- 1Assistance Publique des Hôpitaux de Paris, APHP, Hôpital Saint Antoine, Neurology Department, Paris, France
- 2Sorbonne Université and INSERM, Épidémiologie des maladies Allergiques et Respiratoires, Institut Pierre Louis d'Epidémiologie et Santé Publique, Paris, France
- 3Atmospheric Modelling and Environmental Mapping Unit, INERIS, BP2, Verneuil-en-Halatte, France
- 4Sorbonne Universités, Brain and Spine Institute, ICM, Hôpital de la Pitié Salpêtrière, Inserm UMR-S 1127, CNRS UMR 7225, Paris, France
Objective: Particulate matter (PM) of aerodynamic diameter smaller than 10 μm (PM10) has been associated with multiple sclerosis (MS) relapse. However, the impact of smaller PM with a greater ability to penetrate human organism has never been assessed. We evaluated the impact of PM smaller than 2.5 μm (PM2.5) on the risk of MS relapse.
Material and Methods: In a case-crossover study, we included 2,109 consecutive hospitalizations likely due to MS relapse in day hospital in 5 MS centers in the Paris area from January 2009 to December 2013. For each hospitalization, the natural logarithm of the average weekly PM2.5 concentrations (μg/m3) at the patient's residence address during each of the 6 weeks (week[0] to week[−5]) preceding admission was compared with the concentration during the previous week, using a conditional logistic regression adjusted on temperature, flu-like syndrome rate, pollen count, and holiday period.
Results: PM2.5 average concentration during week[−3] was significantly associated with the risk of hospitalization for MS relapse [OR = 1.21 (CI 1.01;1.46)]. The association was stronger in patients younger than 30 years [OR=1.77 (CI 1.10; 2.83)].
Conclusion: Our study demonstrates an association between exposure to PM2.5 and MS relapse, particularly in young people.
Introduction
Ambient air pollution, and especially particulate matter (PM), has emerged as a global major health concern, leading to the death of 8.9 million people worldwide (1).
PM defined as any gaseous or solid particle suspended in the air is subdivided according to size: respirable (PM10, diameter <10 μm), fine (PM2.5, diameter <2.5 μm), and ultrafine (PM0.1, diameter <0.1 μm). PM10 and PM2.5 often derive from different emissions sources and also have different chemical compositions. Emissions from combustion of gasoline, oil, diesel fuel, or wood produce much of the PM2.5 pollution found in outdoor air, as well as a significant proportion of PM10. PM10 also includes dust from construction sites, landfills, and agriculture, wildfires and brush/waste burning, industrial sources, wind-blown dust from open lands, pollen, and fragments of bacteria (2). The various PMs also differ regarding their potential to penetrate the human body, as both PM2.5 and PM0.1 can reach the plasmatic circulation, whereas PM0.1 may also be able to reach the CNS through the blood-brain barrier (BBB) (3, 4) and the olfactory bulb (5).
PM2.5 exposure was suggested to be associated with the onset of Alzheimer disease (6), stroke (7), amyotrophic lateral sclerosis (8), and MS (9, 10). Several mechanisms have been evoked to explain this relationship: systemic and cerebral inflammation, oxidative stress, and breakdown of the blood-brain barrier (11). PM10 has also been suggested as a risk factor for MS relapse (12–14) and new lesion formation (15), but the effect of PM2.5 exposure on MS relapse risk has never been explored. However, PM2.5 composition differs from PM10 and can penetrate deeper in the lung, therefore inducing a stronger alveolar and systemic inflammation (16). We conducted here a study to determine the specific impact of PM2.5 on MS relapse. As MS frequently starts in young adults with more inflammatory phenotypes in younger subjects, we then selectively investigated whether the relation between PM2.5 and MS relapse depended on age.
Methods
Definition of Hospitalizations Related to MS Relapses
A case-crossover design has been chosen to investigate the association between PM2.5 concentrations and MS relapses, thus making every patient his/her own control, at different periods of exposure. The study area was Ile de France, comprising the city of Paris and surrounding areas, for a total of around 12 million inhabitants.
Hospitalizations were identified using the French administrative database called “Programme de Médicalisation des Sytèmes d'Information (PMSI)” in which the main diagnosis of every hospitalization is referenced. For the purpose of the present study, inclusion criteria targeted hospitalizations of patients whose personal address was located in Ile de France, with a day-hospital stay of 3 to 6 days, with principal hospitalization diagnosis of MS (G35) or non-carcinologic chemotherapy associated with MS (Z512, related diagnosis G35), from January 1, 2009 to December 31, 2013. During this period, relapse treatment by methylprednisolone was only administered IV and mostly through serial day-hospital for 3 to 5 days.
As no specific diagnosis code exists for MS relapse, we built a temporal algorithm in order to exclude hospitalizations for patients with MS without relapse. Criteria used in the algorithm were as follows: (1) MS hospitalizations separated by <7 days were considered as the same hospitalization; (2) for each patient, if two MS hospitalizations were separated by more than 7 days but <75 days, these hospitalizations were considered as repeated hospitalizations; (3) if a patient had three or more consecutive repeated hospitalizations for MS, then all these hospitalizations were excluded, because they were likely to be related to programmed disease-modifying treatments and not to specific treatment for relapse; and (4) if a patient had only two repeated MS hospitalizations, then only the second hospitalization was excluded.
A random validity check of data was made on 100 hospitalizations at Saint Antoine hospital and showed that 88% were related to real relapses; others were diagnosis work-up and progressive aggravation without relapse. In the subset of Saint Antoine, the median delay between symptom onset and hospitalization was 13 days (first quartile 7 days, third quartile 30 days).
PM Exposure and Other Variables
The patients' home addresses and the date of hospitalization were recorded using the administrative database of each hospital. PM2.5 concentrations (μg/m3) data were obtained through the high spatial resolution CHIMERE air quality model (17), an Eulerian offline chemistry-transport model, on a daily basis at a 1-km resolution, accounting for primary pollutant emissions, meteorological fields, topography, and chemical boundary conditions (17). The average weekly PM2.5 concentrations were estimated during each of the 6 weeks (week[0] to week[−5]) preceding admission.
Potential confounders of the relationship between PM2.5 and MS according to the literature consisted in temperature, influenza epidemics, pollen counts, and holiday periods. Meteorological parameters were obtained from the French meteorological service Méteo France. Data on influenza-like infection were obtained from the French Sentinel Network (18): the flu-like syndrome weekly rate was determined as the number of visits per week for influenza symptoms (fever, cough, sore throat, etc.) to general practitioner belonging to the French Sentinel Network. Pollen counts were obtained from the Réseau National de Surveillance Allergique (19). Holiday periods were defined as the two last weeks of December (Christmas holidays) and the whole months of July and August (summer holidays).
Statistical Analysis
The main objective was to determine whether PM2.5 concentration was statistically associated with the risk of MS relapse using a case-crossover design. The secondary objective consisted in exploring the influence of age on the association between PM2.5 and MS relapse through stratification.
In the case-crossover study, each patient was his/her own control. In evaluating the impact of PM2.5 on MS relapse risk, the concentration was assessed by week before hospitalization, week[0] corresponding to the 7 days, ended by the hospitalization admission date. We used the natural logarithm of PM2.5 weekly exposure because it complied with the normality required by the analysis. The natural logarithm (Ln) of the average weekly PM2.5 concentrations for each of the 6 weeks (week[0] to week[−5]) preceding hospitalization was compared to the concentration during the previous week (e.g., PM2.5 exposure at week[0] was compared to PM2.5 concentration at week[−1], PM2.5 concentration at week[−1] was compared to PM2.5 concentration at week[−2], etc.) using a conditional logistic regression, adjusted for average weekly temperature, flu-like syndrome rate, pollen count, categorized as quartiles, and holiday period (“yes” or “no”).
Successively, in order to compare our findings with previous studies exploring PM10 exposure, we analyzed the impact of weekly PM10 exposure on MS relapse risk with the same methodology as that for PM2.5 using conditional logistic regression adjusted on the same confounders allowing to compare the impact and temporal relationship of PM10 and PM2.5 on MS relapse.
Lastly, we evaluated the influence of age on the association between PM2.5 and relapse. We thus evaluated the association during weeks significantly associated with relapses in different classes of age (under 30 years, 30–40 years, 40–50 years, over 50 years).
As MS treatment modifications can influence MS relapse, we did a sensitivity analysis, excluding patients who have switched MS treatments during the 6 weeks before hospitalization. This sensitivity analysis was performed for patients hospitalized at Saint-Antoine Hospital in which medical data were available in our local EDMUS (European Database on Multiple Sclerosis).
This observational study was based on data extracted from the French administrative database (Programme de Médicalisation des Sytèmes d'Information) in strict compliance with the French reference methodology established by the French National Commission on Informatics and Liberty (CNIL) (CNIL authorization for declaration 2060974, Ref MMF/CRX/AE171388), in accordance with the French law, including the GPRD (General Practice Research Database). All statistics were made on Stata 14 software.
Results
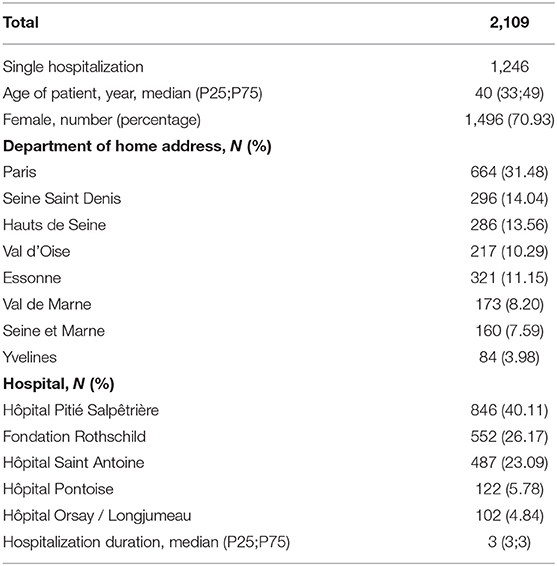
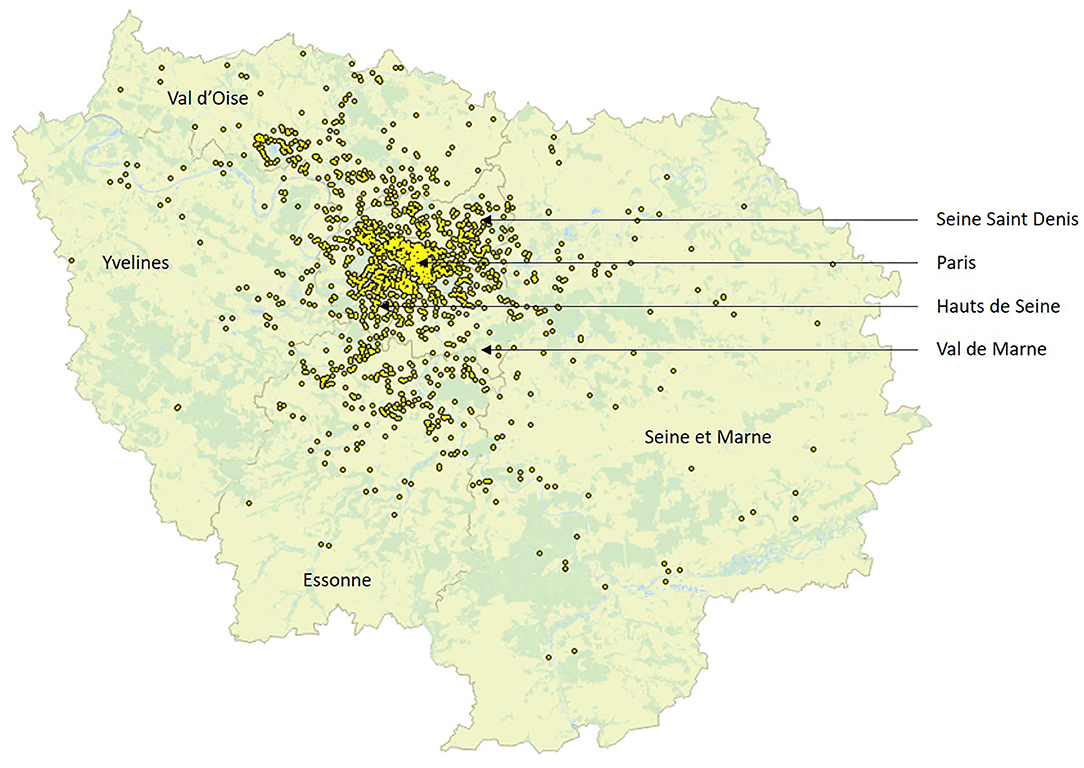
A total of 2,109 hospitalizations likely due to MS relapse were included, as described in Table 1 and Figure 1. The median age of the patients was 40 years; 344, 684, 589, and 492 were, respectively, under 30, 30–40, 40–50, and older than 50 years. Thirty-one percent of the hospitalized patients were living in Paris, while the remaining patients were allocated in other departments of Ile de France.
The monthly distribution of the 2,109 hospitalizations revealed a peak in October (239 hospitalizations), a high rate during winter months (209 in January, 211 in February, and 209 in March), and a decreasing rate in summer (172 in June and 165 in July), with a nadir in August (85 hospitalizations). The mean PM2.5 concentrations of patients with addresses in Ile de France were maximal in the cold period (from October to March, mean PM2.5 22.45 [confidence interval 21.79;23.11] μg/m3) and minimal in the warm period [from April to September, mean PM2.5 14.99 (CI 14.50;15.48) μg/m3]. The median (25th; 75th percentile) PM2.5 concentrations for week[−5] to week[0] were 15.09 (10.73;23.27), 14.88 (10.69;23.35), 15.66 (11.20;23.20), 15.76 (11.04;23.88), 15.46 (11.24;24.45), and 16.08 (11.21;24.55) μg/m3, respectively.
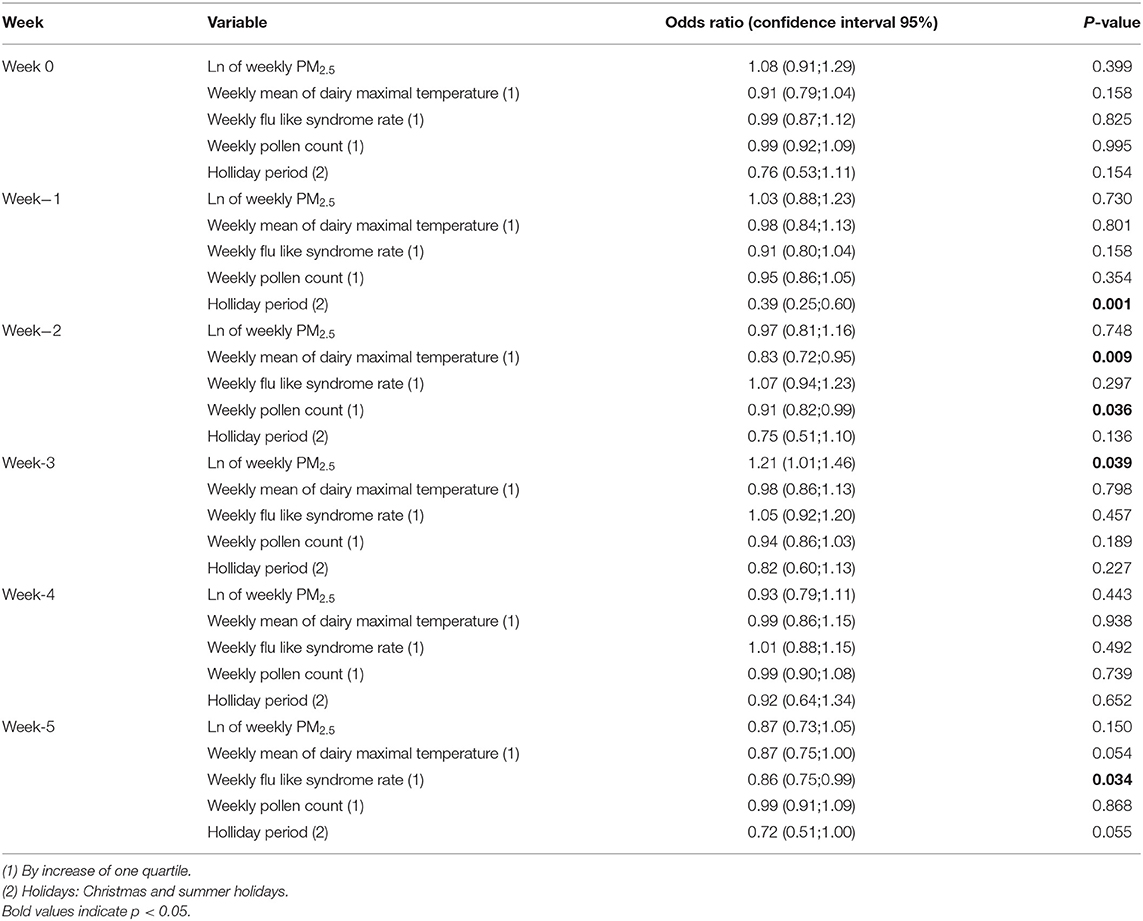
As shown in Table 2 and Figure 2, after adjustment for average weekly temperature, flu-like syndrome rate, pollen count, and holiday period, the PM2.5 concentration 3 weeks (week[-3]) before hospitalization (from day 21 to day 28 before hospitalization) was significantly associated with an increased risk of hospitalization likely due to MS relapse compared to PM2.5 concentration 4 weeks before hospitalization (odds ratio [OR] 1.21 [confidence interval {CI} 1.01;1.46]). No significant association was observed during the other weeks.
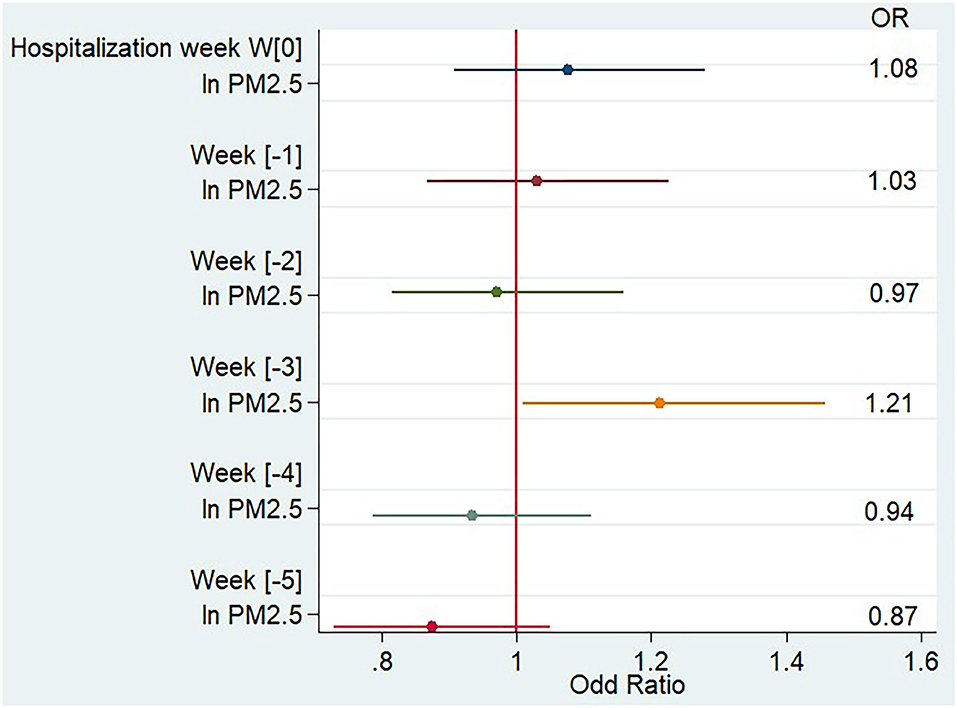
Figure 2. Impact of PM2.5 weekly concentration on the risk of multiple sclerosis relapse, multivariate analysis.
The impact of PM10 exposure on MS relapse risk was equivalent to the one observed for PM2.5 exposure, with a significant association only observed in week[−3] [OR 1.29 (CI 1.02;1.62)].
When we looked at the PM2.5 effect by class of age, as shown in Figure 3, we noticed that PM2.5 association with relapse was greater and only significant in younger patients, as the OR for the 344 hospitalizations of patients younger than 30 years was 1.77 (CI 1.10;2.83). When we restricted the analysis to patients younger than 25 years during week[−3], the OR for MS relapse increased to 3.10 (CI 1.27;7.57).
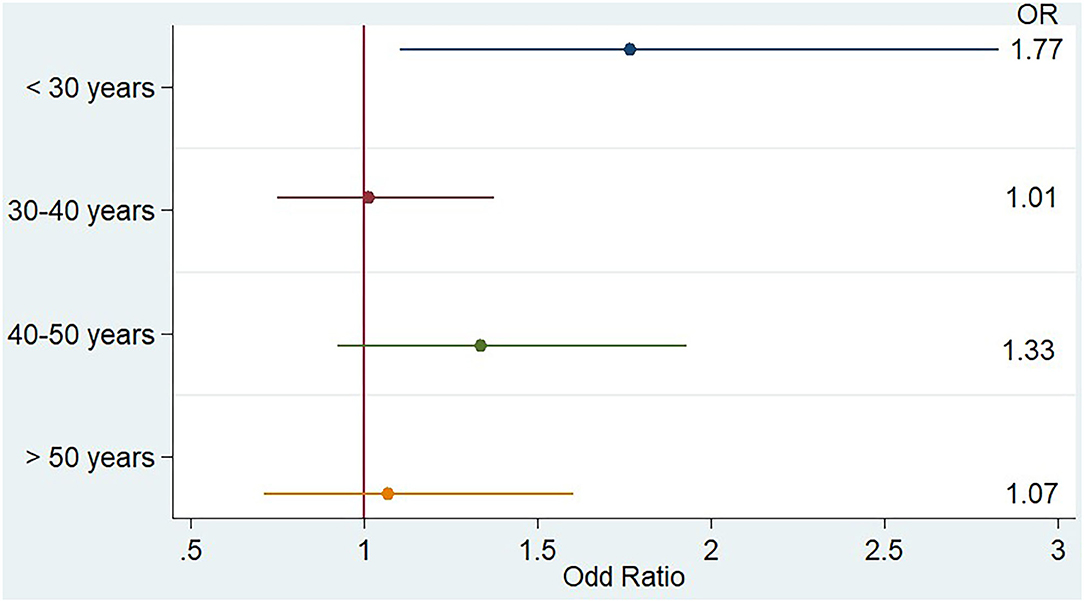
Figure 3. Impact of PM2.5 weekly concentration during week[-3] on the risk of multiple sclerosis relapse stratified by age, multivariate analysis.
A sensitivity analysis was made on patients hospitalized at Saint Antoine Hospital, of which 373 concerned patients included in our local EDMUS. Seventeen (4.5%) of these concerned patients who have initiated a new treatment during the 6 weeks before the hospitalization and were excluded. We performed the same analysis on the remaining 356 hospitalizations. PM2.5 remained associated with MS during week[-3] [OR 1.68 (CI 1.06; 2.66), p = 0.025].
Discussion
In this retrospective study including 2,109 hospitalizations likely due to MS relapse, PM2.5 concentration was associated with hospitalizations, and the association was stronger in patients younger than 30 years.
We found a specific temporal relationship between PM2.5 concentration and hospitalization likely due to relapses for the week[-3] preceding hospitalization, suggesting that several weeks may be required following PM2.5 exposure for the immune response to be orchestrated and generate new lesions underlying relapses. In the subset made in Saint Antoine hospital where the relapses were verified, the first symptoms related to the relapse appeared 13 days before hospitalization, highlighting a delay between the first symptoms onset and hospitalization. This means that finally the delay between pollution peak and relapses may be shorter than 3 weeks. As MS lesions can take several days or weeks to form, first involving activation of the peripheral immune system, subsequently inducing microglial activation, oxidative stress, blood-brain barrier disruption, and demyelination (20), PM2.5 exposure may contribute to either the early immune system activation or to later stages of MS lesion formation, preceding clinical relapse appearance. Interestingly, a short-term impact of PM10 was suggested by two French studies (14, 21). In the former study, MS relapses were associated with PM10 during the cold season, the control days having been chosen to be ±35 days relative to the case (relapse) day. The latter study confirmed the first results but through a multipollutant analysis that also indicated ozone exposure during days 1–3 before the relapse symptom onset as a risk factor during the hot season (21).
In our study, both PM2.5 and PM10 exposures were associated with a similar increase in MS relapse risk during week[−3]. As PM2.5 is part of PM10, this result is not surprising and suggests that most of the effect may be mediated by PM2.5. A multivariate analysis including both PM2.5 and PM10 could not be performed due to the collinearity of both variables that are closely related in the air quality CHIMERE model.
So far, the role of PM2.5 has only been explored in the case of MS incidence and prevalence but not of relapse, with conflicting results. A strong positive correlation between PM2.5 exposure and MS prevalence was found in the province of Padova (9, 10), contrasting an absence of relation in studies having considered MS incidence in adults (22–24). However, in a case–control study of pediatric MS, worsening air quality significantly impacted the odds for MS (OR = 2.83; 95%CI 1.5, 5.4 for patients living <20 miles from a referral center, and OR = 1.61; 95%CI 1.2, 2.3 for those who resided ≥20 miles from a referral center).
Previous pathophysiological studies have supported a key role played by PM2.5 that would predominantly involve a proinflammatory effect. Unlike PM10, PM2.5 can reach pulmonary alveoli, where they are incorporated by alveolar macrophages, promoting macrophage production of inflammatory cytokines such as interleukin-12 and subsequently pushing T lymphocyte differentiation into Th1 profile (25) and systemic inflammation (26). Part of T lymphocyte activation may therefore be triggered in the lung, followed by a migration step toward the CNS. This sequence of events has been described as the “brain–lung axis” (27), which is considered as one of the main explanations of the negative impact of smoking on MS (28). Supporting this hypothesis, the systemic inflammation induced by carbon nanoparticles in rodents could only be observed when particles were administered by inhalation and not when they were administered by intra-arterial infusion (29). More recently, Andrea Cortese and colleagues highlighted in MS patients that PM10 exposure 15 days before blood sampling was associated with the production of Th-17 polarizing interleukin from monocyte derived dendritic cell and an overexpression of CCR-6 CD4+ T circulating cells, which may facilitate their entry to the CNS (30). The time for systemic immune response to be orchestrated and generate posterior damage in the CNS following the “brain–lung axis” could therefore explain the observed delay between PM2.5 exposure and relapse onset.
Systemic and cerebral oxidative stress are also enhanced by particulate matter exposure (31, 32), inducing mitochondrial dysfunction, involved in the initiation of MS lesion formation (“prephagocytic” lesions) (33). Therefore, oxidative stress and mitochondrial dysfunction could be another mechanism of brain damage related to PM exposure, acting synergistically with brain inflammation.
A “direct” toxic effect of PM2.5 on the nervous system may also be hypothesized and contribute to MS relapses, but would imply that part of the PM can reach the brain. In rodents, only PM0.1 can reach the brain essentially through the olfactive nerve, as shown by Elder et al. (5). An alternative way, involving a translocation of PM from the lung to systemic circulation and to the CNS, is still debated, and it has been estimated that ~13% of inhaled nanoparticles are able to translocate from the lung to the blood (34). Such a translocation from the blood to the CNS is considered poorly effective in non-MS patients (5) due to the integrity of the brain-blood barrier. However, in diseases characterized by brain–blood barrier dysfunction such as MS, this way could be more relevant, as highlighted in the EAE model, where intravenously injected nanoparticles incorporated to macrophages and neutrophils were found in the cerebral white matter (4).
In humans, a relationship between air pollution exposure and cerebral inflammation has been previously suggested in a Mexican study comparing the level of inflammatory biomarkers in the CSF of children living in a highly polluted area compared with others living in a less polluted city (35). This study showed that macrophage inhibitory factors interleukin 2 and 6 were significantly increased in the CSF of children living in a more polluted area.
Young people were particularly impacted by PM2.5 concentration in our study, although the confidence interval of the observed association was broad (CI 1.06; 2.66), probably because of the relative small number of hospitalized patients younger than 30 years (N = 344). This result may be explained in two ways. First, young age is associated with a more inflammatory and relapsing disease and with a more frequent breakdown of the blood-brain barrier (36), so that young patients may be more sensitive to environmental aggression. For other neurological diseases such as stroke, age was also found to be negatively associated with the impact of air pollution exposure (37). Second, employment is extremely rapidly declining in MS patients: in a study in Sweden, 23.7% of patients under 34 years received a disability pension, but this ratio climbed to 48.9% for those aged from 35 to 44 years and near 70% for patients over 44 years (38). Accordingly, in a longitudinal study in France, only 54.4% of MS patients were still working 7 years after MS diagnosis (39). Therefore, young people may be more likely to remain active on the labor market. Working in Ile de France often involves a long journey from home to work, and thus an increase in exposure to air pollution, which could explain the strength of the observed association in young people. In the study of Roux et al. (14) in the Strasbourg Area, there was also a trend for an increase in the PM10 concentration association with MS relapse for younger people [the OR for patients under 30 years was 1.48 (CI 0.91; 2.40) and for 30–40 years was 1.65 (CI 1.02; 2.68), while the OR for those older than 40 years was only 1.23 (0.82; 1.84)].
This study has some limitations. Due to the retrospective administrative database collection of data, we cannot assume that all the hospitalizations were linked to MS relapse. However, in our verification sample subset in Saint Antoine Hospital, we found nearly 90% of “true relapses” in the included hospitalizations. Second, the day hospital recruitment that has been chosen may miss part of the hospitalizations for both severe relapses (that required complete hospitalization) and mild relapses (that did not require day hospitalization for methylprednisolone). We could not stratify the analysis based on MS treatment, smoking status, or vitamin D level, but the case-crossover design allowed us to take into account these interindividual differences because each patient was his/her own control. Adjustment on influenza infection was based on ecological measures because we did not have individual data on this variable. Moreover, the time from symptom onset to hospitalization date was variable (median 13 days but first quartile 7 days, and third quartile 30 days in the verification subset of Saint-Antoine Hospital), inducing a temporal dispersion of the period of interest. However, this temporal dispersion should have attenuated the observed relationship, which remained significant in our study. Of note, air pollution was estimated at the address of included patients, and a substantial number of included patients could have passed the weeks before hospitalization elsewhere. Finally, this method of estimation does not account for the exposure during daily journeys and exposure to indoor air pollution.
Despite the mentioned limitations, our study found an association between PM2.5 concentration and the risk of MS relapse. Through technical improvements in the collection and analysis of air pollution data, future studies should investigate the specific effect of each component of fine particle and include ambulatory measurement in order to guide long-term measures to address the issue of air quality.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
Ethical review and approval was not required for the study on human participants in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author Contributions
EJ, BS and IA-M were responsible for study conception, design, supervision, interpretation of data, and drafting of the manuscript. EJ was responsible for statistical analysis. BD was responsible for air pollution data management. AC was responsible for air pollution data. All authors contributed to the article and approved the submitted version.
Conflict of Interest
EJ reports reimbursement for conference registration fees, travel expenses, and accommodation from Sanofi Genzyme, outside the submitted work. BS has received fees for advisory boards and lectures from Genzyme, Merck-Serono, Novartis, Teva, and Biogen, and research support from Roche, Genzyme, and Merck-Serono.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We are grateful to Lucie Ronceray, from Medical Information Department, Hospital Saint Antoine, for the encoding of the PMSI request; to Michel Thibaudon from Réseau National de Surveillance Aérobiologique (R.N.S.A.) for having provided us with pollen counts; and to Denis Cendrier from Météo France for having provided us with meteorological data. We thank Cassie Hague for editorial assistance. We thank Pr Catherine Lubetzki from Assistance Publique des Hôpitaux de Paris (APHP), Groupe Hospitalier Pitié-Salpêtrière, Neurology Department, Paris, France; Olivier Gout from Hôpital Fondation Adolphe de Rothschild, Neurology Department, Paris, France; Philippe Niclot from Hôpital René-Dubos, Neurology Department, Pontoise, France; and Michele Levasseur, Groupe Hospitalier Nord-Essonne, Neurology Department, Orsay, France, for their contribution to this study.
References
1. Burnett R, Chen H, Szyszkowicz M, Fann N, Hubbell B, Pope CA, et al. Global estimates of mortality associated with long-term exposure to outdoor fine particulate matter. Proc Natl Acad Sci USA. (2018) 115:9592–7. doi: 10.1289/isesisee.2018.S02.04.33
2. Brunekreef B, Forsberg B. Epidemiological evidence of effects of coarse airborne particles on health. Eur Respir J. (2005) 26:309–18. doi: 10.1183/09031936.05.00001805
3. Calderón-Garcidueñas L, Solt AC, Henríquez-Roldán C, Torres-Jardón R, Nuse B, Herritt L, et al. Long-term air pollution exposure is associated with neuroinflammation, an altered innate immune response, disruption of the blood-brain barrier, ultrafine particulate deposition, and accumulation of amyloid beta-42 and alpha-synuclein in children and young adults. Toxicol Pathol. (2008) 36:289–310. doi: 10.1177/0192623307313011
4. Kirschbaum K, Sonner JK, Zeller MW, Deumelandt K, Bode J, Sharma R, et al. In vivo nanoparticle imaging of innate immune cells can serve as a marker of disease severity in a model of multiple sclerosis. Proc Natl Acad Sci USA. (2016) 113:13227–32. doi: 10.1073/pnas.1609397113
5. Elder A, Gelein R, Silva V, Feikert T, Opanashuk L, Carter J, et al. Translocation of inhaled ultrafine manganese oxide particles to the central nervous system. Environ Health Perspect. (2006) 114:1172–8. doi: 10.1289/ehp.9030
6. Tsai T-L, Lin Y-T, Hwang B-F, Nakayama SF, Tsai C-H, Sun X-L, et al. Fine particulate matter is a potential determinant of Alzheimer's disease: A systemic review and meta-analysis. Environ Res. (2019) 177:108638. doi: 10.1016/j.envres.2019.108638
7. Yuan S, Wang J, Jiang Q, He Z, Huang Y, Li Z, et al. Long-term exposure to PM2.5 and stroke: a systematic review and meta-analysis of cohort studies. Environ Res. (2019) 177:108587. doi: 10.1016/j.envres.2019.108587
8. Seelen M, Toro Campos RA, Veldink JH, Visser AE, Hoek G, Brunekreef B, et al. Long-term air pollution exposure and amyotrophic lateral sclerosis in netherlands: a population-based case-control study. Environ Health Perspect. (2017) 125:097023. doi: 10.1289/EHP1115
9. Tateo F, Grassivaro F, Ermani M, Puthenparampil M, Gallo P. PM2.5 levels strongly associate with multiple sclerosis prevalence in the Province of Padua, Veneto Region, North-East Italy. Mult Scler Houndmills Basingstoke Engl. (2019) 25:1719–27. doi: 10.1177/1352458518803273
10. Bergamaschi R, Monti MC, Trivelli L, Mallucci G, Gerosa L, Pisoni E, et al. PM2.5 exposure as a risk factor for multiple sclerosis. An ecological study with a Bayesian mapping approach. Environ Sci Pollut Res Int. (2020) 28:2804–9. doi: 10.1007/s11356-020-10595-5
11. Noorimotlagh Z, Azizi M, Pan H-F, Mami S, Mirzaee SA. Association between air pollution and Multiple Sclerosis: a systematic review. Environ Res. (2020) 196:110386. doi: 10.1016/j.envres.2020.110386
12. Oikonen M, Laaksonen M, Laippala P, Oksaranta O, Lilius E-M, Lindgren S, et al. Ambient air quality and occurrence of multiple sclerosis relapse. Neuroepidemiology. (2003) 22:95–9. doi: 10.1159/000067108
13. Angelici L, Piola M, Cavalleri T, Randi G, Cortini F, Bergamaschi R, et al. Effects of particulate matter exposure on multiple sclerosis hospital admission in Lombardy region, Italy. Environ Res. (2016) 145:68–73. doi: 10.1016/j.envres.2015.11.017
14. Roux J, Bard D, Le Pabic E, Segala C, Reis J, Ongagna J-C, et al. Air pollution by particulate matter PM10 may trigger multiple sclerosis relapses. Environ Res. (2017) 156:404–10. doi: 10.1016/j.envres.2017.03.049
15. Bergamaschi R, Cortese A, Pichiecchio A, Berzolari FG, Borrelli P, Mallucci G, et al. Air pollution is associated to the multiple sclerosis inflammatory activity as measured by brain MRI. Mult Scler Houndmills Basingstoke Engl. (2018) 24:1578–84. doi: 10.1177/1352458517726866
16. Xing Y-F, Xu Y-H, Shi M-H, Lian Y-X. The impact of PM2.5 on the human respiratory system. J Thorac Dis. (2016) 8:E69–74. doi: 10.3978/j.issn.2072-1439.2016.01.19
17. Menut L, Bessagnet B, Khvorostyanov D, Beekmann M, Blond N, Colette A, et al. CHIMERE 2013: a model for regional atmospheric composition modelling. Geosci Model Dev. (2013) 6:981–1028. doi: 10.5194/gmd-6-981-2013
18. Réseau Sentinelles > France > Accueil. Available online at: https://websenti.u707.jussieu.fr/sentiweb/ (accessed December 7, 2019).
19. Accueil — Le Réseau National de Surveillance Aérobiologique — RNSA. Available online at: https://www.pollens.fr/ [accessed December7, 2019).
20. Absinta M, Nair G, Sati P, Cortese ICM, Filippi M, Reich DS. Direct MRI detection of impending plaque development in multiple sclerosis. Neurol Neuroimmunol Neuroinflammation. (2015) 2:e145. doi: 10.1212/NXI.0000000000000145
21. Jeanjean M, Bind M-A, Roux J, Ongagna J-C, de Sèze J, Bard D, et al. Ozone, NO2 and PM10 are associated with the occurrence of multiple sclerosis relapses. Evidence from seasonal multi-pollutant analyses. Environ Res. (2018) 163:43–52. doi: 10.1016/j.envres.2018.01.040
22. Chen H, Kwong JC, Copes R, Tu K, Villeneuve PJ, van Donkelaar A, et al. Living near major roads and the incidence of dementia, Parkinson's disease, and multiple sclerosis: a population-based cohort study. Lancet Lond Engl. (2017) 389:718–26. doi: 10.1016/S0140-6736(16)32399-6
23. Bai L, Burnett RT, Kwong JC, Hystad P, van Donkelaar A, Brook JR, et al. Long-term exposure to air pollution and the incidence of multiple sclerosis: a population-based cohort study. Environ Res. (2018) 166:437–43. doi: 10.1016/j.envres.2018.06.003
24. Palacios N, Munger KL, Fitzgerald KC, Hart JE, Chitnis T, Ascherio A, et al. Exposure to particulate matter air pollution and risk of multiple sclerosis in two large cohorts of US nurses. Environ Int. (2017) 109:64–72. doi: 10.1016/j.envint.2017.07.013
25. Miyata R, van Eeden SF. The innate and adaptive immune response induced by alveolar macrophages exposed to ambient particulate matter. Toxicol Appl Pharmacol. (2011) 257:209–26. doi: 10.1016/j.taap.2011.09.007
26. Nemmar A, Holme JA, Rosas I, Schwarze PE, Alfaro-Moreno E. Recent advances in particulate matter and nanoparticle toxicology: a review of the in vivo and in vitro studies. BioMed Res Int. (2013) 2013:279371. doi: 10.1155/2013/279371
27. Odoardi F, Sie C, Streyl K, Ulaganathan VK, Schläger C, Lodygin D, et al. T cells become licensed in the lung to enter the central nervous system. Nature. (2012) 488:675–9. doi: 10.1038/nature11337
28. Rosso M, Chitnis T. Association between cigarette smoking and multiple sclerosis: a review. JAMA Neurol. (2019) 77:245–53. doi: 10.1001/jamaneurol.2019.4271
29. Ganguly K, Ettehadieh D, Upadhyay S, Takenaka S, Adler T, Karg E, et al. Early pulmonary response is critical for extra-pulmonary carbon nanoparticle mediated effects: comparison of inhalation versus intra-arterial infusion exposures in mice. Part Fibre Toxicol. (2017) 14:19. doi: 10.1186/s12989-017-0200-x
30. Cortese A, Lova L, Comoli P, Volpe E, Villa S, Mallucci G, et al. Air pollution as a contributor to the inflammatory activity of multiple sclerosis. J Neuroinflammation. (2020) 17:334. doi: 10.1186/s12974-020-01977-0
31. Wei H, Feng Y, Liang F, Cheng W, Wu X, Zhou R, et al. Role of oxidative stress and DNA hydroxymethylation in the neurotoxicity of fine particulate matter. Toxicology. (2017) 380:94–103. doi: 10.1016/j.tox.2017.01.017
32. Ehsanifar M, Tameh AA, Farzadkia M, Kalantari RR, Zavareh MS, Nikzaad H, et al. Exposure to nanoscale diesel exhaust particles: Oxidative stress, neuroinflammation, anxiety and depression on adult male mice. Ecotoxicol Environ Saf. (2019) 168:338–47. doi: 10.1016/j.ecoenv.2018.10.090
33. Haider L, Fischer MT, Frischer JM, Bauer J, Höftberger R, Botond G, et al. Oxidative damage in multiple sclerosis lesions. Brain J Neurol. (2011) 134(Pt 7):1914–24. doi: 10.1093/brain/awr128
34. Péry ARR, Brochot C, Hoet PHM, Nemmar A, Bois FY. Development of a physiologically based kinetic model for 99m-technetium-labelled carbon nanoparticles inhaled by humans. Inhal Toxicol. (2009) 21:1099–107. doi: 10.3109/08958370902748542
35. Calderón-Garcidueñas L, Cross JV, Franco-Lira M, Aragón-Flores M, Kavanaugh M, Torres-Jardón R, et al. Brain immune interactions and air pollution: macrophage inhibitory factor (MIF), prion cellular protein (PrP(C)), Interleukin-6 (IL-6), interleukin 1 receptor antagonist (IL-1Ra), and interleukin-2 (IL-2) in cerebrospinal fluid and MIF in serum differentiate urban children exposed to severe vs. low air pollution. Front Neurosci. (2013) 7:183. doi: 10.3389/fnins.2013.00183
36. Kalincik T, Vivek V, Jokubaitis V, Lechner-Scott J, Trojano M, Izquierdo G, et al. Sex as a determinant of relapse incidence and progressive course of multiple sclerosis. Brain J Neurol. (2013) 136(Pt 12):3609–17. doi: 10.1093/brain/awt281
37. Yitshak Sade M, Novack V, Ifergane G, Horev A, Kloog I. Air Pollution and Ischemic Stroke Among Young Adults. Stroke. (2015) 46:3348–53. doi: 10.1161/STROKEAHA.115.010992
38. Tinghög P, Hillert J, Kjeldgård L, Wiberg M, Glaser A, Alexanderson K. High prevalence of sickness absence and disability pension among multiple sclerosis patients: a nationwide population-based study. Mult Scler Houndmills Basingstoke Engl. (2013) 19:1923–30. doi: 10.1177/1352458513488234
Keywords: multiple sclerosis, relapse, air pollution, particulate matter 2.5 μm, young
Citation: Januel E, Dessimond B, Colette A, Annesi-Maesano I and Stankoff B (2021) Fine Particulate Matter Related to Multiple Sclerosis Relapse in Young Patients. Front. Neurol. 12:651084. doi: 10.3389/fneur.2021.651084
Received: 08 January 2021; Accepted: 13 April 2021;
Published: 21 May 2021.
Edited by:
Valentina Tomassini, University of Studies G. d'Annunzio Chieti and Pescara, ItalyReviewed by:
Andreia Barroso, Brigham and Women's Hospital and Harvard Medical School, United StatesCarlo Pozzilli, Sapienza University of Rome, Italy
Copyright © 2021 Januel, Dessimond, Colette, Annesi-Maesano and Stankoff. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Edouard Januel, ZWRvdWFyZC5qYW51ZWwmI3gwMDA0MDthcGhwLmZy
†These authors have contributed equally to this work
 Edouard Januel
Edouard Januel Boris Dessimond
Boris Dessimond Augustin Colette
Augustin Colette Isabella Annesi-Maesano
Isabella Annesi-Maesano Bruno Stankoff
Bruno Stankoff