Connolly SJ, Ezekowitz MD, Yusuf S, Eikelboom J, Oldgren J, Parekh A, et al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. (2009) 361:1139–51. doi: 10.1056/NEJMoa0905561
9. Jin C, Huang RJ, Peterson ED, Laskowitz DT, Hernandez AF, Federspiel JJ, et al. Intravenous tpa (tissue-type plasminogen activator) in patients with acute ischemic stroke taking non-vitamin k antagonist oral anticoagulants preceding stroke. Stroke. (2018) 49:2237–40. doi: 10.1161/STROKEAHA.118.022128
10. Giustozzi M, Verso M, Agnelli G, Becattini C. Reversal of dabigatran-associated bleeding using idarucizumab: review of the current evidence. J Thromb Thrombolysis. (2017) 44:527–35. doi: 10.1007/s11239–017-1555–4
11. Pretnar Oblak J, Sabovic M, Frol S. Intravenous thrombolysis after idarucizumab application in acute stroke patients—a potentially increased sensitivity of thrombi to lysis? J Stroke Cerebrovasc Dis. (2019) 28:768–73. doi: 10.1016/j.jstrokecerebrovasdis.2018.11.019
12. Touzé E, Gruel Y, Gouin-Thibault I, De Maistre E, Susen S, Sie P, et al. Intravenous thrombolysis for acute ischaemic stroke in patients on direct oral anticoagulants. Europ J Neurol. (2018) 25:747–52. doi: 10.1111/ene.13582
13. Kermer P, Eschenfelder CC, Diener HC, Grond M, Abdalla Y, Althaus K, et al. Antagonizing dabigatran by idarucizumab in cases of ischemic stroke or intracranial hemorrhage in germany – a national case collection. Int J Stroke. (2017) 12:383–91. doi: 10.1177/1747493017701944
14. Vosko MR, Bocksrucker C, Drwiła R, Dulíček P, Hauer T, Mutzenbach J, et al. Real-life experience with the specific reversal agent idarucizumab for the management of emergency situations in dabigatran-treated patients: a series of 11 cases. J Thromb Thromb. (2017) 43:306–17. doi: 10.1007/s11239–017-1476–2
15. Pikija S, Sztriha LK, Sebastian Mutzenbach J, Golaszewski SM, Sellner J. Idarucizumab in dabigatran-treated patients with acute ischemic stroke receiving alteplase: a systematic review of the available evidence. CNS Drugs. (2017) 31:747–57. doi: 10.1007/s40263–017-0460-x
16. Mutzenbach JS, Pikija S, Otto F, Halwachs U, Weymayr F, Sellner J. Intravenous thrombolysis in acute ischemic stroke after dabigatran reversal with idarucizumab – a case report. Ann Clin Transl Neurol. (2016) 3:889–92. doi: 10.1002/acn3.346
17. Kafke W, Kraft P. Intravenous thrombolysis after reversal of dabigatran by idarucizumab: a case report. Case Rep Neurol. (2016) 8:140–4. doi: 10.1159/000447531
18. Facchinetti R, DeGuidi G, Pitoni F, Ricci G, Lippi G. Rapid and well tolerated action of idarucizumab for antagonizing dabigatran in a patient needing urgent thrombolysis: a case report. Blood Coagul Fibri. (2017) 28:576–9. doi: 10.1097/MBC.0000000000000634
19. Agosti S, Casalino L, Rocci E, Zaccone G, Rota E. Successful intravenous thrombolysis for ischemic stroke after reversal of dabigatran anticoagulation with idarucizumab: a case report. J Med Case Rep. (2017) 11:224. doi: 10.1186/s13256–017-1404–2
20. Berrouschot J, Stoll A, Hogh T, Eschenfelder CC. Intravenous thrombolysis with recombinant tissue-type plasminogen activator in a stroke patient receiving dabigatran anticoagulant after antagonization with idarucizumab. Stroke. (2016) 47:1936–8. doi: 10.1161/STROKEAHA.116.013550
21. Frol S, Šabovič M, Popovič KŠ, Oblak JP. Revascularization outcomes following acute ischemic stroke in patients taking direct oral anticoagulants: a single hospital cohort study. J Thromb Thromb. (2020) 51:194–202. doi: 10.1007/s11239–020-02168–7
22. Giannandrea D, Caponi C, Mengoni A, Romoli M, Marando C, Gallina A, et al. Intravenous thrombolysis in stroke after dabigatran reversal with idarucizumab: case series and systematic review. J Neurology, Neurosurg Psychiatry. (2019) 90:619–23. doi: 10.1136/jnnp-2018–318658
23. Hieber M, Hollasch H, Heck D, Mächtel M, Geisen U, Niesen WD, et al. Reversal of dabigatran using idarucizumab: single center experience in four acute stroke patients. J Thromb Thromb. (2018) 46:12–5. doi: 10.1007/s11239–018-1658–6
24. Šanák D, Jakubíček S, Cerník D, Herzig R, Kunáš Z, Mikulík R, et al. Intravenous thrombolysis in patients with acute ischemic stroke after a reversal of dabigatran anticoagulation with idarucizumab: a real-world clinical experience. J Stroke Cerebrovasc Dis. (2018) 27:2479–83. doi: 10.1016/j.jstrokecerebrovasdis.2018.05.004
25. Alvarez Bravo G, Orts Castro E, Carvalho Monteiro G, López Zuazo I. Intravenous fibrinolysis in ischemic stroke of large vessel after reversing effect of dabigatran with idarucizumab. J Stroke Cerebrovasc Dis. (2017) 26:e192–e3. doi: 10.1016/j.jstrokecerebrovasdis.2017.04.032
26. Barber PA, Wu TY, Ranta A. Stroke reperfusion therapy following dabigatran reversal with idarucizumab in a national cohort. Neurology. (2020) 94:e1968–e72. doi: 10.1212/WNL.0000000000009155
27. Baule A, Cabigiosu F, Zanda B, Sanna A, Mongili C, Manca A. Thrombolysis in acute ischemic stroke after idarucizumab for dabigatran etexilate reversal in elderly: a case report. J Vasc Interv Neurol. (2018) 10:15–7.
28. Beharry J, Waters MJ, Drew R, Fink JN, Wilson D, Campbell BCV, et al. Dabigatran reversal before intravenous tenecteplase in acute ischemic stroke. Stroke. (2020) 2020:1616–9. doi: 10.1161/STROKEAHA.119.028327
29. Binet Q, Hammer FD, Rocrelle O, Peeters A, Scavée C, Hermans C. Systemic thrombolysis and endovascular thrombectomy in severe acute ischemic stroke after dabigatran reversal with idarucizumab. Clin Case Rep. (2018) 6:698–701. doi: 10.1002/ccr3.1446
30. Bissig D, Manjunath R, Traylor BR, Richman DP, Ng KL. Acute stroke despite dabigatran anticoagulation treated with idarucizumab and intravenous tissue plasminogen activator. J Stroke Cerebrovasc Dis. (2017) 26:e102–e4. doi: 10.1016/j.jstrokecerebrovasdis.2016.12.037
31. Candelaresi P, Iannuzzi A, Servillo G, Gottilla R. Left atrial appendage thrombus on full-dose dabigatran treatment: a case report. Eur Heart J Case Rep. (2020) 4:1–4. doi: 10.1093/ehjcr/ytaa057
32. Fang CW, Tsai YT, Chou PC, Chen HM, Lu CM, Tsao CR, et al. Intravenous thrombolysis in acute ischemic stroke after idarucizumab reversal of dabigatran effect: analysis of the cases from taiwan. J Stroke Cerebrovasc Dis. (2019) 28:815–20. doi: 10.1016/j.jstrokecerebrovasdis.2018.11.029
33. Harsha KJ, Thirunavukkarasu S, Butcher K. Acute stroke thrombolysis following dabigatran reversal using idarucizumab. Neurology India. (2019) 67:568–70. doi: 10.4103/0028–3886.258032
34. Hieber M, Bardutzky J. Immediate reversal of dabigatran by idarucizumab prior to laboratory and imaging results in acute stroke. Front Neurol. (2019) 10:230. doi: 10.3389/fneur.2019.00230
35. Hosoki S, Takagi M, Yamagami H, Ando D, Toyoda K, Koga M. Paradoxical elevation of plasma dabigatran after reversal with idarucizumab in stroke thrombolysis. J Neurol. (2018) 265:2451–453. doi: 10.1007/s00415–018-9011–8
36. Jala S, O'Brien E. Treatment with intravenous alteplase for acute ischemic stroke after reversal of dabigatran with idarucizumab: a case study. J Neurosci Nurs. (2019) 51:21–5. doi: 10.1097/JNN.0000000000000412
37. Küpper C, Feil K, Klein M, Feuerecker R, Lücking M, Thanbichler F, et al. Idarucizumab administration in emergency situations: the munich registry of reversal of pradaxa® in clinical routine (mr repair). J Neurol. (2019) 266:2807–11. doi: 10.1007/s00415–019-09492-w
38. Lin YT, Lai YJ, Lai TH. Idarucizumab for intravenous thrombolysis and endovascular thrombectomy in acute stroke: a case report. J Emerg Med. (2020) 58:e113–e6. doi: 10.1016/j.jemermed.2019.09.040
39. Lo WT, Ng KF, Chan SC, Kwok VW, Fong CS, Chan ST, et al. Intravenous stroke thrombolysis after reversal of dabigatran effect by idarucizumab: First reported case in hong kong. Hong Kong Med J. (2018) 24:81–3. doi: 10.12809/hkmj166231
40. Loh CH, Herkes G. Successful thrombolysis for acute ischaemic stroke after reversal of dabigatran etexilate with idarucizumab. BMJ Case Rep. (2019) 12:e229128. doi: 10.1136/bcr-2018–229128
41. Maramattom B, Thomas J. Reversal of anticoagulation effect of dabigatran with idarucizumab, for thrombolysis in acute ischemic stroke: inimicus inimico amicus. Ann Indian Acad Neurol. (2019) 22:515–7. doi: 10.4103/aian.AIAN_536_18
42. Meyer D, Chu F, Derry K, Hailey L. Acute reversal of dabigatran with idarucizumab for intravenous thrombolysis as acute stroke treatment. J Clin Neurosci. (2019) 59:355–7. doi: 10.1016/j.jocn.2018.09.027
43. Ng FC, Bice J, Rodda A, Lee-Archer M, Crompton DE. Adverse clinical outcomes after dabigatran reversal with idarucizumab to facilitate acute stroke thrombolysis. J Neurol. (2017) 264:591–4. doi: 10.1007/s00415–017-8410–6
44. Ohtani T, Sintoku R, Yajima T, Kaneko N. Successful thrombolytic therapy with recombinant tissue plasminogen activator in ischemic stroke after idarucizumab administration for reversal of dabigatran: a case report. J Med Case Rep. (2019) 13:390. doi: 10.1186/s13256–019-2326-y
45. Ohya Y, Makihara N, Wakisaka K, Morita T, Ago T, Kitazono T, et al. Thrombolytic therapy in severe cardioembolic stroke after reversal of dabigatran with idarucizumab: case report and literature review. J Stroke Cerebrovasc Dis. (2018) 27:e128–e31. doi: 10.1016/j.jstrokecerebrovasdis.2018.02.025
46. Pásztor M, Bereczki D, Szakács Z, May Z. Systemic thrombolysis after the administration of idarucizumab in acute ischemic stroke. Ideggyogyaszati Szemle. (2017) 70:284–8. doi: 10.18071/isz.70.0284
47. Renard A, Mallecourt C, Wilhlem L, Combes E, Foucher S, Arteaga C. Intravenous thrombolysis and thrombectomy in stroke after dabigatran reversal by idarucizumab. Presse Med. (2018) 47:401–4. doi: 10.1016/j.lpm.2018.02.009
48. Schäfer N, Müller A, Wüllner U. Systemic thrombolysis for ischemic stroke after antagonizing dabigatran with idarucizumab—a case report. J Stroke Cerebrovasc Dis. (2016) 25:e126–e7. doi: 10.1016/j.jstrokecerebrovasdis.2016.05.006
49. Schulz JG, Kreps B. Idarucizumab elimination of dabigatran minutes before systemic thrombolysis in acute ischemic stroke. J Neurol Sci. (2016) 370:44. doi: 10.1016/j.jns.2016.09.010
50. Ting A, Venkat AR, Gawarikar Y, Patel R. Reversal of dabigatran with idarucizumab in hyperacute stroke: a new paradigm? Med J Austr. (2019) 210:302–303.e301. doi: 10.5694/mja2.50122
51. Tireli D, He J, Nordling MM, Wienecke T. Systemic thrombolysis in acute ischemic stroke after dabigatran etexilate reversal with idarucizumab—a case report. J Stroke Cerebrovasc Dis. (2017) 26:e123–e5. doi: 10.1016/j.jstrokecerebrovasdis.2017.03.039
52. Tsai LK, Lin HJ, Chua SK, Liao PC, Yang YP, Chou PC, et al. Real-world experience with idarucizumab to reverse anticoagulant effect in dabigatran-treated patients: report of 11 cases from taiwan. J Stroke Cerebrovasc Dis. (2018) 27:e27–e33. doi: 10.1016/j.jstrokecerebrovasdis.2017.09.044
53. Tsai YT, Hsiao YJ, Tsai LK, Yen PS, Lin FY, Lu CH, et al. Idarucizumab-facilitated intravenous thrombolysis in acute stroke with dabigatran: Two cases with hemorrhagic transformation. J Neurol Sci. (2018) 388:155–7. doi: 10.1016/j.jns.2018.03.021
54. Turine G, Peeters A, Hermans C, Eeckhoudt S, Duprez T. Intravenous thrombolysis after reversal of dabigatran by idarucizumab: a moment to be a pioneer. Acta Neurol Bel. (2017) 117:753–5. doi: 10.1007/s13760–017-0751–5
55. von Wowern F, Brizzi M, Holst J. Reversal of the anticoagulation effects of dabigatran etexilate by idarucizumab in three patients needing urgent surgical intervention and one case of intravenous thrombolysis in ischaemic stroke. Eur J Case Rep Intern Med. (2017) 4:000569. doi: 10.12890/2017_000569
56. Vukorepa G, Devedija S, Crnjaković M, Karakaš M, Cuk Ž. Successful intravenous thrombolysis in acute ischemic stroke after reversal of dabigatran etexilate with idarucizumab at the end of therapeutic time window. Turk J Emerg Med. (2020) 20:90–92. doi: 10.4103/2452–2473.285015
57. Zhao H, Coote S, Pesavento L, Jones B, Rodrigues E, Ng JL, et al. Prehospital idarucizumab prior to intravenous thrombolysis in a mobile stroke unit. Int J Stroke. (2019) 14:265–269. doi: 10.1177/1747493018790081
58. Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: the prisma statement. Ann Internal Med. (2009) 151:264–9, W264. doi: 10.7326/0003–4819-151–4-200908180–00135
59. Murad MH, Sultan S, Haffar S, Bazerbachi F. Methodological quality and synthesis of case series and case reports. BMJ Evidence-Based Med. (2018) 23:60–3. doi: 10.1136/bmjebm-2017–110853
60. Tse DM, Young L, Ranta A, Barber PA. Intravenous alteplase and endovascular clot retrieval following reversal of dabigatran with idarucizumab. J Neurol Neurosurg Psychiatry. (2018) 89:549–50. doi: 10.1136/jnnp-2017–316449
61. Cappellari M, Forlivesi S, Squintani GM, Facchinetti R, Bovi P. Intravenous thrombolysis for stroke after dabigatran reversal with idarucizumab: an update. J Thromb Thromb. (2017) 43:528–9. doi: 10.1007/s11239–017-1485–1
62. Gawehn A, Ayari Y, Heuschkel C, Kaste M, Kermer P. Successful thrombolysis with recombinant tissue plasminogen activator after antagonizing dabigatran by idarucizumab: a case report. J Med Case Rep. (2016) 10:1–3. doi: 10.1186/s13256–016-1050–0
63. Laxamana LC, Co COC, Yu JRT, Mojica CV, Iboleon-Dy MAM, Domingo AMC, et al. Dabigatran reversal with idarucizumab preceding thrombolysis in an octogenarian patient with chronic kidney disease and acute stroke: a case report. Clin Therap. (2020) 42:1840–5. doi: 10.1016/j.clinthera.2020.07.006
64. Emberson J, Lees KR, Lyden P, Blackwell L, Albers G, Bluhmki E, et al. Effect of treatment delay, age, and stroke severity on the effects of intravenous thrombolysis with alteplase for acute ischaemic stroke: a meta-analysis of individual patient data from randomised trials. Lancet. (2014) 384:1929–35. doi: 10.1016/S0140–6736(14)60584–5
65. Keselman B, Gdovinová Z, Jatuzis D, Melo TPE, Vilionskis A, Cavallo R, et al. Safety and outcomes of intravenous thrombolysis in posterior versus anterior circulation stroke: results from the safe implementation of treatments in stroke registry and meta-analysis. Stroke. (2020) 51:876–82. doi: 10.1161/STROKEAHA.119.027071
66. Ammollo CT, Semeraro F, Incampo F, Semeraro N, Colucci M. Dabigatran enhances clot susceptibility to fibrinolysis by mechanisms dependent on and independent of thrombin-activatable fibrinolysis inhibitor. J Thromb Haemost. (2010) 8:790–8. doi: 10.1111/j.1538–7836.2010.03739.x
Keywords: dabigatran, idarucizumab, ischemic stroke, intravenous thrombolysis, outcome
Citation: Frol S, Sagris D, Pretnar Oblak J, Šabovič M and Ntaios G (2021) Intravenous Thrombolysis After Dabigatran Reversal by Idarucizumab: A Systematic Review of the Literature. Front. Neurol. 12:666086. doi: 10.3389/fneur.2021.666086
Received: 09 February 2021; Accepted: 03 May 2021;
Published: 03 June 2021.
Edited by:
Robin Lemmens, University Hospitals Leuven, BelgiumReviewed by:
Alexander Tsiskaridze, Tbilisi State University, GeorgiaKen Butcher, University of Alberta, Canada
Copyright © 2021 Frol, Sagris, Pretnar Oblak, Šabovič and Ntaios. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Senta Frol, c2VudGFmcm9sQGdtYWlsLmNvbQ==
†These authors have contributed equally to this work
 Senta Frol
Senta Frol Dimitrios Sagris
Dimitrios Sagris Janja Pretnar Oblak1
Janja Pretnar Oblak1 George Ntaios
George Ntaios