
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Neurol. , 06 May 2021
Sec. Neurotrauma
Volume 12 - 2021 | https://doi.org/10.3389/fneur.2021.660885
Background: Chronic subdural hematomas (cSDH) are increasingly prevalent worldwide with the increased aging population and anticoagulant use. Different surgical, medical, and endovascular treatments have had varying success rates. Primary neurosurgical interventions include burr hole drainage of the cSDH and mini-craniotomies/craniotomies with or without fenestration of the inner membrane. A key assessment of the success or failure of cSDH treatments has been symptomatic recurrence rates which have historically ranged from 5 to 30%. Pre-operative prediction of the inner subdural membrane by CT scan was used to guide our decision to perform mini-craniotomies. Release of the inner membrane facilitates the expansion of the brain and likely improves glymphatic flow.
Methods: Consecutive mini-craniotomies (N = 34) for cSDH evacuation performed by a single neurosurgeon at a quaternary academic medical center/Level I trauma center from July 2018-September 2020 were retrospectively reviewed. Patient characteristics [age, gender, presenting GCS, GOS, initial CTs noting the inner subdural membrane, midline shift (MLS), cSDH width, inner membrane fenestration, cSDH recurrence, post-operative seizures, infections, length of stay] were extracted from the EMR.
Results: Twenty nine patients had mini-craniotomies as primary treatment of the cSDH. Mean age = 68.9 ± 19.7 years (range 22–102), mean pre-operative GCS = 14.5 ± 1.1, mean MLS = 6.75 ± 4.2 mm, and mean maximum thickness of cSDH = 17.7 ± 6.0 mm. Twenty four were unilateral, five bilateral, 34 total craniotomies were performed. Thirty three had inner membrane signs on pre-operative head CTs and an inner subdural membrane was fenestrated in all cases except for the one craniotomy that didn't show these characteristic CT findings. Mean operating time = 79.5 ± 26.0 min. Radiographic and clinical improvement occurred in all patients. Mean improvement in MLS = 3.85 ± 2.69. There were no symptomatic recurrences, re-operations, surgical site infections, or deaths during the 6 months of follow-up. One patient was treated for post-operative seizures with AEDs for 6 months.
Conclusion: Pre-operative CT scans demonstrating inner subdural membranes may guide one to target the treatment to allow release of this tension band. Mini-craniotomy with careful fenestration of the inner membrane is very effective for this. Brain re-expansion and re-establishment of normal brain interstitial flow may be important in long term outcomes with cSDH and may be related to the recent interests in brain glymphatics and dural lymphatics.
Chronic subdural hematomas are becoming increasingly common worldwide as the population ages and are more frequently on anticoagulants. It has been estimated that the overall incidence is 1.7 to 20.6 per 100,000 population (1–3). This is a common neurosurgical problem and it has been estimated that it will likely be the most common cranial neurosurgical procedure by the year 2025 (4, 5). Additionally, there are long term sequelae of both operated and unoperated cSDH. In a long-term retrospective study (1990–2015) in Finland, the patients with cSDH had continuous excess mortality up to 20 years after the diagnosis (2). Additionally, the presence of the cSDH has been associated with neuronal degeneration and cerebral atrophy as measured on serial imaging studies. In addition to the local mass effect, this suggests the presence of inflammatory and neurotoxic sequelae of the cSDH with or without surgery (6, 7). There is a high incidence of dementia and other cognitive and emotional sequelae in patients with cSDH (8). Whether this is an expected finding in this age group or whether this is related to the cSDH has been debated (2).
Blood in the potential space between the arachnoid and the dura elicits a profound inflammatory response generating a highly vascular outer subdural membrane affixed to the dura and an inner collagenous membrane up against the brain pia arachnoid (Figures 1A–C). The outer membrane is the driving force for the growth of the cSDH, and it can be vascular and thick with abundant inflammatory cells (Figure 1B) (9). The inner membrane is thin and relatively avascular and acellular (Figure 1C). Detailed ultrastructural studies have demonstrated the collagen matrix and tight junctions between some of the cells in this membrane (10, 11). The blood break down products are encapsulated between the outer and inner membranes, and as the collection increases in size, the underlying brain parenchyma is compromised and symptoms may ensue depending on the location. This may include headaches, weakness, cognitive decline, and seizures. The outer subdural membrane is very active and has been shown to have high levels of VEGF, inflammatory factors, and autophagy signaling factors (12–14).
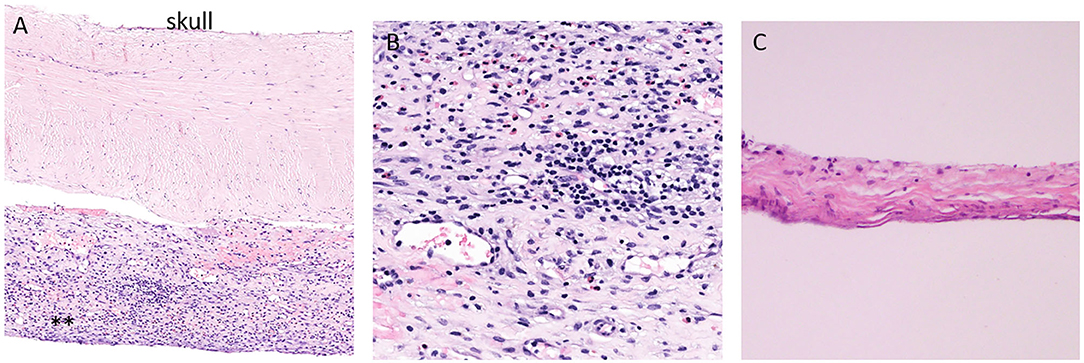
Figure 1. Histopathology of the dura and adjacent outer and inner subdural membrane. (A). H and E stain of the dura with adjacent outer membrane (**). (B). Higher magnification view of the outer subdural membrane demonstrating the high number of inflammatory cells. (C). H and E stain of the inner membrane. Note the thin and avascular and rather acellular appearance.
Treatment options for cSDH include surgery (15–17), observation and medications such as dexamethasone (12, 18–20) and endovascular obliteration of the meningeal artery (21–25). There have been numerous studies examining the different procedures. In recent years, the burr hole drainage of cSDH has been the most commonly used treatment modality. Advocates argue that the population with the cSDH are generally older patients with many medical co-morbidities and the minimization of the time under anesthesia has many advantages (26–28). However, it is difficult to visualize the septations, vessels, and the inner membrane. There have been several studies demonstrating that endoscopic assisted burr hole drainage of the cSDH aids in the visualization of formed or sequestered hematomas as well as septations and membranes (29).
Critics of craniotomies for cSDH argue that the operative time is higher and there is more morbidity and mortality in comparison to burr hole drainage (15, 30). Reports of craniotomies for cSDH frequently include both large and mini-craniotomies. The reports are mixed as to whether there is fenestration of the inner membrane (5). Manipulation of the inner membrane is done with care to prevent damage to the underlying pia-arachnoid and cortical vessels.
Release of the compressed pia-arachnoid is nicely accomplished via craniotomy, evacuation of the chronic subdural hematoma, and fenestration of the inner membrane. We present our experience with this technique in 29 patients (34 craniotomies because of bilateral representation in five) and highlight the nuances of the procedure that may restore and re-establish the brain glymphatic and dural lymphatic circulation. Pre-operative CT scans demonstrating the inner subdural membrane were particularly helpful in guiding the decision to proceed with the mini-craniotomy.
This was a retrospective study of patients who had undergone a mini-craniotomy with evacuation of the cSDH from June 2018 until September 2020 at a single institute. These were consecutive cases performed by the same attending neurosurgeon at a quaternary academic medical center and American College of Surgeons Verified Level I Trauma Center. IRB approval of the study and IRB waiver of consent was obtained because of the retrospective nature of the study. IRB review included ethical review of the study and there were no ethical issues identified. Selection of patients for the mini-craniotomy included the delineation of the inner subdural membrane on the pre-operative CT scan, radiographic findings suggesting break down products of mixed densities, mass effect and neurological symptoms. Additional patients done during this period were excluded from the analysis if they had had a prior cSDH drained at an outside facility or at our facility, thus treatment of persistent or recurrent cSDH were not included in this analysis largely because of the heterogenous nature of the cSDH after prior treatment(s). cSDH drained via burr or twist drill holes were not included in this study as the intent of this analysis was to focus on the inner membrane and the effect(s) of opening it. Also, the difficulty and safety associated with opening the inner subdural membrane is examined. Bilateral mini-craniotomies were included in this analysis; however, they were not included if there was burr hole drainage on one side and mini-craniotomy on the contralateral side or if the craniotomies were done at separate settings. Thus, the total number of mini-craniotomies in this evaluation was greater than the number of patients.
Each case was examined for the patient age, gender, initial GCS, CT and MRI (where available), appearance of cSDH, maximum width of the cSDH, midline shift before and after surgery, mechanism of injury, use of anti-coagulants, fenestration of the inner and outer cSDH membranes, CT resolution of the cSDH, any recurrence of cSDH, improvement if any in the midline shift, any re-operations for recurrence, final GOS, complications (seizures, surgical site infections, new neurological deficits, death within 6 months).
Particular attention was paid to the presence of the identification of the inner membrane on the pre-operative CT scan. This is generally defined as thin hyperdense lines that parallel the skull vault and cerebrum (31). Axial and coronal views were examined for this. Frequently if there is a good deal of mass effect of the cSDH, the limiting inner membrane creates a concave appearance for the cSDH.
The mini-craniotomy was planned to be centered over the epicenter of the cSDH at its point of maximum width. The incision was an L-shaped incision and the bone flap, oblong in shape, was 3–4 cm in maximum dimension. Very careful hemostasis was achieved of the epidural space and the dura to ensure that there was no run down of blood at the time of the closure. The dura was opened in a cruciate fashion taking care to note and preserve the outer membrane, which was opened sharply after the dural leaflets were tacked back (Figure 2A). Fenestration of the outer membrane was limited to that which was immediately beneath the dural opening. Edges of the outer membrane were coagulated. No effort was made to “chase” the outer membrane beneath the edges of the bone flap. The liquified cSDH and any subacute and partially formed blood clot was irrigated out with warm sterile normal saline. Any septations or intra-hematoma bridging vessels were coagulated and cut and dissected free. The inner translucent membrane that was adjacent to the underlying pia arachnoid was gently lifted up with the application of low suction and a microhook. The inner membrane was opened sharply with a #11 blade being particularly cautious to avoid any underlying vessels (Figure 2B). The edges of the inner membrane were lifted up and the multiple fenestrations were done in a radial fashion several centimeters away from the entry point using a small Metzenbaum scissors. The edges of the inner membrane were coagulated with bi-polar coagulation to ensure that there was no bleeding into the cavity. The cavity was carefully inspected and irrigated. Particular care was taken to avoid any disruption of the arachnoid membrane so that any of the toxic bi-products of the cSDH did not enter the subarachnoid space. Thus, this helped to mitigate propagation of the inflammatory process of the cSDH. Because the plane was very well-identified between the inner membrane and the arachnoid layer, this was readily accomplished in all cases. The dura was re-approximated in a non-water tight fashion. Gaps in the dura were covered with gel-foam. The bone flap was secured in the usual manner with plating systems. The subdural space was filled with warm normal saline prior to the closure of the skin with a specific effort to eliminate any air in the subdural space. A subgaleal medium hemovac drain was placed and tunneled out through the skin.
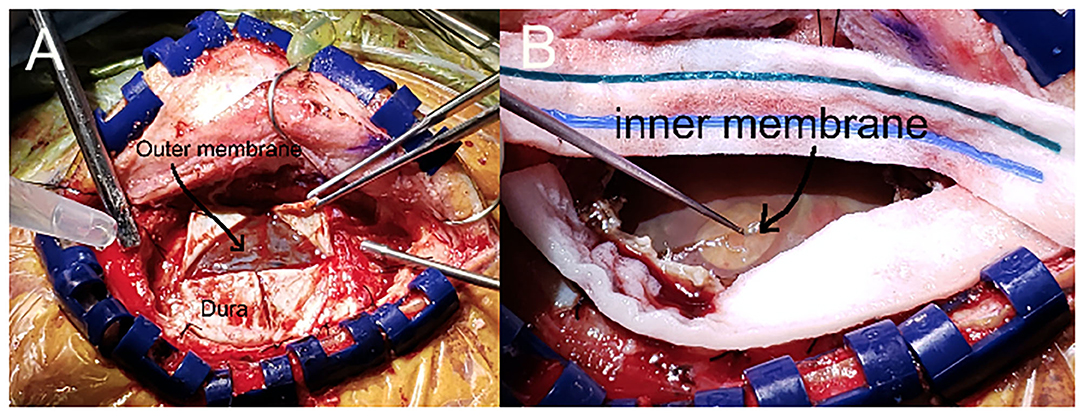
Figure 2. Intraoperative view of the mini-craniotomy demonstrating the inner and outer membrane. (A). Mini-craniotomy demonstrating the retraction of the dural leaflets and the intact outer membrane. (B). The outer membrane has been opened and the cSDH has been removed. Note the gentle lifting up of the opaque yet translucent inner membrane with the microhook that enables the fenestration of the inner membrane.
After the patient was extubated, a CT scan of the head was done when the patient transitioned from the recovery room to their ICU or floor bed. This occurred within 6 h of reaching the recovery room. The patient was nursed overnight with the head of bed at 0–10 degrees and given a 100% non-rebreather oxygen treatment for 6 h if there was any residual pneumocephalus or if the brain had not fully expanded. Physical and occupational therapy teams worked with the patients starting on post-operative day #1. The hemovac drain was maintained under self-suction until the drain output was <30 cc/24 h or at 3 days (whichever came first) at which point the drain was removed at the bedside taking care to avoid allowing air to track back into the subdural space. An absorbable purse-string suture was placed to close the exit site.
Patients that had residual air or blood products had follow up CT scans of the head 24 h later and 1–2 days later until either the residual blood and air were stable or the brain had re-expanded to a near normal state. Patients that did not have any residual subdural fluid/blood or any neurological issues did not have further CT scans of the head. Patients with residual subdural fluid/blood at the time of discharge had f/u CT scans at their 4–6 week routine clinic follow-up.
The patients were followed as part of routine care and were given outpatient clinic follow-ups at 1 month, 3 months and 6 months after discharge from the hospital. If the patient did not have full brain re-expansion or if there was any question of residual blood in the subdural space on the last in house CT scan, and if there was a need to restart anti-coagulation, a CT scan was done prior to the resumption of anticoagulants which was 2–3 weeks after the surgery.
The salient features of our 29 patients that underwent the 34 mini-craniotomies for cSDH are summarized in Tables 1–3. Five patients had bilateral mini-craniotomies. The M:F was 2.6:1. The mean age was 68.9 (S.D. = 19.7) with a range of 22–102 years. Mean initial GCS was 14.5 (S.D. = 1.1) and the mean GOS was 4.9 (S.D. = 0.44). The mean thickness of the cSDH was 17.7 mm (S.D. = 4.2) and the mean mid line shift was 6.75 mm (S.D. = 4.2).
Based on the pre-operative CT scans, nine of the collections were pure cSDH, 13 were considered subacute, and 12 had an acute component in addition to the cSDH (acute on chronic). Fifteen of the patients were on anticoagulants: three on Eliquis, two on coumadin, and 10 on antiplatelet therapy. Twenty-two of the patients had a clear history of TBI in the 1–3 months prior to their presentation to us. Three were spontaneous and 4 were of unknown etiology. Fourteen patients had the obliteration of the subdural space with re-expansion of the brain within 7 days (early resolution of the cSDH). Fifteen had resolution in a delayed fashion (>28 days).
Operative time for the procedure was measured from the time of the skin incision to the final closure. This was 79.5 min (S.D. = 26.11). Where a patient had bilateral craniotomies, the total operative time was divided by two. All of the patients except one had a well-identified inner membrane that was fenestrated at the time of surgery. This was the one patient that did not have a well-defined membrane on the pre-operative CT scan of the head.
The average length of stay after the evacuation of the cSDH was 4.6 days (S.D. = 3.8), which included one patient that remained in the hospital for 22 extra days due to social issues and the need to identify a socially safe place to live. When the analysis was performed without this patient, the average post-operative length of stay was 3.85 days (S.D. = 2.0).
There were no radiographic or symptomatic recurrence of cSDH that occurred during the follow-up period of 6 months. There were no in-hospital deaths. The mean GOS was 4.9 +/−0.44 at the time of discharge from the hospital. One patient was treated for seizures for 6 months (Table 4).
In this paper, we present our experience using the mini-craniotomy for the evacuation of cSDH. There have been several reports advocating for this technique primarily because of the improved visualization and the ability to address intracapsular septations and organized clot (16, 17, 32, 33). Figure 3 is a depiction of the relationship between the dura, the outer membrane and the hematoma and the inner membrane and the view that can be afforded via a mini-craniotomy. In Figure 4, we can see in the coronal view the encapsulation of the chronic breakdown products of blood as well as the compression upon the underlying cortex. The pia arachnoid is not in direct contact with the toxic blood products as the inner membrane acts as a barrier (12). The specific fenestration of the inner membranes was recently examined and found to result in statistically improved brain expansion when compared to the standard double burr hole technique (34). Recent studies have indicated that one of the most significant factors in the recurrence rate of cSDHs is the amount of pneumocephalus and the rate of expansion of the brain. Early expansion within 7 days was correlated with a decrease in the recurrence rate (35). The immediate mean MLS improvement in our patients was nearly 4 mm.
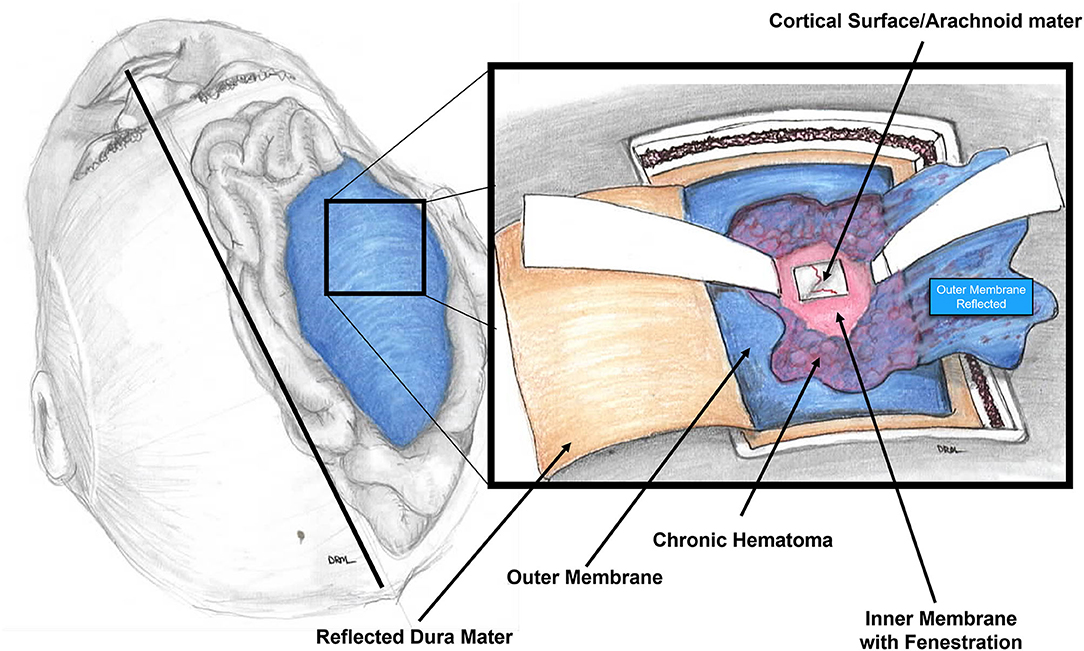
Figure 3. Diagram of the intraoperative view of the mini-craniotomy. The dura is retracted and the outer membrane is depicted with the underlying chronic subdural hematoma. A fenestration of the inner membrane is portrayed. The underlying pia-arachnoid membrane on gross examination is normal appearing.
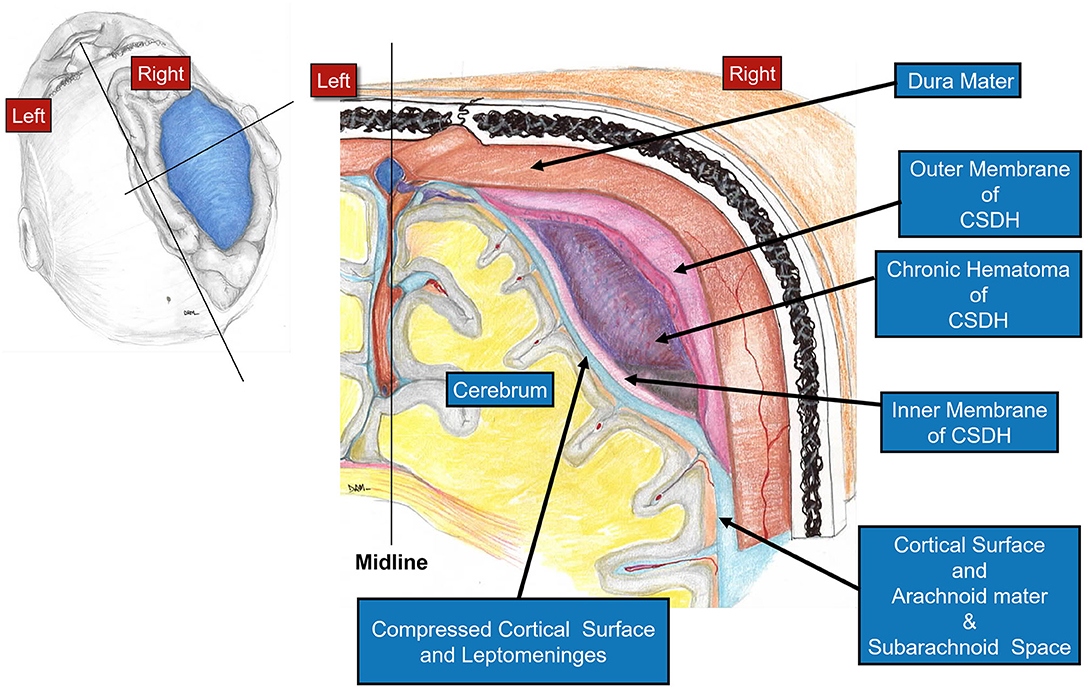
Figure 4. Diagram showing a coronal view through the cSDH. Note the cross-sectional appearance of the layers with the outer membrane adjacent to the dura and the thinner inner membrane pressing upon the pia-arachnoid. The compression upon the underlying cortex affects the flow of interstitial fluid through the brain and the glymphatic system.
Although our study is small and retrospective, we were able to achieve 0% symptomatic recurrence, and 0% mortality. Key to the procedure is the identification of the inner subdural membrane and the wide fenestration of the membrane. This inner membrane can be delineated on pre-operative CT scans and MRI scans (Figure 5). At the time of surgery, we found that the membrane was generally translucent yet xanthochromic in coloration and was pressed up against the pia arachnoid (Figure 2). The thin membrane is seen in Figure 1C which demonstrates its avascular, acellular, and largely collagenous nature. In all of our cases (except the one case where there no inner membrane identified), the inner membrane was not adherent to the cortex and a plane was readily developed between the inner membrane and the pia-arachnoid layer which was not violated. We specifically avoided opening the arachnoid to decrease the chance that the inflammatory breakdown products of the cSDH might aggravate the subarachnoid contents. However, the inner membrane, despite it being thin has tensile strength that is released with fenestration. This allows the brain to re-expand more readily, and this is particularly important in the aged brain which may have underlying atrophy (5, 34). The relationship of the inner membrane to the cortical surface is depicted in Figures 6, 7. No effort was made to remove the inner membrane or strip it off of the pia-arachnoid. Careful fenestration under direct vision (by looking under the bone flap using loupes and a headlight) was done to avoid damage to any vessels. The radial fenestration of the inner membrane allowed for the brain expansion to occur.
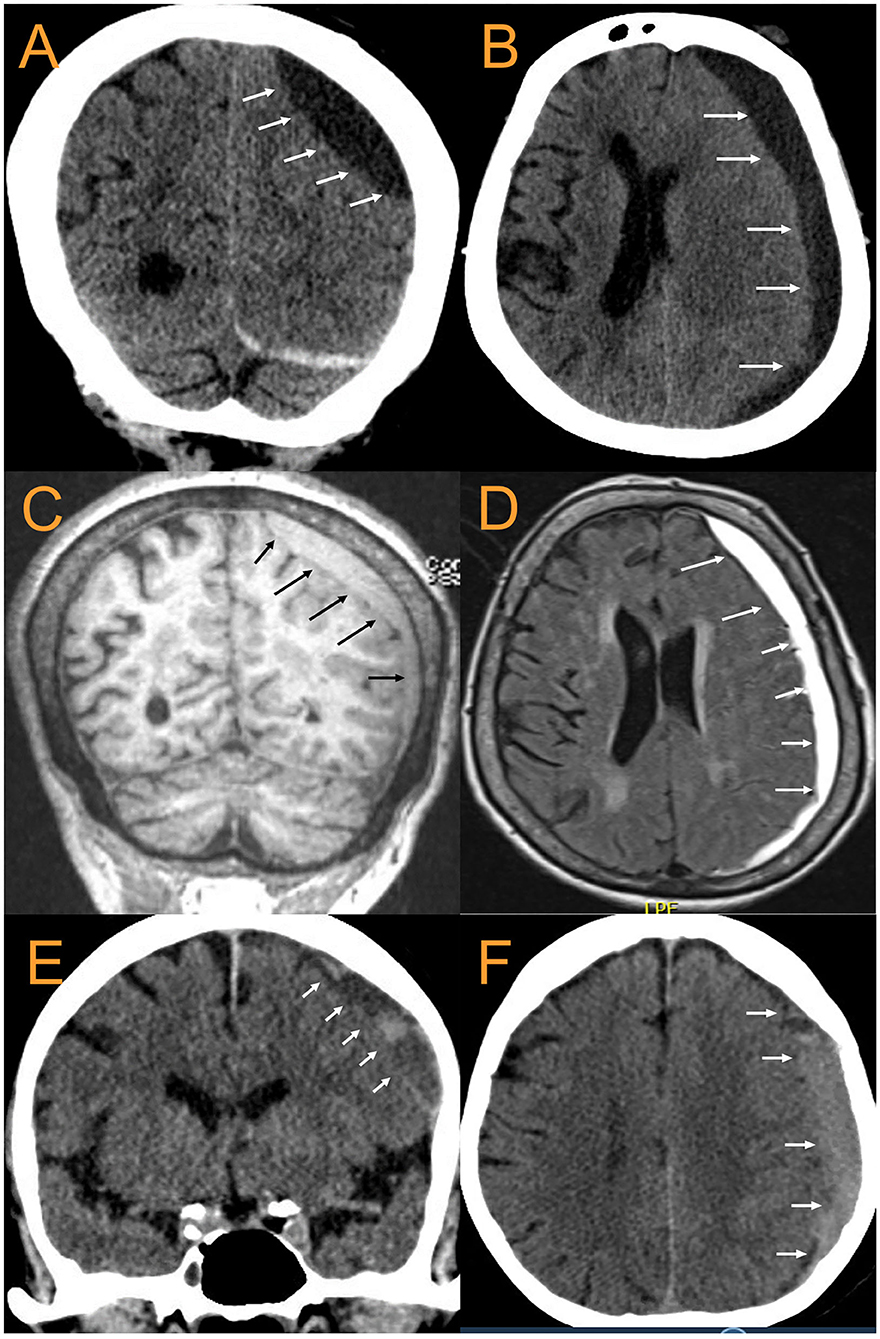
Figure 5. CT and MRI examples of cSDH. 88 yo F with largely chronic SDH with small acute component. (A). coronal CT, (B). Axial CT, (C). MPR coronal MRI, (D). T2-FLAIR Axial MRI. The arrows point to the inner subdural membrane which can be seen delineating a boundary between the cSDH and the pia -arachnoid. (E,F): 79 yoM with subacute cSDH. Once again, the arrows point out the location of the inner subdural membrane.
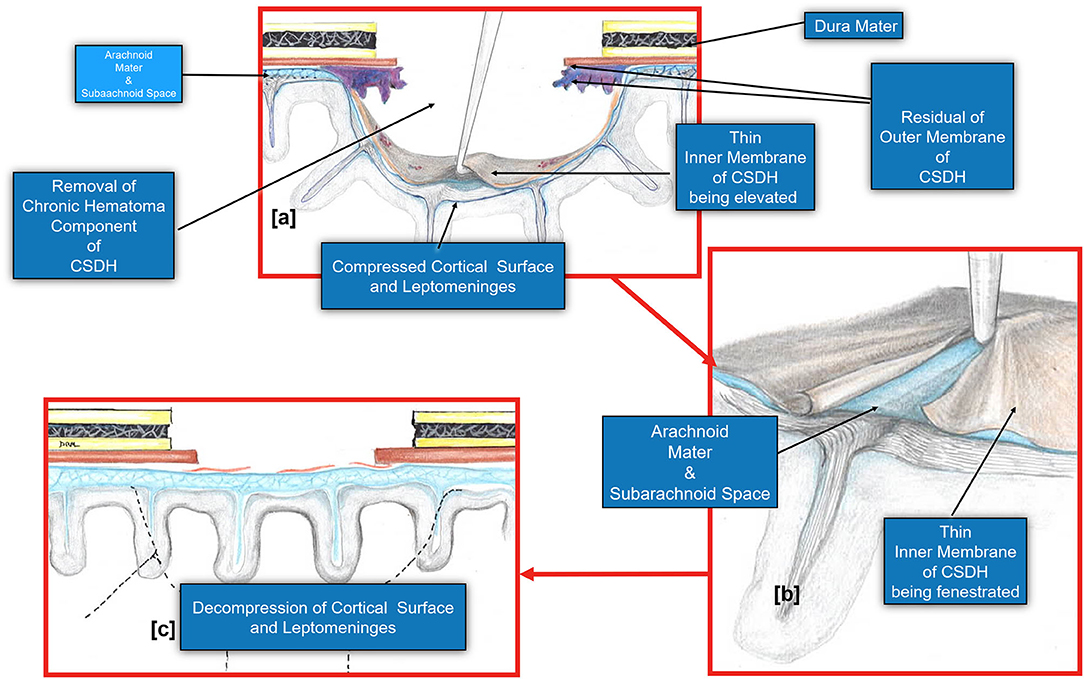
Figure 6. Diagram demonstrating the fenestration of the inner cSDH membrane. (a). The inner subdural membrane is elevated off of the pia-arachnoid using a microhook or by applying suction with a Rhoton #5 sucker. (b). Once this is lifted, a plane is readily developed between the pia-arachnoid and the membrane. A series of cuts may be made extending out radially to complete the fenestration. (c). The fenestration of the inner membrane allows the decompression of the cortical surface and the pia arachnoid. Note that the pia-arachnoid is not opened and rarely is scared to the inner membrane. Occasionally we have noted the accumulation of xanthochromic fluid that has accumulated between the inner membrane and pia arachnoid. This is irrigated out.
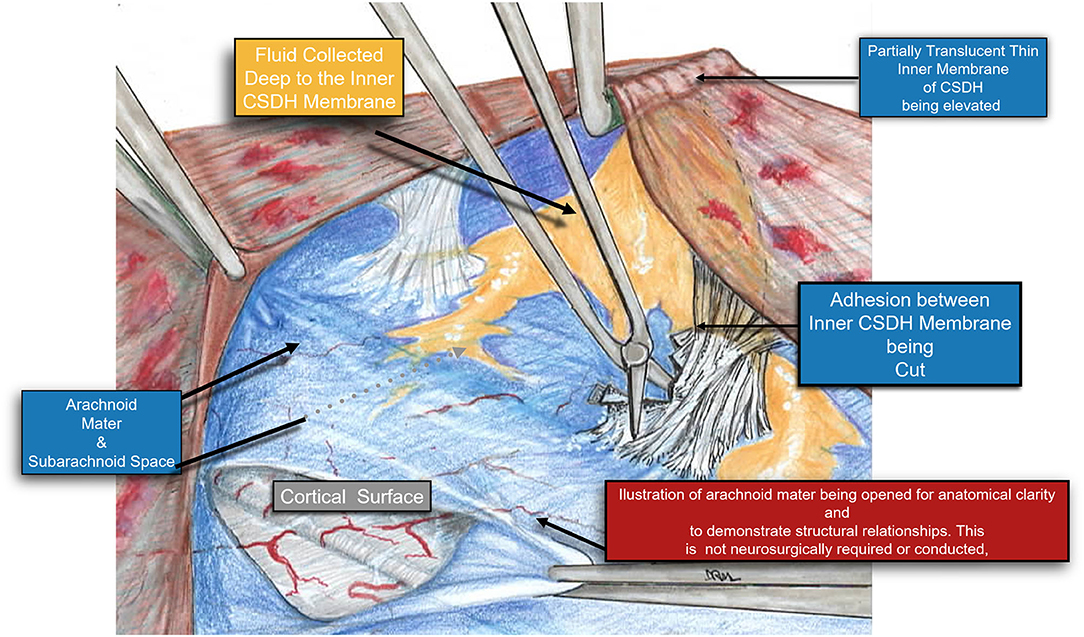
Figure 7. A Higher magnification diagram demonstrating the relationship of the inner cSDH membrane to the pia-arachnoid and the underlying cortex. Adhesions between the pia-arachnoid and the inner subdural membrane were very rare. When encountered these were easily released by cutting them with a microscissors. It is also possible to plan the fenestrations around these areas of adhesion, thus still affecting the release of the tension band of the inner membrane upon the underlying cortex.
Figure 8 is a demonstration of an 83 yo patient with a cSDH that had very nice expansion of the brain after craniotomy and fenestration within 1–2 h of the surgery. In the post-operative CT scan, there is very little intracranial air seen as every effort was made to fill up the entire subdural space with normal saline irrigation during closure. This is particularly important to allow the brain to re-expand in a liquid medium (36).
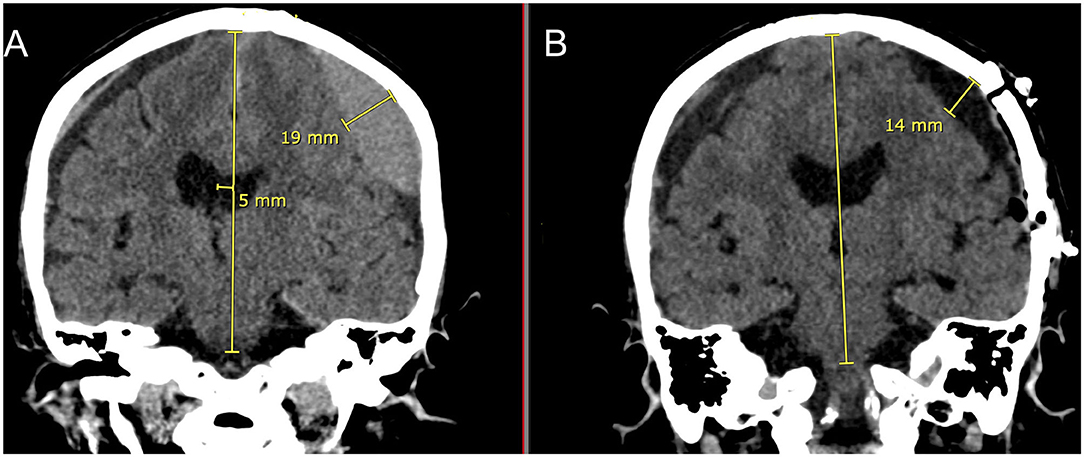
Figure 8. Eighty three yo F who suffered a ground level fall who was on aspirin. She had mild headaches and speech difficulties with a right upper extremity pronator drift. (A): Preoperative coronal CT scan demonstrating a left sided cSDH with midline shift. Note the lentiform shape afforded by the inner subdural membrane that is displacing the underlying cortex. There is a smaller right sided acute on chronic SDH. (B): Post-operative coronal CT scan done within 1–2 h of reaching the recovery room. Note the decrease in the mass effect and midline shift with near complete removal of the cSDH and re-expansion of the brain on the left. There is very little intracranial air. On the right sided there is increased prominence of the subdural space and the acute on chronic subdural hematoma.
There is much interest in the long-term sequelae of both operated and non-operated cSDH. Several studies have demonstrated the association of cognitive decline and cerebral atrophy with cSDH (2, 6, 8). Clearly a rather robust inflammatory response of the brain to the initial SDH is elicited and persists (7, 12). Recent trials have been directed at attenuating this response with dexamethasone or atorvastatin (19, 20, 37). There has also been much recent interest in middle meningeal artery (MMA) embolization for the treatment of cSDH. Recent series have demonstrated its efficacy in the stabilization and decrease in size of cSDH after upfront treatment (23–25, 31, 38). The attraction of these embolization techniques is the relative minimal invasive nature which is particularly of concern in the elderly and frail. Most reports are cautious about using MMA up front in cases where there is significant mass effect or neurological deficits. A midline shift of >5 mm or motor strength of 4/5 contralateral to the cSDH has been used as a guideline to avoid MMA embolization (25, 38). As seen in Table 1, all of our patients except for two presented with symptoms/complaints that were concerning enough that they came to our ED. Also, as seen in Table 2, the mean MLS for our patients was 6.75 mm. The MMA embolization has a delayed effect with an average time to resolution of MLS to be 46 days for MLS <5 mm and 51 days for MLS>5 mm (38). The recent findings with MMA embolization suggest that the mechanism is via devascularization of the outer membranes. The finding of embolization material in the inner membrane raises the question of whether or not devitalization of this membrane may also be important in decreasing the size of the cSDH (14, 39).
Clearly the cSDH can exert direct focal pressure on the underlying brain (Figure 4) which may lead to impaired neural dysfunction and long-term neural degeneration. The goal of the surgical treatment whether via craniotomy or burr holes is to decompress the brain and to eliminate the toxic effects of the blood break down products. It is difficult to open the inner subdural membrane via a burr hole and create a wide fenestration. The benefit of the immediate release of constraints of the inner membrane that are possible via the craniotomy are not appreciated with the burr hole method. Over time, because of the thin nature of the inner membrane and because of the outward pressure of the brain, the inner membrane may break up spontaneously. Nonetheless, this is dependent upon the underlying brain. An elderly, somewhat atrophic, brain is unlikely to have the ability to spontaneously disrupt the inner membrane.
The presence of the cSDH likely disrupts the normal flow of brain interstitial fluid because of the local mass effect. There has been much recent interest in the importance of this flow of fluid through the brain via the glymphatics and the dural lymphatics (40–42). If the inner membrane is not fenestrated, this itself will act as a constraint on the brain which may continue to affect the glymphatics. Finally, if the brain is unable to expand and if the pia-arachnoid does not contact the dural lymphatics, this may also affect CSF turnover (43, 44). There is increasing evidence that the glymphatic and the dural lymphatic systems are important for clearing potential neurodegenerative toxins such as Tau and Amyloid beta from the brain (43, 45, 46). Aberrancies in their clearance and that of other proteins has been postulated to be a factor in the development of dementia. Further studies into the relationship between cSDH and long term outcomes depending on the type of surgery or procedure performed will help us to better tailor the procedure to optimize the long-term neurological outcomes.
Limitations of the study include the small sample size and the retrospective nature of the study. This is a single center study examining the results of a single neurosurgeon with follow-up that is limited to 6 months. Additionally, the effect on the long-term cognitive outcome of these patients was not explored. More detailed longitudinal studies of the rate of cerebral atrophy in patients undergoing the mini-craniotomies vs. burr hole drainage need to be done to determine if the theoretical benefits that we propose are realized.
Mini-craniotomy with careful fenestration of the inner membrane is very effective in the treatment of cSDH with a very low recurrence rate. There are a variety of methods to treat cSDH, the key ones with their advantages and disadvantages are outlined in Table 5. Re-expansion of the brain and re-establishment of normal brain interstitial fluid flow may be important in the success of this procedure and may be related to the recent interests in brain glymphatics and dural lymphatics. This is an area for future research and studies as the number of patients with cSDH and neurodegenerative disorders continues to increase.
The datasets generated for this article are not readily available because of patient confidentiality and HIPPA restrictions. De-identified data may be made available upon request. Requests to access the datasets should be directed to Jeff Chen, SmVmZmV3YzFAaHMudWNpLmVkdQ==.
The studies involving human participants were reviewed and approved by University of California Irvine IRB board. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
JC, JX, and DT contributed the writing, conception, and data analysis. DM provided all of the drawn diagrams. MP-R provided the pathology pictures. All authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
1. Rauhala M, Helen P, Huhtala H, Heikkila P, Iverson GL, Niskakangas T, et al. Chronic subdural hematoma-incidence, complications, and financial impact. Acta Neurochir (Wien). (2020) 162:2033–43. doi: 10.1007/s00701-020-04398-3
2. Rauhala M, Helen P, Seppa K, Huhtala H, Iverson GL, Niskakangas T, et al. Long-term excess mortality after chronic subdural hematoma. Acta Neurochir (Wien). (2020) 162:1467–78. doi: 10.1007/s00701-020-04278-w
3. Feghali J, Yang W, Huang J. Updates in chronic subdural hematoma: epidemiology, etiology, pathogenesis, treatment, and outcome. World Neurosurg. (2020) 141:339–45. doi: 10.1016/j.wneu.2020.06.140
4. Sahyouni R, Goshtasbi K, Mahmoodi A, Tran DK, Chen JW. Chronic Subdural Hematoma: a Historical and Clinical Perspective. World Neurosurg. (2017) 108:948–53. doi: 10.1016/j.wneu.2017.09.064
5. Sahyouni R, Mahboubi H, Tran P, Roufail JS, Chen JW. Membranectomy in chronic subdural hematoma: meta-analysis. World Neurosurg. (2017) 104:418–29. doi: 10.1016/j.wneu.2017.05.030
6. Bin Zahid A, Balser D, Thomas R, Mahan MY, Hubbard ME, Samadani U. Increase in brain atrophy after subdural hematoma to rates greater than associated with dementia. J Neurosurg. (2018) 129:1579–87. doi: 10.3171/2017.8.JNS17477
7. Jarrott B, Williams SJ. Chronic brain inflammation: the neurochemical basis for drugs to reduce inflammation. Neurochem Res. (2016) 41:523–33. doi: 10.1007/s11064-015-1661-7
8. Moffatt CE, Hennessy MJ, Marshman LAG, Manickam A. Long-term health outcomes in survivors after chronic subdural haematoma. J Clin Neurosci. (2019) 66:133–7. doi: 10.1016/j.jocn.2019.04.039
9. Katsuki M, Kakizawa Y, Wada N, Yamamoto Y, Uchiyama T, Nakamura T, et al. Endoscopically observed outer membrane color of chronic subdural hematoma and histopathological staging: white as a risk factor for recurrence. Neurol Med Chir (Tokyo). (2020) 60:126–35. doi: 10.2176/nmc.oa.2019-0203
10. Yamashima T. The inner membrane of chronic subdural hematomas: pathology and pathophysiology. Neurosurg Clin N Am. (2000) 11:413–24. doi: 10.1016/S1042-3680(18)30103-7
11. Schachenmayr W, Friede RL. The origin of subdural neomembranes. I. Fine structure of the dura-arachnoid interface in man. Am J Pathol. (1978) 92:53–68.
12. Edlmann E, Giorgi-Coll S, Whitfield PC, Carpenter KLH, Hutchinson PJ. Pathophysiology of chronic subdural haematoma: inflammation, angiogenesis and implications for pharmacotherapy. J Neuroinflammation. (2017) 14:108. doi: 10.1186/s12974-017-0881-y
13. Osuka K, Watanabe Y, Usuda N, Aoyama M, Takeuchi M, Takayasu M. Expression of autophagy signaling molecules in the outer membranes of chronic subdural hematomas. J Neurotrauma. (2019) 36:403–7. doi: 10.1089/neu.2018.5626
14. Moshayedi P, Liebeskind DS. Middle meningeal artery embolization in chronic subdural hematoma: implications of pathophysiology in trial design. Front Neurol. (2020) 11:923. doi: 10.3389/fneur.2020.00923
15. Shim YW, Lee WH, Lee KS, Kim ST, Paeng SH, Pyo SY. Burr hole drainage versus small craniotomy of chronic subdural hematomas. Korean J Neurotrauma. (2019) 15:110–6. doi: 10.13004/kjnt.2019.15.e25
16. Al-Jehani H, Petrecca K. The case for mini-craniotomy in the management of chronic subdural hematoma. Acta Neurochir (Wien). (2013) 155:189–90. doi: 10.1007/s00701-012-1539-2
17. Van Der Veken J, Duerinck J, Buyl R, Van Rompaey K, Herregodts P, D'Haens J. Mini-craniotomy as the primary surgical intervention for the treatment of chronic subdural hematoma–a retrospective analysis. Acta Neurochir (Wien). (2014) 156:981–7. doi: 10.1007/s00701-014-2042-8
18. Huang J, Gao C, Dong J, Zhang J, Jiang R. Drug treatment of chronic subdural hematoma. Expert Opin Pharmacother. (2020) 21:435–44. doi: 10.1080/14656566.2020.1713095
19. Allison A, Edlmann E, Kolias AG, Davis-Wilkie C, Mee H, Thelin EP, et al. Statistical analysis plan for the Dex-CSDH trial: a randomised, double-blind, placebo-controlled trial of a 2-week course of dexamethasone for adult patients with a symptomatic chronic subdural haematoma. Trials. (2019) 20:698. doi: 10.1186/s13063-019-3866-6
20. Hutchinson PJ, Edlmann E, Bulters D, Zolnourian A, Holton P, Suttner N, et al. Trial of dexamethasone for chronic subdural hematoma. N Engl J Med. (2020) 383:2616–27. doi: 10.1056/NEJMoa2020473
21. Pouvelle A, Pouliquen G, Premat K, Chougar L, Lenck S, Degos V, et al. Larger middle meningeal arteries on computed tomography angiography in patients with chronic subdural hematomas as compared with matched controls. J Neurotrauma. (2020) 37:2703–8. doi: 10.1089/neu.2020.7168
22. Ban SP, Hwang G, Byoun HS, Kim T, Lee SU, Bang JS, et al. Middle meningeal artery embolization for chronic subdural hematoma. Radiology. (2018) 286:992–9. doi: 10.1148/radiol.2017170053
23. Link TW, Boddu S, Paine SM, Kamel H, Knopman J. Middle meningeal artery embolization for chronic subdural hematoma: a series of 60 cases. Neurosurgery. (2019) 85:801–7. doi: 10.1093/neuros/nyy521
24. Catapano JS, Ducruet AF, Nguyen CL, Cole TS, Baranoski JF, Majmundar N, et al. A propensity-adjusted comparison of middle meningeal artery embolization versus conventional therapy for chronic subdural hematomas. J Neurosurg. (2021). doi: 10.3171/2020.9.JNS202781. [Epub ahead of print].
25. Kan P, Maragkos GA, Srivatsan A, Srinivasan V, Johnson J, Burkhardt JK, et al. Middle meningeal artery embolization for chronic subdural hematoma: a multi-center experience of 154 consecutive embolizations. Neurosurgery. (2021) 88:268–77. doi: 10.1093/neuros/nyaa379
26. Bartek J Jr., Sjavik K, Kristiansson H, Stahl F, Fornebo I, Forander P, et al Predictors of recurrence and complications after chronic subdural hematoma surgery: a population-based study. World Neurosurg. (2017) 106:609–14. doi: 10.1016/j.wneu.2017.07.044
27. Bartek J Jr., Sjavik K, Stahl F, Kristiansson H, Solheim OO, Gulati S, et al Surgery for chronic subdural hematoma in nonagenarians: a Scandinavian population-based multicenter study. Acta Neurol Scand. (2017) 136:516–20. doi: 10.1111/ane.12764
28. Regan JM, Worley E, Shelburne C, Pullarkat R, Watson JC. Burr hole washout versus craniotomy for chronic subdural hematoma: patient outcome and cost analysis. PLoS ONE. (2015) 10:e0115085. doi: 10.1371/journal.pone.0115085
29. Zhang J, Chen J. The therapeutic effects of craniotomy versus endoscopic-assisted trepanation drainage for isolated chronic subdural haematoma (ICSH): a single-centre long-term retrospective comparison study. Brain Res Bull. (2020) 161:94–7. doi: 10.1016/j.brainresbull.2020.04.014
30. Lega BC, Danish SF, Malhotra NR, Sonnad SS, Stein SC. Choosing the best operation for chronic subdural hematoma: a decision analysis. J Neurosurg. (2010) 113:615–21. doi: 10.3171/2009.9.JNS08825
31. Joyce E, Bounajem MT, Scoville J, Thomas AJ, Ogilvy CS, Riina HA, et al. Middle meningeal artery embolization treatment of nonacute subdural hematomas in the elderly: a multiinstitutional experience of 151 cases. Neurosurg Focus. (2020) 49:E5. doi: 10.3171/2020.7.FOCUS20518
32. Mahmood SD, Waqas M, Baig MZ, Darbar A. Mini-craniotomy under local anesthesia for chronic subdural hematoma: an effective choice for elderly patients and for patients in a resource-strained environment. World Neurosurg. (2017) 106:676–9. doi: 10.1016/j.wneu.2017.07.057
33. Matsumoto H, Hanayama H, Okada T, Sakurai Y, Minami H, Masuda A, et al. Which surgical procedure is effective for refractory chronic subdural hematoma? Analysis of our surgical procedures and literature review. J Clin Neurosci. (2018) 49:40–7. doi: 10.1016/j.jocn.2017.11.009
34. Kocaman U, Yilmaz H. Description of a modified technique (mini craniotomy-basal membranotomy) for chronic subdural hematoma surgery and evaluation of the contribution of basal membranotomy performed as part of this technique to cerebral expansion. World Neurosurg. (2019) 122:e1002–e6. doi: 10.1016/j.wneu.2018.10.196
35. Jang KM, Choi HH, Mun HY, Nam TK, Park YS, Kwon JT. Critical depressed brain volume influences the recurrence of chronic subdural hematoma after surgical evacuation. Sci Rep. (2020) 10:1145. doi: 10.1038/s41598-020-58250-w
36. Chavakula V, Yan SC, Huang KT, Liu J, Bi WL, Rozman P, et al. Subdural pneumocephalus aspiration reduces recurrence of chronic subdural hematoma. Oper Neurosurg (Hagerstown). (2020) 18:391–7. doi: 10.1093/ons/opz193
37. Fan YS, Wang B, Wang D, Xu X, Gao C, Li Y, et al. Atorvastatin combined with low-dose dexamethasone for vascular endothelial cell dysfunction induced by chronic subdural hematoma. Neural Regen Res. (2021) 16:523–30. doi: 10.4103/1673-5374.293152
38. Gomez-Paz S, Akamatsu Y, Salem MM, Enriquez-Marulanda A, Robinson TM, Ogilvy CS, et al. Upfront middle meningeal artery embolization for treatment of chronic subdural hematomas in patients with or without midline shift. Interv Neuroradiol. (2020). doi: 10.1177/1591019920982816. [Epub ahead of print].
39. Saito H, Tanaka M, Hadeishi H. Angiogenesis in the septum and inner membrane of refractory chronic subdural hematomas: consideration of findings after middle meningeal artery embolization with low-concentration n-butyl-2-cyanoacrylate. NMC Case Rep J. (2019) 6:105–10. doi: 10.2176/nmccrj.cr.2018-0275
40. Iliff JJ, Goldman SA, Nedergaard M. Implications of the discovery of brain lymphatic pathways. Lancet Neurol. (2015) 14:977–9. doi: 10.1016/S1474-4422(15)00221-5
41. Louveau A, Smirnov I, Keyes TJ, Eccles JD, Rouhani SJ, Peske JD, et al. Structural and functional features of central nervous system lymphatic vessels. Nature. (2015) 523:337–41. doi: 10.1038/nature14432
42. Louveau A, Nau JY. Nervous and lymphatic system communicate with each other. “Zyka virus” unravels its mystery. Rev Med Suisse. (2015) 11:1462–3.
43. Tamura R, Yoshida K, Toda M. Current understanding of lymphatic vessels in the central nervous system. Neurosurg Rev. (2020) 43:1055–64. doi: 10.1007/s10143-019-01133-0
44. Glinskii OV, Huxley VH, Xie L, Bunyak F, Palaniappan K, Glinsky VV. Complex non-sinus-associated pachymeningeal lymphatic structures: interrelationship with blood microvasculature. Front Physiol. (2019) 10:1364. doi: 10.3389/fphys.2019.01364
45. Iliff JJ, Chen MJ, Plog BA, Zeppenfeld DM, Soltero M, Yang L, et al. Impairment of glymphatic pathway function promotes tau pathology after traumatic brain injury. J Neurosci. (2014) 34:16180–93. doi: 10.1523/JNEUROSCI.3020-14.2014
Keywords: chronic subdural hematoma, neurosurgery, dural lymphatics, glymphatics, mini-craniotomy, fenestration, inner membrane
Citation: Chen JW, Xu JC, Malkasian D, Perez-Rosendahl MA and Tran DK (2021) The Mini-Craniotomy for cSDH Revisited: New Perspectives. Front. Neurol. 12:660885. doi: 10.3389/fneur.2021.660885
Received: 29 January 2021; Accepted: 25 March 2021;
Published: 06 May 2021.
Edited by:
Gregory W. J. Hawryluk, University of Manitoba, CanadaReviewed by:
Andrew Ajisebutu, University of Manitoba, CanadaCopyright © 2021 Chen, Xu, Malkasian, Perez-Rosendahl and Tran. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jefferson W. Chen, SmVmZmV3YzFAaHMudWNpLmVkdQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.