
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Neurol. , 08 December 2021
Sec. Multiple Sclerosis and Neuroimmunology
Volume 12 - 2021 | https://doi.org/10.3389/fneur.2021.664596
This article is part of the Research Topic Disease Modifying Therapies in Multiple Sclerosis View all 25 articles
 Riccardo Garbo1*
Riccardo Garbo1* Daniela Cutuli2
Daniela Cutuli2 Simone Lorenzut2
Simone Lorenzut2 Gian Luigi Gigli1,3
Gian Luigi Gigli1,3 Daniele Bagatto4
Daniele Bagatto4 Mariarosaria Valente1,3
Mariarosaria Valente1,3Cladribine is an effective disease-modifying treatment for relapsing-remitting multiple sclerosis that acts as an immune reconstitution therapy and is administered in a pulsed manner. Despite its efficacy, severe disease reactivation early after treatment represents a serious clinical problem, and clear evidence to guide the management of such a situation is lacking. Here, we describe the case of a patient experiencing considerable disease activity during the 1st year after the initiation of cladribine treatment. The patient was switched to alemtuzumab and, therefore, received double immune reconstitution therapy. Data regarding this approach are lacking, and real-world observations may be of interest. Despite achieving good control of disease activity, we observed several serious infectious complications. Our results suggest that sequential immune reconstitution therapies may be effective; however, at the price of higher susceptibility to infections.
Cladribine and alemtuzumab have proven to be effective treatments for relapsing-remitting multiple sclerosis (RRMS), and both act as immune reconstitution therapies administered in a pulsed manner (1–3). Disease activity may occur early after the first course of treatment. However, this does not necessarily imply a treatment failure that requires further modifications to the treatment strategy. For this reason, drug response evaluation is generally performed at least a few months after the second drug course (4). Nevertheless, relevant disease activity early after a treatment course of one of these drugs may sometimes represent a serious clinical problem, potentially leading to permanent disability. In the CLARITY trial, interferon beta-1a rescue therapy was used (1). However, evidence of managing such a problem is scarce, subsequently leading to different clinical choices in a real-world setting (5). Here, we report a case of considerable ongoing disease activity after the first course of cladribine treatment, which was managed with alemtuzumab administration. Data regarding this sequence of therapies, which act through immune system depletion and reconstitution, are lacking, and real-world observations are, therefore, of interest. After alemtuzumab treatment, the patient achieved disease stability; however, several infectious complications were observed. This suggests that this sequential treatment strategy can be applied but warrants caution and careful monitoring.
Here, we report the case of a 42-year-old patient diagnosed with RRMS at the age of 24 years, which was treated with different disease-modifying therapies. In 2012, after 2 years of natalizumab treatment, the patient was switched to fingolimod because of the high risk of progressive multifocal leukoencephalopathy. The patient remained stable until March 2017, when MRI progression was observed followed by a clinical relapse during the subsequent year.
Considering the presence of relevant disease activity, after discussing possible alternatives with the patient and considering an anti-JC virus (JCV) antibody index of 3.72, in May 2018, fingolimod therapy was discontinued and 9 weeks later, after lymphocyte count (ALC) recovery, oral cladribine was started. The patient received 1.75 mg/kg of cladribine and completed the first treatment course. The expanded disability status scale (EDSS) score at therapy initiation was 2.0, the ALC was 1,380 cells/μl, and baseline control MRI did not show any new lesions or contrast enhancement.
The patient consulted us in February 2019, reporting a slowly progressive somatosensory symptomatology over the previous month, which was considered as a relapse with no impact on permanent disability. However, MRI was performed and revealed four new demyelinating lesions, two of which presented with contrast enhancement. In July 2019, a new MRI scan was obtained, revealing five new cerebral enhancing lesions (Figure 1). We decided to switch therapy from cladribine to alemtuzumab. Therefore, the patient did not receive the second course of cladribine. The first course of alemtuzumab was administered in September 2019, when the ALC returned to the normal range (1,060/μl). The patient received 200 mg oral acyclovir twice daily for a month after infusions.
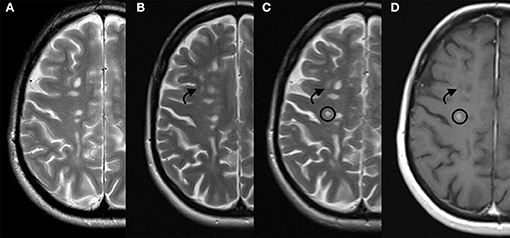
Figure 1. Patient's disease progression on MRI. From the left to the right: T2 weighted sequences respectively on June 2018 (A), February 2019 (B), and July 2019 (C), and; contrast enhanced T1 weighted sequence on July 2019 (D). Arrow indicates a new demyelinating lesion and circle indicates a new demyelinating lesion presenting contrast enhancement. MRI, magnetic resonance imaging.
After starting alemtuzumab, the patient did not present any clinical relapses, radiologic signs of disease activity, or worsening EDSS score until January 2021. Despite good disease control, the patient experienced various infectious complications. In November 2019, she was treated with oral amoxicillin/clavulanate to address an upper airway infection. In December 2019, the patient was hospitalized on a precautionary basis because of A/H3 influenza infection; however, she did not require treatment. At the end of January, she received oral antibiotic treatment for upper airway infection. In February 2020, the patient presented with dermatomal varicella zoster virus reactivation, which required hospitalization and intravenous acyclovir.
The second course of alemtuzumab was administered in September 2020. A few days after treatment, the patient was again hospitalized for Escherichia coli-related left pyelonephritis, with findings of a duplicated ureter, and was successfully treated with antibiotics. Case timeline is provided in Figure 2.
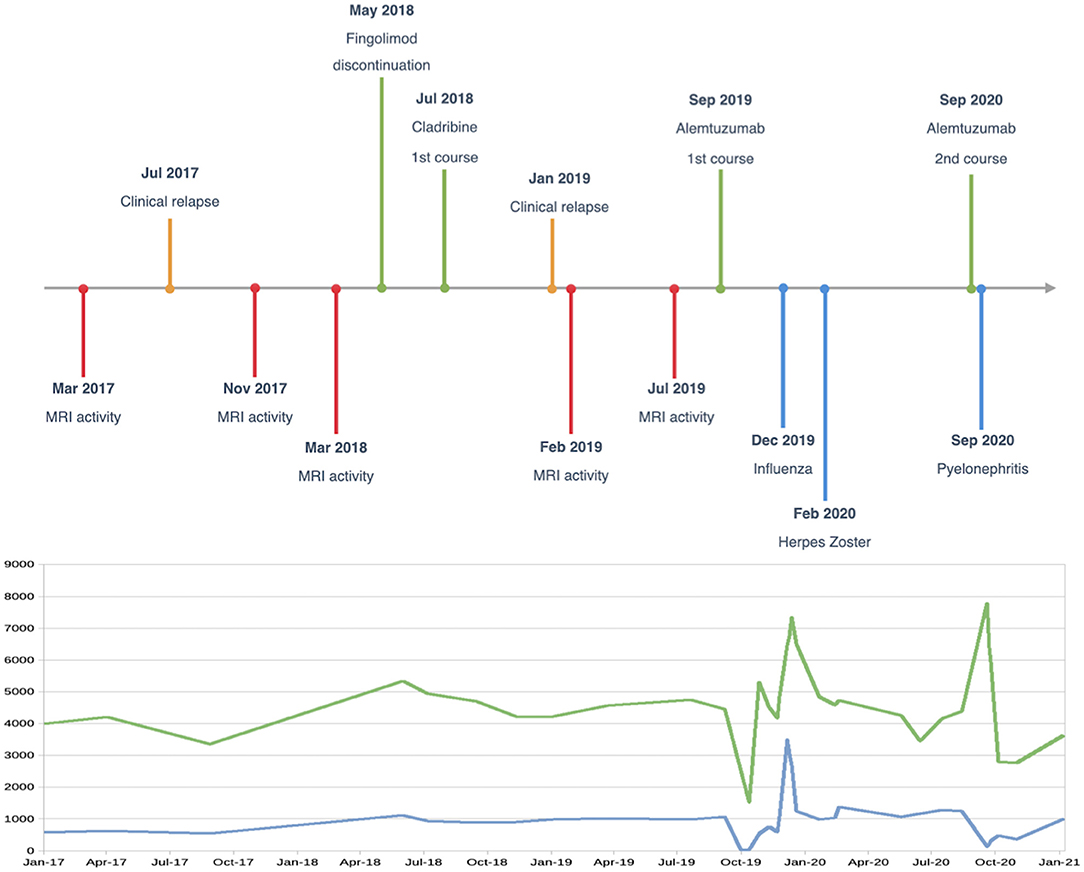
Figure 2. Case timeline, and white blood cell (green line) and lymphocyte (blue line) changes over time (cells/μl).
Written informed consent was obtained from the patient for the use of clinical data and imaging studies.
In the current report, we describe the management of ongoing disease activity in the 1st year after cladribine initiation in a patient previously treated with fingolimod. Although cladribine has been proved to be effective in highly active multiple sclerosis (6, 7), it might not be sufficient to control inflammatory activity after fingolimod withdrawal, as recently reported in other cases (8–10). Some indirect comparisons suggest that cladribine and fingolimod have similar efficacy (7, 11). However, it has been postulated that lymphocytes entrapped in the lymph nodes due to fingolimod action could evade depletion provoked by subsequent immune reconstitution therapies (9). In our case, cladribine was initiated only after ALC recovery.
Management of considerable disease activity that appears early after the administration of an immune reconstitution therapy course is challenging without strong evidence to guide clinical decisions. Regarding alemtuzumab, some cases of severe reactivation after the first treatment course have been described with different management strategies, including continuation of scheduled therapy (12) or administration of a B-cell depleting agent, such as rituximab (13, 14) or ocrelizumab (15). Both these strategies have been proven to be effective and safe. In more aggressive cases, autologous stem cell transplantation could be an option as well (16). Regarding cladribine, very scarce data are available in the literature, and switching to another highly effective therapy, as in our case, is thought to be a reasonable option (17). In the CLARITY trial, rescue therapy with interferon beta-1a could be applied for patients with highly active disease (1), and 2.5% of the patients in the cladribine 3.5 mg/kg group received this treatment (18). In the reported case, interferon therapy was not considered as the patient had already received it in the past, without successful disease activity control.
Treatment with natalizumab, fingolimod, rituximab, ocrelizumab, and autologous stem cell transplantation has also been reported in the 1st year after cladribine initiation, but without outcome details (5, 8, 9).
Both alemtuzumab and cladribine cause lymphocyte depletion. The extent of B cell reduction is quite similar among the two treatments, but with a slower repopulation rate under cladribine administration (19). Alemtuzumab provokes a more rapid lymphocyte depletion, has a broader degree of action, and causes a more profound and durable reduction of CD4+ and CD8+ T cells compared to cladribine (19). Unfortunately, since lymphocyte subset monitoring is not routinely required in clinical practice, we measured them only at a few time points. This makes these measurements of scarce interest, as no trend after treatments or correlation with disease activity or infectious complications could be identified. However, given the previously mentioned pharmacodynamics of these treatments along with other multiple sclerosis treatments, ALC may be of limited utility, and immunophenotyping may be helpful in guiding treatment decisions in the future.
Regarding efficacy, no head-to-head comparisons exist between cladribine and alemtuzumab, and the results from a network meta-analysis did not reveal any differences in the outcome measures (6). Longer follow-up will be required to assess the long-term efficacy of alemtuzumab in the reported case. However, breakthrough disease activity observed after cladribine initiation was rapidly and effectively controlled with the new subsequent immune reconstitution treatment. With this approach, there may be an augmented risk of side effects due to the additional action on the immune system. In trials of alemtuzumab, the more frequently observed infections included upper airway infections, influenza, herpetic virus infections, and urinary tract infections, as observed in our present case (20). Other opportunistic infections have been observed mostly within months after treatment initiation (21). In addition, an increased risk of herpes zoster infection has been reported in association with cladribine (18). Along with the infections reported during alemtuzumab treatment, an additional risk caused by previous cladribine exposure should also be considered in our patient. We waited for ALC normalization before alemtuzumab administration, but ALC was anyway lower than the levels observed before cladribine initiation. However, the status of ALC before an alemtuzumab treatment course does not predict any subsequent infection risk (20). Depletion of CD8+ T cells has been suggested to be associated with an increased risk of viral infection after alemtuzumab treatment (22). Although cladribine has a small effect on naive and memory CD8+ T cell counts, recovery at week 48 was minimal for naïve CD8+ T-cells and did not occur for memory CD8+ T cells in clinical trials (23). However, this aspect could be negligible considering the more profound T cell depletion induced by alemtuzumab.
In conclusion, alemtuzumab proved to be effective at controlling severe disease activity that appeared early after cladribine administration. However, the observation of different infectious complications warrants caution and a discussion about pharmacological prophylaxis for intercurrent infections. A longer follow-up and the description of similar cases may be helpful in the assessment of the efficacy and safety of sequential immune reconstitution therapies.
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.
Ethical review and approval was not required for the study on human participants in accordance with the local legislation and institutional requirements. The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
RG: investigation and writing the original draft. DC: investigation, conceptualization, writing, reviewing, and editing. SL: conceptualization, writing, reviewing, and editing. GG: supervision, writing, reviewing, and editing, as well as resources. DB: visualization. MV: supervision, writing, reviewing, and editing. All authors contributed to the manuscript and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
We would like to thank Editage (www.editage.com) for English language editing.
1. Giovannoni G, Comi G, Cook S, Rammohan K, Rieckmann P, Sørensen P, et al. A placebo-controlled trial of oral cladribine for relapsing multiple sclerosis. N Engl J Med. (2010) 362:416–26. doi: 10.1056/NEJMoa0902533
2. Cohen J, Coles A, Arnold D, Confavreux C, Fox E, Hartung H, et al. Alemtuzumab vs. interferon beta 1a as first-line treatment for patients with relapsing-remitting multiple sclerosis: a randomised controlled phase 3 trial. Lancet. (2012) 380:1819–28. doi: 10.1016/S0140-6736(12)61769-3
3. Coles A, Twyman C, Arnold D, Cohen J, Confavreux C, Fox E, et al. Alemtuzumab for patients with relapsing multiple sclerosis after disease-modifying therapy: a randomised controlled phase 3 trial. Lancet. (2012) 380:1829–39. doi: 10.1016/S0140-6736(12)61768-1
4. Yamout B, Sahraian M, Bohlega S, Al-Jumah M, Goueider R, Dahdaleh M, et al. Consensus recommendations for the diagnosis and treatment of multiple sclerosis: 2019 revisions to the MENACTRIMS guidelines. Mult Scler Relat Disord. (2020) 37:101459. doi: 10.1016/j.msard.2019.101459
5. Lizak N, Hodgkinson S, Butler E, Lechner-Scott J, Slee M, McCombe P, et al. Real-world effectiveness of cladribine for Australian patients with multiple sclerosis: an MSBase registry substudy. Mult Scler. (2020) 27:465–74. doi: 10.1177/1352458520921087
6. Siddiqui M, Khurana I, Budhia S, Hettle R, Harty G, Wong S. Systematic literature review and network meta-analysis of cladribine tablets versus alternative disease-modifying treatments for relapsing–remitting multiple sclerosis. Curr Med Res Opin. (2017) 34:1361–71. doi: 10.1080/03007995.2017.1407303
7. Signori A, Saccà F, Lanzillo R, Maniscalco G, Signoriello E, Repice A, et al. Cladribine vs. other drugs in MS. Neurol Neuroimmunol. (2020) 7:e878. doi: 10.1212/NXI.0000000000000878
8. Cellerino M, Bonavita S, Ferrero M, Inglese M, Boffa G. Severe disease activity in MS patients treated with cladribine after fingolimod withdrawal. J Neurol Sci. (2020) 418:117156. doi: 10.1016/j.jns.2020.117156
9. Radlberger R, Sakic I, Moser T, Pilz G, Harrer A, Wipfler P. Immune phenotyping study revealing caveats regarding a switch from fingolimod to cladribine. Mult Scler Relat Disord. (2021) 48:102727. doi: 10.1016/j.msard.2020.102727
10. Coss-Rovirosa F, Salado-Burbano J, Casallas-Vanegas A, Caire-Herrera L, Gómez-Figueroa E, Flores-Rivera J. Severe fingolimod rebound syndrome after switching to cladribine treatment. Mult Scler Relat Disord. (2020) 40:101938. doi: 10.1016/j.msard.2020.101938
11. Bartosik-Psujek H, Kaczyński Ł, Górecka M, Rolka M, Wójcik R, Zieba P, et al. Cladribine tablets vs. other disease-modifying oral drugs in achieving no evidence of disease activity (NEDA) in multiple sclerosis–a systematic review and network meta-analysis. Mult Scler Relat Disord. (2021) 49:102769. doi: 10.1016/j.msard.2021.102769
12. Schwenkenbecher P, Deppe J, Hümmert M, Jacobs R, Bronzlik P, Stangel M, et al. Management of MS-relapse during alemtuzumab therapy: is it really B-cell-mediated? Mult Scler Relat Disord. (2018) 19:6–7. doi: 10.1016/j.msard.2017.10.014
13. Haghikia A, Dendrou C, Schneider R, Grüter T, Postert T, Matzke M, et al. Severe B-cell-mediated CNS disease secondary to alemtuzumab therapy. Lancet Neurol. (2017) 16:104–6. doi: 10.1016/S1474-4422(16)30382-9
14. Wehrum T, Beume L, Stich O, Mader I, Mäurer M, Czaplinski A, et al. Activation of disease during therapy with alemtuzumab in 3 patients with multiple sclerosis. Neurology. (2018) 90:e601–5. doi: 10.1212/WNL.0000000000004950
15. Vališ M, Ryška P, Halúsková S, Klímová B, Pavelek Z. Highly active RRMS and ocrelizumab after failure of alemtuzumab therapy. BMC Neurol. (2020) 20:202. doi: 10.1186/s12883-020-01789-y
16. Boffa G, Sbragia E, Raiola A, Varaldo R, Capello E, Gallo P, et al. Autologous hematopoietic stem cell transplantation following alemtuzumab therapy in aggressive multiple sclerosis: a report of three cases. Mult Scler. (2020) 2020:135245852091481. doi: 10.1177/1352458520914818
17. Meuth SG, Bayas A, Kallmann B, Kleinschnitz C, Linker R, Rieckmann P, et al. Long-term management of multiple sclerosis patients treated with cladribine tablets: an expert opinion. Expert Opin Pharmacother. (2020) 21:1965–9. doi: 10.1080/14656566.2020.1792885
18. Giovannoni G. Cladribine to treat relapsing forms of multiple sclerosis. Neurother J Am Soc Exp Neurother. (2017) 14:874–87. doi: 10.1007/s13311-017-0573-4
19. Baker D, Herrod S, Alvarez-Gonzalez C, Zalewski L, Albor C, Schmierer K. Both cladribine and alemtuzumab may effect MS via B-cell depletion. Neurol Neuroimmunol. (2017) 4:e360. doi: 10.1212/NXI.0000000000000360
20. Wray S, Havrdova E, Snydman D, Arnold D, Cohen J, Coles A, et al. Infection risk with alemtuzumab decreases over time: pooled analysis of 6-year data from the CAMMS223, CARE-MS I, and CARE-MS II studies and the CAMMS03409 extension study. Mult Scler. (2018) 25:1605–17. doi: 10.1177/1352458518796675
21. Hartung H, Mares J, Barnett M. Alemtuzumab: rare serious adverse events of a high-efficacy drug. Mult Scler. (2020) 26:737–40. doi: 10.1177/1352458520913277
22. Baker D, Herrod S, Alvarez-Gonzalez C, Giovannoni G, Schmierer K. Interpreting lymphocyte reconstitution data from the pivotal phase trials of alemtuzumab. J Am Med Assoc Neurol. (2017) 74:961. doi: 10.1001/jamaneurol.2017.0676
Keywords: multiple sclerosis, cladribine, alemtuzumab, immune reconstitution therapy, case report
Citation: Garbo R, Cutuli D, Lorenzut S, Gigli GL, Bagatto D and Valente M (2021) Opportunities and Obstacles Associated With Sequential Immune Reconstitution Therapy for Multiple Sclerosis: A Case Report. Front. Neurol. 12:664596. doi: 10.3389/fneur.2021.664596
Received: 05 February 2021; Accepted: 08 November 2021;
Published: 08 December 2021.
Edited by:
Mahsa Ghajarzadeh, Universal Scientific Education and Research Network, IranReviewed by:
Hans-Peter Hartung, Heinrich Heine University of Düsseldorf, GermanyCopyright © 2021 Garbo, Cutuli, Lorenzut, Gigli, Bagatto and Valente. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Riccardo Garbo, cmljY2FyZG8uZ2FyYm9Ab3V0bG9vay5pdA==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.