- 1Stroke Unit, Neurology Unit, Department of Neuroscience, Ospedale Civile Baggiovara, Azienda Ospedaliera Universitaria di Modena, Modena, Italy
- 2Laboratory of Clinical Pathology and Toxicology, Department of Laboratory Medicine, Ospedale Civile Baggiovara, Azienda Ospedaliera Universitaria di Modena, Modena, Italy
- 3Istituto di Ricovero e Cura a Carattere Scientifico (IRCCS) Istituto delle Scienze Neurologiche di Bologna, Department of Neurology and Stroke Center, Maggiore Hospital, Bologna, Italy
- 4Department of Biomedical, Metabolic and Neural Sciences, University of Modena and Reggio Emilia, Modena, Italy
Background: Platelet function testing is a valid tool to investigate the clinical response to antiplatelet therapy in different clinical settings; in particular, it might supply helpful information in patients with cerebrovascular disease. Oral antiplatelet treatment, such as Aspirin (ASA) and Clopidogrel, is the gold standard in secondary stroke prevention of non-cardiogenic ischemic stroke; conversely, its application as a primary prevention therapy is not routinely recommended in patients with vascular risk factors. Multiple electrode platelet aggregometry (MEA) impedance aggregometer is a validated device to test platelet inhibition induced by ASA or Clopidogrel.
Case Report: We report the case of a 78-year-old patient without relevant clinical history, taking ASA as primary prevention strategy, who was admitted for sudden onset of dysarthria and left facial hyposthenia during physical effort. Brain CT revealed two small subcortical bilateral spontaneous intracranial hemorrhages. Platelet aggregometry with MEA performed upon admission revealed a very strong platelet inhibition induced by ASA (result of the ASPI Test was 5 U, consistent with an ultra-responsiveness to ASA, and the cutoff value of correct responsiveness is <40 U). MRI at longitudinal follow-up revealed the presence of two small cavernous angioma underlying hemorrhagic spots.
Conclusion: The evaluation of platelet reactivity in stroke patients undergoing antiplatelet therapies, not commonly performed in clinical practice, could be useful to optimize prevention strategies; the verification of the biological effectiveness of ASA or Clopidogrel could be a valid tool in the definition of each patient's risk profile, particularly in patients with cerebrovascular disease known to be at increased risk for both hemorrhagic and thrombotic complications.
Background
International leading guidelines strongly recommended antiplatelet therapy in secondary prevention of non-cardiogenic strokes, as it is associated with an estimated reduction of relative risk of stroke or death on average by about 22% (1, 2). Conversely, the use of pharmacological strategy for primary cardiovascular prophylaxis, including stroke prevention, is still a debated topic (3). It is mandatory to improve the control of modifiable risk factors, such as hypertension and diabetes, but antiplatelet agents have no clear indications (3). Recently, the ACC/AHA guideline suggests to address primary prevention with low-dose Aspirin daily treatment to selected patients between 40 and 79 years of age, who are at higher risk for ischemic vascular event, but not at increased bleeding risk (4).
Therefore, the use of Aspirin might be reasonable only for people whose 10-year vascular risk is notable (at least higher than 10%) for the benefits to outweigh the risks associated with treatment. In particular, the association of diabetes mellitus with other high-risk conditions has been considered for primary prevention strategies (1, 3, 4).
Platelet function testing is a valid tool to investigate the clinical response to antiplatelet therapy in different clinical settings; several clinical and biological mechanisms for antiplatelet “resistance” or, conversely, “ultra-responsiveness” have been supposed (incongruent dose, poor compliance, genetic polymorphisms, baseline hyperactivity, and/or accelerated platelet turnover) (5–7). Thus, the possibility of testing the biological effectiveness of antiplatelet medications in vascular patients could be potentially useful for promptly detecting any relevant clinical problems, including safety in ultra-responder patients (8). However, the implementation of platelet function testing in routine clinical practice is not widely supported, mainly due to a lack of consensus on the effective improvement of clinical outcome with tailored therapy; other studies conversely debated the usefulness of platelet function monitoring, particularly in terms of reliability of results between different tests available (9, 10).
Within impedance aggregometers, the device “Multiple Electrode Platelet Aggregometry” (MEA, Multiplate Analyzer®, Roche Diagnostics International Ltd., CH-6343 Rotkreuz, Switzerland) (11, 12) showed correlation with the estimates of the antiplatelet effect of Clopidogrel and ASA obtained by other methods (13). Platelet aggregometry is a function test based on the stimulation of platelet–platelet aggregation with various agonists [adenosine diphosphate (ADP), arachidonic acid (ASPI), and thrombin receptor-activating peptide (TRAP)] and can be used to monitor the effects of antiplatelet agents, classified into three groups regarding their mechanism of action (thromboxane inhibitors—Aspirin, ASA, ADP receptor antagonists—Clopidogrel, and glycoprotein IIb/IIIa inhibitors). A comprehensive overview of platelet activation pathways is summarized in Figure 1A. According to the principles of impedance aggregometry, Multiplate Analyzer® assessed residual platelet function in whole blood of patients undergoing antiplatelet therapy; every test is performed in a single-use test cell, which incorporates two independent impedance metal sensors. After the addition of specific agonists (ADP, ASPI, and TRAP), the platelet–platelet aggregation is induced and real-time recording starts. The ADP Test reagent contains ADP, which triggers platelet activation via different ADP receptors, the most important of which is blocked by Clopidogrel (14). The ASPI Test reagent contains arachidonic acid, whose activation pathway is blocked by ASA (15); TRAP aggregation test is used to obtain a platelet aggregation measure relatively independent of others, supporting the proper sample preparation. Once activation of platelet aggregation starts on metal sensors, the electrical resistance increases; the resistance change is transformed to arbitrary aggregation units (AUs) and plotted against time. The area under the aggregation curve (AUC) quantifies the aggregation response, expressed in units (U; 1 U corresponds to 10 AU*min) (Figure 1B). Cutoff value of the ASPI Test indicating correct responsiveness to ASA is <40 U (16), while values under 30 U indicate strong enzymatic inhibition and higher risk of bleeding (17).
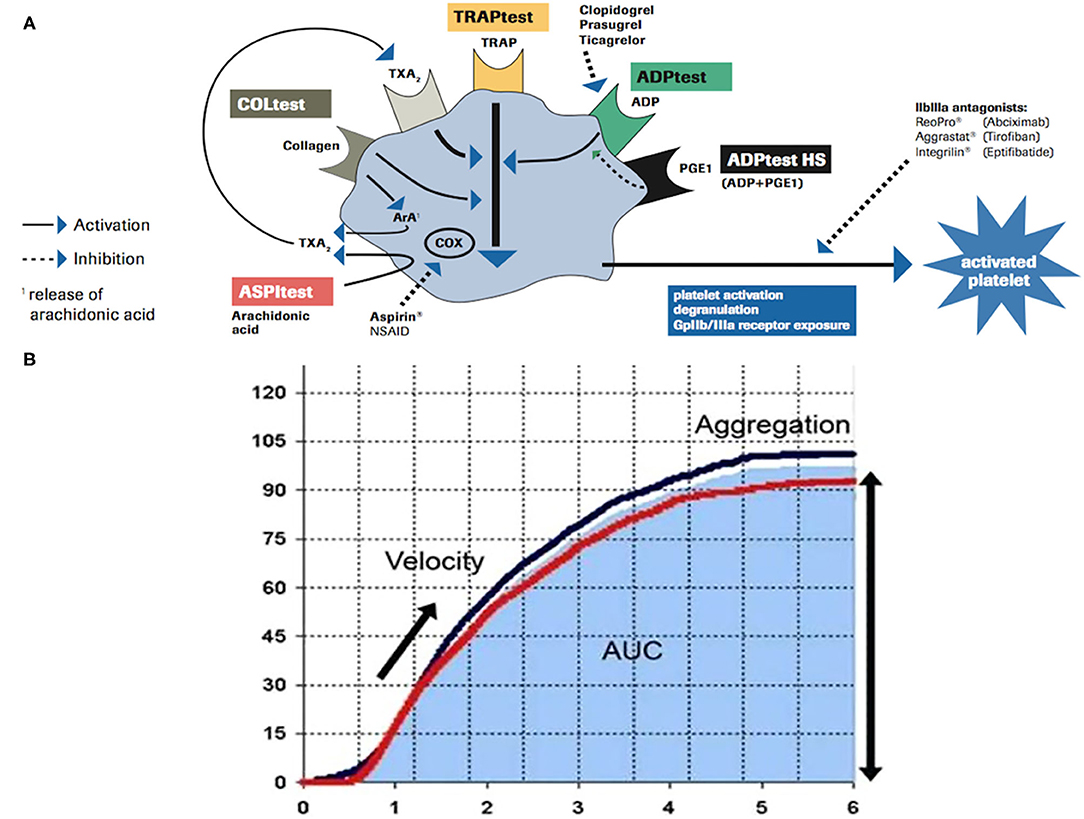
Figure 1. (A) Schematic overview of platelet activation/inhibition pathways and impedance aggregometry tests (ADP Test, ASPI Test, and TRAP Test). (B) Graphic presentation of platelet–platelet aggregation induced during each test; platelet responsiveness is quantified by the area under the curve (AUC*min). Modified from Roche Diagnostics International.
Case Presentation
A 78-year-old man without relevant clinical history was admitted in the Stroke Unit for sudden onset of slurred speech and left oral rhyme deviation during physical effort, without headache and/or limb weakness. Patient's past medical history reported bilateral neurosensory hypoacusis, previous cataract surgery, and carpal tunnel syndrome surgically treated. Pharmacological anamnesis revealed daily treatment with Aspirin 100 mg as a vascular primary prevention strategy, started 3 months before.
Neurological examination showed paralysis of right VII cranial nerve, right deviation of protruded tongue, and mild dysarthria (NIH stroke scale 2/42). Brain CT revealed multiple chronic lacunar infarctions of basal ganglia bilaterally, and two acute small intraparenchymal hemorrhages, within post-rolandic subcortical region on the right side (Figure 2A) and pre-rolandic subcortical region on the left side (Figure 2B); CT angiography showed mild carotid and vertebral atherosclerosis, and no vascular malformation (Figure 2C).
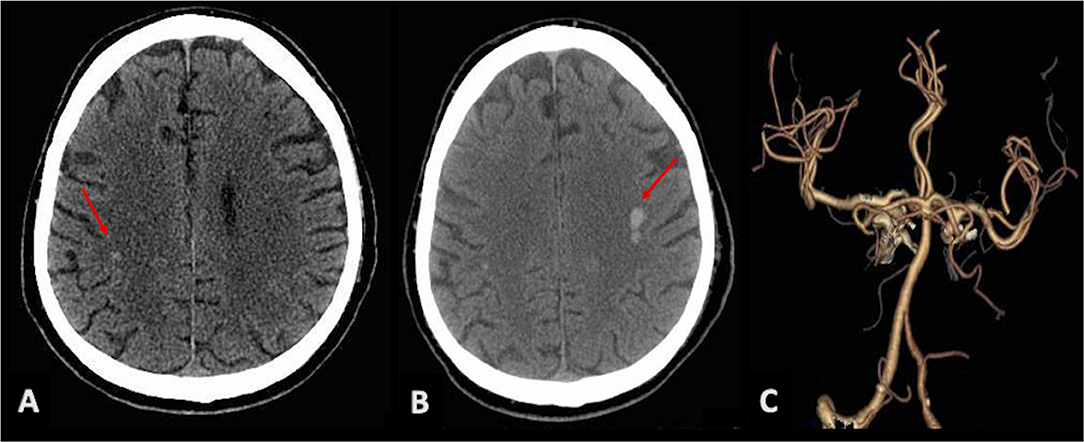
Figure 2. Brain CT scans showing bilateral intraparenchymal hyperdense lesions (red arrows): a small hemorrhagic spot in the post-rolandic area on the right (A) and a greater hematoma in the left pre-rolandic area (B). (C) CTA with no evidence of vascular malformations.
Aspirin therapy was immediately discontinued. Intensive monitoring in the Stroke Unit and cardiac ultrasound revealed an unknown arterial hypertension, with a chronic hypertensive cardiopathy. Target therapy with ACE inhibitors (Enalapril 20 mg once daily) was started, with blood pressure normalization. Multiplate® platelet function analysis performed upon admission revealed a very strong platelet inhibition induced by ASA; the area under the aggregation curve (AUC) on the ASPI Test was 5 U, consistent with an ultra-responsiveness to ASA, with normal platelet aggregation induced by other agonists on the ADP Test and TRAP Test (Figure 3).
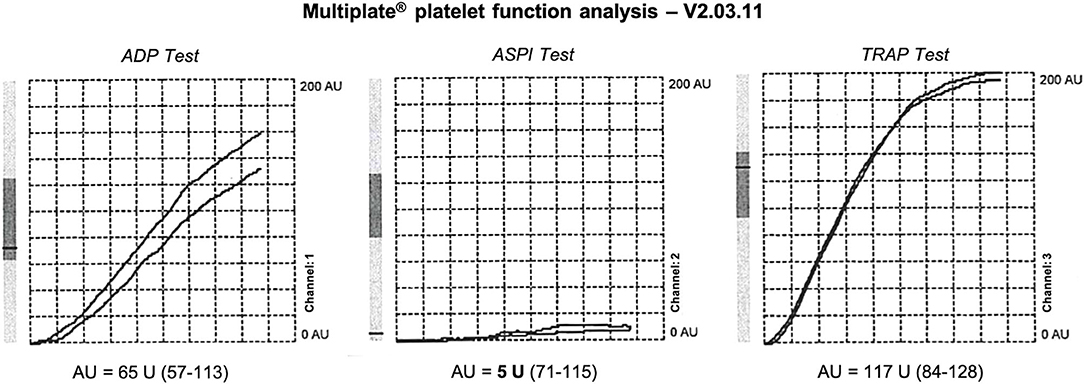
Figure 3. Platelet function testing performed upon admission during Aspirin therapy. Marked reduction of AUC on ASPI Test, 5 U, expressing a strong platelet inhibition induced by ASA (ultra-responder patient). Expected values in healthy individuals are in brackets.
Neurological examination of patients at discharge was completely normalized. Due to the “atypical” locations of intraparenchymal hematomas, we performed a brain MRI at longitudinal follow-up in order to exclude non-hypertensive causes of bleeding. Gradient-echo T2*-weighted sequences revealed two small roundish lesions, in the anatomical site of bilateral subcortical hematomas, with minute central nucleus of methemoglobin and dark hemosiderin rim, and without surrounding edema, consistent with cavernous venous malformations (Figure 4A). Multiple similar but smaller cavernous angiomas were detected throughout subcortical white matter on both sides, particularly in temporal and occipital lobes, and in the area of basal ganglia (Figures 4B,C). On differential diagnosis, T2 and FLAIR sequences excluded findings suggestive of other conditions, as possible cerebral amyloid angiopathy; no evidence of significant subcortical leukoencephalopathy was detected besides lacunar microinfarcts in the region of basal ganglia bilaterally, and no signs consistent with superficial siderosis were detected.
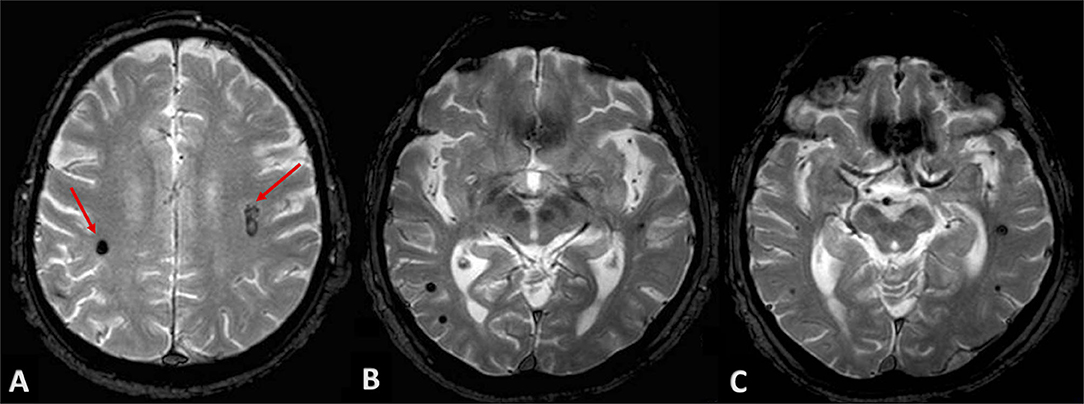
Figure 4. Patient's MRI at follow-up. Gradient-echo T2*-weighted sequences revealed roundish lesions underlying well-known intraparenchymal hemorrhages (A, red arrows), with classic magnetic resonance appearance of cavernous venous malformations. (B,C) show multiple and similar lesions throughout subcortical white matter and basal ganglia, bilaterally.
Discussion
We presented a case of a previous healthy patient, admitted for intracerebral atypical hemorrhages, taking no medications except ASA in primary prevention. Diagnostic workup revealed a condition of unrecognized arterial hypertension, and the presence of multiple intracerebral cavernous venous malformations, some of which with acute bleeding. Symptomatic hemorrhagic complication occurs as a clinical manifestation of cavernous angioma in 25% of cases (18), but the annual average rate of bleeding is reported to be lower in patients without history of prior hemorrhage (19). However, rupture rate rises in patients with associated condition at risk of bleeding, such as hypertension. Many studies suggest the likely safety of antiplatelet medications in patients with cerebral cavernous malformations (19), but outside of randomized controlled protocols (20).
The role of antiplatelet agents for the primary prevention of cardiovascular disease, including stroke, is still widely debated, due to the delicate balance between efficacy and safety in patients without established previous vascular events. Several randomized clinical trials showed that Aspirin is effective in the reduction of recurrence risk, with a tolerable increase of bleeding complications; thus, international practice guidelines strongly recommended ASA in secondary prevention of vascular diseases, as ischemic stroke or myocardial infarction (1). Regarding primary prevention, diverging results have contributed to unclear indications about antiplatelet therapy, which is not routinely recommended, primarily due to safety (4). Therefore, in clinical practice, ASA treatment should be tailored on each patient's risk profile (e.g., associations of diabetes mellitus and other high-risk conditions) and might be reasonable only in case of a notable 10-year risk of primary vascular events (3, 4).
The possibility to test the biological effectiveness of antiplatelet agents, with platelet function testing devices such as Multiplate Analyzer®, might supply helpful information to clinicians, primarily to assess the responsiveness to ASA or Clopidogrel in ischemic stroke patients. Nevertheless, it might be a valid tool in the stratification of patient's risk profile as well, while considering the safety of a primary prevention regimen, particularly in the presence of clinical conditions associated with an increased risk of hemorrhagic complications.
However, longitudinal studies are needed to assess whether aggregometry might supply individualized information and whether it can be considered a valid tool in the development of tailored therapies, as the main limitation of its implementation in everyday clinical practice.
Conclusion
Our report illustrates the potential clinical benefit of platelet function testing in patients undergoing antiplatelet therapy, with particularly useful application in the definition of patient's risk profile in case of primary prevention treatment with Aspirin. However, RCTs and longitudinal studies are needed to assess whether routine platelet function monitoring might be considered a decision-making tool for clinicians, both in patients with vascular diseases subjected to secondary prevention therapy and during the evaluation of safety profile of antiplatelet treatment in selected patients deserving of pharmacological primary prevention therapy.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
Written informed consent was obtained from the individual for the publication of any potentially identifiable images or data included in this article.
Author Contributions
FR, GB, and AZ: manuscript drafting/revising, data acquisition, and final revision. NL: data acquisition. LV: manuscript revising and data acquisition. FN: manuscript revising. LC: manuscript revising and data acquisition. SM: manuscript drafting/revising and final revision. All authors approved the final version.
Conflict of Interest
AZ has received funding for speaker honoraria and consulting fees from Boehringer-Ingelheim and speaker honoraria from Cerenovus.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Goldstein LB, Bushnell CD, Adams RJ, Appel LJ, Braun LT, Chaturvedi S, et al. Guidelines for the primary prevention of stroke: a guideline for healthcare professionals from the american heart association/american stroke association. Stroke. (2011) 42:517–84. doi: 10.1161/STR.0b013e3181fcb238
2. Antithrombotic Trialists C. Collaborative meta-analysis of randomised trials of antiplatelet therapy for prevention of death, myocardial infarction, and stroke in high risk patients. BMJ. (2002) 324:71–86. doi: 10.1136/bmj.324.7329.71
3. Meschia JF, Bushnell C, Boden-Albala B, Braun LT, Bravata DM, Chaturvedi S, et al. Guidelines for the primary prevention of stroke: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. (2014) 45:3754–832. doi: 10.1161/STR.0000000000000046
4. Arnett DK, Blumenthal RS, Albert MA, Buroker AB, Goldberger ZD, Hahn EJ, et al. 2019 ACC/AHA guideline on the primary prevention of cardiovascular disease: a report of the American College of Cardiology/American Heart Association Task Force on clinical practice guidelines. Circulation. (2019) 140:e596–646. doi: 10.1016/j.jacc.2019.03.010
5. Sofi F, Marcucci R, Gori AM, Giusti B, Abbate R, Gensini GF. Clopidogrel non-responsiveness and risk of cardiovascular morbidity. An updated meta-analysis. Thromb Haemost. (2010) 103:841–8. doi: 10.1160/TH09-06-0418
6. Hankey GJ, Eikelboom JW. Aspirin resistance. Lancet. (2006) 367:606–17. doi: 10.1016/S0140-6736(06)68040-9
7. Wang TH, Bhatt DL, Topol EJ. Aspirin and clopidogrel resistance: an emerging clinical entity. Eur Heart J. (2006) 27:647–54. doi: 10.1093/eurheartj/ehi684
8. Rosafio F, Lelli N, Mimmi S, Vandelli L, Bigliardi G, Dell'Acqua ML, et al. Platelet function testing in patients with acute ischemic stroke: an observational study. J Stroke Cerebrovasc Dis. (2017) 26:1864–73. doi: 10.1016/j.jstrokecerebrovasdis.2017.04.023
9. Polzin A, Helten C, Dannenberg L, Mourikis P, Naguib D, Achilles A, et al. Platelet reactivity in patients on aspirin and clopidogrel therapy measured by a new bedside whole-blood assay. J Cardiovasc Pharmacol. (2019) 73:40–7. doi: 10.1097/FJC.0000000000000631
10. Helten C, Naguib D, Dannenberg L, Pöhl M, Ayhan A, Hohlfeld T, et al. Platelet function testing: dead or alive. J Thromb Haemost. (2018) 16:984–6. doi: 10.1111/jth.13997
11. Toth O, Calatzis A, Penz S, Losonczy H, Siess W. Multiple electrode aggregometry: a new device to measure platelet aggregation in whole blood. Thromb Haemost. (2006) 96:781–8. doi: 10.1160/TH06-05-0242
12. Mueller T, Dieplinger B, Poelz W, Calatzis A, Haltmayer M. Utility of whole blood impedance aggregometry for the assessment of clopidogrel action using the novel multiplate analyzer–comparison with two flow cytometric methods. Thromb Res. (2007) 121:249–58. doi: 10.1016/j.thromres.2007.03.022
13. Siller-Matula JM, Gouya G, Wolzt M, Jilma B. Cross validation of the multiple electrode aggregometry. A prospective trial in healthy volunteers. Thromb Haemost. (2009) 102:397–403. doi: 10.1160/TH08-10-0669
14. ADPtest Package Insert (06673686001V2). Roche Diagnostics GmbH. Available online at: https:/diagnostics.roche.com/global/en/productsinstruments/multiplate-6-analyzer.html#productInfo
15. ASPItest Package Insert (06673821001V2). Roche Diagnostics GmbH. Available online at: https:/diagnostics.roche.com/global/en/productsinstruments/multiplate-6-analyzer.html#productInfo
16. Al-Azzam SI, Alzoubi KH, Khabour O, Alowidi A, Tawalbeh D. The prevalence and factors associated with aspirin resistance in patients premedicated with aspirin. Acta Cardiol. (2012) 67:445–8. doi: 10.1080/AC.67.4.2170686
17. von Pape KW, Dzijan-Horn M, Bohner J, Spannagl M, Weisser H, Calatzis A. Control of aspirin effect in chronic cardiovascular patients using two whole blood platelet function assays. Pfa-100 and multiplate. Hamostaseologie. (2007) 27:155–60; quiz: 161–2. doi: 10.1055/s-0037-1616905
18. Akers A, Al-Shahi Salman R, Awad IA, Dahlem K, Flemming K, Hart B, et al. Synopsis of guidelines for the clinical management of cerebral cavernous malformations: consensus recommendations based on systematic literature review by the angioma alliance scientific advisory board clinical experts panel. Neurosurgery. (2017) 80:665–80. doi: 10.1093/neuros/nyx091
19. Zyck S, Gould GC. Cavernous venous malformation. Treasure Island, FL: StatPearls Publishing (2020).
Keywords: case report, aggregometry, antiplatelet therapy, primary prevention, intracerebral hemorrhage
Citation: Rosafio F, Bigliardi G, Lelli N, Vandelli L, Naldi F, Ciolli L, Meletti S and Zini A (2021) Why Me? To Be an Ultra-Responder to Antiplatelet Therapy: A Case Report. Front. Neurol. 12:663308. doi: 10.3389/fneur.2021.663308
Received: 02 February 2021; Accepted: 28 June 2021;
Published: 10 August 2021.
Edited by:
Laszlo Szapary, University of Pécs, HungaryReviewed by:
Lina Palaiodimou, University General Hospital Attikon, GreeceLisa Kristina Dannenberg, University Hospital of Düsseldorf, Germany
Copyright © 2021 Rosafio, Bigliardi, Lelli, Vandelli, Naldi, Ciolli, Meletti and Zini. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Andrea Zini, YW5kcmVhLnppbmlAbWUuY29t
 Francesca Rosafio1
Francesca Rosafio1 Andrea Zini
Andrea Zini