- 1Neuropsychology Unit, IRCCS Istituto Centro San Giovanni di Dio Fatebenefratelli, Brescia, Italy
- 2IRCCS, Fondazione don Carlo Gnocchi—ONLUS, Milan, Italy
- 3Macroattività Ambulatoriale Complessa (MAC) Memory Clinic and Molecular Markers Laboratory, IRCCS Istituto Centro San Giovanni di Dio Fatebenefratelli, Brescia, Italy
- 4Alzheimer's Research Unit, Macroattività Ambulatoriale Complessa (MAC) Memory Clinic, IRCCS Istituto Centro San Giovanni di Dio Fatebenefratelli, Brescia, Italy
Background: In recent years, emphasis has been placed on cognitive enhancement to stimulate cognitive abilities and prevent functional decline. Considering that traditional face-to-face interventions can be very expensive and are not accessible to all individuals, the need to transfer care from the clinic to the patient's home is evident. In this regard, cognitive tele-enhancement interventions have received increased attention.
Aim: The aim of this review was to provide an overview of protocols that apply remotely controlled cognitive training with individualized feedback on performance by the therapist in healthy older adults or participants with subjective memory complaints.
Methods: Out of 35 articles assessed for eligibility, eight studies were identified. Of the selected studies, five included cognitively healthy older adults, while three included participants with subjective memory complaints.
Results: Most of the reviewed studies showed beneficial effects of cognitive tele-enhancement interventions, reporting improvements in memory, sustained attention, working memory, executive functions, and language abilities. Moreover, reductions in anxiety and depression symptomatology levels, as well as in subjective memory difficulties, were described in some of the studies.
Conclusions: Cognitive tele-enhancement treatment could be a good alternative to face-to-face intervention. This literature review highlights the importance of applying preventive cognitive interventions to subjects with initial subjective memory complaints. Remote modalities seem to facilitate the application of such interventions.
Introduction
The world's population is aging, requiring us to face new challenges. Improvements in medical care have led to a consistent increase in the average age, and it is estimated that the elderly population will increase in the future (1, 2). In Europe, the proportion of people aged 65 or over is expected to double in the next 25 years (3).
Healthy cognitive functioning is a primary condition of well-being, independence, and successful aging (4–6). In recent years, promoting active and healthy aging has become an important challenge to ensure good physical, social, and cognitive functioning. However, aging is often associated with cognitive decline related to impairment of memory, language, judgment, and executive functions (7). In light of these considerations, it is obvious that the most at-risk population is elderly individuals, who often report memory complaints in the absence of objective deficits from neuropsychological assessment and psychometric tests (8). Although these cases have been extensively described, it is only in recent years that this condition, better known as “subjective memory complaints” (SMCs), has received increasing interest as a consequence of greater attention in characterizing the preclinical stages of Alzheimer's disease (AD) (9). Indeed, many epidemiological studies have shown that this category of patients is associated with a higher risk of developing mild cognitive impairment (MCI) or AD (10).
At present, pharmacological therapies to prevent cognitive decline have not had great success, and, consequently, much emphasis has been placed on cognitive training to stimulate cognitive abilities and prevent functional decline. The term “brain plasticity” is used to refer to the ability of the human brain to develop continuously through daily experience and the active maintenance of cognitive functions (11–13) and, from a neurorehabilitative perspective, to respond to intrinsic or extrinsic stimuli by reorganizing its structure, function, and connections (14). Several studies have indicated a wide range of activities that can induce brain plasticity; among these, physical exercise and cognitive stimulation were the most suitable (15, 16). Cognitive training refers to a standardized and systematic set of exercises aimed at stimulating specific cognitive domains: attention, memory, language, and executive functions (17, 18). Cognitive training is a promising approach for slowing cognitive decline and provides the potential to generalize the benefits to other domains that are not directly stimulated and to maintain the results over time (19–24). Traditional forms of cognitive programs are delivered in person by a therapist, but traditional face-to-face treatment can be very expensive and is not easily accessible due to several barriers, such as difficulty in reaching the therapist's office, physical or financial difficulties, or lack of caregivers (25). In the last few years, the need to transfer care from the clinic to the patient's home to overcome these barriers and to introduce sustainable and accessible care treatments for all has become increasingly evident (26–28). The World Health Organization defines “telemedicine” as “the delivery of health care services, where distance is a critical factor, by all health care professionals using information and communication technologies for the exchange of valid information for diagnosis, treatment and prevention of disease and injuries, research and evaluation, and for the continuing education of health care providers, all in the interest of advancing the health of individuals and their communities” (29).
A subcategory of the broader concept of telemedicine is telerehabilitation. The term telerehabilitation commonly refers to any type of home-based intervention in which digital technology (mobile phones, video, sensors, online platforms, etc.) allows for a double communication loop between the hospital and the patient (30). The presence of the “double loop” represents an essential requirement of telerehabilitation that enables both remote monitoring of patient performance and responding with appropriate feedback (26, 30). Recently, with the advancement of technology and following the elimination of constraints due to temporal and spatial limits, “cognitive telerehabilitation” has become more popular in the fields of cognitive assessment and/or intervention for patients with neurological disorders (26, 27, 31, 32). Dodakian et al. (33) tested an asynchronous home-based telerehabilitation program in patients with stroke and found good compliance by participants; likewise, a randomized controlled trial involving subjects with MCI or mild-to-moderate AD used a tablet to deliver an asynchronous, personalized rehabilitation plan with both cognitive and aerobic physical exercise providing feedback through weekly phone calls with the aim of observing the efficacy of a technology-enhanced home care service to preserve cognitive and motor levels of functioning (34). Most importantly, as Cotelli et al. (31) showed in their systematic review, telerehabilitation can be as effective as face-to-face interventions. Many studies have shown that a key component of the successful application of cognitive telerehabilitation systems is the use of feedback from the therapist on the performance ability of patients, which has a positive influence on their performance, engagement, and motivation (35). In fact, clinicians, even if at distance, using synchronous (in which patient and clinician perform activities in real-time, for example, through videoconference) or asynchronous (in which patient and therapist work offline with virtual therapists or digital contents) treatment modalities, can directly work with patients to set goals that are reasonable and achievable. The possibility of digital contents, both to collect several parameters associated with user's performance and to implement adaptive training, allows reaching a balance between individual resources and the difficulty level of rehabilitative activities also in the asynchronous telerehabilitation model. Moreover, the asynchronous modality permits the patient to organize their time to dedicate some to rehabilitation with a certain degree of freedom, allowing the therapist to prescribe high-intensity training for the long-term in a wide number of older adults (34, 36). In this way clinicians can help subjects believe they have the skills and ability to reach their goals and, finally, provide positive reinforcement (37) using online or offline monitoring and feedback modalities.
Although this training was initially mainly dedicated to neurological patients, the focus has shifted to the prevention of cognitive decline (38, 39). Accordingly, a less investigated but certainly no less interesting area is the application of tele-enhancement cognitive interventions following the positive trajectory of aging in older adults who are not necessarily subject to cognitive and/or physical decline. In light of new technologies, cognitive training can now be administered remotely as a nonpharmacological treatment in patients with dementia (31) and as a preventative intervention in healthy adults. Thus, the aim of this review was to identify and provide an overview of treatments in which the double loop communication approach was implemented (i.e., cognitive training remotely controlled by a therapist and in which feedback on training performance was given) in healthy older adults and in participants with SMC.
Review of the Literature
Search Strategies and Study Selection Criteria
We searched the literature in the Medline (PubMed) database using the following terms: “[Telehealth OR Tele enhancement OR (Online AND training) OR (home AND computer OR web)] AND (Cognitive AND intervention) AND [(healthy AND aging) OR (older AND adults) OR (subjective AND memory AND complaints)].” We reviewed all titles and abstracts and examined all relevant original research articles. Additionally, the references of the articles were examined to identify possible additional sources. Only English-language articles were selected (see flowchart in Figure 1). For study selection, we primarily analyzed the title and abstract of the paper. From this first analysis, animal studies, reports of secondary data, such as meta-analyses, reviews, or letters, usability studies, and study protocols were excluded. Thereafter, we proceeded to read the full texts of the remaining articles. The following criteria were applied in the final selection of the studies: (a) original research; (b) conducted on cognitively healthy older adult subjects and/or participants with SMC; (c) comprising at-home tele-enhancement cognitive interventions; (d) providing at least one cognitive outcome; (e) comprising a group level analysis; and (f) published before September 29, 2020. Moreover, studies that included only participants with objective cognitive decline or subjects with other neurological or psychiatric disorders, as well as studies in which cognitive training was not administrated, were not selected for our review. In addition, we excluded studies that did not investigate cognitive training gains through quantitative or subjective cognitive measures. Based on the concept of tele-enhancement, moreover, we also excluded studies that did not provide the possibility for the research assistant to remotely monitor the training and give personalized feedback on training performance to the participant (see in Figure 1).
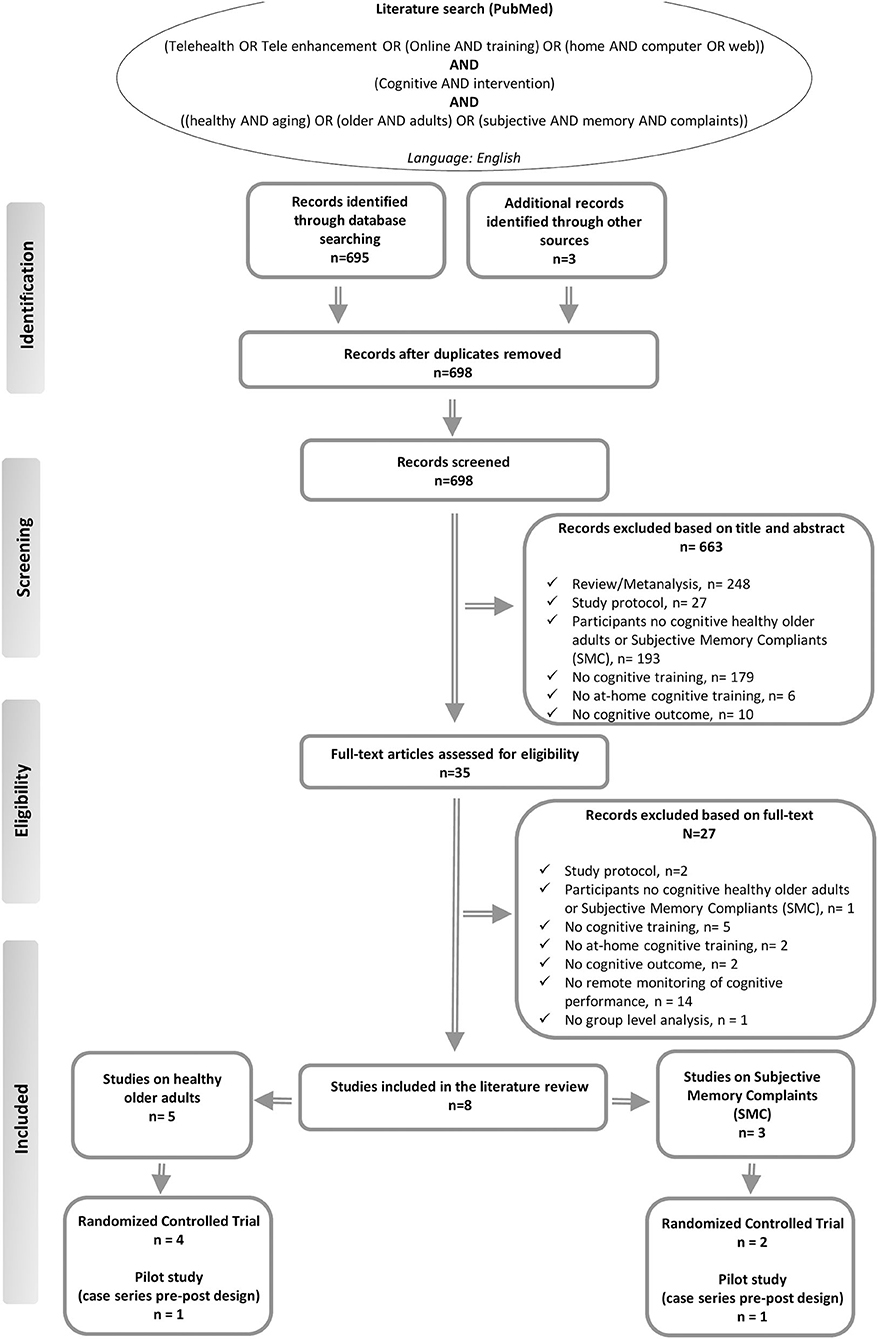
Figure 1. Summary of the literature search—PRISMA flow diagram (48).
Baseline Characteristics of Included Studies
Out of 35 full-text articles assessed for eligibility, only eight studies published between 2012 and 2019 fulfilled the criteria for inclusion in this literature review. Of the selected studies, five included cognitively healthy older adults, while three included participants with SMC. In all selected studies, at least one group in which the participants' training performances were remotely monitored by the therapist was present.
Summarizing, a total of 461 cognitively healthy older adults were enrolled in five selected studies (see Table 1).

Table 1. Studies that have assessed the effects of cognitive tele-enhancement interventions on cognitive functions in healthy older adults.
Four out of five studies on cognitively healthy older adults are Randomized Controlled Trial (RCT) and have an active or passive control group; whereas one is a pilot study (case series pre-post design) without a control group (40). In all these studies, the effects of a single-user and asynchronous tele-enhancement approach based on computerized cognitive training were investigated. Moreover, the tele-enhancement interventions were remotely offline controlled in all studies. Regarding the feedback, it was provided offline in most of the studies included, except for Rebok et al. (18), where a facilitator was available by phone or Internet for assistance during the training. The effects of the computerized cognitive treatment were evaluated on neuropsychological test; moreover, subjective measures were included in the studies of Rebok et al. (18), Brehmer et al. (20), and Vermeij et al. (40). Furthermore, all the selected works investigated the maintenance of the training gains over time (from 1 to 6 months follow-up).
Regarding the studies on SMC participants, a total of 175 subjects were enrolled in the three selected articles (see Table 2). Two randomized controlled trials with at least a control group (active or passive) (38, 41) and one pilot study (case series pre-post design) without a control group were included (42). All the selected studies investigated the effectiveness of a tele-enhancement approach based only on computerized cognitive training, except for the study of Pereira-Morales et al. (41), in which one experimental group (IPP, Integrated Psycho-stimulation Program group) received computerized cognitive training associated with traditional pen and paper cognitive training. In addition, in all studies, the modality for tele-enhancement intervention was single-user and asynchronous with remote offline monitoring and feedback. Furthermore, all studies examined the training effects on cognitive objective and subjective measures. Finally, none of the included studies investigated the long-term effects of the treatment.
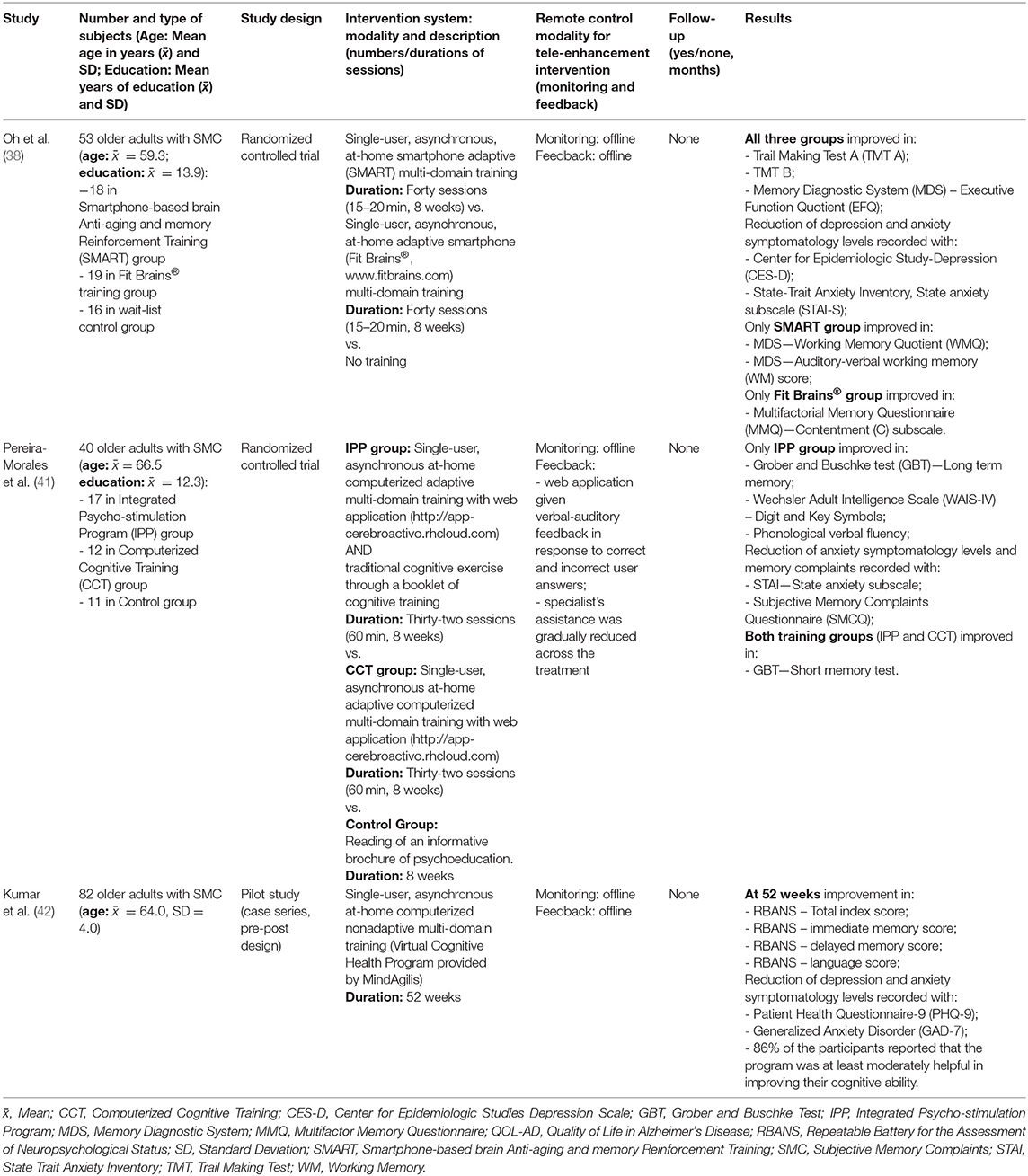
Table 2. Studies that assessed the effects of tele-enhancement cognitive interventions on cognitive functions in subjects with subjective memory complaints.
Healthy Older Adults
In recent years, cognitive tele-enhancement interventions in healthy older adults based on remote at-home training assistance have become more popular.
A randomized controlled trial of Brehmer et al. (20) investigated the effects of 5 weeks of intensive domain-general adaptive (e.g., individualized adjustment of task difficulty levels) WM training in comparison to low-level practice (constant low task difficulty level) WM training in younger (age: 20–30 years, education: 15.0 years) and older participants (age: 60–70 years, mean of education: 15.4 years). The tele-enhancement approach consisted of a WM training (Cogmed QM software (https://www.cogmed.com) practiced at-home, administrated for 20–25 sessions over 5 weeks (single-user type and asynchronous). In particular, the training involved the following tasks: (i) maintenance of multiple stimuli at the same time, (ii) short delays during which the representation of stimuli should be held in WM, and (iii) unique sequencing of stimuli order in each trial. Remote offline monitoring was performed and offline feedback on the training performance was provided once a week via email for all participants. The benefits of the WM training were evaluated assessing the performance obtained during training and the score achieved in a series of neuropsychological measures. Gains were assessed using criterion, near-transfer, and far-transfer tasks before training, after 5 weeks of intervention, as well as after a 3-month time interval. Group-level analyses showed that younger and older adults improved their performance across the sessions of adaptive WM training. Both younger and older adults gained more in criterion (Digit Span backward and Span Board forward) and near-transfer tasks (Digit Span forward and Span Board backward) after adaptive WM training in comparison to low-level practice WM training. Regarding far-transfer tasks, similar performance improvements for the adaptive and the low-level practice training were observed for tests of interference control (Stroop-color word) and reasoning (Raven's Progressive Matrices). Both younger and older adults gained more after adaptive WM training than after low-level practice WM training in a test measuring sustained attention (PASAT, Paced Auditory Serial Addition Task). Moreover, the individuals that underwent the adaptive WM training reported a higher reduction of memory complaints on the cognitive functioning scale (CFQ). Overall, at post-treatment assessment, younger participants showed greater improvement than older adults in all neuropsychological measures. Interestingly, the observed results were maintained across 3-month follow-up, re-administering the same tests of the baseline.
In a randomized controlled trial, Anguera et al. (43) assessed whether the application of an adaptive custom-designed 3D videogame (Neuroracer), developed to challenge perceptual discrimination and visuomotor tracking, led to enhancements of cognitive control abilities in a sample of healthy older adults (age: 60–85 years, mean of education: 16.8 years). In total, 46 participants were randomized into three groups: Multitasking Training (MTT; n = 16), Singletask Training (STT; n = 15), and No-Contact Control group (NCC; n = 15). For participants in the experimental group (MTT) and in the active control group (STT) a tele-enhancement approach based on Neuroracer was administrated over 12 at-home sessions (over 4 weeks). The modality of the training was single-user, asynchronous, and adaptive (e.g., the difficulty level was adjusted to the individual performance across the training). However, the groups differed for the tasks proposed during the training: the MTT group played only the “Sign and Drive” condition (respond as rapidly as possible to the appearance of a sign only when a green circle was present while maintaining a car in the center of a winding road using a joystick); whereas the STT group performed “Sign Only” condition or “Drive Only” condition. Remote offline monitoring was delivered and participants were contacted weekly by phone or email to encourage and discuss in an offline way their training results. Otherwise, the participants in the NCC group did not receive any treatment. The effects of the training were investigated on training performance measures and on cognitive control domain tasks after 4 weeks from baseline (corresponded to the end of the treatment for MTT and STT groups), and at 6-month follow-up, re-administering the same tests of the baseline. At the group level, the analysis showed that both MTT and STT equally improved their performances across the training sessions. However, at post-training assessment only the MTT group significantly improved in Delayed-recognition working memory and in sustained attention tasks (Test of Variables of Attention, T.O.V.A). Likewise, only the MTT group maintained their improvements on training performance at 6-month follow-up.
In a pilot study (case series pre-post design), Vermeij et al. (40) applied a WM training (Cogmed QM software, https://www.cogmed.com) in 23 healthy older adults (age: 63–81 years, mean of education: 14.4 years) and 18 MCI subjects (age: 59–85 years, mean of education: 13.6 years). Specifically, all participants received 25 sessions (over 5 weeks) of an at-home tele-enhancement approach, administrated in single-user, asynchronous, and adaptive modalities (e.g., the difficulty level was adjusted to the performance of the individual participant by increasing or decreasing the number of items). A control group (active or passive) was not included. Regarding the WM training program, it included a total of 12 exercises (i.e., recall a series of verbally presented numbers and hidden numbers in reverse order; recall a series of verbally presented letters; recall verbally presented letters and their location; recall a series of locations presented, rotating 4 × 4 grid, rotating 3D grid, rotating cube, and rotating circle; recall the order in which moving objects lit up; recall and sort numbers in a 4 × 4 grid; recall the order of presented circles and respond within a certain time frame). In total, eight tasks were chosen for each training session. Remote offline monitoring and weekly offline feedback (given by telephone) were performed. The changes in performance achieved during training as well as on neuropsychological tests (both near-transfer and far-transfer tasks) were considered to quantify the training gains at the end of the treatment and at a 3-month follow-up. During cognitive assessments, parallel versions of tests were administered when available. Furthermore, before the beginning of the treatment, each participant was asked to formulate three personalized goals concerning attention-related or WM-related daily activities. At the end of the treatment and during the follow-up visit each participant was asked to rate his/her personalized goals' performance on a scale from 1 (very far from achieving the goal) to 10 (goal completely achieved), as a subjective measure of training gains. In addition, participants completed the Cognitive Failures Questionnaire in order to evaluate daily life difficulties. Group-level analysis revealed an improvement of training performance for both groups, with larger gains in healthy older adults than MCI. However, both groups showed comparable training gains on near-transfer tasks (WAIS-III Digit Span forward, WAIS-III Digit Span backward, and WMS-III Spatial Span backward) at the post-training assessment. Specifically, healthy older adults showed an improvement on Digit Span forward and on Spatial Span backward tasks, while Digit Span backward and Spatial Span backward scores were enhanced in MCI. Regarding the far-transfer effect, in both healthy older adults and MCI subjects, significant gains on the Ruff Figural Fluency Task (RFFT) were observed. Moreover, both groups reported the achievement of the three personalized goals. The gains on near-transfer tasks and on subjective memory measures were maintained at 3-month follow-up for both groups, whereas only healthy older group maintained the training benefit on RFFT (far-transfer task) at follow-up. Otherwise, at an individual level, a limited number of participants showed reliable training gains only on near-transfer tasks.
In a double-blind randomized controlled trial, Buitenweg et al. (21) investigated whether a 12-week tele-enhancement approach based on a computerized cognitive flexibility training (using games available on website www.braingymmer.com), impacted the cognitive functions in 139 healthy older adults (age: 60–80 years; mean of education: 5.8 years). Participants practiced a single-user type and asynchronous at-home training (60 sessions of 30 min over 12 weeks). Remote offline monitoring was performed, and offline feedback was given weekly or biweekly via telephone. Subjects were randomly assigned to three groups: Frequent Switching (FS, n = 56), Infrequent Switching (IS, n = 33), or Mock Training (MT, n = 50). Specifically, for participants in the experimental groups (FS and IS) adaptive games of reasoning, working memory, and attention were selected, but they differed for the frequency of task switching: in each training session, the FS group was asked to perform 10 games of 3 min each, while the IS group was requested to play three games of 10 min each. Otherwise, for subjects in the active control group (MT group), nonadaptive games were chosen, which put minimal demands on executive functions (three games of 10 min each/session). The effects on training performance, as well as on neuropsychological measures, were evaluated after the training and at the 1-month follow-up. In all assessments, parallel tests were used when available. In this study, principal and secondary analyses were conducted, and in both cases the level of significance was adjusted for multiple comparisons (Bonferroni corrections). At the group level, an improvement of training performance across the sessions was found for all groups, although FS and IS groups more significantly improved than the MT group. In addition, all the three groups significantly improved their performances on switching tasks (switch task and dual task), on cognitive flexibility tests (Delis–Kaplan Executive Function System, D-KEFS Trail Making Test-4, TMT-4, and TMT-B- online version), on psychomotor speed measures (Drag-and-drop; Drag-to-grid; Click task; and TMT-A-online version), on processing speed tasks (Digit Symbol Coding, DSC; DSC—online version; Tower of London, ToL), on reasoning measure (Shipley Institute of Living Scale, SILS), on working memory (PASAT), and on verbal long term memory tests (Rey Auditory Verbal Learning Test, RAVLT). The gains in most of the tests that improved post-training were maintained at 1-month follow-up for all groups.
Recently, Rebok et al. (18) in a pilot randomized controlled trial compared the effects of a web-based and classroom-based memory training (10 sessions of 60–75 min of mnemonic skills training over 5–6 weeks). Healthy older adult participants (mean age: 71.1 years; mean of education: 15.5 years) were randomized into three groups: web-based (n = 70), classroom (n = 69), and wait-list control (n = 69) groups. An at-home, single-user type and asynchronous tele-enhancement approach using Active Memory Works (AMW) was administrered to the web-based group. Remote online monitoring was performed and a facilitator was available by phone or Internet for assistance. For the classroom group, online monitoring was applied, and a facilitator was available during in-person mnemonic skills training aimed at remembering lists and sequences of items, text materials, and main ideas and details of stories and other text-based information. The content of the web-based and of the classroom group was matched for the first five sessions focusing on strategy training and for the last five sessions incorporating review and practice of previously learned techniques. Otherwise, the participants in the wait-list control group did not receive any treatment. All three groups completed assessment on objective and subjective measures of cognitive functioning and on everyday functioning scales at baseline, after 6 weeks (corresponded to the end of the treatment for web-based and classroom groups) and at 6-month follow-up. In order to reduce the learning effect, outcome measures included also parallel versions of the Rey Auditory Learning Test (RAVLT) and of the Rivermead Behavioral Memory Test (RBMT). The group-level analysis showed that participants, regardless of group assignment, achieved a significant improvement on objective cognitive tests (RAVLT, RBMT—Paragraph Recall; Fluency test; Animal naming; Word series; and Digit Symbol Substitution test, DSST) and on subjective memory scales (Metamemory in Adulthood, MIA and Memory Functioning Questionnaire, MFQ). The gains were maintained for all three groups at the 6-month follow-up. Moreover, the web-based and classroom-based training groups reported comparable satisfaction with the program. In conclusion, this study suggested that web-based training is an acceptable and feasible mode to provide memory training in healthy older adults and its effects are comparable to in-person training.
Subjective Memory Complaint
Investigation of the efficacy of cognitive training delivered remotely has also been extended to subjects who experience a subjective decline in cognitive functions.
In this regard, Oh et al. (38) in a randomized controlled trial study, examined the effects of 8 weeks of a tele-enhancement approach based on smartphone cognitive training in participants that reported memory difficulties at Subjective Memory Complaints Questionnaire (SMCQ). A total of 53 SMC adults (age: 50–68 years; education: 6–18 years) were randomly assigned to one of three groups: Smartphone-based brain Anti-aging and Memory Reinforcement Training group (SMART, n = 18), Fit Brains® group (n = 19), and wait-list control group (n = 16). For SMART and Fit Brains® groups the intervention was administrated on smartphone for 40 sessions over 8 weeks and was in single-user and in asynchronous modality. Remote offline monitoring was performed and offline feedback was provided weekly via phone calls. Specifically, the SMART training included two nonadaptive and eight adaptive tasks aimed to improve attention, memory, and working memory abilities; whereas the Fit Brains® treatment (www.fitbrains.com) included adaptive tasks for several cognitive domains (e.g., attention, memory, and logic). Otherwise, no cognitive intervention was provided to participants in the wait-list control group. Training gains were evaluated on neuropsychological tests and on self-reported questionnaires on subjective memory complaints, depression, and anxiety. Group-level analyses showed that all participants, regardless of group assignment, improved in terms of the Trail Making Test (part A and B) and the MDS—Executive Functions Quotient (EFQ). Moreover, we found a decrease in depression and anxiety levels, evaluated with the Center for Epidemiologic Study-Depression (CES-D), and State-Trait Anxiety Inventory, State anxiety subscale (STAI-S) scales, respectively. Only the SMART group significantly improved their performance on WM tests (Memory Diagnostic System, MDS—Working Memory Quotient, WMQ and MDS—Auditory-verbal working memory, WM score). Otherwise, a significant improvement on a self-report questionnaire [Multifactorial Memory Questionnaire, MMQ—Contentment (C) subscale] was found only in the Fit Brains® group.
In a randomized controlled trial study, Pereira-Morales et al. (41) investigated the effectiveness of a computerized cognitive training program in a group of 40 older adults with subjective memory complaints, evidenced at the SMCQ (mean age: 66.5 years; mean of education: 12.3 years). In this study, participants were randomly assigned to an Integrated Psychostimulation Program (IPP, n = 17), to a Computerized Cognitive Training (CCT, n = 12), or to a control group (n = 11). All groups performed their activities over 8 weeks in at-home, single-user, and asynchronous modalities. Only for experimental groups (IPP and CCT) the computerized training was adaptive (e.g., adjustment of difficulty level on user's was performances) and remote offline monitoring was performed. Verbal-auditory feedback was given by web application and specialist's assistance was gradually reduced across the treatment sessions. The IPP group underwent a tele-enhancement approach based on computerized cognitive training associated with traditional pen and paper cognitive training. The IPP training session comprised 60 min of a multi-domain (e.g., orientation, attention, memory, and executive functions) computerized cognitive training through a web application (http://app-cerebroactivo.rhcloud.com) associated with 30 min of traditional pen and paper exercises with a booklet of cognitive training (BCT). Otherwise, the CCT group received 60 min of a tele-enhancement approach based only on computerized cognitive training, using the same web application used for the IPP group. Finally, the control group was asked to read a psychoeducational informative brochure. Following the intervention, psychological, and neuropsychological measures, were administered and analyzed using group-level analyses and multiple testing correction (Bonferroni). The results indicated that the IPP group significantly improved on long-term memory (Grober and Buschke test, GBT), processing speed (Wechsler Adult Intelligence Scale, WAIS-IV—Digit and Key Symbols), and phonological verbal fluency measures. Moreover, this group showed a reduction of anxiety symptomatology level (STAI) and of memory complaints (SMCQ). Both IPP and CCT groups showed a significant improvement on a short-memory test (GBT). Otherwise, for the control group, no statistical changes were found on cognitive and clinical measures. In conclusion, these findings demonstrated that intensive training that combined computerized cognitive training and traditional cognitive training led to significant improvements in cognitive function and psychological well-being.
Kumar et al. (42), in a pilot study (case series pre-post design), evaluated in a group of 82 older adults with subjective cognitive decline (SMC, age: 60.0–74.9) the impact of an intensive (52 weeks) multidomain lifestyle intervention (Virtual Cognitive Health, VC Health program) on cognitive functions and mental health. Participants were enrolled in the study if reported subjective cognitive decline as recorded on the Subjective Cognitive Decline Questionnaire, SCD-9. A control group (active or passive) was not included. In particular, the VC Health program consisted of a tele-enhancement approach based on computerized training focused on nutrition, physical exercise, and cognitive training, designed to prevent or delay cognitive decline and impairment in older at-risk adults. More specifically, the computerized cognitive training program (provided by MindAgilis; London, England) was nonadaptive and was focused on processing speed, executive functions, working memory, episodic memory, and mental speed. Moreover, it was administrated at-home and was single-user type and asynchronous. Furthermore, remote offline monitoring and offline feedback (via telephone, email, and/or text messaging) were performed. The effects were evaluated during the treatment (at 24 weeks) and post-training (after 52 weeks) in terms of cognitive and mental health measures, using alternate forms of the tests when available (i.e., Repeatable Battery for the Assessment of Neuropsychological Status, RBANS). Group-level analysis showed a decrease in depression symptoms level (as measured by Patient Health Questionnaire, PHQ-9) at 24 weeks. At the end of the treatment (52 weeks) a significant improvement on RBANS-Total Index score and on immediate memory, language, and delayed memory RBANS sub-Index scores were observed. Furthermore, a decrease in depression (PHQ-9) and anxiety levels (Generalized Anxiety Disorder, GAD-7) was also recorded. Lastly, the 86% (51/59) of the participants who completed the treatment (n = 59) reported that the VC Health program was at least moderately helpful in improving their cognitive ability.
Discussion
To our knowledge, there are currently no other reviews that have investigated the use of cognitive enhancement treatments carried out with remote control on cognitively healthy older adults or on SMC subjects. The main purpose of the present review was to highlight studies that have used tele-enhancement and where the therapist supplied feedback on training performance to provide a general overview of treatments conducted at patient homes with remote control in healthy older adults and SMC subjects.
Only eight studies that adopted at-home tele-enhancement cognitive interventions were included in this review; specifically, five focused on cognitively healthy older adults, while three focused on participants with SMC. More specifically, only studies with double-loop communication between the clinic and home were considered because the presence of feedback on cognitive performance seems to have many benefits, especially in terms of participant motivation (41).
The results were heterogeneous due to some substantial differences between the studies. The cognitive enhancement protocols adopted by the studies included in this review focused primarily on the enhancement of different cognitive domains. In particular, some studies applied an intervention directed to specific cognitive function (18, 20, 40, 43) while the others reported the effects of a multi-domain intervention aimed at enhancing working memory, attention, reasoning, executive functions, episodic memory, and orientation (21, 38, 41, 42).
Regarding the studies on healthy older adults, three studies investigated the effects of a tele-enhancement intervention using memory training (18, 20, 40): one study applied a computerized cognitive training directed to cognitive control abilities (43), while another one applied a multi-domain cognitive training (21). Despite the differences in methodologies, types of intervention, and sample sizes, all investigations on cognitively healthy older adults found evidence for an immediate improvement on objective and/or subjective cognitive outcome measures and reported long-term effects during follow-up evaluations, demonstrating general maintenance of improvements (from 1 to 6 months).
Regarding the studies on participants with SMC, all three studies focused on the application of multi-domain tele-enhancement interventions and reported improvements in different cognitive domains, such as memory, executive functions, processing speed, and language (38, 41, 42). Moreover, a reduction in anxiety and depression symptomatology levels, as well as in subjective memory difficulties, was described in all studies (38, 41, 42). Unfortunately, none of the studies involving participants with subjective memory complaints assessed the maintenance of cognitive tele-enhancement effects over time.
However, a strength of most of the included studies was the implementation of adaptive training, which allows for reaching a balance between individual abilities and the difficulty level of rehabilitative activities, implementing a personalized treatment (20, 21, 38, 40, 41, 43). Although these results are of considerable interest, it is important to note some study limitations.
The existing research reflects a general lack of randomized clinical trials, a small sample size, a great diversity of technology utilized, and lack of common outcome measures. Moreover, selected studies were widely different in study design and cognitive treatments (number, duration and frequency of sessions, mode of tele-enhancement intervention delivery, monitoring, and feedback modality). In addition, only a few studies applied a multiple comparisons correction in the statistical analyses (21, 41). Taken together, these differences make it difficult to compare the results of the studies and to draw a final conclusion. In addition, the characterization of the cognitive profiles of the enrolled subjects was not always detailed. Since the literature has shown that subjective cognitive decline and mild cognitive impairment are associated with an increased risk of developing dementia (44–47), it is necessary to investigate in depth the cognitive profile and the presence of subjective cognitive complaints in the recruited subjects. This evidence underlines the need for future well-designed studies. Despite these critical issues, telerehabilitation appears to be an excellent opportunity for intervention to strengthen cognitive functions, with results comparable to face-to-face rehabilitation interventions. Telerehabilitation presents new opportunities to enhance cognition in older adults and in subjects with subjective memory complaints. Future studies will involve a large cohort of subjects and will allow us to evaluate whether tele-enhancement approaches could be as effective as face-to-face intervention.
Author Contributions
CA, EC, MS, EG, FB, FR, GB, OZ, RM, and MC: conception and methodology and writing—review and editing. CA, EC, RM, and MC: data curation. CA, EC, MS, EG, RM, and MC: writing—original draft preparation. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Italian Ministry of Health (Ricerca Corrente) and by Lombardy Region (Announcement POR-FESR 2014-2020—Azione I.1.B.1.3) within the project named Smart&TouchID.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Luy M, Di Giulio P, Di Lego V, Lazarevič P, Sauerberg M. Life expectancy: frequently used, but hardly understood. Gerontology. (2020) 66:95–104. doi: 10.1159/000500955
2. US Census Bureau. Older People Projected to Outnumber Children for First Time in US History. National Population Projections (2018).
4. Baltes PB, Smith J. New frontiers in the future of aging: from successful aging of the young old to the dilemmas of the fourth age. Gerontology. (2003) 49:123–35. doi: 10.1159/000067946
5. Clarke C, Woods B, Moniz-Cook E, Mountain G, Øksnebjerg L, Chattat R, Diaz A, et al. Measuring the well-being of people with dementia: a conceptual scoping review. Health Qual Life Outcomes. (2020) 18:249. doi: 10.1186/s12955-020-01440-x
6. Simon SS, Tusch ES, Feng NC, Håkansson K, Mohammed AH Daffner KR. Is computerized working memory training effective in healthy older adults? Evidence from a multi-site, randomized controlled trial. J Alzheimers Dis. (2018) 65:931–49. doi: 10.3233/JAD-180455
8. Vannini P, Hanseeuw B, Munro CE, Amariglio RE, Marshall GA, Rentz DM, et al. Hippocampal hypometabolism in older adults with memory complaints and increased amyloid burden. Neurology. (2017) 88:1759–67. doi: 10.1212/WNL.0000000000003889
9. Mendonça MD, Alves L, Bugalho P. From subjective cognitive complaints to dementia: who is at risk? A Systematic Review. Am J Alzheimers Dis Other Demen. (2016) 31:105–14. doi: 10.1177/1533317515592331
10. Perrotin A, La Joie R, de La Sayette V, Barré L, Mézenge F, Mutlu J, et al. Subjective cognitive decline in cognitively normal elders from the community or from a memory clinic: differential affective and imaging correlates. Alzheimers Dement. (2017) 13:550–60. doi: 10.1016/j.jalz.2016.08.011
11. Lee Y. The recent decline in prevalence of dementia in developed countries: implications for prevention in the Republic of Korea. J Korean Med Sci. (2014) 29:913–8. doi: 10.3346/jkms.2014.29.7.913
12. Nussbaum PJG. Brain health: bridging neuroscience to consumer application. J Am Soc Aging. (2011) 35:6–12.
13. Cabeza R, Albert M, Belleville S, Craik FIM, Duarte A, Grady CL, et al. Maintenance, reserve and compensation: the cognitive neuroscience of healthy ageing. Nat Rev Neurosci. (2018) 19:701–10. doi: 10.1038/s41583-018-0068-2
14. Cramer SC, Sur M, Dobkin BH, O'Brien C, Sanger TD, Trojanowski JQ, et al. Harnessing neuroplasticity for clinical applications. Brain. (2011) 134:1591–609. doi: 10.1093/brain/awr039
15. Bherer L. Cognitive plasticity in older adults: effects of cognitive training and physical exercise. Ann N Y Acad Sci. (2015) 1337:1–6. doi: 10.1111/nyas.12682
16. Iordan AD, Cooke KA, Moored KD, Katz B, Buschkuehl M, Jaeggi SM, et al. Neural correlates of working memory training: evidence for plasticity in older adults. Neuroimage. (2020) 217:116887. doi: 10.1016/j.neuroimage.2020.116887
17. National Academies of Sciences Engineering and Medicine. Preventing Cognitive Decline and Dementia: A Way Forward. Washington, DC: National Academies Press (2017).
18. Rebok GW, Tzuang M, Parisi JM. Comparing web-based and classroom-based memory training for older adults: the ACTIVE Memory Works™ Study. J Gerontol B Psychol Sci Soc Sci. (2020) 75:1132–43. doi: 10.1093/geronb/gbz107
19. Berry AS, Zanto TP, Clapp WC, Hardy JL, Delahunt PB, Mahncke HW, et al. The influence of perceptual training on working memory in older adults. PLoS One. (2010) 5:e11537. doi: 10.1371/journal.pone.0011537
20. Brehmer Y, Westerberg H, Bäckman L. Working-memory training in younger and older adults: training gains, transfer, and maintenance. Front Hum Neurosci. (2012) 6:63. doi: 10.3389/fnhum.2012.00063
21. Buitenweg JIV, van de Ven RM, Prinssen S, Murre JMJ, Ridderinkhof KR. Cognitive flexibility training: a large-scale multimodal adaptive active-control intervention study in healthy older adults. Front Hum Neurosci. (2017) 11:529. doi: 10.3389/fnhum.2017.00529
22. Hertzog C, Kramer AF, Wilson RS, Lindenberger U. Enrichment effects on adult cognitive development: can the functional capacity of older adults be preserved and enhanced? Psychol Sci Public Interest. (2008) 9:1–65. doi: 10.1111/j.1539-6053.2009.01034.x
23. Mahncke HW, Connor BB, Appelman J, Ahsanuddin ON, Hardy JL, Wood RA, et al. Memory enhancement in healthy older adults using a brain plasticity-based training program: a randomized, controlled study. Proc Natl Acad Sci U S A. (2006) 103:12523–8. doi: 10.1073/pnas.0605194103
24. Simon SS, Castellani M, Belleville S, Dwolatzky T, Hampstead BM, Bahar-Fuchs A. The design, evaluation, and reporting on non-pharmacological, cognition-oriented treatments for older adults: results of a survey of experts. Alzheimers Dement (N Y). (2020) 6:e12024. doi: 10.1002/trc2.12024
25. Kueider AM, Parisi JM, Gross AL, Rebok GW. Computerized cognitive training with older adults: a systematic review. PLoS One. (2012) 7:e40588. doi: 10.1371/journal.pone.0040588
26. Peretti A, Amenta F, Tayebati SK, Nittari G, Mahdi SS. Telerehabilitation: review of the state-of-the-art and areas of application. JMIR Rehabil Assist Technol. (2017) 4:e7. doi: 10.2196/rehab.7511
27. Rogante M, Grigioni M, Cordella D, Giacomozzi C. Ten years of telerehabilitation: a literature overview of technologies and clinical applications. NeuroRehabilitation. (2010) 27:287–304. doi: 10.3233/NRE-2010-0612
28. Topol E. The Topol Review: Preparing the Healthcare Workforce to Deliver the Digital Future. Health Education England (2019).
29. World Health Organization. Telemedicine: Opportunities and Developments in Member States. Report on the Second Global Survey on eHealth (2010).
30. Di Tella S, Pagliari C, Blasi V, Mendozzi L, Rovaris M, Baglio F. Integrated telerehabilitation approach in multiple sclerosis: a systematic review and meta-analysis. J Telemed Telecare. (2020) 26:385–99. doi: 10.1177/1357633X19850381
31. Cotelli M, Manenti R, Brambilla M, Gobbi E, Ferrari C, Binetti G, Cappa SF. Cognitive telerehabilitation in mild cognitive impairment, Alzheimer's disease and frontotemporal dementia: a systematic review. J Telemed Telecare. (2019) 25:67–79. doi: 10.1177/1357633X17740390
32. How TV, Hwang AS, Green REA, Mihailidis A. Envisioning future cognitive telerehabilitation technologies: a co-design process with clinicians. Disabil Rehabil Assist Technol. (2017) 12:244–61. doi: 10.3109/17483107.2015.1129457
33. Dodakian L, McKenzie AL, Le V, See J, Pearson-Fuhrhop K, Burke Quinlan E, et al. A home-based telerehabilitation program for patients with stroke. Neurorehabil Neural Repair. (2017) 31:923–33. doi: 10.1177/1545968317733818
34. Realdon O, Rossetto F, Nalin M, Baroni I, Cabinio M, Fioravanti R, et al. Technology-enhanced multi-domain at home continuum of care program with respect to usual care for people with cognitive impairment: the Ability-TelerehABILITation study protocol for a randomized controlled trial. BMC Psychiatry. (2016) 16:425. doi: 10.1186/s12888-016-1132-y
35. Matamala-Gomez M, Maisto M, Montana JI, Mavrodiev PA, Baglio F, Rossetto F, et al. The role of engagement in teleneurorehabilitation: a systematic review. Front Neurol. (2020) 11:354. doi: 10.3389/fneur.2020.00354
36. Isernia S, Pagliari C, Jonsdottir J, Castiglioni C, Gindri P, Gramigna C, et al. Efficiency and patient-reported outcome measures from clinic to home: the human empowerment aging and disability program for digital-health rehabilitation. Front Neurol. (2019) 10:1206. doi: 10.3389/fneur.2019.01206
37. Suter P, Suter WN, Johnston D. Theory-based telehealth and patient empowerment. Popul Health Manag. (2011) 14:87–92. doi: 10.1089/pop.2010.0013
38. Oh SJ, Seo S, Lee JH, Song MJ, Shin MS. Effects of smartphone-based memory training for older adults with subjective memory complaints: a randomized controlled trial. Aging Ment Health. (2018) 22:526–34. doi: 10.1080/13607863.2016.1274373
39. Burton RL, O'Connell ME. Telehealth rehabilitation for cognitive impairment: randomized controlled feasibility trial. JMIR Res Protoc. (2018) 7:e43. doi: 10.2196/resprot.9420
40. Vermeij A, Claassen JA, Dautzenberg PL, Kessels RP. Transfer and maintenance effects of online working-memory training in normal ageing and mild cognitive impairment. Neuropsychol Rehabil. (2016) 26:783–809. doi: 10.1080/09602011.2015.1048694
41. Pereira-Morales AJ, Cruz-Salinas AF, Aponte J, Pereira-Manrique F. Efficacy of a computer-based cognitive training program in older people with subjective memory complaints: a randomized study. Int J Neurosci. (2018) 128:1–9. doi: 10.1080/00207454.2017.1308930
42. Kumar S, Tran J, Moseson H, Tai C, Glenn JM, Madero EN, et al. The impact of the virtual cognitive health program on the cognition and mental health of older adults: pre-post 12-month pilot study. JMIR Aging. (2018) 1:e12031. doi: 10.2196/12031
43. Anguera JA, Boccanfuso J, Rintoul JL, Al-Hashimi O, Faraji F, Janowich J, et al. Video game training enhances cognitive control in older adults. Nature. (2013) 501:97–101. doi: 10.1038/nature12486
44. Jessen F, Amariglio RE, van Boxtel M, Breteler M, Ceccaldi M, Chételat G, et al. A conceptual framework for research on subjective cognitive decline in preclinical Alzheimer's disease. Alzheimers Dement. (2014) 10:844–52. doi: 10.1016/j.jalz.2014.01.001
45. Petersen RC, Roberts RO, Knopman DS, Boeve BF, Geda YE, Ivnik RJ, et al. Mild cognitive impairment: ten years later. Arch Neurol. (2009) 66:1447–55. doi: 10.1001/archneurol.2009.266
46. Petersen RC, Smith GE, Waring SC, Ivnik RJ, Tangalos EG, Kokmen E. Mild cognitive impairment: clinical characterization and outcome. Arch Neurol. (1999) 56:303–8. doi: 10.1001/archneur.56.3.303
47. Yaffe K, Petersen RC, Lindquist K, Kramer J, Miller B. Subtype of mild cognitive impairment and progression to dementia and death. Dement Geriatr Cogn Disord. (2006) 22:312–9. doi: 10.1159/000095427
Keywords: cognitive, telerehabilitation, tele-enhancement, healthy older adults, subjective memory complaints
Citation: Alaimo C, Campana E, Stoppelli MR, Gobbi E, Baglio F, Rossetto F, Binetti G, Zanetti O, Manenti R and Cotelli M (2021) Cognitive Tele-Enhancement in Healthy Older Adults and Subjects With Subjective Memory Complaints: A Review. Front. Neurol. 12:650553. doi: 10.3389/fneur.2021.650553
Received: 07 January 2021; Accepted: 03 June 2021;
Published: 05 July 2021.
Edited by:
Nicola Smania, University of Verona, ItalyReviewed by:
Renata Avila, University of São Paulo, BrazilAlana Xavier Batista, University of São Paulo, Brazil
Copyright © 2021 Alaimo, Campana, Stoppelli, Gobbi, Baglio, Rossetto, Binetti, Zanetti, Manenti and Cotelli. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Rosa Manenti, cm1hbmVudGlAZmF0ZWJlbmVmcmF0ZWxsaS5ldQ==
†These authors have contributed equally to this work
 Cristina Alaimo
Cristina Alaimo Elena Campana
Elena Campana Maria Rachele Stoppelli1
Maria Rachele Stoppelli1 Elena Gobbi
Elena Gobbi Francesca Baglio
Francesca Baglio Federica Rossetto
Federica Rossetto Orazio Zanetti
Orazio Zanetti Rosa Manenti
Rosa Manenti Maria Cotelli
Maria Cotelli